Abstract
Brain amyloid-β (Aβ) peptide accumulation and aggregation are critical events in the pathogenesis of Alzheimer disease. Increasing evidence has demonstrated that LRP1 is involved in Alzheimer disease pathogenesis. The physiological ligands of LRP1, including apoE, play significant roles in the cellular clearance of Aβ. The receptor-associated protein (RAP) is a specialized chaperone for members of the low density lipoprotein receptor family. RAP shares structural and receptor-binding properties with apoE. Here, we show that RAP binds to both Aβ40 and Aβ42 in a concentration-dependent manner and forms complexes with them. Fluorescence-activated cell sorter analysis showed that RAP significantly enhances the cellular internalization of Aβ in different cell types, including brain vascular smooth muscle, neuroblastoma, glioblastoma, and Chinese hamster ovary cells. This effect of RAP was confirmed by fluorescence microscopy and enzyme-linked immunosorbent assay. RAP binds to both LRP1 and heparin; however, the ability of RAP to enhance Aβ cellular uptake was blocked by heparin and heparinase treatment but not by LRP1 deficiency. Furthermore, the effects of RAP were significantly decreased in heparan sulfate proteoglycan-deficient Chinese hamster ovary cells. Our findings reveal that RAP is a novel Aβ-binding protein that promotes cellular internalization of Aβ.
INTRODUCTION
Increasing evidence demonstrates that members of the low density lipoprotein receptor (LDLR)2 family play important roles in the pathogenesis of Alzheimer disease (AD). These receptors play roles in the metabolism of the amyloid precursor protein (APP) and amyloid-β (Aβ) peptides (1–3). The accumulation and aggregation of Aβ cleaved from APP in the brain are thought to be the critical steps in the pathogenesis of AD (1–3). In particular, LRP1 (LDLR-related protein 1) facilitates APP endocytic trafficking and processing to Aβ (4–6). LRP1 also mediates the metabolism of Aβ in neurons (7), glial cells (8, 9), and brain vessels (10–12). The physiological ligands of LRP1, in particular apoE and α2-macroglobulin, function as Aβ chaperones and facilitate Aβ clearance (1, 13).
The 39-kDa receptor-associated protein (RAP) is a specialized chaperone for members of the LDLR family, including LRP1 (14, 15). Whereas most other endoplasmic reticulum (ER) chaperones function primarily in substrate folding, RAP functions in both receptor folding (16) and subsequent trafficking (14) by blocking premature ligand binding during receptor maturation. RAP binds to LRP1 at multiple sites (14, 15) with high affinity (KD = 1–10 nm) (17). It also has been used extensively as an antagonist for LRP1. RAP was initially discovered as a protein that co-purifies with LRP1 from human placenta (18, 19). Structurally, human RAP is composed of 323 amino acids and has three independent domains (14) connected by flexible linkers (20). The C-terminal domain (domain 3) of RAP mediates folding and trafficking of receptors, whereas the N-terminal domains (domains 1 and 2) control the binding of certain ligands to receptors (21). The N-terminal domains of RAP and apoE both contain a four-helix bundle that includes a receptor-binding site, whereas their C-terminal domains are flexible (22). Additionally, both RAP and apoE contain clusters of basic amino acid residues that mediate binding to both receptors and heparin (23–26). Thus, RAP shares structural and receptor-binding properties with apoE.
In our studies of the cellular metabolism of Aβ, we noticed that RAP facilitates Aβ uptake. We hypothesized that RAP is a novel Aβ-binding protein. In this study, we tested potential interaction Aβ and RAP in vitro. We also analyzed the effect of exogenous RAP on Aβ cellular uptake using human brain vascular smooth muscle cells (HBVSMC), mouse neuroblastoma N2a cells, human glioblastoma U87 cells, and human neuroblastoma SH-SY5Y cells because these cells have been demonstrated to play roles in Aβ cellular uptake (7, 8, 10). We found that RAP interacts with Aβ and promotes its cellular uptake and that cell-surface heparan sulfate proteoglycan (HSPG) might mediate this function.
EXPERIMENTAL PROCEDURES
Materials
Aβ40, Aβ42, and 5(6)-carboxyfluorescein (FAM)-labeled Aβ40 were purchased from AnaSpec (San Jose, CA). Aβ peptides were dissolved in dimethyl sulfoxide at 200 μm and kept in −80 °C before use. Recombinant RAP and Mesd (mesoderm development) were produced and purified as described previously (27, 28).
Gel Filtration Chromatography
Aβ40 (500 nm) was incubated in phosphate-buffered saline (PBS) with or without 2.5 μm RAP for 2 h at 37 °C. Samples (500 μl) were injected onto a Superdex 75 10/30 HR column (GE Healthcare) attached to a fast protein liquid chromatography system with 50 mm ammonium acetate (pH 8.5) as the elution buffer. Fractions of 500 μl each were collected at a flow rate of 0.5 ml/min, and the concentration of Aβ within each fraction was determined by sandwich enzyme-linked immunosorbent assay (ELISA) using anti-Aβ40 antibody 2G3 for capturing and biotin-conjugated antibody 3D6 for detection (29). Additionally, some fractions were analyzed by 12.5% SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride-nitrocellulose membrane (Immobilon-P) for Western blot analysis using anti-Aβ antibody 6E10 or anti-RAP antibody.
Detection of the Aβ·RAP Complex
Aβ (1 μm) was incubated with or without RAP (100–1000 nm) for 1 h at 37 °C in PBS. For cross-linking of Aβ with RAP, 4 μl of glutaraldehyde (0.625% diluted from a 25% stock) was added to samples (40 μl), followed by the addition of 11 μl of NaBH4 (6.6 mg/ml in 0.1 m NaOH) to stabilize the Schiff base formed between the aldehyde and amines. After incubation for 10 min at room temperature, 20 μl of SDS-PAGE sample buffer containing 10% 2-mercaptoethanol and 20% sucrose was added (30). Samples were separated by 12.5% SDS-PAGE and Western-blotted using anti-Aβ antibody 6E10 or anti-RAP antibody.
Cell Culture
HBVSMC were purchased from ScienCell (Carlsbad, CA) and cultured under standard culture conditions according to the manufacturer's protocol. Wild-type mouse embryonic fibroblast (MEF) cells (MEF1), LRP1-deficient MEF cells (MEF2), human glioblastoma U87 cells, and human neuroblastoma SH-SY5Y cells were cultured in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum and maintained at 37 °C in humidified air containing 5% CO2 as described previously (31–34). Mouse neuroblastoma N2a cells were cultured in DMEM and Opti-MEM I (1:1) containing 5% fetal bovine serum. All Chinese hamster ovary (CHO) cell lines were grown in Ham's F-12 medium containing 10% fetal bovine serum, 100 units/ml penicillin G, and 100 μg/ml streptomycin sulfate (35).
Detection of Cell-associated Aβ by ELISA
Cells were plated onto 24-well dishes and allowed to grow to 90% confluency. Cells were incubated with Aβ40 or Aβ42 (100 nm) for 1 h at 37 °C in serum-free DMEM. After washing three times with PBS, cells were dissolved in 5 m guanidine in 50 mm Tris-HCl (pH 8.0). Samples were diluted 10-fold in DMEM and analyzed by sandwich ELISA for human Aβ40 (antibody 2G3) and human Aβ42 (antibody 21F12), both detected with biotin-conjugated antibody 3D6.
Aβ Cellular Internalization
Cells were culture on the glass bottom culture dishes (MatTek Corp., Ashland, MA) at 37 °C for at least 24 h before experiments. After incubation with FAM-labeled Aβ40 (500 nm) at 37 °C for 4 h in serum-free DMEM in the presence or absence of RAP (500 nm), the fluorescence of Aβ40 was observed by confocal laser scanning fluorescence microscopy (Model LSM 510 inverted microscope, Carl Zeiss, Jena, Germany) at 488-nm argon excitation using a 510–535-nm bandpass barrier filter.
Knockdown of LRP1 by Small Interfering RNA (siRNA)
Knockdown of LRP1 was performed as described previously (36). Single-stranded, LRP1-specific, sense and antisense RNA oligonucleotides were synthesized by Ambion (Austin, TX). Double-stranded RNA molecules were generated according to the manufacturer's instructions. Cells were transfected with siRNA (120 nm) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's specifications and used for analysis 48 h after transfection.
Fluorescence-activated Cell Sorter (FACS)-based Aβ Internalization Assay
Cells were plated onto 12-well dishes and allowed to grow to 90% confluency. Cells were incubated with FAM-labeled Aβ40 (500 nm) in serum-free DMEM in the presence of nonimmune rabbit IgG or RAP (500 nm). In some experiments, various concentrations of heparin (Elkins-Sinn Inc., Cherry Hill, NJ) and heparinase I (Sigma) were used. Cells were washed three times with PBS and then removed from the plate using cell dissociation solution (Sigma). Cells were centrifuged at 1400 × g for 2 min, resuspended in 100 μl of PBS containing Pronase (0.5 mg/ml; Roche Applied Science), and incubated at 4 °C for 30 min. Cells were washed and resuspended in 300 μl of PBS containing 1.5% fetal bovine serum, 1% NaN3, and 1% paraformaldehyde. Cells (1 × 104) from each sample were analyzed for fluorescence on a FACSCalibur machine (BD Biosciences). Cells without any exposure to FAM-labeled Aβ were used as a control for background fluorescence.
Statistical Analysis
All quantified data represent an average of triplicate samples. Error bars represent S.D. Statistical significance was determined by Student's t test, and p < 0.05 was considered significant.
RESULTS
RAP Binds to Aβ Peptides in Vitro
To examine whether RAP is a binding protein for Aβ, RAP (2.5 μm) was incubated for 2 h at 37 °C with Aβ40 (500 nm) in PBS, and the resulting mixture was subjected to gel filtration chromatography with a Superdex 75 column run on a fast protein liquid chromatography system. After incubation with RAP, Aβ detected by ELISA appeared to separate into two distinct peaks, which corresponded to the void and elution volumes of free Aβ (Fig. 1A, peaks a and b, respectively). In contrast, when Aβ was incubated with buffer alone, only a single peak was detected, which corresponded to the elution volume of free Aβ (Fig. 1A, peak b). To analyze the molecular nature of the Aβ peaks, aliquots of peak fractions were subjected to SDS-PAGE and Western blotting. After column chromatography of the Aβ/RAP mixture, a band was detected by anti-Aβ antibody 6E10 in the void volume peak, consistent with the results from ELISA (Fig. 1B). Although the molecular size of Aβ in the void volume peak (peak a) was the same as that in the elution volume peak (peak b), Western blotting for RAP showed that the void volume peak indeed contained RAP. These results suggest that RAP interacts with Aβ in solution to form molecular complexes; however, these complexes might be separated during SDS-PAGE. To confirm the binding of RAP to Aβ, we incubated Aβ40 or Aβ42 (1 μm) with various concentrations of RAP (100, 200, 500, and 1000 nm) for 1 h at 37 °C in PBS and examined the extent of Aβ·RAP complex formation by Western blotting after cross-linking with glutaraldehyde (Fig. 2). Western blotting for Aβ detected not only an Aβ monomer band but also a new band between the 37- and 50-kDa markers, consistent with the molecular size of the Aβ·RAP complex. The formation of the band depended on the RAP concentration after incubation with Aβ40 (Fig. 2A) or Aβ42 (Fig. 2B). Next, we confirmed the existence of Aβ·RAP complexes by Western blotting for Aβ and RAP (Fig. 2C). RAP (1 μm) was incubated with or without Aβ40 (1 μm) for 1 h at 37 °C in PBS, followed by cross-linking with glutaraldehyde and Western blotting for Aβ. The same membrane was analyzed using anti-RAP antibody after reblotting (Fig. 2C, right panel). Although Western blotting for Aβ showed two bands in the Aβ/RAP mixture sample, no band was detected in the RAP-alone sample (Fig. 2C, left panel), indicating that anti-Aβ antibody does not recognize RAP. On the other hand, anti-RAP antibody recognized the upper RAP band (37–50 kDa) but not the lower Aβ band (Fig. 2C, left panel). These results indicate that RAP forms complexes with both Aβ40 and Aβ42 in a concentration-dependent manner. The Aβ·RAP complex detected by anti-Aβ antibody was less abundant than the same complex detected by anti-RAP antibody. This might be due to the difference in sensitivity of these antibodies.
FIGURE 1.
Interaction between RAP and Aβ. A, Aβ40 (500 nm) was incubated with either 2.5 μm RAP (●) or buffer alone (○) for 2 h at 37 °C and subjected to Superdex 75 gel filtration chromatography. The concentrations of Aβ in every other fraction were determined by ELISA. B, selected fractions after fast protein liquid chromatography were subjected to 12.5% SDS-PAGE and analyzed by Western blotting (WB) for Aβ and RAP. Arrows a and b indicate the first and second peaks from A, respectively.
FIGURE 2.
Complex formation between RAP and Aβ. Aβ40 (1000 nm; A) and Aβ42 (1000 nm; B) were incubated with increasing concentrations of RAP (50, 100, 200, 500, and 1000 nm) for 1 h at 37 °C in PBS. Samples were analyzed by 12.5% SDS-PAGE after cross-linking with glutaraldehyde, followed by Western blotting (WB) for Aβ. C, the Aβ·RAP complex was detected by Western blotting for Aβ and RAP. RAP (1 μm) was incubated with or without 1 μm Aβ40 for 1 h at 37 °C in PBS, and the sample was analyzed after cross-linking with glutaraldehyde, followed by Western blotting for Aβ (left panel). The same membrane was analyzed using anti-RAP antibody after reblotting (right panel).
RAP Enhances Aβ Association with HBVSMC
To investigate whether RAP enhances Aβ cellular uptake, we used HBVSMC. Aβ deposition is often detected in the smooth muscle layer of the small artery as cerebral amyloid angiopathy in AD patients, and smooth muscle cells have been demonstrated to internalize Aβ in vitro (10). Aβ40 or Aβ42 (100 nm) was added to HBVSMC in culture in the presence of RAP at various concentrations (50–1000 nm), and the amount of cell-associated Aβ was analyzed by ELISA after incubation for 1 h at 37 °C. When cells were incubated with 100 nm Aβ40 for 1 h at 37 °C, 0.3% of the added Aβ40 was associated with HBVSMC. After incubation of Aβ40 with RAP, the association of Aβ with cells was increased in a concentration-dependent manner (Fig. 3A). Similar results were obtained for Aβ42 (Fig. 3A). Cell-associated Aβ40 and Aβ42 in the presence of RAP at 500 nm were increased to 2.4- and 2.0-fold of the levels without RAP, respectively (Fig. 3B). These results indicate that RAP enhances the association of both Aβ40 and Aβ42 with HBVSMC in a concentration-dependent manner.
FIGURE 3.
Increase in cell-associated Aβ by RAP in brain vascular smooth muscle cells. A, effect of RAP on the cellular association of Aβ in HBVSMC. Cells were incubated with Aβ40 (100 nm) or Aβ42 (100 nm) in the absence or presence of increasing concentrations of RAP (50, 100, 200, 500, and 1000 nm) for 1 h at 37 °C in serum-free DMEM. The amounts of cell-associated Aβ40 (○) and Aβ42 (●) were measured by ELISA. B, amounts of cell-associated Aβ40 and Aβ42 in HBVSMC measured by ELISA. Cells were incubated with Aβ40 (100 nm) or Aβ42 (100 nm) in the absence (gray bars) or presence (black bars) of 500 nm RAP. Error bars represent the mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01 (significantly different from the amount of cell-associated Aβ without RAP).
RAP Enhances Aβ Internalization by HBVSMC
To confirm the ability of RAP to promote Aβ internalization, HBVSMC were cultured with fluorescently labeled Aβ40 and analyzed by confocal laser scanning microscopy. When cells were incubated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C, Aβ40 was detected in the intracellular space in a punctate pattern, likely in endosomal and/or lysosomal compartments (Fig. 4, A and B), indicating that HBVSMC can be used as an in vitro model to study Aβ internalization. In the presence of 500 nm RAP, the internalization of FAM-labeled Aβ40 was significantly increased (Fig. 4, C and D). We found that RAP had no effect on the uptake of fluorescently labeled transferrin, used as a control (supplemental Fig. 1). These results indicate that RAP enhances the internalization of Aβ40 in HBVSMC.
FIGURE 4.
Enhancement of Aβ cellular uptake by RAP in brain vascular smooth muscle cells. The effect of RAP on the cellular uptake of Aβ in HBVSMC was analyzed using confocal laser scanning microscopy. HBVSMC were treated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in serum-free DMEM. Aβ was detected in the intracellular space in a punctate pattern (A and B). In the presence of 500 nm RAP, the cellular uptake of Aβ was significantly enhanced (C and D). The upper and lower panels are FAM-labeled Aβ40 and merged images with Nomarski images, respectively. Scale bars = 20 μm.
Next, FACS was used to quantify the amount of internalized Aβ40 in HBVSMC. Cells were incubated with FAM-labeled Aβ, treated with Pronase to remove cell-surface Aβ, and then analyzed for fluorescence intensity by FACS. FACS analysis revealed that the peak of fluorescence intensity of cells was shifted to the right after incubation with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in the presence of 500 nm nonimmune rabbit IgG as a control (Fig. 5A, upper panel), indicating that cell fluorescence intensity was increased by endocytosed FAM-labeled Aβ40. Although RAP itself did not show any effect on cell fluorescence intensity, the peak of fluorescence intensity in the presence of 500 nm RAP was shifted further to the right after incubation with 500 nm FAM-labeled Aβ40 (Fig. 5A, lower panel), indicating that RAP enhances the internalization of Aβ. When the changes in mean cell fluorescence intensity were quantified, cell-internalized Aβ40 in the presence of RAP (500 nm) was significantly increased to 5.6-fold of that in the presence of IgG, whereas the LRP5/LRP6-specific chaperone Mesd (28) did not show any significant effect to enhance the cellular uptake of Aβ (Fig. 5B). Together, these results clearly indicate that RAP enhances the internalization of Aβ in HBVSMC.
FIGURE 5.
Increase in Aβ internalization by RAP in brain vascular smooth muscle cells. The effects of RAP on the internalization of Aβ in HBVSMC were analyzed by FACS. HBVSMC were incubated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in serum-free DMEM in the presence of nonimmune rabbit IgG, Mesd, or RAP (500 nm). A, shown are histograms of cell fluorescence intensity after incubation with or without FAM-labeled Aβ40 in the presence of IgG (upper panel), Mesd (middle panel), or RAP (lower panel). The x axis represents relative fluorescence intensity, and the y axis represents cell number. AU, arbitrary units. B, the change in mean cell fluorescence intensity from background fluorescence was quantified as the amount of internalized FAM-labeled Aβ40. The bars indicate the amount of internalized Aβ in HBVSMC after incubation with IgG, Mesd, or RAP. Error bars represent the mean ± S.D. (n = 3). N.S., not significant; **, p < 0.01 (significantly different from the amount of Aβ after incubation with IgG as a control).
RAP Enhances Aβ Internalization in N2a and U87 Cells
To investigate the function of RAP in Aβ internalization in other cell types, we used the mouse neuroblastoma cell line N2a and the human glioblastoma cell line U87. Neurons and glial cells, as well as smooth muscle cells, are known to internalize Aβ (7, 8). N2a cells were incubated with 500 nm FAM-labeled Aβ40 for 24 h at 37 °C in the presence of 500 nm RAP or IgG as a control, and the uptake of Aβ was assessed by FACS. Aβ internalization was significantly (9.3-fold) higher in the presence of RAP compared with the control (Fig. 6A). In the case of U87 cells, although RAP significantly increased Aβ uptake to 1.4-fold of the control (Fig. 6B), the extent of this increase was lower compared with HBVSMC and N2a cells. These results indicate that RAP can enhance Aβ cellular uptake in neuronal cells, glial cells, and smooth muscle cells; however, the ability of RAP to promote Aβ uptake varies depending on the cell type.
FIGURE 6.

Increase in Aβ internalization by RAP in neuronal cells and glial cells. Mouse neuroblastoma N2a (A) and human glioblastoma U87 (B) cells were incubated with FAM-labeled Aβ40 (500 nm) at 37 °C in serum-free DMEM in the presence or absence of RAP (500 nm). N2a and U87 cells were incubated for 24 and 4 h, respectively. Error bars represent the mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01 (significantly different from the amount of Aβ after incubation with IgG as a control).
RAP Mediates the Cellular Uptake of Aβ in an LRP1-independent Manner
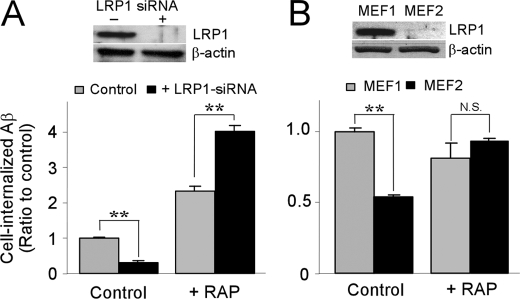
LRP1 is a major Aβ-scavenging receptor (1), and RAP has a high affinity for LRP1. Therefore, we next investigated whether LRP1 is involved in the function of RAP to enhance Aβ cellular uptake. To analyze the role of LRP1 in RAP-mediated Aβ internalization, HBVSMC were transfected with LRP1 siRNA using Lipofectamine 2000 and used for analysis 48 h after transfection. The expression of LRP1 was significantly suppressed by siRNA (Fig. 7A, inset). LRP1-suppressed HBVSMC were incubated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in the presence of 500 nm RAP or IgG as a control, and the internalization of Aβ was assessed by FACS. Knockdown of LRP1 suppressed Aβ internalization to 30% of that in HBVSMC treated with the vehicle Lipofectamine 2000 (Fig. 7A). However, RAP further enhanced Aβ internalization in LRP1-deficient HBVSMC compared with HBVSMC treated with Lipofectamine 2000 (Fig. 7A). These results indicate that RAP increases Aβ cellular uptake in an LRP1-independent manner.
FIGURE 7.
RAP promotes Aβ cellular uptake independently of LRP1. A, HBVSMC without (gray bars) or with (black bars) LRP1 knockdown by siRNA were incubated with FAM-labeled Aβ40 (500 nm) for 4 h at 37 °C in serum-free DMEM in the presence or absence of RAP (500 nm).Western blotting (inset) showed that LRP1 expression was effectively suppressed by siRNA transfection. B, wild-type MEF1 (gray bars) and LRP1-deficient MEF2 cells (black bars) were incubated with FAM-labeled Aβ40 (500 nm) for 4 h at 37 °C in serum-free DMEM in the presence of nonimmune rabbit IgG or RAP (500 nm). Western blotting (inset) showed that LRP1 was completely deficient in MEF2 cells. Error bars represent the mean ± S.D. (n = 3). N.S., not significant; **, p < 0.01 (significantly different from the amount of Aβ after incubation with IgG as a control).
To further examine the role of LRP1 in RAP-promoted Aβ uptake, we next used wild-type MEF1 and LRP1-deficient MEF2 cells (37, 38). MEF1 or MEF2 cells were incubated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in the presence of 500 nm RAP or IgG as a control, and the uptake of Aβ was assessed by FACS. Aβ internalization in MEF2 cells was decreased to 54% of that in MEF1 cells (Fig. 7B). Although RAP showed no enhancement of Aβ internalization in MEF1 cells, Aβ internalization in MEF2 cells was significantly (1.7-fold) higher in the presence of RAP compared with the control (Fig. 7B). These results further confirm that RAP increases Aβ cellular uptake in an LRP1-independent manner.
HSPG Is Involved in the RAP-mediated Cellular Uptake of Aβ
RAP has a high affinity for heparin (24). Therefore, we next investigated whether HSPG is involved in the function of RAP to enhance Aβ cellular uptake. To analyze the role of HSPG in RAP-mediated Aβ internalization, HBVSMC and SH-SY5Y cells were incubated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in the presence of 500 nm RAP with or without heparin, and the internalization of Aβ was assessed by FACS. Heparin (15 units/ml = 100 μg/ml) did not interfere with the interaction between RAP and Aβ40 (Fig. 8A). Although RAP significantly increased Aβ internalization in both HBVSMC (Fig. 8B) and SH-SY5Y cells (Fig. 8C), heparin suppressed the function of RAP in a concentration-dependent manner (Fig. 8D). In addition, heparinase treatment also significantly decreased the function of RAP (Fig. 8E). These results suggest that RAP increases Aβ cellular uptake in an HSPG-dependent manner.
FIGURE 8.
Heparin inhibits RAP function to promote Aβ cellular uptake. A, Aβ40 (1 μm) was incubated with or without RAP (1 μm) in the presence of heparin (15 units/ml = 100 μg/ml) for 1 h at 37 °C in PBS and analyzed by 12.5% SDS-PAGE after cross-linking with glutaraldehyde, followed by Western blotting for Aβ. HBVSMC (B) and SH-SY5Y cells (C) were incubated with FAM-labeled Aβ40 (500 nm) with or without RAP (500 nm) for 4 h at 37 °C in serum-free DMEM in the presence or absence of heparin (15 units/ml). **, p < 0.01 (significantly different from the amount of Aβ after incubation with IgG as a control). D, various concentrations of heparin (0.15 milliunits/ml to 15 units/ml) were used in HBVSMC (○) and SH-SY5Y cells (●) for incubation with FAM-labeled Aβ40 (500 nm) plus RAP (500 nm). E, cells were incubated with heparinase I (5 units/ml) for 2 h at 37 °C in serum-free DMEM, and internalization of Aβ was analyzed in the presence of RAP. Error bars represent the mean ± S.D. (n = 3). **, p < 0.01 (significantly different from the amount of RAP-mediated Aβ internalization without heparinase treatment as a control).
To further examine the role of HSPG in RAP-promoted Aβ uptake, we next used wild-type CHO-K1 cells, CHO-M1 cells deficient in N-acetylglucosaminyltransferase/glucuronyltransferase (required for heparan sulfate (HS) biosynthesis) (39), and CHO-745 cells deficient in xylosyltransferase (required for both HS and chondroitin sulfate biosynthesis). CHO cells (40) were incubated with 500 nm FAM-labeled Aβ40 for 4 h at 37 °C in the presence of 500 nm RAP, and the uptake of Aβ was assessed by FACS. Aβ internalization was significantly (2.4-fold) higher in the presence of RAP compared with the control in CHO-K1 cells (Fig. 9). However, the function of RAP in Aβ internalization in CHO-M1 and CHO-745 cells was suppressed to 23 and 13% of that in CHO-K1 cells, respectively (Fig. 9A). Heparin suppressed the function of RAP in CHO-K1 cells in a concentration-dependent manner but showed no effect in CHO-M1 cells (Fig. 9B). These results strongly suggest that proteoglycan may mediate the function of RAP to increase Aβ cellular uptake. The difference between CHO-M1 and CHO-745 cells was small compared with that between CHO-K1 and CHO-M1 cells, indicating that HSPG may be the predominant cell-surface proteoglycan that mediates RAP function.
FIGURE 9.
RAP promotes Aβ cellular uptake through HSPG. A, CHO-K1 (wild-type), CHO-M1 (HS-deficient), and CHO-745 (HS- and chondroitin sulfate-deficient) cells were incubated with FAM-labeled Aβ40 (500 nm) for 4 h at 37 °C in serum-free DMEM in the presence RAP (500 nm). **, p < 0.01 (significantly different from the amount of internalized Aβ in CHO-K1 cells after incubation with IgG as a control). B, various concentrations of heparin (0.15 milliunits/ml to 15 units/ml) were used in CHO-K1 (●) and CHO-M1 (○) cells for incubation with FAM-labeled Aβ40 (500 nm) plus RAP (500 nm). Error bars represent the mean ± S.D. (n = 3).
DISCUSSION
AD is a progressive neurodegenerative disorder in which the aggregation and deposition of Aβ are central for its pathogenesis (1–3). Aβ is cleaved from APP in the ER, trans-Golgi network, plasma membrane, and endosome and then secreted to the extracellular space (41–43). It has been suggested that the cell quality control mechanisms by chaperones prevent intracellular aggregation and deposition of Aβ in the healthy brain. Although several cytosolic chaperones (44, 45) bind to Aβ in vitro, they are predicted to rarely interact with Aβ under physiological conditions. In this study, we revealed that an ER chaperone, RAP, binds to both Aβ40 and Aβ42 and forms complexes with them. Under physiological conditions, RAP functions as a chaperone within the early compartments of the secretary pathway. Immunoelectron analysis revealed that RAP localizes mainly within the ER (70%) and early Golgi compartments (24%) and less frequently on the cell surface or within compartments of the endocytic pathway (14, 31). Because RAP possesses an ER retention signal sequence (HNEL), it is efficiently retrieved from the Golgi back to the ER (14, 46). However, overexpression of RAP has been shown to induce the saturation of the retrieval system, resulting in the secretion of RAP into the extracellular space (47). Thus, it may be possible that RAP could be secreted when its expression is up-regulated under certain physiological or pathological conditions such as AD, suggesting that RAP might encounter Aβ in the extracellular space. Pandey et al. (48) showed that a polymorphism of the LRPAP1 gene encoding RAP protein is involved in the susceptibility to degenerative dementias. The frequency of the DD genotype and D allele of the LRPAP1 gene was significantly higher in degenerative dementias than in controls. The odds ratios for the risk of degenerative dementia were estimated to 3.76 for the DD genotype and 2.80 for the D allele (48). Additional studies are necessary to confirm this result; however, it suggests that RAP likely plays some roles in AD pathogenesis.
RAP might also play roles in APP folding, trafficking, and/or processing (49). The ability of RAP to bind Aβ suggests that RAP might also bind to APP and regulate its trafficking and processing. Additionally, RAP regulates the maturation and trafficking of LRP1, facilitating APP endocytic trafficking and processing to Aβ (50). Therefore, a RAP dysfunction may lead to a disturbance in APP and Aβ metabolism, resulting in Aβ deposition. Consistent with this hypothesis, when RAP-deficient mice were crossed with human APP transgenic mice, the amount of Aβ deposition was increased (51), suggesting a central role for RAP in Aβ metabolism and AD pathogenesis. Recently, Xu et al. (52) revealed that partial reductions in RAP enhance Aβ deposition in the APPswe/PS1dE9 model of AD. Because partial RAP deficiency did not significantly affect the levels of LRP1 and SorLA/LR11, RAP may directly regulate APP and/or Aβ metabolism in vivo.
In addition to the ability of RAP to bind Aβ, we demonstrated that exogenous RAP enhances the cellular uptake of Aβ in different cell types, including HBVSMC, neuroblastoma cells, glioblastoma cells, and CHO cells. RAP is widely used to antagonize ligand interactions with members of the LDLR family (15). In several studies, RAP was used to antagonize LRP1-mediated Aβ uptake (53, 54). Although these studies focused on endothelial cells and used low concentrations of Aβ, their results are opposite from ours. They showed that RAP prevents Aβ uptake and clearance in vitro and in vivo. This discrepancy may be explained as follows. 1) The ability of RAP to regulate Aβ cellular uptake may be different depending on the cell type; 2) the effect of RAP may be different depending on the Aβ concentration; and 3) RAP may block LRP1-mediated Aβ uptake when the Aβ·LRP1 ligand complex is produced. Our results strongly indicate that RAP has to be used very cautiously as an antagonist of LRP1 when the cellular uptake of Aβ is investigated. Vascular smooth muscle cells, neurons, and glial cells can internalize Aβ (7, 8, 10), which is predicted to be mainly degraded. However, when the levels of internalized Aβ exceed the cellular capacity to degrade internalized Aβ, it may accumulate and deposit intracellularly, resulting in cell death. Therefore, we analyzed the cell toxicity of enhancement of Aβ uptake by RAP using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. We found no significant differences in cell viability in the presence or absence of RAP (supplemental Fig. 2), suggesting that RAP enhances the cellular clearance of Aβ without significant effects on cell viability under these experimental conditions. The accumulation of Aβ in the extracellular space is thought to trigger its aggregation and deposition, which contribute to AD and cerebral amyloid angiopathy. Therefore, the function of RAP to enhance the cellular clearance of Aβ might be protective against the pathogenesis of AD and cerebral amyloid angiopathy.
Unexpectedly, RAP enhances Aβ cellular uptake in an LRP1-independent manner. In LRP1-suppressed HBVSMC and LRP1-deficient MEF cells (MEF2), the effect of RAP was strongly detected. Moreover, RAP increased the internalization of Aβ in N2a cells, which express little LRP1 or LDLR (55). These findings suggest that the role of RAP in facilitating Aβ uptake is independent of the LDLR family members, despite the high affinities of RAP for them. Previous studies have demonstrated that RAP is a heparin-binding protein (24, 56). Three basic amino acid clusters (R282VSRSREK289, R203LRR206, and R314ISRAR319) within domain 3 of RAP contribute to high affinity binding to both RAP and LRP1 (24). HSPG is ubiquitously expressed on the cell surface in almost all mammalian cells (57). Therefore, we focused on RAP interaction with HSPG and demonstrated that HSPG is involved in the ability of RAP to promote Aβ cellular uptake. We found that the ability of RAP to promote Aβ uptake varies depending on the cell type. Analysis of HS from different mammalian tissues revealed that HS biosynthesis differs depending upon the tissue (58). The cell type discrepancies in RAP-mediated Aβ uptake may be due to the differences in the cell-specific HS structure. In addition, it is well known that interactions with the cell-surface HSPGs glypican and syndecan are required for the internalization of several cationic peptides (59). Glypican is glycosylphosphatidylinositol-anchored, and syndecan has a transmembrane domain (59). Among them, GPC1 (glypican-1) and SDC3 (syndecan-3) are major HSPGs in the adult brain (60, 61); therefore, we investigated the association of these HSPGs in RAP function. GPC1 and SDC3 in glial cells have been shown to be associated with Aβ deposits in AD (62). Although knockdown of GPC1 and SDC3 decreased the internalization of Aβ in HBVSMC in the absence of RAP, no inhibitory effect on RAP-mediated Aβ uptake was detected (supplemental Fig. 3). These results suggest that multiple HSPGs mediate the function of RAP. When one HSPG is suppressed, other HSPGs may compensate. The other possibility is that other HSPGs on the cell surface or HSPGs in the extracellular matrix (e.g. perlecan, agrin, and collagen XVIII) may be involved in RAP function. Further studies are needed to characterize HSPGs that mediate the RAP function.
In this study, we demonstrated that RAP binds to Aβ and enhances its cellular uptake. Because the structure of each domain of RAP has already been determined (20, 63, 64), it may be possible to develop novel RAP-mimicking drugs to increase the cellular uptake of Aβ for AD therapy. Our findings thus provide a new insight into the molecular mechanism of AD pathogenesis and a potential therapeutic strategy for AD.
Supplementary Material
Acknowledgment
We thank Dr. Lijuan Zhang for providing several cell lines for this study.
This work was supported, in whole or in part, by National Institutes of Health Grants AG027924 and AG031784 (to G. B.). This work was also supported by a Zenith Fellows award from the Alzheimer's Association (to G. B.) and by fellowships from the Uehara Memorial Foundation, the American Health Assistance Foundation, and the American Heart Association (to T. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- LDLR
- low density lipoprotein receptor
- AD
- Alzheimer disease
- APP
- amyloid precursor protein
- Aβ
- amyloid-β
- RAP
- receptor-associated protein
- ER
- endoplasmic reticulum
- HBVSMC
- human brain vascular smooth muscle cells
- HSPG
- heparin sulfate proteoglycan
- FAM
- 5(6)-carboxyfluorescein
- PBS
- phosphate-buffered saline
- ELISA
- enzyme-linked immunosorbent assay
- MEF
- mouse embryonic fibroblast
- DMEM
- Dulbecco's modified essential medium
- CHO
- Chinese hamster ovary
- siRNA
- small interfering RNA
- FACS
- fluorescence-activated cell sorter
- HS
- heparan sulfate.
REFERENCES
- 1.Bu G., Cam J., Zerbinatti C. (2006) Ann. N.Y. Acad. Sci. 1086, 35–53 [DOI] [PubMed] [Google Scholar]
- 2.Jaeger S., Pietrzik C. U. (2008) Curr. Alzheimer Res. 5, 15–25 [DOI] [PubMed] [Google Scholar]
- 3.Marzolo M. P., Bu G. (2009) Semin. Cell Dev. Biol. 20, 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulery P. G., Beers J., Mikhailenko I., Tanzi R. E., Rebeck G. W., Hyman B. T., Strickland D. K. (2000) J. Biol. Chem. 275, 7410–7415 [DOI] [PubMed] [Google Scholar]
- 5.Pietrzik C. U., Busse T., Merriam D. E., Weggen S., Koo E. H. (2002) EMBO J. 21, 5691–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cam J. A., Bu G. (2006) Mol. Neurodegener. 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu Z., Strickland D. K., Hyman B. T., Rebeck G. W. (1999) J. Neurochem. 73, 1393–1398 [DOI] [PubMed] [Google Scholar]
- 8.Wyss-Coray T., Loike J. D., Brionne T. C., Lu E., Anankov R., Yan F., Silverstein S. C., Husemann J. (2003) Nat. Med. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 9.Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Liao Z., Lieberburg I., Motter R., Mutter L., Soriano F., Shopp G., Vasquez N., Vandevert C., Walker S., Wogulis M., Yednock T., Games D., Seubert P. (1999) Nature 400, 173–177 [DOI] [PubMed] [Google Scholar]
- 10.Urmoneit B., Prikulis I., Wihl G., D'Urso D., Frank R., Heeren J., Beisiegel U., Prior R. (1997) Lab. Investig. 77, 157–166 [PubMed] [Google Scholar]
- 11.Kanekiyo T., Bu G. (2009) Future Neurol. 4, 55–65 [Google Scholar]
- 12.Bu G. (2009) Nat. Rev. Neurosci. 10, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zlokovic B. V. (2005) Trends Neurosci. 28, 202–208 [DOI] [PubMed] [Google Scholar]
- 14.Bu G., Geuze H. J., Strous G. J., Schwartz A. L. (1995) EMBO J. 14, 2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bu G., Marzolo M. P. (2000) Trends Cardiovasc. Med. 10, 148–155 [DOI] [PubMed] [Google Scholar]
- 16.Bu G., Rennke S. (1996) J. Biol. Chem. 271, 22218–22224 [DOI] [PubMed] [Google Scholar]
- 17.Iadonato S. P., Bu G., Maksymovitch E. A., Schwartz A. L. (1993) Biochem. J. 296, 867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashcom J. D., Tiller S. E., Dickerson K., Cravens J. L., Argraves W. S., Strickland D. K. (1990) J. Cell Biol. 110, 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., Argraves W. S. (1990) J. Biol. Chem. 265, 17401–17404 [PubMed] [Google Scholar]
- 20.Lee D., Walsh J. D., Migliorini M., Yu P., Cai T., Schwieters C. D., Krueger S., Strickland D. K., Wang Y. X. (2007) Protein Sci. 16, 1628–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obermoeller L. M., Warshawsky I., Wardell M. R., Bu G. (1997) J. Biol. Chem. 272, 10761–10768 [DOI] [PubMed] [Google Scholar]
- 22.Hatters D. M., Peters-Libeu C. A., Weisgraber K. H. (2006) Trends Biochem. Sci 31, 445–454 [DOI] [PubMed] [Google Scholar]
- 23.Wilson C., Wardell M. R., Weisgraber K. H., Mahley R. W., Agard D. A. (1991) Science 252, 1817–1822 [DOI] [PubMed] [Google Scholar]
- 24.Melman L., Cao Z. F., Rennke S., Marzolo M. P., Wardell M. R., Bu G. (2001) J. Biol. Chem. 276, 29338–29346 [DOI] [PubMed] [Google Scholar]
- 25.Libeu C. P., Lund-Katz S., Phillips M. C., Wehrli S., Hernáiz M. J., Capila I., Linhardt R. J., Raffaï R. L., Newhouse Y. M., Zhou F., Weisgraber K. H. (2001) J. Biol. Chem. 276, 39138–39144 [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi Y., Deguchi N., Takagi C., Tanaka M., Dhanasekaran P., Nakano M., Handa T., Phillips M. C., Lund-Katz S., Saito H. (2008) Biochemistry 47, 6702–6710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warshawsky I., Bu G., Schwartz A. L. (1993) J. Clin. Investig. 92, 937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Chen J., Lu W., McCormick L. M., Wang J., Bu G. (2005) J. Cell Sci. 118, 5305–5314 [DOI] [PubMed] [Google Scholar]
- 29.Zerbinatti C. V., Wozniak D. F., Cirrito J., Cam J. A., Osaka H., Bales K. R., Zhuo M., Paul S. M., Holtzman D. M., Bu G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine H., 3rd (1995) Neurobiol. Aging 16, 755–764 [DOI] [PubMed] [Google Scholar]
- 31.Bu G., Maksymovitch E. A., Geuze H., Schwartz A. L. (1994) J. Biol. Chem. 269, 29874–29882 [PubMed] [Google Scholar]
- 32.Willnow T. E., Herz J. (1994) Methods Cell Biol. 43, 305–334 [DOI] [PubMed] [Google Scholar]
- 33.Ida N., Masters C. L., Beyreuther K. (1996) FEBS Lett. 394, 174–178 [DOI] [PubMed] [Google Scholar]
- 34.Fuentealba R. A., Barría M. I., Lee J., Cam J., Araya C., Escudero C. A., Inestrosa N. C., Bronfman F. C., Bu G., Marzolo M. P. (2007) Mol. Neurodegener. 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avirutnan P., Zhang L., Punyadee N., Manuyakorn A., Puttikhunt C., Kasinrerk W., Malasit P., Atkinson J. P., Diamond M. S. (2007) PLoS Pathog. 3, e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Lu W., Bu G. (2003) FEBS Lett. 555, 346–350 [DOI] [PubMed] [Google Scholar]
- 37.Reblin T., Niemeier A., Meyer N., Willnow T. E., Kronenberg F., Dieplinger H., Greten H., Beisiegel U. (1997) J. Lipid Res. 38, 2103–2110 [PubMed] [Google Scholar]
- 38.Takayama Y., May P., Anderson R. G., Herz J. (2005) J. Biol. Chem. 280, 18504–18510 [DOI] [PubMed] [Google Scholar]
- 39.Broekelmann T. J., Kozel B. A., Ishibashi H., Werneck C. C., Keeley F. W., Zhang L., Mecham R. P. (2005) J. Biol. Chem. 280, 40939–40947 [DOI] [PubMed] [Google Scholar]
- 40.Esko J. D., Stewart T. E., Taylor W. H. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 3197–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S. J., Liyanage U., Bickel P. E., Xia W., Lansbury P. T., Jr., Kosik K. S. (1998) Nat. Med. 4, 730–734 [DOI] [PubMed] [Google Scholar]
- 42.Greenfield J. P., Tsai J., Gouras G. K., Hai B., Thinakaran G., Checler F., Sisodia S. S., Greengard P., Xu H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole S. L., Vassar R. (2007) Mol. Neurodegener. 2, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudva Y. C., Hiddinga H. J., Butler P. C., Mueske C. S., Eberhardt N. L. (1997) FEBS Lett. 416, 117–121 [DOI] [PubMed] [Google Scholar]
- 45.Raman B., Ban T., Sakai M., Pasta S. Y., Ramakrishna T., Naiki H., Goto Y., Rao Ch. M. (2005) Biochem. J. 392, 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bu G., Rennke S., Geuze H. J. (1997) J. Cell Sci. 110, 65–73 [DOI] [PubMed] [Google Scholar]
- 47.Willnow T. E., Sheng Z., Ishibashi S., Herz J. (1994) Science 264, 1471–1474 [DOI] [PubMed] [Google Scholar]
- 48.Pandey P., Pradhan S., Mittal B. (2008) Genes Brain Behav. 7, 943–950 [DOI] [PubMed] [Google Scholar]
- 49.Zheng H., Koo E. H. (2006) Mol. Neurodegener. 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cam J. A., Zerbinatti C. V., Li Y., Bu G. (2005) J. Biol. Chem. 280, 15464–15470 [DOI] [PubMed] [Google Scholar]
- 51.Van Uden E., Mallory M., Veinbergs I., Alford M., Rockenstein E., Masliah E. (2002) J. Neurosci. 22, 9298–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu G., Karch C., Li N., Lin N., Fromholt D., Gonzales V., Borchelt D. R. (2008) PLoS ONE 3, e3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibata M., Yamada S., Kumar S. R., Calero M., Bading J., Frangione B., Holtzman D. M., Miller C. A., Strickland D. K., Ghiso J., Zlokovic B. V. (2000) J. Clin. Investig. 106, 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deane R., Wu Z., Sagare A., Davis J., Du Yan S., Hamm K., Xu F., Parisi M., LaRue B., Hu H. W., Spijkers P., Guo H., Song X., Lenting P. J., Van Nostrand W. E., Zlokovic B. V. (2004) Neuron 43, 333–344 [DOI] [PubMed] [Google Scholar]
- 55.Cuitino L., Matute R., Retamal C., Bu G., Inestrosa N. C., Marzolo M. P. (2005) Traffic 6, 820–838 [DOI] [PubMed] [Google Scholar]
- 56.Warshawsky I., Bu G., Schwartz A. L. (1993) J. Biol. Chem. 268, 22046–22054 [PubMed] [Google Scholar]
- 57.Skidmore M. A., Guimond S. E., Rudd T. R., Fernig D. G., Turnbull J. E., Yates E. A. (2008) Connect. Tissue Res. 49, 140–144 [DOI] [PubMed] [Google Scholar]
- 58.Kreuger J., Spillmann D., Li J. P., Lindahl U. (2006) J. Cell Biol. 174, 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poon G. M., Gariépy J. (2007) Biochem. Soc. Trans. 35, 788–793 [DOI] [PubMed] [Google Scholar]
- 60.Carey D. J. (1997) Biochem. J. 327, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 62.O'Callaghan P., Sandwall E., Li J. P., Yu H., Ravid R., Guan Z. Z., van Kuppevelt T. H., Nilsson L. N., Ingelsson M., Hyman B. T., Kalimo H., Lindahl U., Lannfelt L., Zhang X. (2008) Brain Pathol. 18, 548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen P. R., Ellgaard L., Etzerodt M., Thogersen H. C., Poulsen F. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 7521–7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y., Migliorini M., Walsh J., Yu P., Strickland D. K., Wang Y. X. (2004) J. Biomol. NMR 29, 271–279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.