Abstract
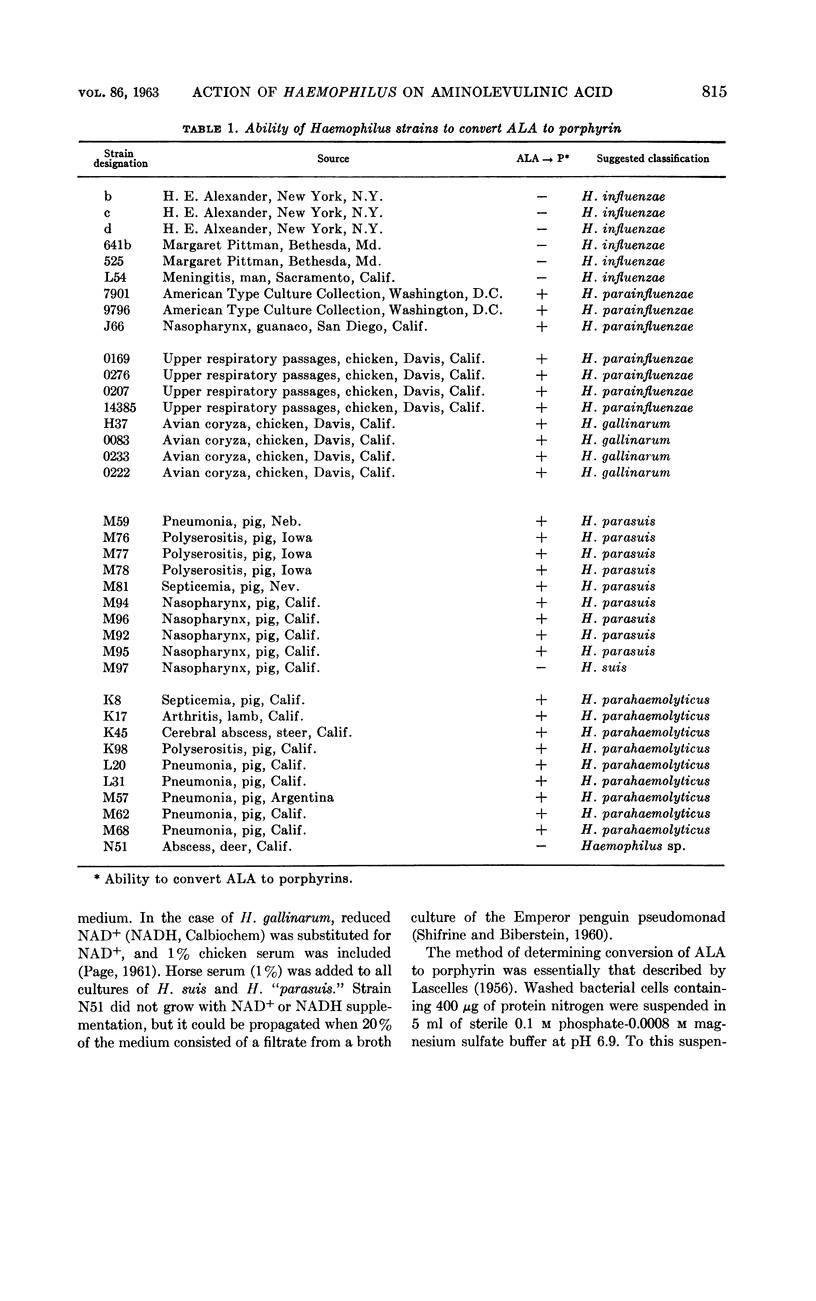
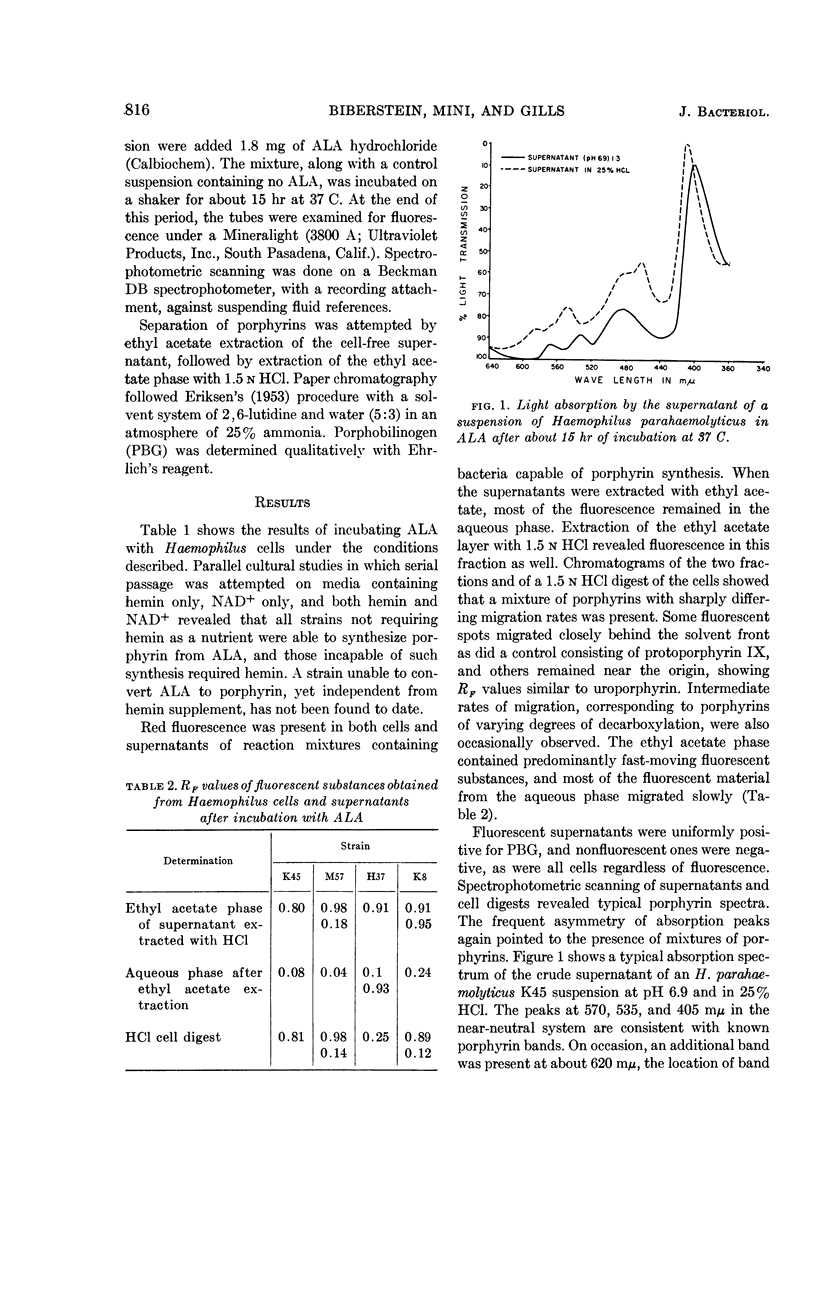
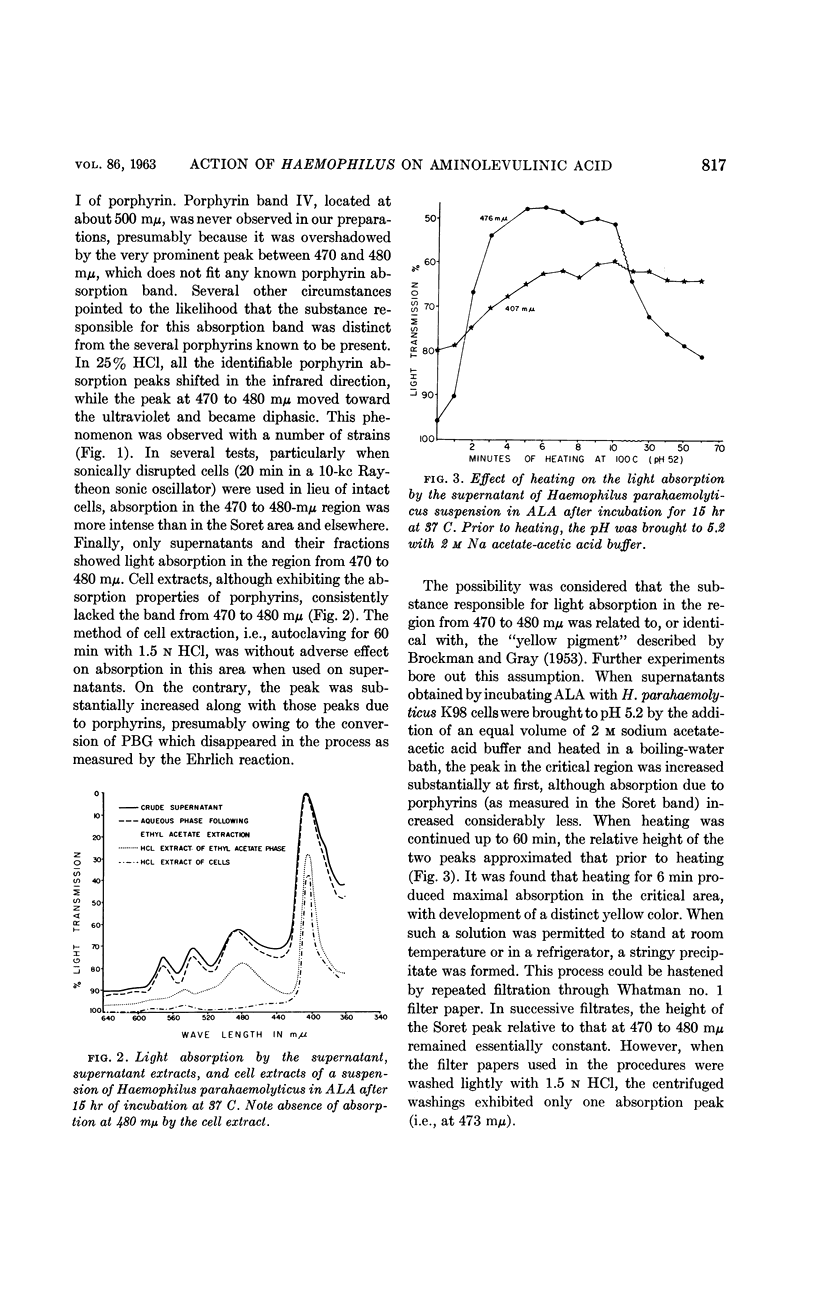
Biberstein, Ernst L. (University of California, Davis), Patricia D. Mini, and Marjorie G. Gills. Action of Haemophilus cultures on δ-aminolevulinic acid. J. Bacteriol. 86:814–819. 1963.—Utilization of δ-aminolevulinic acid (ALA) by strains of Haemophilus recovered from various sources was investigated. With the 37 cultures studied, there was perfect correlation between absence of hemin requirement and ability to convert ALA to porphyrin. A total of 29 strains, including representatives of H. parainfluenzae, H. parahaemolyticus, H. “parasuis,” and H. gallinarum, fell into this group. The remaining eight isolates, which were incapable of porphyrin synthesis from ALA, were strains of H. influenzae, H. suis, and a Haemophilus culture of uncertain classification obtained from a splenic abscess in a deer. In the active preparations, the products of synthesis included a mixture of porphyrins, porphobilinogen (PBG), and a pigment which absorbed light strongly at 470 to 480 mμ. Paper chromatographic studies of fractions of supernatants and cells revealed the presence of porphyrins having RF values similar to those of uro- and protoporphyrins, as well as some intermediate rates of migration probably representing coproporphyrins. Porphyrins were found both intra- and extracellularly, and PBG and the pigment absorbing at 470 to 480 mμ were confined to the supernatant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCKMAN P. E., GRAY C. H. Studies on porphobilinogen. Biochem J. 1953 Apr;54(1):22–29. doi: 10.1042/bj0540022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSEN L. Paper chromatography of porphyrin pigments. Scand J Clin Lab Invest. 1953;5(2):155–157. [PubMed] [Google Scholar]
- GILDER H., GRANICK S. Studies on the Hemophilus group of organisms; quantitative aspects of growth on various porphin compounds. J Gen Physiol. 1947 Nov 20;31(2):103–117. doi: 10.1085/jgp.31.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. A., Shope R. E. SWINE INFLUENZA : II. A HEMOPHILIC BACILLUS FROM THE RESPIRATORY TRACT OF INFECTED SWINE. J Exp Med. 1931 Jul 31;54(3):361–371. doi: 10.1084/jem.54.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE L. A. Haemophilus infections in chickens. I. Characteristics of 12 Haemophilus isolates recovered from diseased chickens. Am J Vet Res. 1962 Jan;23:85–95. [PubMed] [Google Scholar]
- WHITE D. C., GRANICK S. HEMIN BIOSYNTHESIS IN HAEMOPHILUS. J Bacteriol. 1963 Apr;85:842–850. doi: 10.1128/jb.85.4.842-850.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. Respiratory systems in the hemin-requiring Haemophilus species. J Bacteriol. 1963 Jan;85:84–96. doi: 10.1128/jb.85.1.84-96.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


