Abstract
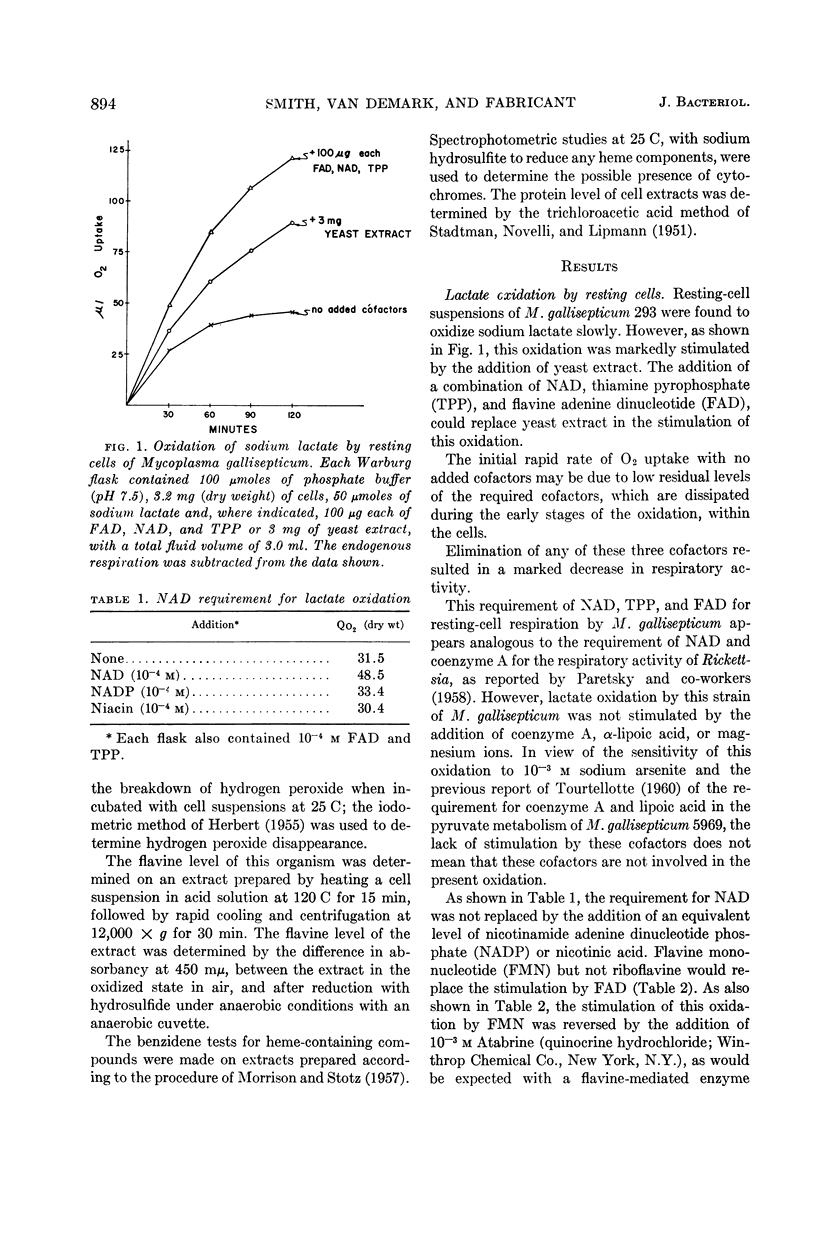
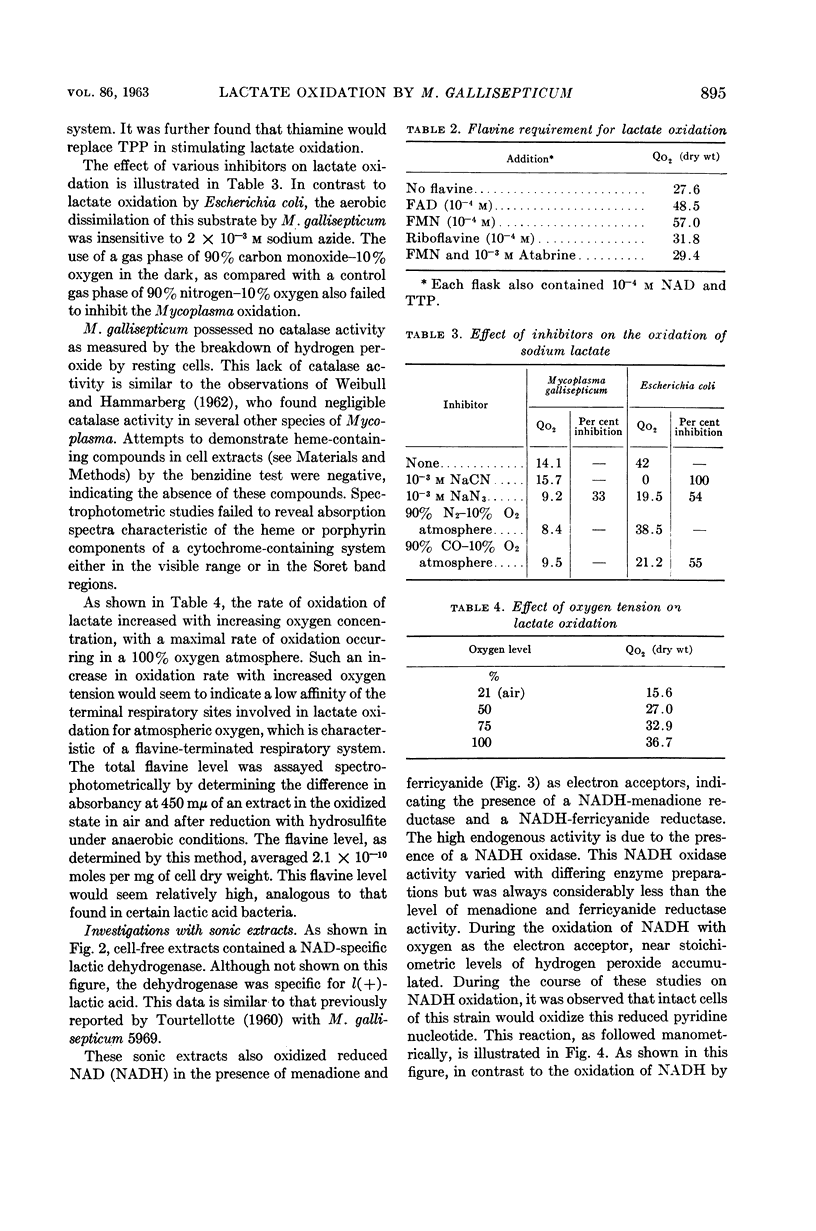
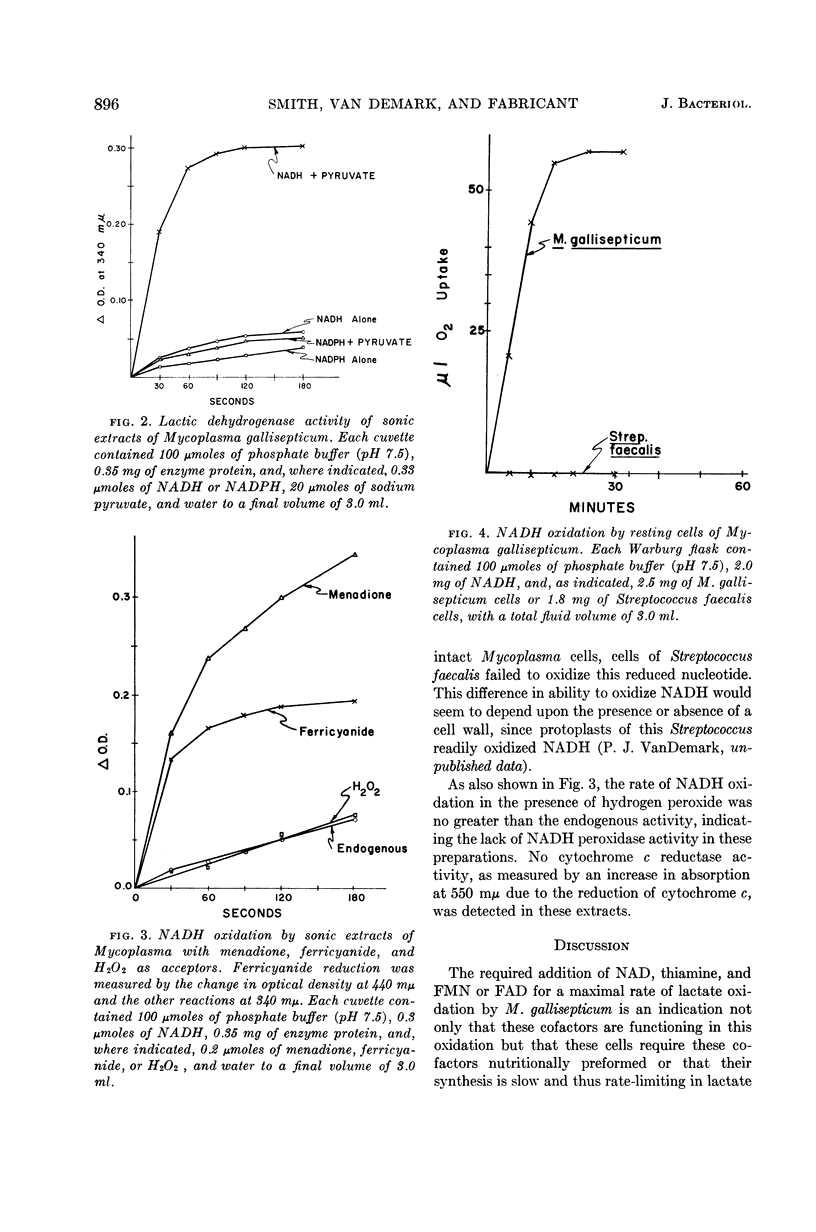
Smith, S. L. (Cornell University, Ithaca, N.Y.), P. J. Van Demark, and J. Fabricant. Respiratory pathways in the Mycoplasma. I. Lactate oxidation by Mycoplasma gallisepticum. J. Bacteriol. 86:893–897. 1963.—Resting cells of Mycoplasma gallisepticum 293 required the addition of nicotinamide adenine dinucleotide, thiamine pyrophosphate, and flavine mononucleotide for the maximal rate of sodium lactate oxidation. Inhibitor studies, as well as spectrophotometric and chemical assays, indicate that the pathway of electron transport to oxygen during lactate oxidation does not involve heme catalysts, and is mediated by flavin-linked enzyme systems. The presence of reduced nicotinamide adenine dinucleotide-specific lactic dehydrogenase, menadione reductase, ferricyanide reductase, and reduced nicotinamide adenine dinucleotide oxidase activities was detected in cell-free extracts. No cytochrome c reductase or reduced nicotinamide adenine dinucleotide peroxidase activity was detected in these extracts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EDWARD D. G., KANAREK A. D. Organisms of the pleuropneumonia group of avian origin: their classification into species. Ann N Y Acad Sci. 1960 Jan 15;79:696–702. doi: 10.1111/j.1749-6632.1960.tb42744.x. [DOI] [PubMed] [Google Scholar]
- MORRISON M., STOTZ E. The extraction and paper chromatography of hemins. J Biol Chem. 1957 Sep;228(1):123–130. [PubMed] [Google Scholar]
- NEIMARK H. C., PICKETT M. J. Products of glucose metabolism by pleuropneumonialike organisms. Ann N Y Acad Sci. 1960 Jan 15;79:531–537. doi: 10.1111/j.1749-6632.1960.tb42719.x. [DOI] [PubMed] [Google Scholar]
- PARETSKY D., DOWNS C. M., CONSIGLI R. A., JOYCE B. K. Studies on the physiology of rickettsiae. I. Some enzyme systems of Coxiella burnetii. J Infect Dis. 1958 Jul-Aug;103(1):6–11. doi: 10.1093/infdis/103.1.6. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The breakdown of pyruvate by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):31–36. [PubMed] [Google Scholar]
- SMITH L. Structure of the bacterial respiratory-chain system. Respiration of Bacillus subtilis spheroplasts as a function of the osmotic pressure of the medium. Biochim Biophys Acta. 1962 Jul 30;62:145–152. doi: 10.1016/0006-3002(62)90499-7. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- WEIBULL C., HAMMARBERG K. Occurrence of catalase in pleuropneumonia-like organisms and bacterial L forms. J Bacteriol. 1962 Sep;84:520–525. doi: 10.1128/jb.84.3.520-525.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


