Abstract
The stereocontrolled total synthesis of 4-hydroxydictyolactone (4), a member of the xenicane diterpene family of natural products, is described. These studies feature the development of the B-alkyl Suzuki cross-coupling reaction for direct access to (E)-cyclononenes from acyclic precursors. The Ireland-Claisen rearrangement is effectively utilized to establish the backbone asymmetry of the contiguous C2, C3, C10 stereotriad of 4. The synthesis strategy has devised an intramolecular Nozaki-Hiyama reductive allylation of a formate ester for the stereoselective formation of five-membered lactols 22. In addition, an internally directed SE' propargylation using allenylmagnesium bromide is described to establish the stereochemistry of the C4 alcohol in 27, and the terminal alkyne is subsequently functionalized via a regioselective syn-silylstannylation to yield 30. Finally, the stereocontrolled phenylselenylation of the ester enolate derived from 43 leads to the desired syn-oxidative elimination to yield the natural product 4.
Introduction
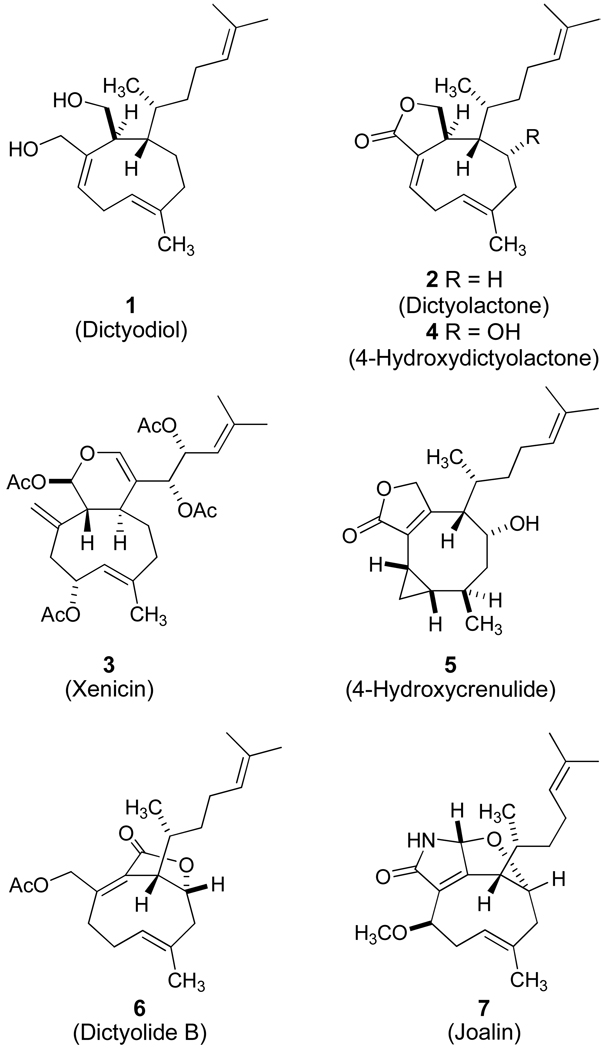
In 1979, Fenical and coworkers reported the isolation of dictyodiol (1), an unusual diterpene from the brown algae, Dictyota crenulata, which exhibited a rare nonconjugated (E),(Z)-cyclononadiene motif.1 These efforts also identified dictyolactone (2) as a related metabolite from the sea hare Aplysia depilans. The investigation followed in the wake of the groundbreaking discovery of xenicin (3) from the soft coral Xenia elongata, which had been unambiguously elucidated by a single crystal X-ray diffraction study.2 Subsequent reports have generally adopted a description of marine natural products displaying a cyclononene framework as examples of the xenicane family.3 Faulkner offered an expansive viewpoint by proposing that five distinct diterpene skeletons could be traced to biosynthetic origins that incorporate xenicane precursors.4 However, Kakisawa and coworkers have proven that the xenicanes from Dictyotaceae algae possess the antipodal configuration as compared to members of the family from soft coral in early studies establishing the tenets of the advanced Mosher ester analysis.5 These findings have been taken into account for the illustration of the structures of Figure 1, and provide a cautionary note for the assignment of the absolute stereochemistry of related metabolites which may or may not be distributed through an aquatic environment of filtering organisms via a common food chain.
Figure 1.
Xenicane and Xenicane-Derived Marine Natural Products
Guella and Pietra first described the isolation and structural elucidation of 4-hydroxydictyolactone (4) from Dictyota ciliolata in 1993 along with the identification of a related metabolite, 4-hydroxycrenulide (5).6 The report also demonstrated that the ultraviolet irradiation of 4 produced a photoisomerization to yield 5. While this transformation formally presents an intramolecular ene process, the concerted pathway would provide for an antarafacial homo[1,5]-hydrogen shift giving rise to the C6 diastereomer of 5. These efforts described the thermal isomerization of 4 leading to the corresponding (Z)-6,7-olefin which did not afford cyclopropane products upon irradiation. Based on their observations, Guella and Pietra speculated that a free radical mechanism may be operative in the formation of 4-hydroxycrenulide (5) and may characterize the increased reactivity of the strained (Z,E)-cyclononadiene ring system of 4-hydroxydictyolactone (4).
Numerous reports provide preliminary accounts of important biological activity among members of the xenicane family. Individual compounds have exhibited antibacterial and antifungal properties,3a ichthyotoxicity,7 and the inhibition of HIV-1 reverse transcriptase.8 Xenicanes, such as dictyotalide B9 (6, Figure 1) display significant levels of cytotoxicity against B16 mouse melanoma cultures whereas florxenilide A,10 a soft coral metabolite, exhibits potent cytotoxicity against human colon cancer cell lines even though these examples represent antipodal subgroups. Joalin (7) is an unusual member of the xenicane family bearing a nitrogen atom in addition to the distinctive bridgehead olefin.11 A recent account has confirmed that several xenicane diterpenes target proliferating cells by the specific induction of apoptosis at micromolar concentrations.12 Thus, this family of natural products may offer a new chemotype for the development of chemotherapeutic agents. A systematic structure-activity evaluation has not been undertaken, and many xenicanes have not been examined.
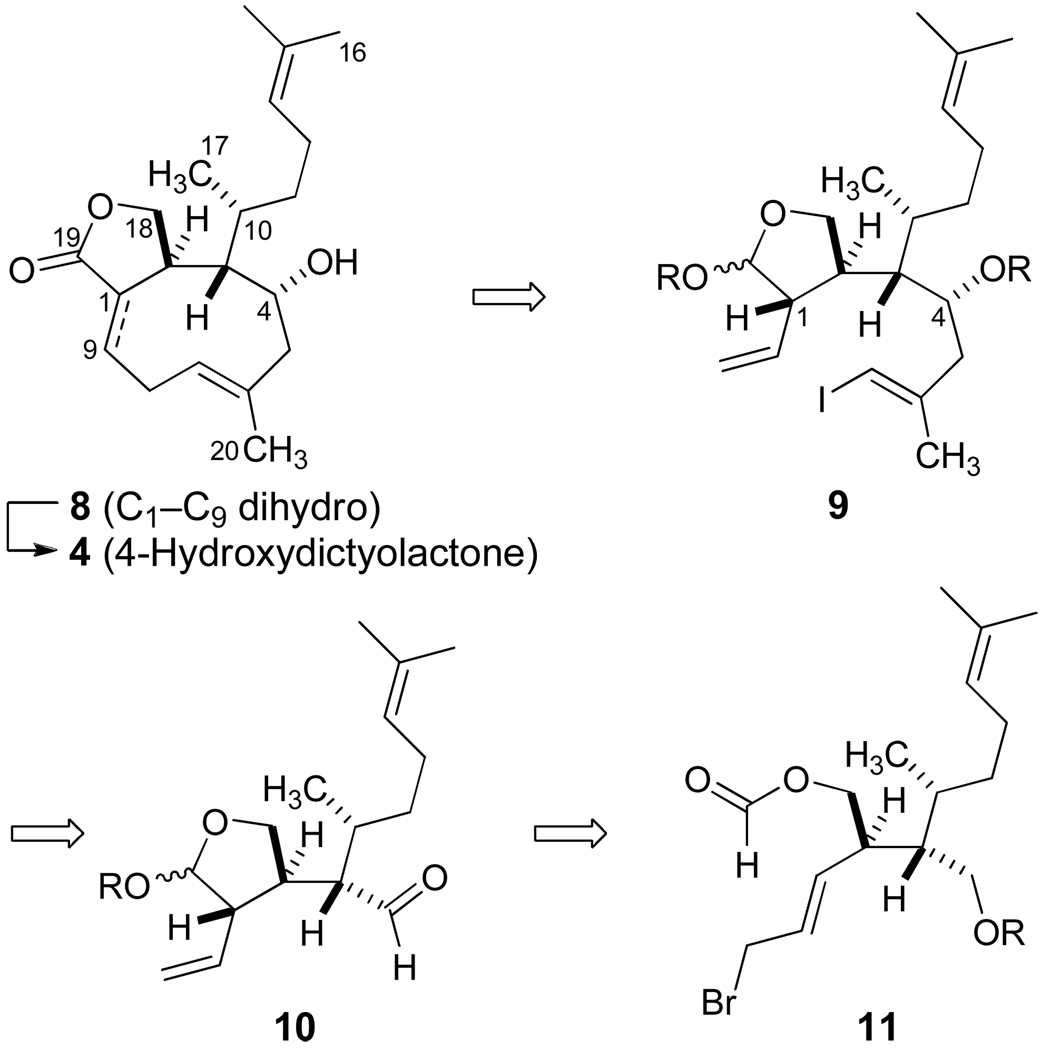
Our plans for the synthesis of 4-hydroxydictyolactone (4) were designed to explore the utility of the B-alkyl Suzuki reaction as a mild palladium-catalyzed cross-coupling event to incorporate the intact (E)-trisubstituted alkene in a direct ring closure of the nine-membered carbocycle (Scheme 1). Our rationale in support of this hypothesis and a preliminary account of our ongoing efforts have recently been described.13 We postulated that the selective hydroboration of 9 would set the stage for the cross-coupling process followed by oxidation to the trans-fused lactone 8. Further oxidation of 8 would introduce the α,β-unsaturation of the natural product 4. Since the irreversible reductive elimination from a coordinated palladium intermediate would provide for the ring closure, we rationalized that the crucial formation of a palladium metallocycle would overcome the entropic features and steric constraints which often dominate processes involving direct closures in nine- and ten-membered carbocycles. The five-membered acetal of 9 was incorporated as an element of conformational bias to aid these efforts. However, our modeling suggested that the stereochemistry at C1 of 9 would play an important role because the corresponding cis-disubstituted tetrahydrofuranyl system imposed significant steric interactions for transition states leading to metallocycle formation.
Scheme 1.
Retrosynthetic Analysis of 4.
General methods for the direct closure of cyclononene systems are uncommon. While new opportunities have explored the synthesis of medium-ring carbocycles using ring-closing metathesis,14 the formation of cyclononenes has presented problems for some RCM strategies.15 On the other hand, two groups have independently described recent results for Nozaki-Hiyama-Kishi cyclizations directed toward pestalotiopsin, a caryophyllene sesquiterpenoid.16 In classic studies by Professor E. J. Corey, the use of the Grob fragmentation was devised to address the synthesis of (±)-caryophyllene.17 This stereocontrolled reaction established an important precedent for the preparation of (E)-cyclononenes via the fragmentation of fused bicyclic systems. Recently, Corey has reported the Grob fragmentation leading to a stable, chiral (E,Z)-cyclononadienone for the enantioselective synthesis of caryophylloids.18 In a similar fashion, the Grob strategy has been successfully applied for total synthesis of the xeniolide, coraxeniolide A.19
The analysis of Scheme 1 readily identified the (E)-alkenyliodide 9 as the penultimate intermediate for a direct cyclization to afford the xenicane framework, and we envisioned the preparation of 9 via two sequential SE' allylation processes. The latter of these events would establish the chirality at C4 and accommodate stereospecific incorporation of the (E)-alkenyl iodide from the aldehyde 10. The initial SE' allylation was projected as a stereocontrolled intramolecular reaction stemming from the reduction of bromide 11 to yield the tetrahydrofuranyl lactol of 10.
Results and Discussion
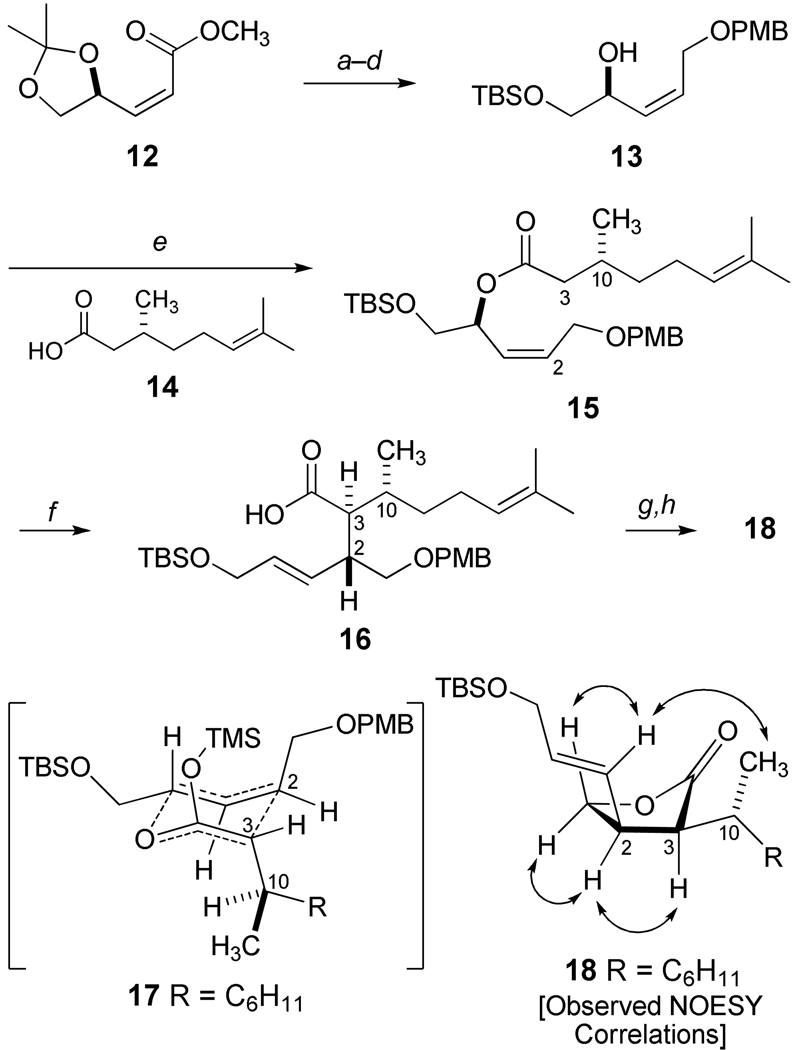
The preparation of chiral, nonracemic 11 of Scheme 1 required a high degree of stereocontrol for the assembly of the contiguous stereotriad presented at C2, C3, and C10. This objective can be problematic for synthesis because the consecutive tertiary centers of asymmetry are uniquely characterized by an arrangement of carbon alkyl substituents. The Claisen rearrangement appeared to be particularly well suited to meet this challenge.20 As illustrated in Scheme 2, the synthesis of the nonracemic Ireland-Claisen precursor 15 began with the known oxidative cleavage of the diacetonide of D-mannitol, and a direct Wittig olefination in methanol yielded a 9:1 (Z:E) ratio of methyl esters leading to 12 (68% yield) after purification by flash chromatography.21 Upon diisobutylaluminum hydride (DIBAL) reduction, the resulting Z-allylic alcohol was protected as its para-methoxybenzyl ether (PMB), and ketal hydrolysis led to O-silylation of the primary alcohol to yield 13. Esterification of 13 with (R)-(+)-citronellic acid (14)22 produced a single diastereomer 15 for subsequent kinetic deprotonation at −78 °C. However, the introduction of 15 into a THF solution containing lithium diisopropylamide (LDA) followed by trimethylsilyl chloride (TMSCl) and Et3N led to substantial amounts of products derived from base-induced elimination which were identified as the TBS ether of (E,E)-5-para-methoxybenzyloxy-2,4-pentadien-1-ol and (R)-(+)-citronellic acid. The inverse addition of LDA into a cold reaction mixture containing 15, TMSCl and Et3N provided excellent conversion to the anticipated E(O)-trimethylsilyl ketene acetal, and heating at 70 °C resulted in the isolation of carboxylic acid 16 (dr 94:6) in high yield. The minimization of steric factors in the chair-like arrangement 17 accounts for the formation of the major diastereomer 16, and the relative assignment of stereochemistry was confirmed by conversion to the cis-disubstituted butyrolactone 18 for NMR studies leading to the observed nuclear Overhauser enhancement correlations (NOESY) illustrated in Scheme 2.
Scheme 2.
Development of the C2, C3, C10 Stereotriad of 16.a
aReagents and Conditions: a) DIBAL, CH2Cl2, −78 °C, 98%; b) PMBCl, NaH, DMF, 0 °C to rt, 97%; c) 1M HCl, MeOH, 100%; d) TBSCl, imidazole, CH2Cl2, 92%; e) 14, EDCI, DMAP, CH2Cl2, 97%; f ) TMSCl, Et3N, −78 °C, then LDA, −78 °C then reflux, 85%, dr = 94:6; g) DDQ, CH2Cl2, H2O, 0 °C; f) EDCI, DMAP, CH2Cl2
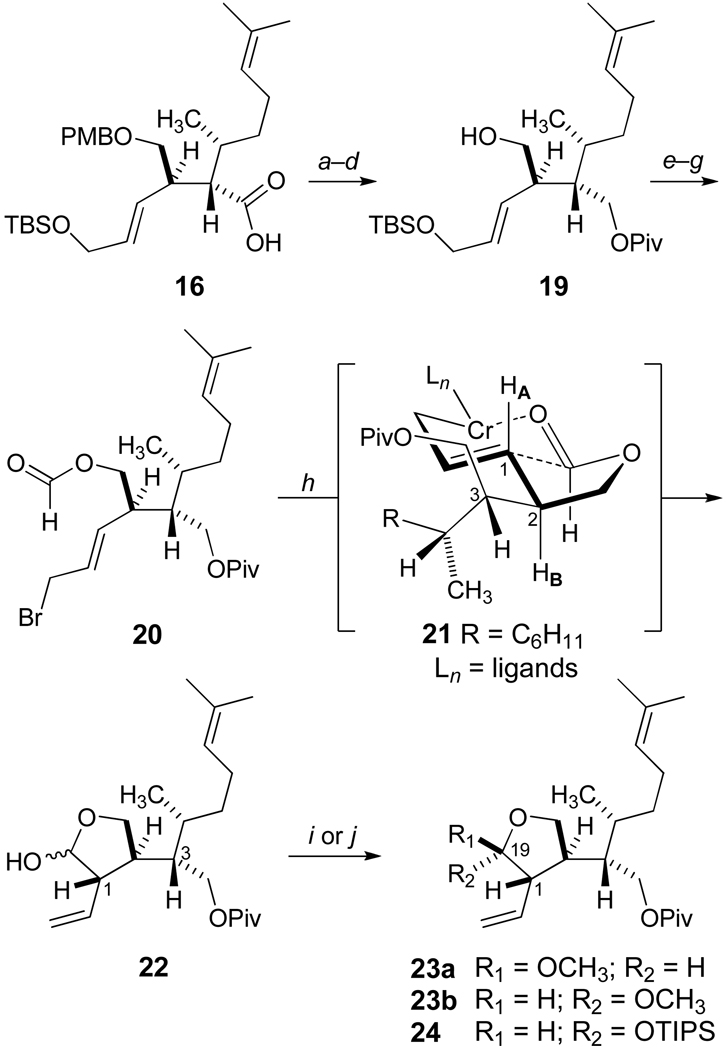
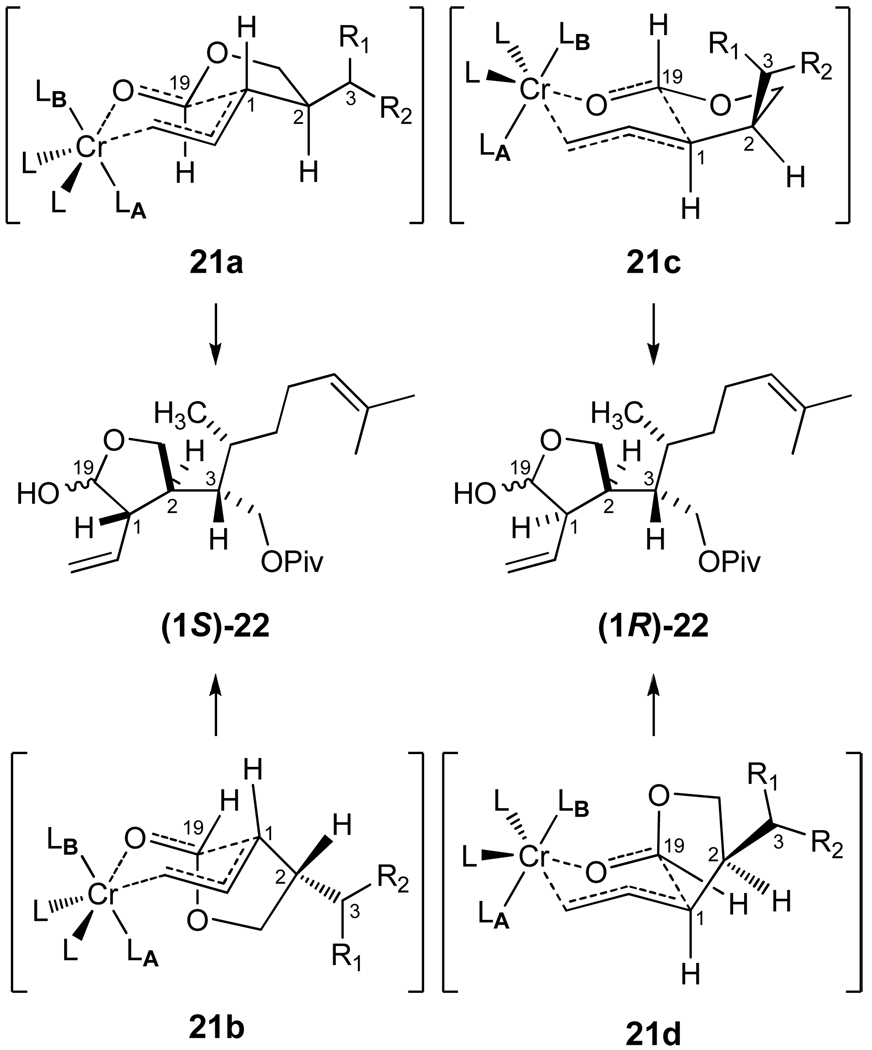
Carboxylic acid 16 was transformed into the pivaloate (Piv) 19 of Scheme 3 in four straightforward steps (70% overall from 16), and esterification with formic acid led to 20 upon introduction of the allylic bromide. Adaptation of the Nozaki-Hiyama conditions23 provided facile intramolecular cyclization to the lactol 22. To the best of our knowledge, Nozaki-Hiyama cyclizations of formate esters have not been previously explored. Our results suggest that this strategy offers versatility and efficiency for the stereoselective synthesis of five and six-membered lactols and related derivatives.24 Indeed, high stereocontrol at C1 was observed for the SE' allylation via internal coordination of the allylchromium species as suggested in 21 by the antiperiplanar disposition of HA and HB.
Scheme 3.
Intramolecular Nozaki-Hiyama Coupling of Formate Ester 20.a
aReagents and Conditions: a) MeI, K2CO3, DMF, 97%; b) DIBAL, CH2Cl2, −78 °C, 100%; c) PivCl, pyr, CH2Cl2, 95%; d) DDQ, pH 7.0 buffer, CH2Cl2, 0 °C, 76%; e) Formic Acid, EDCI, DMAP, CH2Cl2, 96%; f ) Bu4N+ Ph3SiF2−, AcOH, THF, 99%; g) CBr4, PPh3, CH2Cl2; h) CrCl2, THF, 92% (2 steps), dr > 95:5 at C1; i) PPTs, MeOH, 100%; j) TIPSOTf , 2,6-lutidine, CH2Cl2, 0 °C, 90%, dr = 91:9 at C19.
It is well known that crotyl halides undergo reduction to form (E)-allylic chromium(III) reagents regardless of the geometry of the starting butene, and the chromium species coordinate aldehydes for nucleophilic additions via closed six-membered transition states.23 In Scheme 4, four possible arrangements are featured for a detailed analysis of the intramolecular SE' reaction. The octahedral coordination complex of chromium is characterized by a combination of halogen (Cl, Br) and solvent (THF) ligands (L) in addition to the reactive partners of the allylation process. While our analysis does not consider boat-like transition states, two chair-like arrangements, 21a and 21b, may account for the formation of the observed diastereomer (1S)-22. The trans-fused bicyclic 21a favorably illustrates complexation with the carbonyl, which is synclinal with respect to the formate hydrogen, and minimizes nonbonded interactions by pseudoequatorial placement of the highly branched C2 substituent. Although the cis-fused transition state of 21b displays the large pseudoaxial C2 substituent on the convex face of the bicycle, the axial ligand LA may be destabilizing for electronic as well as steric reasons. Our considerations for the diastereofacial reactions leading to the cis-disubstituted lactol (1R)-22 are illustrated in 21c and 21d. The less stable, pseudoaxial disposition of the branched C2 substituent in 21c also appears to present nonbonded interactions with axial LB (THF), and these considerations become more severe in the cis-fused arrangement of 21d. Our spectroscopic characterization of the lactols 22 (1:1 ratio) provided no evidence of the ring-opened hydroxyaldehyde tautomer, and the subsequent quantitative conversion of 22 to the corresponding methyl acetals (Scheme 3) gave an inseparable mixture of diastereoisomers 23a and 23b (dr 58:42). On the other hand, the triisopropylsilyl ether 24 was formed with excellent stereoselectivity (dr 91:9) and provided important advantages for the simplicity of reaction and product analysis in subsequent studies.
Scheme 4.
Transition State Analysis Toward the Formation of 22.
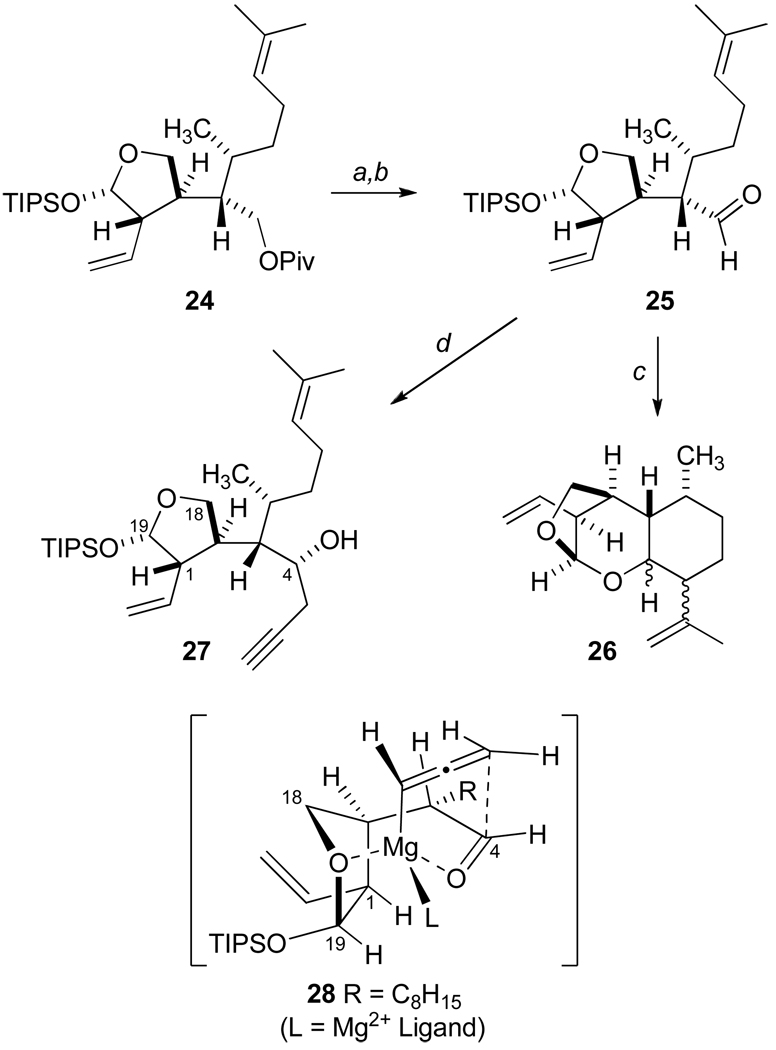
Upon preparation of aldehyde 25 (Scheme 5), we began studies of SE' allylation reactions with Lewis acid activation. Our initial reactions with allylic silanes25 and allenylic stannanes26 were undertaken to probe aspects of inherent diastereofacial selectivity which were not readily apparent from Felkin-Anh modeling of 25. Unfortunately a significant side reaction was encountered with the production of the diastereomeric acetals 26 resulting from a facile Lewis acid-catalyzed intramolecular Prins reaction and ketalization. Attempts to secure C4 stereocontrol using nonracemic allenyl and allyl boron reagents27 displayed poor reactivity toward this sterically congested aldehyde with slow conversion to many products. These observations led to the use of allenylmagnesium bromide28 as a reactive nucleophile which proved to be operationally efficient for preparative scale reactions. Ethereal solutions of the Grignard reagent conveniently gave high yields of SE' propargylation to afford the desired secondary alcohols (96%). Moreover, the carbonyl addition proceeded with good diastereofacial selectivity (dr 84:16), and subsequent flash chromatography led to useful quantities of pure 27. This stereochemical outcome is rationalized by the internal γ-coordination of the divalent magnesium cation for SE' delivery of the allenyl nucleophile via a cyclic six-membered arrangement depicted in 28, and the C4 stereochemistry of the secondary alcohol 27 was assigned by an advanced Mosher ester analysis.5
Scheme 5.
Diastereoselective Propargylation of Aldehyde 25.a
aReagents and Conditions: a) DIBAL, CH2Cl2, −78 °C, 96%; b) TPAP, NMO, 4Å MS, CH2Cl2, 99%; c) SnCl4, CH2Cl2, −78 °C d) Propargyl bromide, Mg0, HgCl2, Et2O, −20 °C, 96%, dr = 84:16.
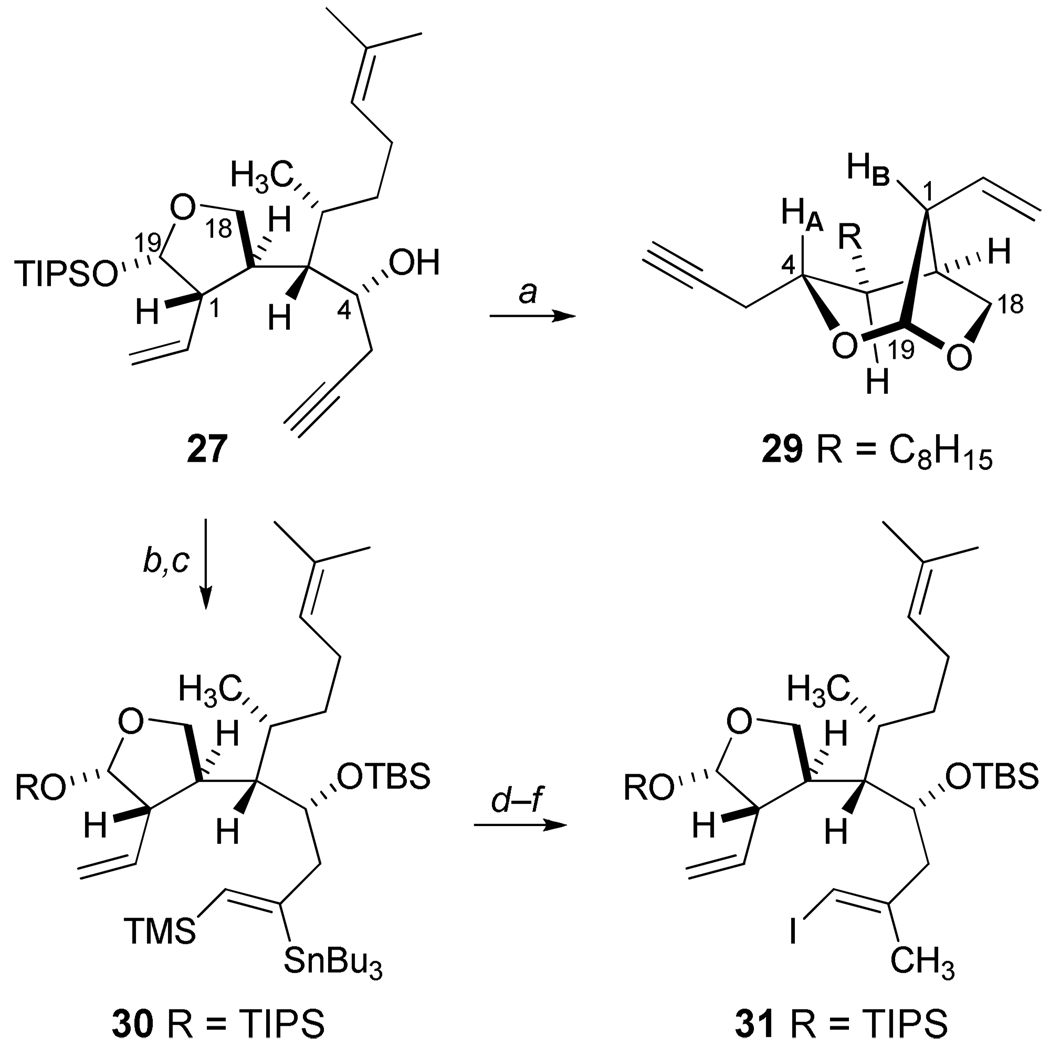
Our plans to utilize homopropargylic alcohol 27 for the stereocontrolled synthesis of the desired (E)-alkenyliodide 31 (Scheme 6) examined the Negishi zirconium-catalyzed carboalumination methodology29 which led to low conversions and a number of side products. A major component of these attempts was isolated and identified as the bicyclic ketal 29 resulting from Lewis acid-catalyzed transketalization. Proton NMR studies demonstrated a distinctive NOESY correlation of HA and HB in 29 which offered additional confirmation of the C4 stereochemical assignment in 27. However, the participation of a proximate propargylic or homopropargylic alcohol is known greatly improve yields in carboalumination reactions.29a,30 Thus, it was not surprising that ketal 29 was found to be unreactive in further reactions to utilize this carboalumination to elaborate the terminal alkyne. In addition, we explored several protecting groups for the C4 alcohol in 27, and we observed very slow, low yielding conversions to the desired alkenyl iodide, as well as other byproducts, in these attempts to apply the Negishi protocol. To resolve these issues, the O-silylation of 27 and subsequent application of a regioselective syn-silylstannylation as described by RajanBabu and coworkers31 was undertaken yielding 30. A convenient three-step protocol from 30 cleanly allowed for the sequential replacement of stannyl and silyl substituents with complete retention of olefin geometry to give the (E)-trisubstituted alkene of 31. Although this sequence has added three steps to our overall route, it is particularly noteworthy that these reactions can be efficiently applied as a general solution which is amenable to preparative scale processes.
Scheme 6.
Functionalization of Alkyne 27.a
aReagents and Conditions: a) A1Me3, Cp2ZrCl2, CH2Cl2, 0 °C, 62%; b) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C, 94%; c) Me3SiSnBu3, Pd(PPh3)4, THF, reflux, 85%; d) I2,2,6-di-t-butyl-4-methylpridine, CH2Cl2, −40 °C, 93%; e) MeLi, CuI, THF, −20 °C, 98%; f ) NIS, CH3CN, rt,82%.
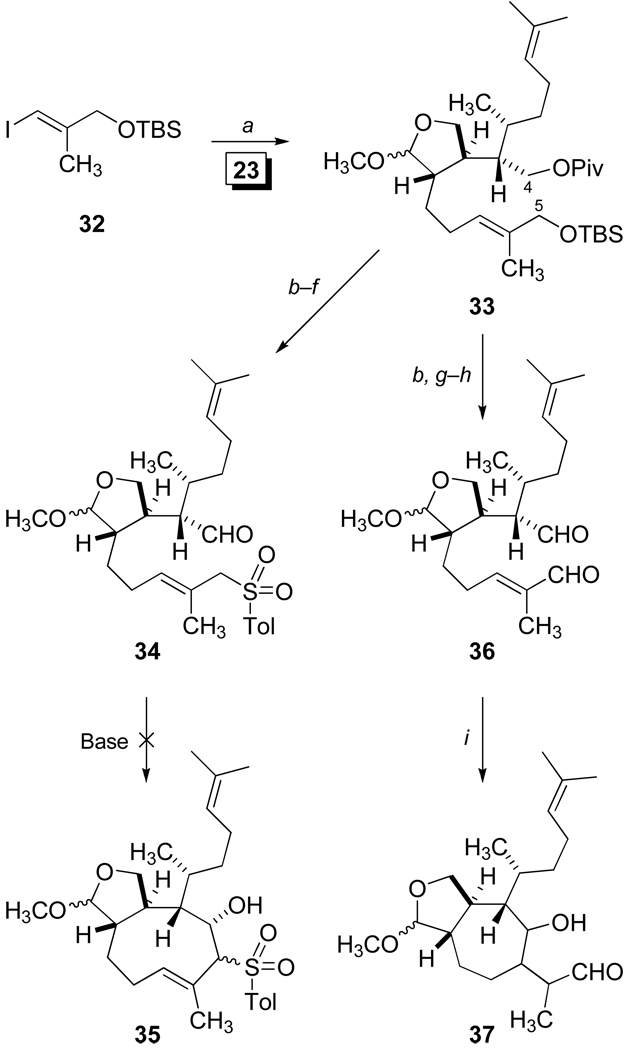
Our studies of the B-alkyl Suzuki cross-coupling reaction32 initially explored the reactivity of alkene 23 (Scheme 3) which was submitted for selective hydroboration at 22 °C. Coupling with the known iodide 3233 (Scheme 7) was observed to give 33 in 60–65% unoptimized yields using PdCl2(dppf), the most widely selected palladium catalyst and reaction conditions for B-alkyl Suzuki processes. The convenient preparation of the diol derivative 33 presented obvious opportunities to explore (E)-cyclononene formation. One approach was examined by the conversion of 33 into the aldehydic sulfone 34 as a precursor for an intramolecular Julia condensation. Our previous syntheses of dolabelladienones have described Julia condensations for direct ring closures leading to the formation of eleven-membered carbocycles.34 In addition, two unrelated examples of the use of α-sulfonyl carbanions in cyclization reactions leading to (E)-cyclononenes have also been reported. A novel intramolecular transacylation strategy was devised for the synthesis of (±)-caryophyllene by Oishi and coworkers,35 and Corey has described the successful capture of a π-allyl palladium intermediate via a β-ketosulfone for the recent total synthesis of antheliolide A.36 Based on these encouraging reports, the allylic sulfone 34 was prepared from 33 (Scheme 7) by desilylation and subsequent displacement using sodium tolylsulfinate prior to deprotection and oxidation. Unfortunately our efforts to obtain the cyclic β-hydroxysulfones 35 upon treatment with various bases only resulted in the recovery of starting 34 in spite of evidence of α-sulfonyl carbanion formation via deuterium incorporation. As an alternative, we examined a reductive coupling strategy toward an effective closure by the straightforward conversion of 33 into the dialdehyde 36 (Scheme 7). Our previous studies for the total synthesis of (+)-4,5-deoxyneodolabelline demonstrated the use of [V2Cl3(THF)6]ZnCl6 for reductive coupling leading to nine-membered syn-diol formation.37 However, the application of this vanadium-based pinacol reaction, in addition to experiments utilizing McMurry conditions,38 with dialdehyde 36 led to the formation of many products. Reactions of samarium diiodide39 with 36 produced the anticipated cycloheptanol 37 as an inseparable mixture of diastereomers.
Scheme 7.
Attempted Cyclization via C4–C5 Bond Formation.a
aReagents and Conditions: a) 23, 9-BBN, THF, rt, then 32, PdCl2(dppf ), Cs2CO3, AsPh3, H2O, DMF, 60% (unopt.); b) Bu4N+ Ph3SiF2−, AcOH, THF, 93%; c) I2, PPh3, imidazole, CH2Cl2; d) Sodium tolylsulf inate, DMF, rt, 86% (2 steps); e) DIBAL, CH2Cl2, −78 °C; f ) Dess-Martin Periodinane, Pyr., CH2Cl2, 94% (2 steps); g) DIBAL, CH2Cl2, −78 °C, 97%;h) Dess-Martin Periodinane, Pyr., CH2Cl2, 93%; i) SmI2, THF, −78 °C.
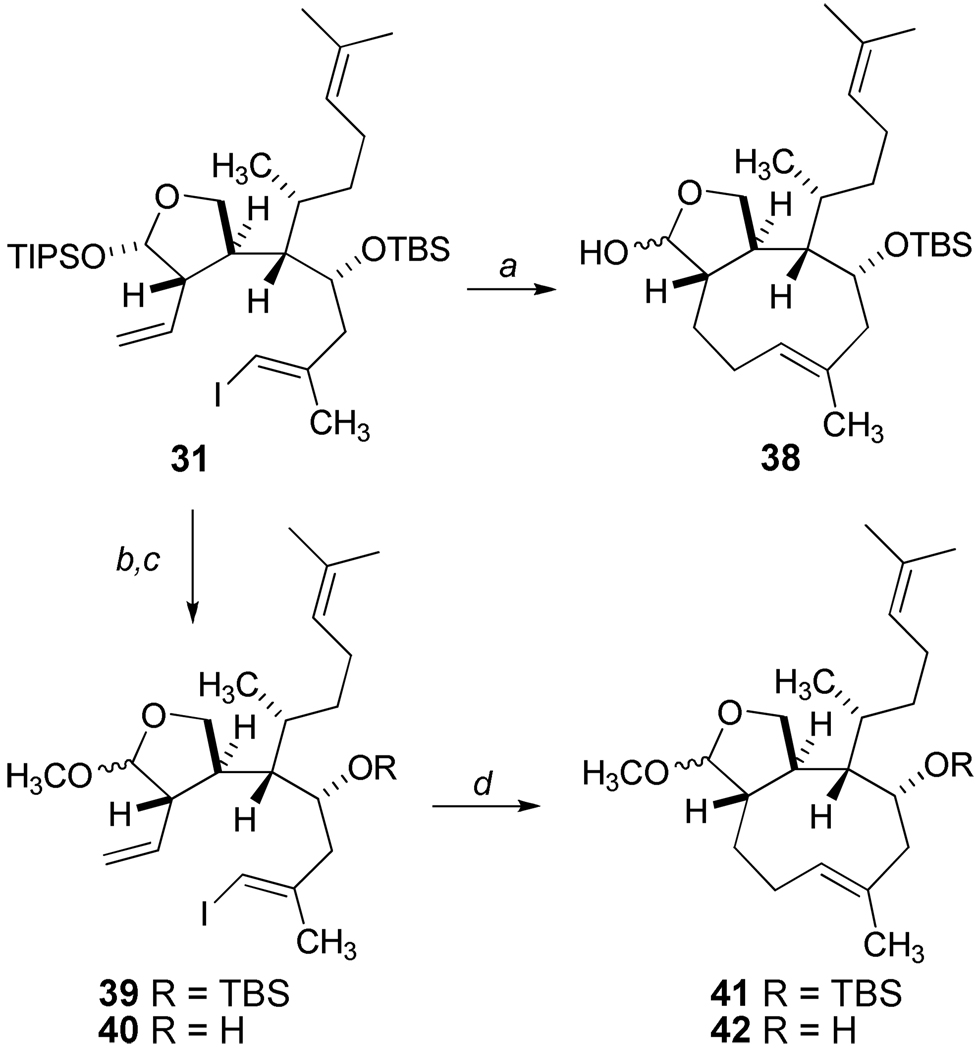
Concomitant studies of the intramolecular B-alkyl Suzuki cross coupling40 of 31 began to show promise (Scheme 8). Our initial reactions produced low yields (10–15%) of (E)-cyclononene product 38, and small improvements were observed with the additions of thallium(I) ethoxide41 and triphenylarsine. However, we noted a substantial difference in the rate of hydroboration of 31 compared to the corresponding methyl acetals 39 (Scheme 8).
Scheme 8.
Initial Studies of B-Alkyl Suzuki Macrocyclization.a
aReagents and Conditions: a) 9-BBN (4 equiv.), THF, 40 °C, then PdCl2(dppf), TlOEt, AsPH3. THF/DMF/H2O (6:3:1), 60 °C; then TBAF, THF,0 °C; b) TBAF, THF 0 °C, 95%; c) PPTs MeOH, rt, 99%;d) 9-BBN (1.2 equiv.), THF,0 °C to rt., then PdCl2(dppf), TlOEt, AsPh3, THF/DMF/H2O (6:3:1), 60 °C.
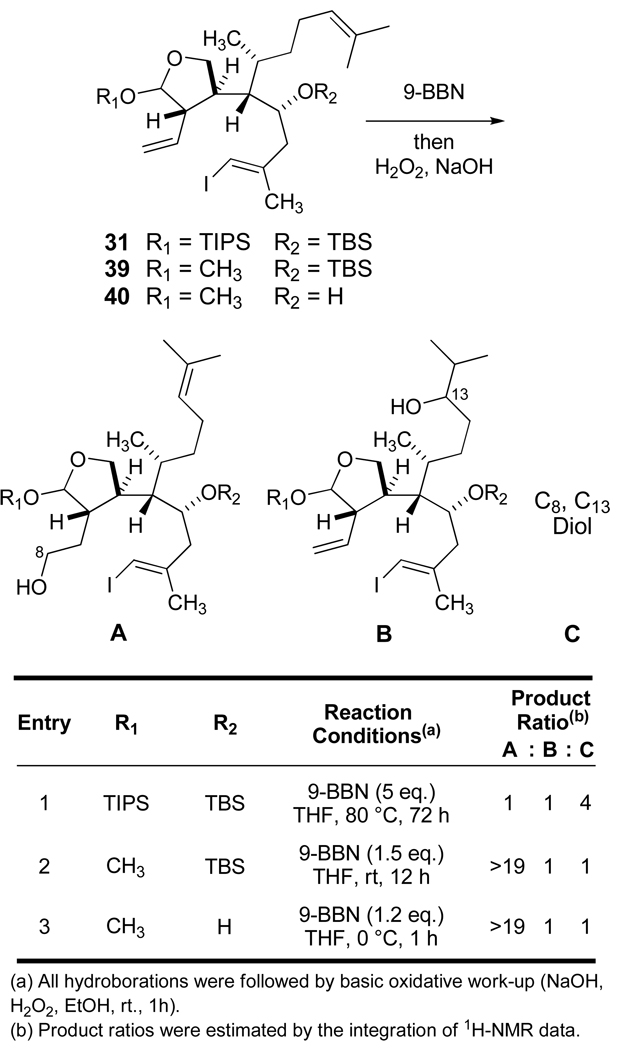
These evaluations are summarized in Figure 2, and illustrate results for hydroboration with complete consumption of starting material followed by the usual basic oxidative quench. The triisopropylsiloxy acetal 31 (entry 1) reacted slowly and required excess reagent at elevated temperature for 72 hours. An analysis of the product distribution confirmed a competing hydroboration of the trisubstituted C13–C14 alkene which led to substantial amounts of the C8, C13 diol (product C). Our modeling of 31 suggested that the remote silyl ethers at C4 and C19 imposed considerable steric hindrance blocking access to each face of the terminal olefin. Indeed, the removal of either of these silyl protecting groups led to more favorable reaction conditions and the chemoselective production of the expected primary alcohol (product A of Figure 2; see entries 2 and 3).
Figure 2.
Hydroboration Studies.
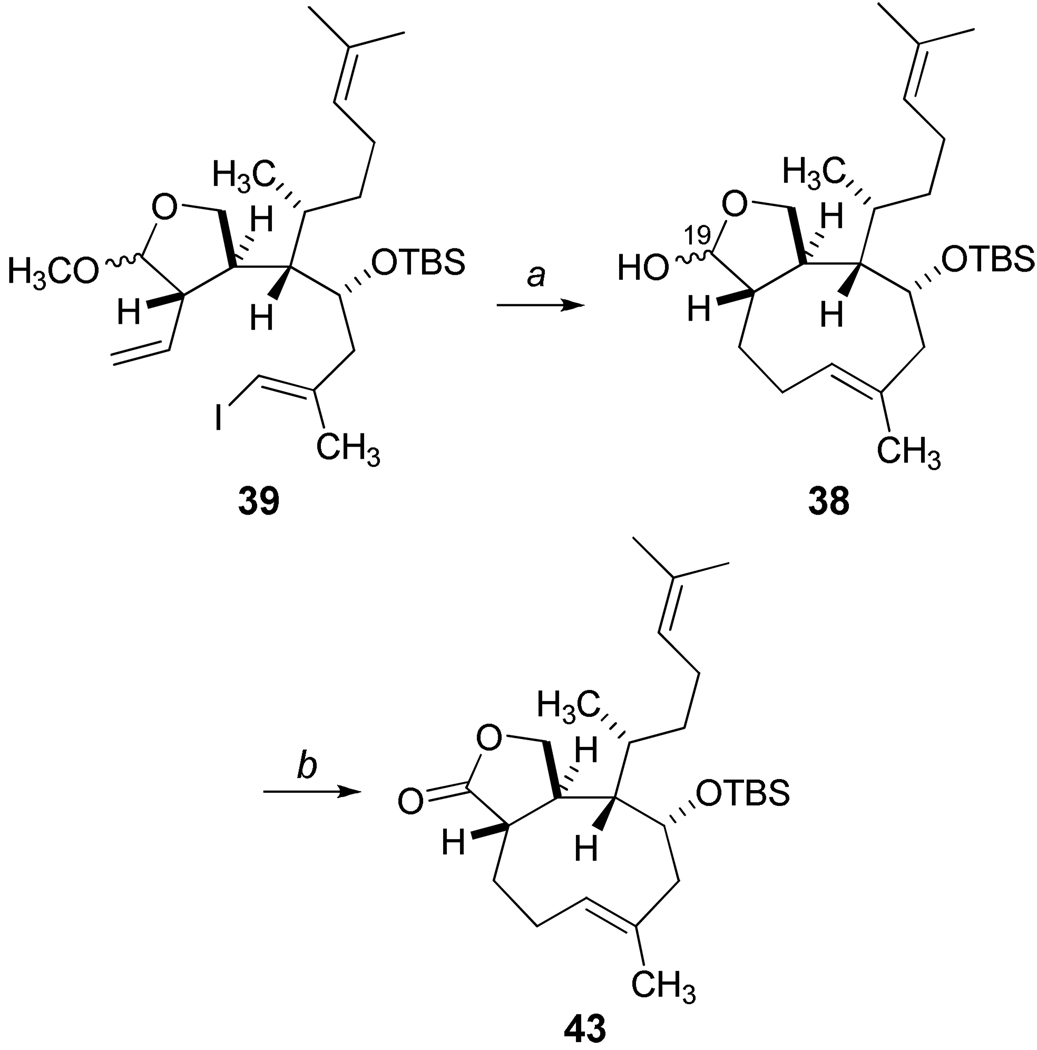
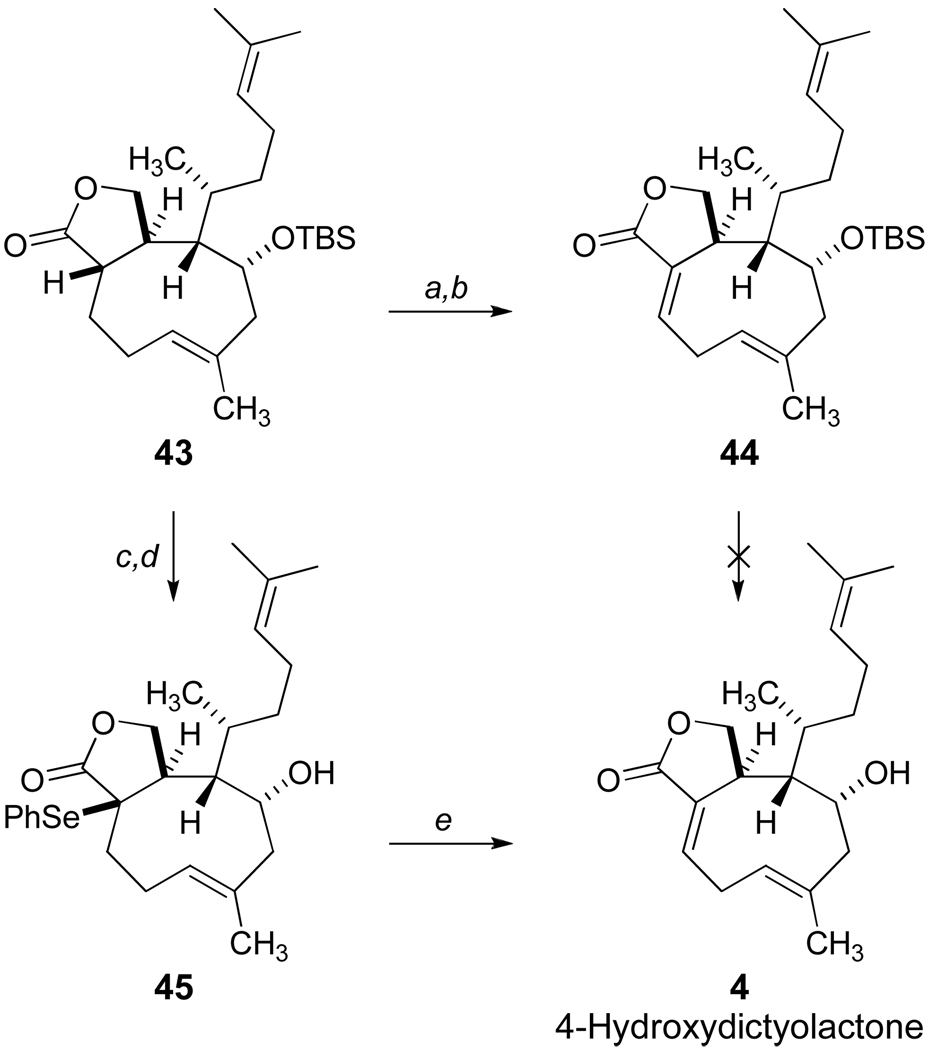
Based on these results, the B-alkyl Suzuki cyclization of 40 (Scheme 8) was examined with an improved outcome which generated approximately 65% mass recovery of the crude product 42 after flash silica gel chromatography. However, the starting alcohol 40 was susceptible to the internal ketalization as previously noted with the isolation of 29 of Scheme 6, and the cyclononene 42 demonstrated instability requiring repeated chromatography with diminishing yields. Thus, optimizations of the cyclization process focused on 39, and modest incremental improvements, including the use of thallium carbonate, aqueous THF (10:1 by volume) and optimized dilution conditions, afforded reproducible and scalable reactions at room temperature leading consistently to 30% yields of pure 41. Higher catalyst loading of PdCl2(dppf) and reaction attempts that screened a selection of other related catalysts did not lead to improved yields. On the other hand, a significant breakthrough was achieved when we explored the use of Pd(PPh3)4 as a catalyst for the Suzuki cyclization event (Scheme 9). Adapting conditions as described by Nakada and coworkers,42 the ring closure of 39 proceeded with surprising efficiency, and the crude product was treated with aqueous acetic acid to yield the diastereomeric lactols 38 (66% yield) after silica gel chromatographic purification. Subsequent oxidation of 38 under mild conditions produced the trans-fused cyclononene lactone 43.
Scheme 9.
Optimization of B-Alkyl Suzuki Cyclization.a
aReagents and Conditions: a) 9-BBN (1.5 quiv.), THF, rt,12 h, then Pd(PPh3)4 (0.5 equiv.), NaOH (5.0 equiv.), CH3CN/H2O (15:1) [0.005M], 85 °C, 18 h; then aq. AcOH, THF, 85 °C, 66%, dr = 80:20 at C19; b) TPAP, NMO, 4 Å mol. sieves, CH2Cl2, 79%.
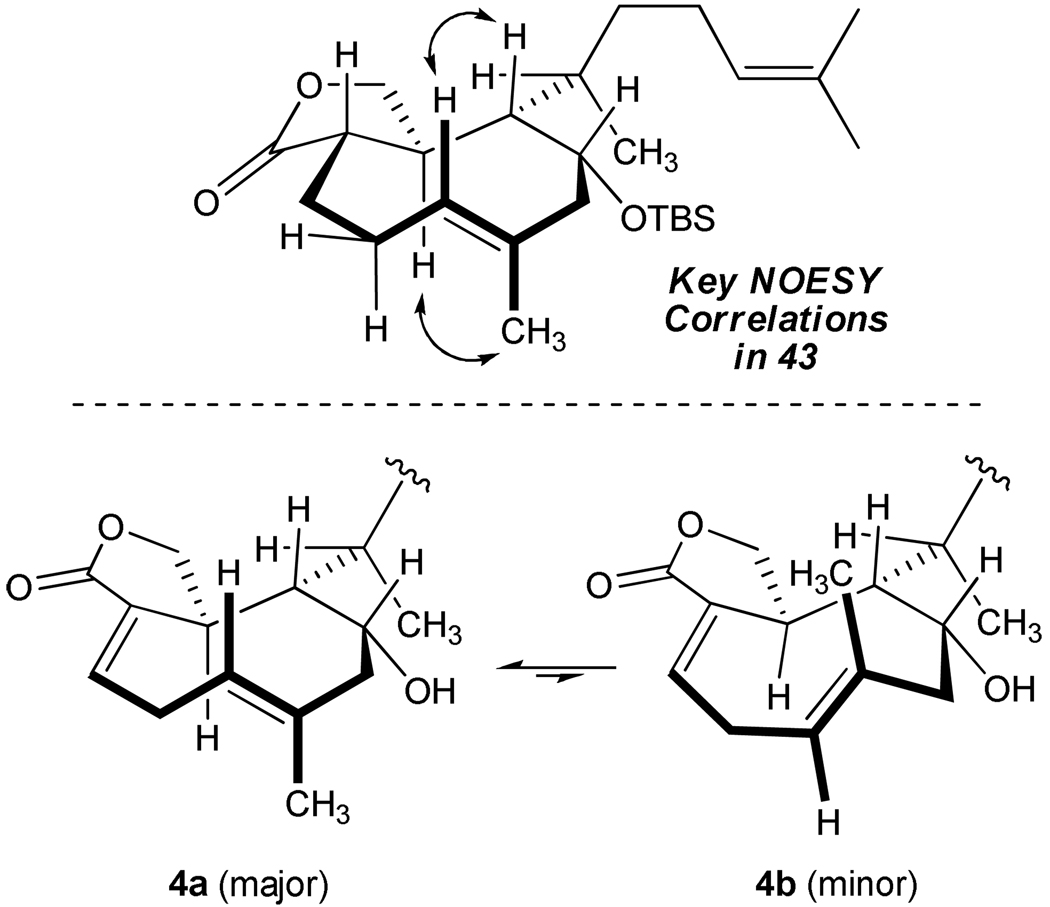
Our studies leading to the characterization of 43 suggested substantial conformational rigidity in this carbocyclic framework. Key NOESY correlations obtained from the 1H-NMR data are illustrated in Figure 3. Our conclusions parallel observations of the natural product itself which exists as two slowly equilibrating ring conformers 4a (major) and 4b (minor) at 23 °C (ratio 95:5).6b
Figure 3.
Ring Conformers of 43 and 4.
The completion of the synthesis of 4-hydroxydictyolactone (4) required a final oxidation for introduction of the C1/C9 unsaturation from cyclononene 43. As shown in Scheme 10, the syn-elimination of an intermediate selenoxide proved to be the most effective choice for this task. In fact, kinetic deprotonation of 43 led to a single phenylselenide (89% yield) and low temperature oxidation quantitatively produced the skipped cyclononadiene 44. Unfortunately, rapid decomposition of 44 was observed under basic conditions of fluoride-induced desilylation whereas 44 was found to be remarkably robust under acidic conditions. Finally, the total synthesis of 4 was completed via the initial deprotection of the C4 silyl ether 43 which was followed by formation of the α-phenylselenide 45 and oxidative elimination. Synthetic 4 proved to be identical in all respects, with the exception of optical rotation data, to naturally occurring 4-hydroxydictyolactone by comparisons with NMR spectra and HRMS data which were kindly provided by Professor Graziano Guella.43
Scheme 10.
Completion of the Total Synthesis of 4.a
aReagents and Conditions: a) LDA, THF, −78 °C, then PhSeBr; b) mCPBA, CH2Cl2, −78 °C, then Et3N, then warm to rt., 89% (2 steps); c) TBAF, THF, 40 °C, 83%; d) LDA, THF, −78 °C, then PhSeBr; e) mCPBA, CH2Cl2, −78 °C, then Et3N, then warm to rt., 55% (2 steps).
In conclusion, we have reported an efficient, enantiocontrolled total synthesis of 4-hydroxydictyolactone (4), a prototypical example of the xenicane family of marine diterpenes. Key features of our investigation include the use of an intramolecular Nozaki-Hiyama reductive SE' allylation of a formate ester for the facile stereoselective synthesis of five-membered lactols as well as an example of γ-chelation for an internally directed SE' propargylation using allenylmagnesium bromide. Finally, our studies have documented the development of the B-alkyl Suzuki cross-coupling reaction as a useful strategy for cyclizations to directly afford complex (E)-cyclononene systems.
Supplementary Material
Acknowledgements
This work is dedicated to Professor E. J. Corey for his pioneering efforts toward the caryophylloid terpenes. We gratefully acknowledge Indiana University for financial support of our work, as well as partial support from the National Institutes of Health (GM-42897).
Footnotes
Supporting Information Available. Full experimental details are included (103 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Finer J, Clardy J, Fenical W, Minale L, Riccio R, Battaile J, Kirkup M, Moore RE. J. Org Chem. 1979;44:2044–2047. [Google Scholar]
- 2.Vanderah DJ, Steudler PA, Ciereszko LS, Schmitz FJ, Ekstrand JD, Van der Helm D. J. Am. Chem. Soc. 1977;99:5780–5784. doi: 10.1021/ja00459a040. [DOI] [PubMed] [Google Scholar]
- 3.Some examples include:Tanaka J, Higa T. Chem. Lett. 1984;13:231–232.Matsumoto T, Enoki N, Ryoichi I. Chem. Lett. 1982;11:1749–1752.Ochi M, Masui N, Kotsuki H, Miura I, Tokoroyama T. Chem. Lett. 1982;11:1927–1930.El-Gamal AAH, Wang S-K, Duh C-Y. J. Nat. Prod. 2006;69:338–341. doi: 10.1021/np058093r.Miyaoka H, Mitome H, Nakano M, Yamada Y. Tetrahedron. 2000;56:7737–7740.
- 4.Faulkner DJ. Nat. Prod. Rep. 1984;1:251–280. [Google Scholar]
- 5.a) Ohtani I, Kusumi T, Ishitsuka MO, Kakisawa H. Tetrahedron Lett. 1989;30:3147–3250. [Google Scholar]; b) Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]
- 6.a) Guella G, Pietra F. J. Chem. Soc., Chem. Comm. 1993;20:1539. [Google Scholar]; b) Guella G, Chiasera G, N’Diaye I, Pietra F. Helv. Chim. Acta. 1994;77:1203–1221. [Google Scholar]
- 7.Miyamoto T, Takenaka Y, Yamada K, Higuchi R. J. Nat. Prod. 1995;58:924–928. doi: 10.1021/np50120a017. [DOI] [PubMed] [Google Scholar]
- 8.This activity is described in a patent report:Ninomya M, Matsuka S, Kawakubo A, Bito N. Jpn. Kokai Tokkyo Koho. 1995 07-285877.
- 9.Ishitsuka MO, Kusumi T, Kakisawa H. J. Org. Chem. 1988;53:5010–5013. [Google Scholar]
- 10.Cheng Y-B, Jang J-Y, Khalil AT, Kuo Y-H, Shen Y-C. J. Nat. Prod. 2006;69:675–678. doi: 10.1021/np058110c. [DOI] [PubMed] [Google Scholar]
- 11.Guella G, N’Diaye I, Chiasera G, Pietra F. J. Chem. Soc. Perkin Trans. 1;1993(14):1545–1546. [Google Scholar]
- 12.Adrianasolo EH, Haramaty L, Degenhardt K, Mathew R, White E, Lutz R, Falkowski P. J. Nat. Prod. 2007;70:1551–1557. doi: 10.1021/np070088v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Our preliminary results appear in the edited transcript of the IUPAC lecture entitled “Studies for the synthesis of marine natural products” presented at the 17th International Conference on Organic Synthesis (ICOS17), June 2008, Daejeon, Korea. See:Williams DR, Walsh MJ, Claeboe CD, Zorn N. Pure Appl. Chem. 2009;81:181–194. doi: 10.1351/PAC-CON-08-07-23.
- 14.a) Crimmins MT, McDougall PJ, Ellis JM. Org. Lett. 2006;8:4079–4082. doi: 10.1021/ol0615782. [DOI] [PubMed] [Google Scholar]; b) Crimmins MT, Brown BH, Plake HR. J. Am. Chem. Soc. 2006;128:1371–1378. doi: 10.1021/ja056334b. [DOI] [PubMed] [Google Scholar]; c) Maier ME. Angew. Chem. Int. Ed. 2000;39:2073–2077. doi: 10.1002/1521-3773(20000616)39:12<2073::aid-anie2073>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Paquette LA, Dong S, Parker GD. J. Org. Chem. 2007;72:7135–7147. doi: 10.1021/jo070862j. [DOI] [PubMed] [Google Scholar]
- 16.a) Takao K, Hayakawa N, Yamada R, Yamaguchi T, Morita U, Kawasaki S, Tadano K. Angew. Chem. Int. Ed. 2008;47:3426–3429. doi: 10.1002/anie.200800253. [DOI] [PubMed] [Google Scholar]; b) Baker TM, Edmonds DJ, Hamilton D, O’Brien CJ, Procter DJ. Angew. Chem. Int. Ed. 2008;47:5631–5633. doi: 10.1002/anie.200801900. [DOI] [PubMed] [Google Scholar]
- 17.Corey EJ, Mitra RB, Uda H. J. Am. Chem. Soc. 1964;86:485–492. [Google Scholar]
- 18.Larionov OV, Corey EJ. J. Am. Chem. Soc. 2008;130:2954–2955. doi: 10.1021/ja8003705. [DOI] [PubMed] [Google Scholar]
- 19.a) Liu G, Smith TC, Pfander H. Tetrahedron Lett. 1995;36:4979–4982. [Google Scholar]; b) Renneberg D, Pfander H, Leumann CJ. J. Org. Chem. 2000;65:9069–9079. doi: 10.1021/jo005582h. [DOI] [PubMed] [Google Scholar]
- 20.For a leading reference:Ireland RE, Wipf P, Armstrong JD. J. Org. Chem. 1991;56:650–657.
- 21.a) Tronchet JMJ, Gentile B. Helv. Chim. Acta. 1979;62:2091–2098. [Google Scholar]; b) Mann J, Weymouth-Wilson AC. Org. Synth. 1997;75:139. [Google Scholar]
- 22.Obtained via oxidation of (R)-(+)-citronellal (TCI, >95% (GC), [α]D20 +12.5° (neat)). See:Muto S, Bando M, Mori K. Eur. J. Org. Chem. 2004;9:1946–1952.
- 23.Okude Y, Hirano S, Hiyama T, Nozaki H. J. Am. Chem. Soc. 1977;99:3179–3181.Buse CT, Heathcock CH. Tetrahedron Lett. 1978;19:1685–1688.Hiyama T, Kimura K, Nozaki H. Tetrahedron Lett. 1981;22:1037–1040.For reviews, see:Cintas P. Synthesis. 1992;3:248–257.Fürstner A. Chem. Rev. 1999;99:991–1046. doi: 10.1021/cr9703360.
- 24.Keck and coworkers have described SmI2-mediated intramolecular reductive cyclization processes to afford 2-alkyl-2-methoxypyranyl motifs. See:Heumann LV, Keck GE. Org. Lett. 2007;9:1951–1954. doi: 10.1021/ol070573h.
- 25.We have explored an SE' allylation process to directly install the desired (E)-trisubstituted alkenylsilane moiety required for 9 of Scheme 1 with a high degree stereocontrol. See:Williams DR, Morales-Ramos ÁI, Williams CM. Org. Lett. 2006;8:4393–4396. doi: 10.1021/ol0613160.
- 26.For reviews of SE' reactions of organostannanes:Marshall JA. Chem. Rev. 1996;96:31–48. doi: 10.1021/cr950037f.Williams DR, Nag PP. Reactions of SE' Substitution for Organostannanes in Organic Synthesis. In: Davies AG, editor. Tin Chemistry. Chichester: John Wiley & Sons; 2008. pp. 515–560.
- 27.a) Ikeda N, Arai I, Yamamoto H. J. Am. Chem. Soc. 1986;108:483–486. doi: 10.1021/ja00263a020. [DOI] [PubMed] [Google Scholar]; b) Corey EJ, Yu CM, Kim SS. J. Am. Chem. Soc. 1989;111:5495–5496. [Google Scholar]
- 28.a) Hopf H, Böhm I, Kleinschroth J. Org. Synth. 1981;60:41. [Google Scholar]; b) Yamamoto H. Comp. Org. Synth. 1991;2:81–98. [Google Scholar]
- 29.a) Rand CL, Van Horn DE, Moore MW, Negishi E. J. Org. Chem. 1981;46:4093–4096. [Google Scholar]; b) Wipf P, Lim S. Angew. Chem. Int. Ed. 1993;32:1068–1071. [Google Scholar]
- 30.Tius MA, Trehan S. J. Org. Chem. 1986;51:765–767. [Google Scholar]
- 31.a) Apte S, Radetich B, Shin S, RajanBabu TV. Org. Lett. 2004;6:4053–4056. doi: 10.1021/ol048265w. [DOI] [PubMed] [Google Scholar]; b) Chenard BL, Laganis ED, Davidson F, RajanBabu TV. J. Org. Chem. 1985;50:3667–3669. [Google Scholar]; c) Mitchell TN, Wickenkamp R, Dicke AR, Schneider U. J. Org. Chem. 1987;52:4868–4874. [Google Scholar]
- 32.Miyaura N, Ishiyama T, Sasaki H, Ishikawa M, Sato M, Suzuki A. J. Am. Chem. Soc. 1989;111:314–321.b) For a review, see:Chemler SR, Trauner D, Danishefsky SJ. Angew. Chem. Int. Ed. 2001;40:4544–4568. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n.
- 33.Lipshutz BH, Lee C-T, Servesko JM. Org. Lett. 2007;9:4713–4716. doi: 10.1021/ol701999h. [DOI] [PubMed] [Google Scholar]
- 34.a) Williams DR, Robinson LA, Nevill CR, Reddy JP. Angew. Chem. Int. Ed. 2007;46:915–918. doi: 10.1002/anie.200603853. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Williams DR. Synthesis Studies of Dolabellanes and Transannular Processes Leading to Related Diterpenes. In: Harmata M, editor. Strategies and Tactics in Organic Synthesis. Vol. 7. Amsterdam: Elsevier; 2008. pp. 243–267. [Google Scholar]
- 35.Ohtsuka Y, Niitsuma S, Tadokoro H, Hayashi T, Oishi T. J. Org. Chem. 1984;49:2326–2332. [Google Scholar]
- 36.Mushti CS, Kim J-H, Corey EJ. J. Am. Chem. Soc. 2006;128:14050–14052. doi: 10.1021/ja066336b. [DOI] [PubMed] [Google Scholar]
- 37.Williams DR, Heidebrecht RW. J. Am. Chem. Soc. 2003;125:1843–1850. doi: 10.1021/ja0279803.b) Generated by reduction of VCl3(THF)3 with zinc dust:Freudenberger JH, Konradi AW, Pedersen SF. J. Am. Chem. Soc. 1989;111:8014–8016.
- 38.McMurry JE, Lectka T, Rico JG. J. Org. Chem. 1989;54:3748–3749.(b) For recent reviews:Ephritikhine M. J. Chem. Soc., Chem. Commun. 1998:2549–2554.
- 39.For a review, see:Molander GA. Chem. Rev. 1992;92:29–68.
- 40.a) Miyaura N, Ishikawa M, Suzuki A. Tetrahedron Lett. 1992;33:2571–2574. [Google Scholar]; b) Kallan NC, Halcomb RL. Org. Lett. 2000;2:2687–2690. doi: 10.1021/ol0062345. [DOI] [PubMed] [Google Scholar]; c) Chemler SR, Danishefsky SJ. Org. Lett. 2000;2:2695–2698. doi: 10.1021/ol0062547. [DOI] [PubMed] [Google Scholar]
- 41.a) Uenishi J, Beau J-M, Armstrong RW, Kishi Y. J. Am. Chem. Soc. 1987;109:4756–4758. [Google Scholar]; b) Frank SA, Chen H, Kunz RK, Schnaderbeck MJ, Roush WR. Org. Lett. 2000;2:2691–2694. doi: 10.1021/ol0062446. [DOI] [PubMed] [Google Scholar]
- 42.Kawada H, Iwamoto M, Utsugi M, Miyano M, Nakada M. Org. Lett. 2004;6:4491–4494. doi: 10.1021/ol0481939. [DOI] [PubMed] [Google Scholar]
- 43.We gratefully acknowledge Professor Graziano Guella of the University of Trento for his timely assistance in providing detailed authentic 1H and 13C NMR spectra and HRMS data for the natural product 4. Although our synthetic material precisely matched the NMR spectra and the HRMS data for 4-hydroxydictyolactone, we report some disparity for the data recorded for optical rotations. Guella and Pietra (ref. 6b) have described the optical rotation of naturally occurring 4 ([α]D25 −247° (c 0.17, CCl4)) whereas Tanaka and Higa (ref. 3a) had previously obtained a sample of 4 ([α]D −153° (c 2.01, CHCl3)) via the hydride reduction and Fetizon oxidation of 4-hydroxydictyodial. Although we have no reason to doubt the purity of our samples (> 95% purity), our optical rotation data for synthetic 4 ([α]D22 −175° (c 0.13, CCl4)) did not agree with the isolation reports.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.