Abstract
Despite converging evidence that major depressive illness is associated with both memory impairment and hippocampal pathology, findings vary widely across studies and it is not known whether these changes are regionally specific. In the present study we acquired brain MRIs (magnetic resonance images) from 31 unmedicated patients with MDD (major depressive disorder; mean age 39.2±11.9 years; 77% female) and 31 demographically comparable controls. Three-dimensional parametric mesh models were created to examine localized alterations of hippocampal morphology. Although global volumes did not differ between groups, statistical mapping results revealed that in MDD patients, more severe depressive symptoms were associated with greater left hippocampal atrophy, particularly in CA1 (cornu ammonis 1) subfields and the subiculum. However, previous treatment with atypical antipsychotics was associated with a trend towards larger left hippocampal volume. Our findings suggest effects of illness severity on hippocampal size, as well as a possible effect of past history of atypical antipsychotic treatment, which may reflect prolonged neuroprotective effects. This possibility awaits confirmation in longitudinal studies.
Keywords: antipsychotic, brain mapping, hippocampus, mood disorder, neuroimaging, subiculum, unipolar depression
Abbreviations: CA, cornu ammonis; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders 4th Edition; HDRS, Hamilton Depression Rating Scales; MDD, major depressive disorder; MRI, magnetic resonance imaging; SCID, Structured Clinical Interview for DSM-IV
INTRODUCTION
Memory deficits are one of the most consistently reported cognitive difficulties in both symptomatic and remitted patients with major depressive illness (Porter et al., 2003; Weiland-Fiedler et al., 2004; Bearden et al., 2006). Given the central role of the hippocampus in the formation and consolidation of new memories (Eichenbaum and Fortin, 2005), as well as its importance for the regulation of motivation and emotion (Davidson et al., 2002), hippocampal pathology is likely to be involved in the pathophysiology of the illness. Indeed, reduced hippocampal volume has been reported by many, but not all, neuroimaging studies of MDD (major depressive disorder) (Campbell and MacQueen, 2004; Videbech and Ravnkilde, 2004). Results of a recent meta-analysis indicated that differences in hippocampal volume were only apparent among MDD patients with a duration of illness longer than 2 years, or who had more than a single disease episode, suggesting that hippocampal volume reductions typically occur after disease onset in MDD patients (McKinnon et al., 2009).
However, a major limitation in most prior investigations is that patients were studied while on a variety of medications. The acute and long-term effects of psychotropic medications on brain structure are not well understood. There is recent evidence for neurotrophic or neuroprotective properties of lithium (Bearden et al., 2008; Yucel et al., 2008a), atypical antipsychotics (Jones et al., 2009; Thompson et al., 2009) and antidepressant medications (Sheline et al., 2003). Also, many patients with mood disorders take multiple medications, at various doses and with variable consistency over the course of the illness, and these effects are very difficult to quantify rigorously.
Finally, the question of regional specificity of hippocampal abnormalities in MDD has rarely been examined. Only one prior study, to our knowledge, has applied three-dimensional mapping methods to examine hippocampal morphology in depressed patients. In this study of patients with elderly depression, Ballmaier et al. (2008) found pronounced, localized surface contractions in patients with late-onset depression, relative to early-onset depression, although differences in overall hippocampal volumes were not detectable. Post-mortem studies have identified neuronal abnormalities in the subiculum, as well as in specific hippocampal subfields, with the most pronounced changes in the CA1 (cornu ammonis 1) region in the brains of depressed individuals (Rosoklija et al., 2000). The CA1 region sends significant output forward to the subiculum, which has direct connections to the entorhinal cortex and the amygdala. It also projects to the ventromedial prefrontal cortex, thalamus, hypothalamus and striatum, structures critically involved in mood regulation (Sapolsky, 2004).
To overcome limitations of prior investigations, we studied a group of relatively young unmedicated patients, diagnosed with MDD with no comorbidities. We used high-resolution MRI (magnetic resonance imaging) and a three-dimensional radial mapping approach to assess subregional structural deformations in the hippocampus. This technique (Thompson et al., 2004a) improves upon other methods in that it visualizes the spatial profile of neuropathological abnormalities, allowing more refined neuroanatomical localization of regionally specific alterations in depressed patients. We hypothesized that unmedicated depressed patients would exhibit localized alterations in hippocampal structure, relative to healthy comparison subjects, which would be most pronounced in the subiculum and CA1 subfields. Secondly, we examined relationships between hippocampal morphology and clinical variables (depression severity, family history, prior medication history and duration of illness). Given prior evidence that antidepressant and antipsychotic medications affect brain structure, we also explored the effects of previous treatment with these medications within the patient sample.
MATERIALS AND METHODS
Subjects
The present study was approved by the University of Pittsburgh Biomedical Institutional Review Board. All subjects provided written informed consent, after study procedures were fully explained. In total 31 unmedicated outpatients with MDD and 31 demographically matched healthy controls were studied (see Table 1). Some subjects in the present study were included in previous reports that focused on other brain structures (Brambilla et al., 2005; Caetano et al., 2006). At the time of participation in the study, all patients were off all psychotropic drugs for at least 2 weeks.
Table 1. Demographics of patients used in the present study.
Values are means±S.D. or percentages (n).
| Demographic | Unmedicated patients with unipolar depression (n = 31) | Healthy comparison subjects (n = 31) | Between-group differences |
| Age (years) | 39.2±11.9 | 36.7±10.7 | F (1,60) = 0.73P = 0.40 |
| Female (n) | 77% (24) | 77% (24) | X2 = 0P = 1.0 |
| Right-handed (n) | 100% (31) | 94% (29) | X2 = 2.07P = 0.49 |
| Education level (years) | 15.4±3.4 | 15.3±2.7 | F (1,60) = 0.55P = 0.40 |
| Race | |||
| Caucasian (n) | 100% (31) | 93% (29) | X2 = 1.0P = 0.60 |
| African-American (n) | 0 | 7% (2) | – |
| Other (n) | 3% (1) | 0 | – |
| HDRS | 11.8±9.1 | – | – |
| Duration of illness (years) | 11.42±10.6 | – | – |
| Age at onset (years) | 27.9±11.6 | – | – |
| Number of episodes | 5.1±5.9 | – | – |
| Current mood state | – | – | |
| Depressed (n) | 65% (20) | – | – |
| Euthymic (n) | 35% (11) | – | – |
| Family history positive (n) | 65% (20) | – | – |
| Medication naïve (n) | 58% (18) | – | – |
| Previous atypical antipsychotic use (n) | 16% (5) | – | – |
| Previous antidepressant use (n) | 42% (13) | – | – |
| Hippocampal volume (mm3) | – | – | |
| Right | 1911.1±280.1 | 1828.9±284.2 | F (1,59) = 2.03P = 0.16 |
| Left | 1885.4±230.8 | 1851.9±326.8 | F (1,59) = 2.26P = 0.14 |
Patients met DSM-IV (Diagnostic and Statistical Manual of Mental Disorders 4th Edition) diagnostic criteria for unipolar MDD, as determined by direct interview with the SCID (Structured Clinical Interview for DSM-IV) (Spitzer et al., 1994). Exclusion criteria were any DSM-IV axis I comorbidity, current medical problems, history of neurological illness, history of head trauma with loss of consciousness, substance or alcohol abuse within the 6 months preceding the study, or history of substance or alcohol dependence at any time. The BRMS (Bech–Rafaelsen Mania Scale) (Bech et al., 1979) and the HDRS (Hamilton Depression Rating Scales) (Hamilton, 1960) were used to rate clinical symptoms, and were administered within a week of the MRI scan. At the time of the MRI scan, 20 subjects (65%) were in a depressed mood state, whereas 11 (35%) were euthymic. Family history information was obtained by directly questioning patients and/or relatives, and by reviewing the medical records. On the basis of this information, first-degree relatives were considered positive for mood disorders if there was a past history of ever having received a diagnosis of unipolar major depression or bipolar disorder by a physician. Patients with at least one first-degree relative with a history of mood disorders were considered familial mood disorder patients.
Healthy controls had no DSM-IV axis I disorders, as determined by direct interview with the SCID-IV-NP (SCID-IV non-patient version). They had no current medical problems and no history of psychiatric disorder among first-degree relatives. The SCID-IV interviews for patients and controls were completed by a trained clinical social worker or a registered nurse at the University of Pittsburgh Outpatient Mood Disorders Clinic. After completion of the SCID-IV interview, psychiatric diagnoses were confirmed by a board-certified study psychiatrist.
MRI procedure
MRI scans were acquired with a 1.5T GE Signa Imaging System running version Signa 5.4.3 software (General Electric Medical Systems). A sagittal scout series was first obtained to verify patient position, image quality and locate a midline sagittal image. A T1-weighted sagittal scout image was obtained for graphical prescription of the coronal and axial images. Three-dimensional gradient echo imaging (Spoiled Gradient Recalled Acquisition, SPGR) was performed in the coronal plane [TR (repetition time), 25 ms; TE (echo time), 5 ms; FOV (field of view), 24 cm; slice thickness, 1.5 mm; NEX (number of excitations), 1; matrix size, 256 mm×192 mm] to obtain 124 images covering the entire brain. Additionally, a double echo-spin echo sequence was used to obtain T2-weighted and proton-density images in the axial plane, to screen for neuroradiological abnormalities.
Anatomical analysis
Individual brain volumes were reformatted in the axial plane, corrected for magnetic field inhomogeneities (Sled and Pike, 1998), resampled into 1-mm isotropic voxels and spatially realigned to the International Consortium for Brain Mapping non-linear average brain template (ICBM152), using FLIRT (available at http://www.fmrib.ox.ac.uk/fsl/). At the same time, image volumes were corrected for head-tilt and alignment with a three-translation and three-rotation rigid-body transformation (without scaling) (Woods et al., 1998), to ensure that brain measurements were not influenced by orientation. The hippocampi from each brain were traced using a software program (Tracer) (Woods, 2003), available at http://www.loni.ucla.edu/Software/Software_Detail.jsp?software_id = 10.
Hippocampi were manually traced bilaterally by a trained image analyst, who was blinded to all demographic variables, and had established excellent reliability with ‘gold standard’ ratings on a training set of six pairs of hippocampi (intraclass correlation coefficient ∼0.90). This level of agreement is comparable with that obtained in prior studies (Becker et al., 2006; Frisoni et al., 2006). Anatomical segmentation was performed using a standard neuroanatomical atlas of the hippocampus (Duvernoy, 1988) according to criteria detailed in Narr et al. (2004). Hippocampal models were delineated in contiguous coronal brain sections using standard guidelines (Pantel et al., 2000), including the hippocampus proper, dentate gyrus and subiculum [see (Becker et al., 2006; Frisoni et al., 2006) for further details]. Hippocampal borders were determined by the temporal horn, choroidal fissure, uncal and ambient cisterns, and the grey/white junction between the subiculum and parahippocampal gyrus. Anatomical landmarks were followed in all three orthogonal viewing planes using interactive segmentation software. Volumes obtained from these tracings were retained for statistical analyses.
To identify regional changes in hippocampal morphology, we used surface-based anatomical mesh modelling methods that allow for precise matching of anatomy between subjects and groups at each hippocampal surface point. To do this, a gridded surface is stretched over the hippocampus, using a rectilinear mesh of equally spaced three-dimensional points along the hippocampal axis and across the upper and lower surfaces. To assess global hippocampal differences, the volumes of these three-dimensional models were measured in cubic millimetres. To measure local differences, a three-dimensional medial curve is defined along the long axis of the hippocampus and radial distance measures (i.e. the distance from homologous hippocampal surface points to the central core of the individual's hippocampal surface model) are estimated, as previously described in Becker et al. (2006). This procedure also allows the averaging of hippocampal surface morphological features across all individuals belonging to a group and records the amount of variation between corresponding surface points relative to the group averages. These methods reveal tissue alterations on the hippocampal surface, e.g. in regions approximately corresponding to the underlying CA1–3 subfields and subiculum/presubiculum (Frisoni et al., 2006; Bearden et al., 2008), and are similar, in some respects, to the high-dimensional computational mapping approach developed for local shape analysis of the hippocampus (Csernansky et al., 2002).
Regressions were performed at each surface point to map linkages between radial size and covariates (i.e. diagnosis, age). In addition, we examined hippocampal parameters both with and without brain size correction in our statistical analyses, given that relationships between hippocampal size and brain size may differ across diagnostic groups, and our goal was to target differences specific to the hippocampus.
Uncorrected two-tailed probability values were mapped on to the averaged hippocampal surface models for the entire group and displayed in three dimensions. As statistical tests were applied at each of the hippocampal surface points, we conducted permutation-based statistics with a threshold of P<0.05 to ensure that the overall pattern of effects in the surface-based maps could not have been observed by chance alone (Thompson et al., 2004b). For this purpose, subjects were randomly assigned to either patient or control groups 100 000 times (while keeping the number of subjects in each group the same), and a new statistical test was performed at each hippocampal surface point for each random assignment. The number of significant results from these randomizations was then compared with the number of significant results in the true assignment to produce a corrected overall significance value for the uncorrected statistical maps. Permutation was conducted both for negative disease effects (control>MDD) and for positive disease effects (MDD>control).
RESULTS
Overall volumetric differences
The two groups did not differ in total brain volume, total grey matter volume or total white matter volume [F (1,60) = 0.11, P = 0.74; F (1,60) = 1.4, P = 0.24; and F (1,60) = 0.67, P = 0.42 respectively]. Hippocampal volumes did not differ significantly between controls and MDD subjects [left: 1851.9±326.8 compared with 1885.4±230.8 respectively, F (1,59) = 2.26, P = 0.14; right: 1828.9±284.2 compared with 1911.1±280.1 respectively, F (1,59) = 2.03, P = 0.16]. These results were not substantively different when controlling for total brain volume [F (1,58) = 1.98, P = 0.12].
Three-dimensional hippocampal maps
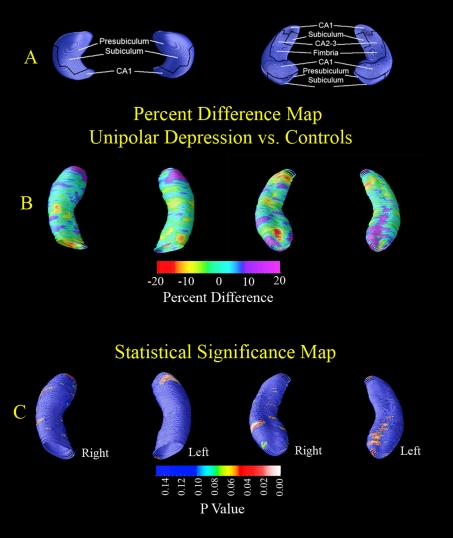
Statistical three-dimensional maps (Figure 1) indicated local differences in hippocampal structure between patients with MDD and control subjects, in terms of percentage difference and statistical significance. Although global volumes did not differ, localized increases (shown in purple) were detected in MDD patients in regions approximately corresponding to the CA1 subfields and portions of the subiculum bilaterally. However, these local differences were not significant following correction for multiple comparisons via permutation analysis (P = 0.10).
Figure 1. Three-dimensional hippocampal maps.
(A) Topographical correspondence of pathology on blank MRI-based models of the hippocampal formation of normal controls, from inferior (left) and superior (right) views. Based on Duvernoy (1988), where neuropathological areas are shown in seven equally spaced coronal slices spanning the entire length of the hippocampus. (B and C) Statistical three-dimensional maps indicate local differences in hippocampal structure between MDD patients and control subjects, in terms of percentage difference (B) and statistical significance (C). Purple colours indicate regions of localized increase in MDD patients compared with controls, whereas red colours indicate relative thinning in MDD patients relative to controls. Although overall volumes did not differ, localized increases (purple colours) were detected in MDD patients, in regions approximately corresponding to the CA1 subfields and portions of the subiculum/presubiculum bilaterally.
Effects of clinical variables
In the overall sample, hippocampal volume was inversely correlated with age (left: r = −0.27, P = 0.03; right: r = −0.32, P = 0.01). Within the depressed group alone, age showed a similar trend-level inverse relationship with hippocampal volume (r = −0.35, P = 0.055). In addition, HDRS scores were inversely correlated with hippocampal volume within the depressed group (r = −0.35, P≤0.05), i.e. a greater severity of depressive symptoms was associated with a lower hippocampal volume. To further examine the relationship between age, depression severity and hippocampal volume, we conducted a multiple regression analysis, using age and HDRS scores as predictors of hippocampal volume. The overall model was highly significant [F (1,28) = 6.31, P = 0.005], and there were significant main effects of both age and HDRS score, indicating that both of these factors made significant independent contributions to hippocampal size. Categorically, those patients who were currently in a depressed mood state (HDRS≥12) had significantly smaller hippocampal volumes than those who were in a remitted state [F (1,29) = 5.05, P = 0.03, eta2 = 0.15]. These results remained significant after controlling for the effects of age (P≤0.05).
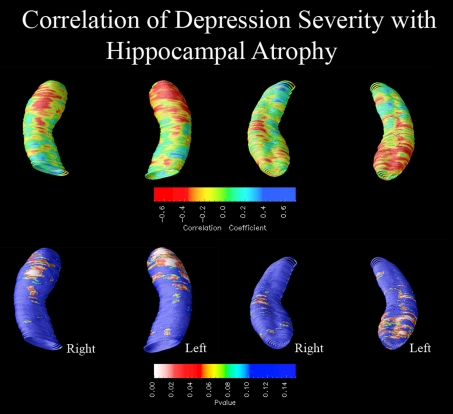
As age and depression severity were correlated, we examined linkages between age-adjusted HDRS score and hippocampal radial distance. Significant inverse correlations, confirmed by permutation tests, were observed between HDRS score and hippocampal surface morphology within the MDD group, such that more severe depression was associated with greater left hippocampal atrophy, particularly in the subiculum and CA1 subfields (permutation-corrected P values: right, P = 0.29; left, P = 0.009; Figure 2).
Figure 2. Correlation of hippocampal morphology with depression severity.
Three-dimensional statistical maps show significant relationships between age-adjusted HDRS score and regional hippocampal atrophy within the depressed group (left-hand depicts inferior view of the hippocampus, right-hand depicts superior view). In the significance maps (bottom panel), red and white colours denote P values ≤0.05. Greater depression severity was associated with greater left hippocampal atrophy, particularly in the subiculum and CA1 subfields (left, Pcorrected = 0.009; right, Pcorrected = 0.29).
Hippocampal structure was not significantly associated with duration of illness, the number of prior mood episodes, family history of mood disorder or past history of antidepressant use (P>0.10, not significant). However, MDD patients who had previously been treated with atypical antipsychotics showed a trend toward larger hippocampi than MDD patients who were antipsychotic-naïve (permutation-corrected P values: left, P = 0.06; right, P = 0.07; see Supplementary Figure S1 at http://www.asnneuro.org/an/001/an001e020add.htm).
DISCUSSION
The hippocampal maps in the present study provide novel findings regarding regional hippocampal alterations in unmedicated patients with major depression. Specifically, overall hippocampal volume was preserved in unmedicated depressed patients, concomitant with localized (non-significant) increases in CA1 subfields and portions of the subiculum bilaterally in depressed patients relative to controls. However, the sample was heterogeneous, and several clinical variables, particularly severity of depression and age, made independent contributions to hippocampal morphology and volume.
The notion that hippocampal volume reduction may be a consequence of depression has been influential in guiding recent animal, postmortem and clinical examinations of the pathophysiological basis of depression (Campbell and MacQueen, 2004). The findings of the present study support this, as we find that increased depression severity was associated with left hippocampal atrophy; however, hippocampal reduction did not characterize this sample of relatively young, currently unmedicated depressed outpatients overall. Indeed, we found a trend toward increased hippocampal volume in unipolar patients previously treated with atypical antipsychotics, which may reflect postulated neuroprotective effects of these agents (Thompson et al., 2009). Although caution is clearly warranted in interpreting these findings, given the small number of subjects previously treated with antipsychotics, these findings suggest that not only current drug treatment status, but past history of psychotropic medication usage, may be important in assessing structural neuroanatomical differences in patients with mood disorders.
In a large sample of bipolar patients, Jones et al. (2009) found that current antipsychotic use was associated with significantly larger temporal white matter volumes; specifically, bipolar subjects taking antipsychotics had larger white matter volumes than bipolar subjects not taking antipsychotics or healthy comparison subjects. Sheline et al. (2003) observed that longer duration of untreated depression was associated with hippocampal volume reduction, which provides some tentative evidence that antidepressants may have a neuroprotective effect. In addition, Frodl et al. (2008) found a significant left hippocampal volume increase in a subgroup of depressed patients who took antidepressants over a 3-year period. In contrast, Yucel et al. (2008b) found that medication-exposed patients with unipolar major depression, and those with multiple episodes, had smaller subgenual prefrontal cortical volumes than patients with no exposure to medication and healthy controls, suggesting that illness burden and short-term medication exposure may mediate brain alterations in anterior cingulate regions. Thus, although previous studies have observed effects of current medication use, this is the first study, to our knowledge, to suggest that prior use of atypical antipsychotics may have a persistent effect on hippocampal structure. While caution is clearly warranted in interpreting these trend-level findings, we felt that their inclusion was important to encourage investigation of such effects in other studies. To confirm this suggestive finding, longitudinal studies that assess the same individuals repeatedly over time are needed.
The results of the present study are generally consistent with a recent meta-analysis (McKinnon et al., 2009), which found that studies including young adult patients showed equivalent hippocampal volumes between MDD patients and controls, which may be due to reduced burden of illness in this population. In addition, other studies are consistent with ours in showing that specific clinical characteristics of the sample may affect neuroanatomical findings (Frodl et al., 2002; Ballmaier et al., 2008; MacQueen et al., 2008). Another recent study of medial temporal structures in major depressive illness (Keller et al., 2008) found that depressed patients with psychosis had a significantly smaller mean amygdala volume relative to depressed patients without psychosis and healthy comparison subjects, but no differences between depressed patients without psychosis and healthy comparison subjects. Similar to our findings, they observed no group differences in hippocampal volume. Nevertheless, our findings contrast with those of Alexander et al. (2005), who studied a comparably sized sample of unmedicated patients with major depressive illness, and found smaller posterior (but not anterior) hippocampal volume in clinically remitted MDD patients as compared with controls. Notable differences between our samples include symptomatic status, as Alexander and colleagues included only clinically remitted patients, whereas 65% of the patients in the present study were in a depressive episode at the time of investigation. In addition, although we had a similar proportion of subjects who were medication-naïve, no subjects in the Alexander et al. (2005) study had been previously treated with antipsychotics.
Hippocampal differences may be somewhat localized and difficult to detect in small, heterogeneous samples using global measures (McDonald et al., 2004). The identification of regional alterations in hippocampal structure may thus help to elucidate the underlying pathophysiological mechanisms associated with depression, and also indicate functional systems that may be selectively disturbed in the illness. Our findings indicated depression-associated atrophy in the left hippocampus that was particularly pronounced in the subiculum and CA1 subfields. Using a rigorous measurement protocol for tracing the hippocampus, Maller et al. (2007) reported differential volume loss in the tail of the hippocampus in MDD patients relative to healthy controls. This region approximately corresponds to the CA1 subfields that we found to be affected by depression severity. Notably, MacQueen et al. (2008) also found that, in patients with recurrent MDD, larger volume in the hippocampal body/tail (but not the head) was associated with better treatment response at 8 weeks, suggesting that localized hippocampal alterations may be associated with clinical response. In a study of late-onset depression using methods similar to ours, Ballmaier et al. (2008) found that regional surface contractions were significantly pronounced in late- relative to early-onset depression, particularly in the anterior of the subiculum and lateral posterior of the CA1 subfield in the left hemisphere. These findings, as well as our own, are consistent with postmortem studies, which have identified neuronal abnormalities in the subiculum in the brains of depressed individuals (Rosoklija et al., 2000), as well as in distinct layers of hippocampal subfields, with most pronounced changes in CA1 regions, followed by CA2 and CA3 subfields (Stockmeier et al., 2004). The CA1 and CA2 subfields may be particularly vulnerable to vascular damage (Duvernoy, 1988), which is consistent with findings of local volume reductions in late-onset depression (Ballmaier et al., 2008) and with the hypothesis that ischaemic small-vessel disease may be implicated in the pathogenesis of elderly depression (Lyness, 2002).
Certain limitations of the present study should be noted. First, only some of the subjects were medication-naïve. Although samples were small for subgroup analyses, we nevertheless found a significant association between regional hippocampal volume reduction and depression severity, as well as a suggestive relationship between prior treatment with atypical antipsychotics and hippocampal volume. As our study was cross-sectional, it cannot be ruled out that the observed group differences were attributable to other factors. However, it is tempting to speculate that these suggestive findings may reflect postulated effects of neuropil increase related to atypical antipsychotic treatment, manifested as subtle volumetric increases on MRI. Using the same methodology for hippocampal mapping in a sample of patients with bipolar disorder, we previously found that unmedicated bipolar patients showed localized deficits in the right hippocampus, in regions corresponding primarily to the CA1 subfields, as compared with both normal controls and lithium-treated bipolar patients (Bearden et al., 2008). Finally, we did not assess neurocognitive function in this sample, so the functional significance of these hippocampal alterations remains to be established.
One prior study (Ballmaier et al., 2008) has assessed the relationship between regional hippocampal morphology and memory performance in elderly depressed patients, using the CVLT (California Verbal Learning Test). This study found a strong correlation between delayed verbal memory and left-sided regional atrophy in the CA1 subfield and subiculum in patients with late-onset depression, which may resemble the patterns found in early Alzheimer's disease. However, these elderly depressed patients did not show deficits on memory measures relative to comparison subjects, suggesting that regional hippocampal atrophy patterns and their associations with memory performance could become apparent before clinical evidence of cognitive decline. Although it is unknown whether this sample of relatively young, unmedicated depressed patients suffered from memory impairment at the time of the present study, the patterns of localized atrophy we found in relation to depression severity are highly consistent with the regional findings of Ballmaier et al. (2008), suggesting that hippocampal CA1 subfields and the subiculum may be particularly vulnerable to the effects of depression. Hypercortisolaemia and ischaemia have both been hypothesized to contribute to hippocampal damage in major depression (MacQueen et al., 2003; Sheline et al., 2003). It has also been proposed that impaired neurogenesis may contribute to mood symptoms in major depression (Sapolsky, 2004). Although admittedly speculative, this may be the mechanism underlying our finding of more pronounced hippocampal deficits associated with increasing depression severity. However, whether these changes are reversible with symptomatic improvement is unknown. Longitudinal studies are clearly needed to better understand the time course of hippocampal changes in relation to symptomatic and cognitive changes in major depressive illness.
Online data
ACKNOWLEDGEMENTS
We wish to thank the study participants for making this work possible.
Footnotes
This work was supported by the National Institutes of Health [grant numbers K23 MH074644-01 (to C.E.B.), MH 01736, MH 30915, RR020571]; the Veterans Administration (VA Merit Review); and the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Foundation (Brazil).
REFERENCES
- Alexander N, Wood S, Bonne O, Nugent A, Luckenbaugh D, Young T, Bain E, Charney D, Drevets W. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Kaur S, Sanches M, Villarreal V, Bowden C, Soares JC. Sources of declarative memory impairment in bipolar disorder: mnemonic processes and clinical features. J Psychiatr Res. 2006;40:47–58. doi: 10.1016/j.jpsychires.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bearden C, Thompson P, Dutton R, Frey B, Peluso M, Nicoletti M, Diershke N, Hayashi K, Klunder A, Glahn D, Brambilla P, Sassi RB, Mallinger AG, Soares JC. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech–Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatr Scand. 1979;59:420–430. doi: 10.1111/j.1600-0447.1979.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Becker JT, Davis SW, Hayashi KM, Meltzer CC, Toga AW, Lopez OL, Thompson PM. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Glahn DC, Balestrieri M, Soares JC. Magnetic resonance findings in bipolar disorder. Psychiatr Clin North Am. 2005;28:443–467. doi: 10.1016/j.psc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshavan MS, Kupfer DJ, Frank E, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller JP, Miller MI. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- Davidson R, Pizzagalli D, Nitschke J, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The Human Hippocampus: An Atlas of Applied Anatomy. Munich, Germany: JF Bergman Verlag; 1988. [Google Scholar]
- Eichenbaum H, Fortin NJ. Bridging the gap between brain and behavior: cognitive and neural mechanisms of episodic memory. J Exp Anal Behav. 2005;84:619–629. doi: 10.1901/jeab.2005.80-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM. In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR-based study. Neuroimage. 2006;32:104–110. doi: 10.1016/j.neuroimage.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Frodl T, Jäger M, Smajstrlova I, Born C, Bottlender R, Palladino T, Reiser M, Möller HJ, Meisenzahl EM. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LD, Payne ME, Messer DF, Beyer JL, Macfall JR, Krishnan KR, Taylor WD. Temporal lobe volume in bipolar disorder: relationship with diagnosis and antipsychotic medication use. J Affect Disord. 2009;114:50–57. doi: 10.1016/j.jad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A, Schatzberg AF. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165:872–880. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness JM. The cerebrovascular model of depression in late life. CNS Spectr. 2002;7:712–715. doi: 10.1017/s1092852900008695. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17:1023–1027. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Pantel J, O'Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, Andreasen NC. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Trans Med Imaging. 1998;17:653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV. New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM. Mapping hippocampal and ventricular change in Alzheimer's disease. Neuroimage. 2004a;22:1754–1566. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Toga A. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage. 2004b;23(Suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Bartzokis G, Hayashi KM, Klunder AD, Lu PH, Edwards N, Hong MS, Yu M, Geaga JA, Toga AW, Charles C, Perkins DO, McEvoy J, Hamer RM, Tohen M, Tollefson GD, Lieberman JA. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19:1107–1123. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, Neumeister A. Evidence for continuing neuropsychological impairments in depression. J Affect Disord. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP. Multitracer: a Java-based tool for anatomic delineation of grayscale volumetric images. Neuroimage. 2003;19:1829–1834. doi: 10.1016/s1053-8119(03)00243-x. [DOI] [PubMed] [Google Scholar]
- Yucel K, Taylor VH, McKinnon MC, Macdonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2008a;33:361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Chahal R, Taylor VH, Macdonald K, Joffe R, MacQueen GM. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008b;33:3157–3163. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.