Abstract
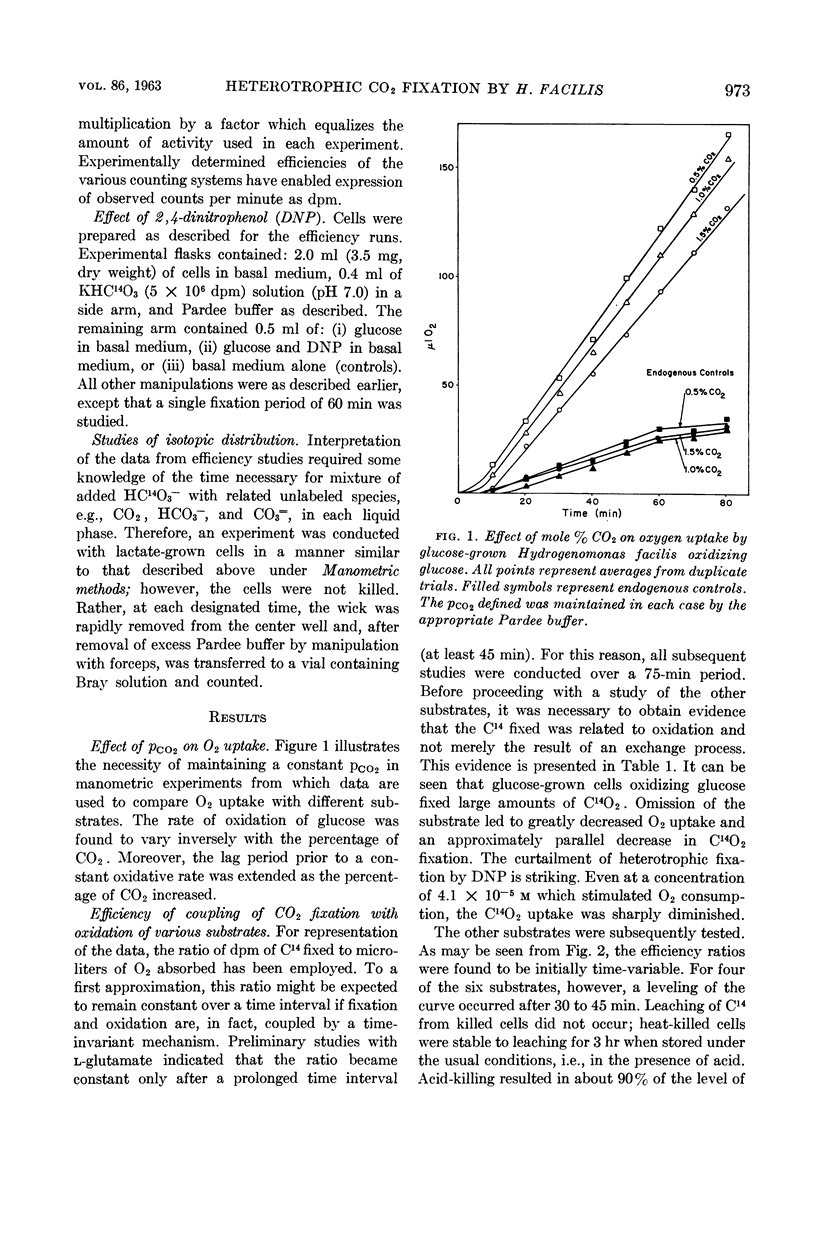
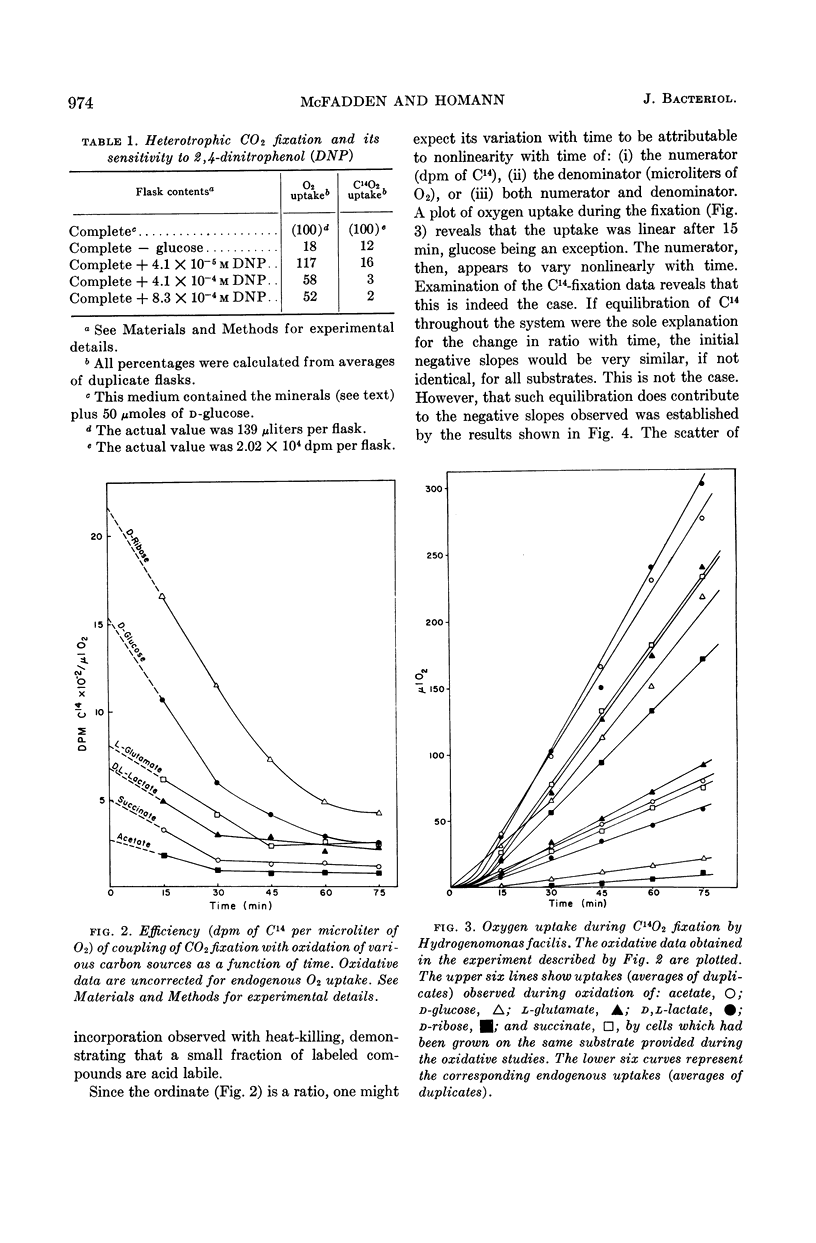
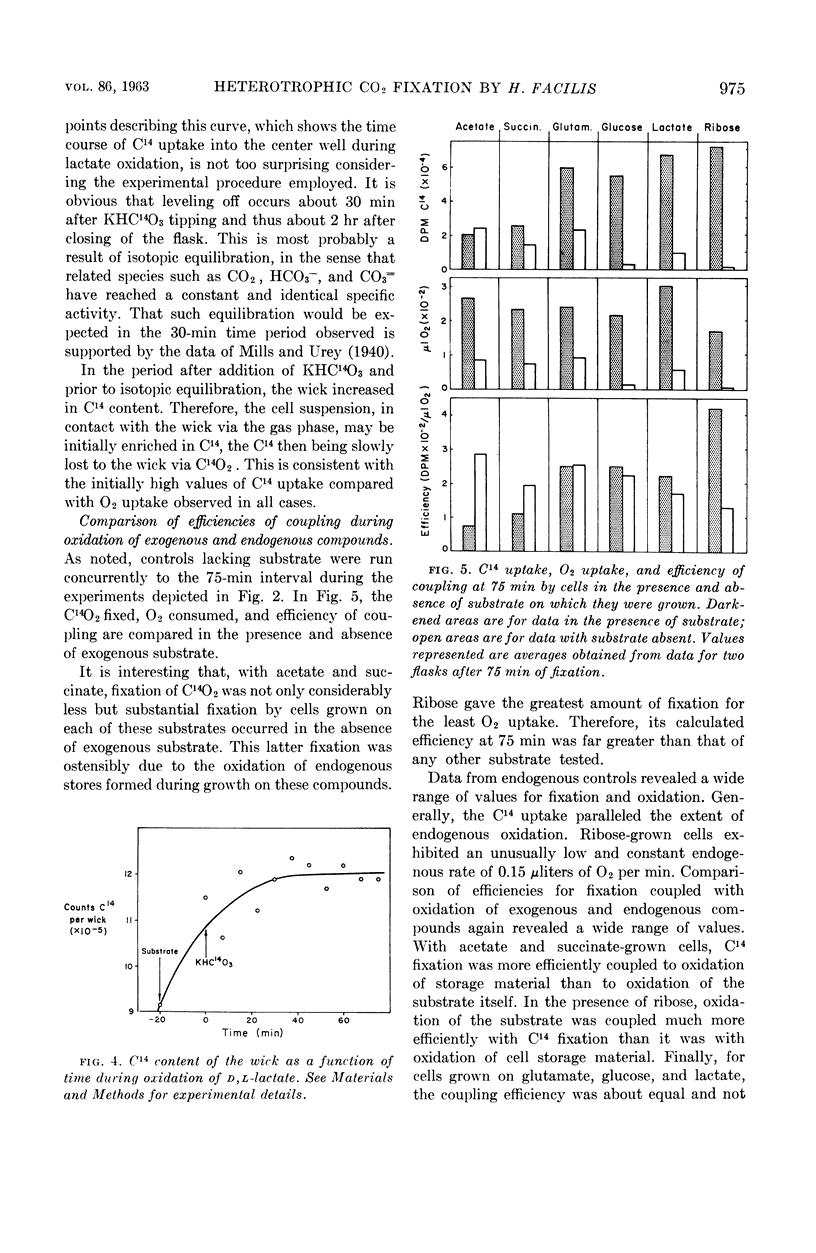
McFadden, Bruce A. (Washington State University, Pullman), and H. Robert Homann. Quantitative studies of the effect of organic substrates and 2,4-dinitrophenol on heterotrophic carbon dioxide fixation in Hydrogenomonas facilis. J. Bacteriol. 86:971–977. 1963.—Whole cells of Hydrogenomonas facilis under heterotrophic conditions fixed levels of C14O2 which depended upon the nature of the carbon source being oxidized. It was established that oxidative rates varied as a function of pCO2. Therefore, all studies were conducted in the presence of 1.5 mole% CO2 in the gas phase. With glucose-grown cells supplied with glucose as substrate, the heterotrophic fixation was curtailed 98% by the addition of 8.3 × 10−4m 2,4-dinitrophenol (DNP). A coupling between reductive fixation of CO2 and heterotrophic oxidation of substrate is consistent with the observed effect of DNP. The efficiency of coupling of fixation with oxidation was studied for acetate, d-glucose, l-glutamate, d,l-lactate, d-ribose, and succinate as substrates. Kinetic studies showed that the efficiency of coupling (expressed as disintegrations per minute of C14 per microliter of O2) was initially time-variable for all substrates; however, it approached a constant value after 30 to 45 min for acetate, glutamate, lactate, and succinate. The initial variation of the ratio with time was due primarily to C14O2 uptake, which was nonlinear with time. Control studies in the absence of exogenous substrate indicated that CO2 fixation may also be linked to oxidation of endogenous stores accumulated during heterotrophic growth. d-Ribose appears to be the most promising substrate for short-term fixation studies owing to the rapid incorporation of C14 and the unusually low endogenous fixation rate by cells grown on ribose. Calculations reveal that, after isotopic equilibrium has occurred, the amount of CO2 utilized during glucose oxidation is almost 50% of O2 uptake during the same interval. Even during succinate oxidation, which was shown to be coupled much less effectively with CO2 fixation, the CO2 utilized during the same interval is 8% of O2 uptake.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., BOLTON E. T., ALDOUS E. Utilization of carbon dioxide in the synthesis of proteins by Escherichia coli. I. J Biol Chem. 1952 Sep;198(1):165–172. [PubMed] [Google Scholar]
- ATKINSON D. E., MCFADDEN B. A. Use of membrane filters in the measurement of biological incorporation of radioactive isotopes. J Bacteriol. 1956 Jan;71(1):123–124. doi: 10.1128/jb.71.1.123-124.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMANN F. H., TOWNE J. C., BURRIS R. H. Assimilation of carbon dioxide by hydrogen bacteria. J Biol Chem. 1958 Jan;230(1):13–24. [PubMed] [Google Scholar]
- CALVIN M. The path of carbon in photosynthesis. Science. 1962 Mar 16;135(3507):879–889. doi: 10.1126/science.135.3507.879. [DOI] [PubMed] [Google Scholar]
- KELLY R. G., PEETS E. A., GORDON S., BUYSKE D. A. Determination of C-14 and H3 in biological samples by Schoeniger combustion and liquid scintillation techniques. Anal Biochem. 1961 Jun;2:267–273. doi: 10.1016/s0003-2697(61)80010-9. [DOI] [PubMed] [Google Scholar]
- LAFFERTY R. M. [Carbon dioxide fixation by organotropic bacteria]. Arch Mikrobiol. 1963;44:373–405. [PubMed] [Google Scholar]
- McFADDEN B. A. Some products of C1402 fixation by Hydrogenomonas facilis. J Bacteriol. 1959 Mar;77(3):339–343. doi: 10.1128/jb.77.3.339-343.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLEAN D. J., ROBINSON N. H., PURDIE E. F. The influence of the metabolic state and of the medium on carbon dioxide fixation by Serratia marcescens. J Bacteriol. 1951 May;61(5):617–626. doi: 10.1128/jb.61.5.617-626.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ A., BOVELL C., Jr Growth and hydrogenase activity of a new bacterium, Hydrogenomonas facilis. J Bacteriol. 1952 Jan;63(1):87–98. doi: 10.1128/jb.63.1.87-98.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., LAFFERTY R., STELLMACH-HELWIG R. [Quantitative measurements on CO2 incorporation by organotrophic bacteria]. Arch Mikrobiol. 1961;38:55–72. [PubMed] [Google Scholar]


