Abstract
Background/Aim
Slc26a6 (PAT1, CFEX) is a major chloride/base exchanger located on the apical membrane of the kidney proximal tubule. The purpose of the present study was to examine the effect of Slc26a6 deletion on the apical Na+/H+ exchanger 3 (NHE3) in the straight segment (S3) of the proximal tubule, which is the major site for the reabsorption of filtered chloride in the kidney.
Methods
The proximal tubule S3 segment was perfused and the intracellular pH and apical Na+/H+ exchanger activity and expression were measured.
Results
In the proximal tubule straight segments that were microperfused in vitro, baseline intracellular pH, measured by BCPCF-AM, was 7.10 ± 0.02 in Slc26a6–/– and 7.33 ± 0.02 in Slc26a6+/+ animals, a significant reduction in Slc26a6 mutant mice (p < 0.00001). The activity of the apical Na+/H+ exchanger was 0.49 ± 0.02 pH units/min in Slc26a6+/+ and 0.26 ± 0.03 pH units/min in Slc26a6–/– animals, a significant reduction in Slc26a6–/– mice (p < 0.0001). Formate-induced intracellular alkalinization, which is mediated via NHE3, was significantly blunted in Slc26a6–/– animals, with an alkalinization magnitude of 0.16 pH unit in Slc26a6–/– versus 0.37 in Slc26a6+/+ animals (p < 0.00001, n = 5 separate animals). Angiotensin II stimulation of NHE3 activity was intact in Slc26a6–/– animals. Buffering capacity was comparable in Slc26a6+/+ and Slc26a6–/– mice. Immunoblotting and immunofluorescent labeling demonstrated comparable NHE3 abundance and distribution in kidney proximal tubules of Slc26a6+/+ and Slc26a6–/– mice.
Conclusion
In conclusion, Slc26a6 deletion downregulates the apical Na+/H+ exchanger activity in the straight segment of the proximal tubule. The absence of a significant renal sodium loss in Slc26a6-null mice, despite NHE3 downregulation in the in vitro perfused tubules, points to possible activation of signaling pathways that can stimulate the apical Na+/H+ exchanger in vivo.
Key Words: Chloride absorption; Bicarbonate absorption; Na+/H+ exchanger 3; SLC26A6 (PAT1, CFEX) anion exchanger
Introduction
SLC26A6 (PAT1, CFEX) is a member of a large, conserved family of anion exchangers (SLC26) that encompasses at least 10 distinct genes [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. SLC26A6 shows significant homology with other members of the SLC26 family, including DRA (downregulated in adenoma; SLC26A3), pendrin (SLC26A4) and SLC26A9, all known chloride/base/bicarbonate exchangers [9, 11]. In humans, SLC26A6 maps to chromosome 3 and encodes a 738-amino acid protein [9]. Immunohistochemical studies localized SLC26A6 to the apical membranes of the kidney proximal tubule [11] and villi of the duodenum [13]. Functional studies in in vitro expression systems demonstrated that SLC26A6 can mediate Cl–/HCO3–, Cl–/oxalate, Cl–/hydroxyl and Cl–/formate exchanges [9, 11, 13, 15, 16], all known anion exchange activities described in apical membranes of the kidney proximal tubule and/or small intestine [17,18,19]. Microperfusion experiments demonstrated that oxalate-stimulated NaCl absorption is abolished and the apical Cl–/base exchanger activity is decreased in microperfused proximal tubules of Slc26a6-null mice [20], indicating that Slc26a6 is a major apical chloride/base exchanger in the kidney proximal tubule [20].
The kidney proximal tubule is comprised of 3 anatomically and physiologically distinct segments, with the first 2 segments (S1 and S2) predominantly involved in bicarbonate reabsorption and the last segment (S3) mainly responsible for the reabsorption of filtered chloride [21]. The chloride reabsorption in the S3 segment occurs via parallel activation of apical Na+/H+ exchange and Cl–/base exchange [17, 18]. The purpose of the present study was to examine the effect of Slc26a6 deletion on the apical Na+/H+ exchanger (NHE) activity in the S3 segment of microperfused kidney proximal tubule.
Experimental Procedures
Animals
Slc26a6–/– and Slc26a6+/+ littermate mice [20] were bred from Slc26a6 heterozygote (+/–) breeding pairs. Animals were 3–4 months old and were predominantly male. They were euthanized with the use of anesthetics (pentobarbital sodium) according to the institutional guidelines and approved protocols.
Proximal Tubule Isolation, in vitro Microperfusion and Intracellular pH Measurement
Isolation and microperfusion of proximal tubules in Slc26a6+/+ mice and Slc26a6–/– mice were performed according to established protocols and as described [19, 20]. Intracellular pH (pHi) was measured using 2',7'-bis-(3-carboxypropyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCPCF-AM), as described before [19, 20, 22, 23]. The probe was excited at 488 and 440 nm and emission was measured at 520 nm. Regions of interest were placed in the individual cells and several cells per single tubule analyzed [24]. Only one tubule per animal was analyzed. Digitized images were analyzed with the Attograph software (BD Bioscience, Rockville, Md., USA). Intracellular calibration was performed with the high K+/nigericin method [19, 20, 22, 23].
The apical NHE was assayed as the rate of pHi acidification [change in pHi per change in time (dpHi/dt)] upon the removal of perfusate sodium as well as the rate of intracellular alkalinization (dpHi/dt) upon the return of perfusate sodium. The switching of the perfusate from the sodium-containing to sodium-free solution results in the reversal of the apical NHE direction and causes the exchange of luminal acid for cellular sodium. One tubule per animal was used. The replacement of the sodium-free with the sodium-containing perfusate results in intracellular alkalinization by exchanging the cellular acid for the luminal sodium. The rate of NHE activity was measured as the steep phase of the recovery from acidic pHi. For microperfusion, the following solutions were used for baseline pHi measurement: for experiments without CO2/HCO3–, both the perfusate and bath solution consisted of 140 mM NaCl, 2.5 mM K2HPO4, 1.2 mM MgSO4, 5.5 mMD-glucose, 6 mML-alanine, 5 mM HEPES, and 2 mM CaCl2; for experiments with CO2/HCO3–, the perfusate and bath solution consisted of 115 mM NaCl, 25 mM NaHCO3 2.5 mM K2HPO4, 1.2 mM MgSO4, 5.5 mMD-glucose, 6 mML-alanine, 1 mM Na citrate, 4 mM Na lactate, and 2 mM CaCl2; for experiments without bicarbonate, the solutions were gassed with 100% O2, and for experiments with bicarbonate, the solutions were gassed with 95% O2/5% CO2. All solutions had a pH value of 7.4 at 37°C. The rationale for using early proximal tubule perfusate in the S3 segment was to avoid changes in pHi, as late proximal tubule perfusate has a lower bicarbonate concentration and thus a lower pH value (approx. 6.8). Introduction of this latter solution to the tubule lumen lowers the pHi and, therefore, artificially alters proton and bicarbonate gradients. For apical NHE activity measurement, the perfusate was switched to a Na-free perfusate in which NaCl was replaced with tetramethylammonium chloride (TMACl). For formate stimulation of apical Na+/H+ exchange, 100 μM formate was added to the bath and pHi was monitored. The intracellular alkalinization of proximal tubule cells in response to formate exposure is mediated via apical Na+/H+ exchanger 3 (NHE3) [24]. Formate was added only to the basolateral side in order to prevent any interference of formate uptake by the apical Slc26a6, which has been shown to transport formate in vitro [11]. All pHi measurements were performed in the absence of CO2/HCO3– in the media.
For apical Cl–/OH– exchange measurement, the perfusate was sequentially switched to chloride-free and chloride-containing solutions [19, 20]. Exposing the tubule to a chloride-free perfusate results in intracellular alkalinization and switching back to a chloride-containing solution results in the return of the alkaline pH to baseline. The Cl–/OH– exchanger activity was assessed by the rate of the intracellular alkalinization upon switching to the chloride-free solution. For chloride-free experiments, chloride-containing solutions were replaced with gluconate and calcium was adjusted at 4 mM.
Buffer Capacity
The intrinsic cell buffer capacity (βi) was measured with the NH4Cl prepulse technique according to established protocols [24]. We used Na-free solutions to block sodium-dependent transporters. NaCl was replaced with the equimolar amount of TMACl. DIDS, at 1 mM, was added to the bath and luminal side to keep the chloride-dependent transporters inactive. In NH4Cl-containing solutions, 20 mM NH4Cl replaced equimolar concentration of TMACl. Equivalent base flux (EBF) was calculated as dpHi/dt × βi × V and expressed in picomoles per millimeter of tubule per minute, where βi is the intrinsic cell buffer capacity in bicarbonate-free solutions. V designates cell volume per tubule length calculated according to the formula V = π[(do)2 – (di)2]/4. do and di stand for the outer and inner tubule diameter. Tubule diameters were measured at ×4,000 magnification with an eyepiece reticle.
Immunoblot Analysis of NHE3
Brush border membrane proteins were isolated from the kidney cortex according to established protocols [25], resolved by SDS-PAGE (50 μg/lane) and transferred to the nitrocellulose membrane. The membrane was blocked with 5% milk proteins, and then incubated for 6 h with antibodies against NHE3 [26]. The secondary antibody was a donkey anti-rabbit IgG conjugated to horseradish peroxidase (Pierce, Rockford, Ill., USA). The results were visualized using a chemiluminescence method (SuperSignal Substrate, Pierce, Rockford, Ill., USA) and captured on light-sensitive imaging film (Kodak, Rochester, N.Y., USA). β-Actin abundance was determined by western blotting and the results were expressed as NHE3/β-actin.
Immunofluorescence Labeling Studies
Mice were euthanized and kidneys were removed, cut in tissue blocks, and fixed in formaldehyde solution overnight at 4°C [27, 28]. The tissue was frozen on dry ice, and 6-μm sections were cut with a cryostat and stored at −80°C until used. For staining, cryosections were washed twice in 0.01 M PBS (pH 7.4) and blocked with 10% goat serum for 30 min at room temperature. Cells were permeabilized in 0.3% Triton X-100/PBS solution for 45–60 min. Primary antibodies against NHE3 were diluted 1:40 in 0.3% Triton X-100/PBS solution and applied to sections overnight at room temperature. Sections treated with the primary antibodies were rinsed twice in 0.01 M PBS for 10 min and then incubated with a secondary antibody for 2 h at room temperature. Alexa Fluor 488 (green) or Alexa Fluor 568 (red) goat anti-rabbit antibody was used at 1/200 as secondary antibodies. After washing, sections were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif., USA). Sections were examined on the epifluorescent microscope Eclipse 600 (Nikon Bioscience, Melville, N.Y., USA) equipped with a SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, Mich., USA).
RNA Isolation and Northern Blot Hybridization
Total cellular RNA was extracted from kidneys according to established methods, quantitated spectrophotometrically, and stored at −80°C. Total RNA samples (30 μg/lane) were fractionated on a 1.2% agarose/formaldehyde gel, transferred to Magna NT nylon membranes, cross-linked by UV light and baked. Hybridization was performed according to established protocols [29]. The membranes were washed, blotted dry and exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif., USA). A 32P-labeled NHE8 or renin cDNA fragment was used for northern hybridizations. For NHE8, a PCR fragment encoding the nucleotides 396–1116 from mouse cDNA (Genbank No. NM_148929) was used. The primer sequence for the mouse NHE8 was comprised of NHE8–396: TCCTTCTCTTGCTCCCACCTATTATC and NHE8–1116: CCAAGAAATGCAAACACACACG. For renin, a PCR fragment encoding the nucleotides 1033–1277 from mouse cDNA was used.
Materials
32P-dCTP was purchased from New England Nuclear (Boston, Mass., USA). Nitrocellulose filters and other chemicals were from Sigma Chemical Co. (St. Louis, Mo., USA). RadPrime DNA labeling kit was purchased from Gibco BRL (Gaithersburg, Md., USA), and BCECF was from Molecular Probes Inc. (Eugene, Oreg., USA).
Statistical Analyses
Values are expressed as mean ± SEM. Statistical analysis was performed using the Student t test or ANOVA; p < 0.05 was considered statistically significant.
Results
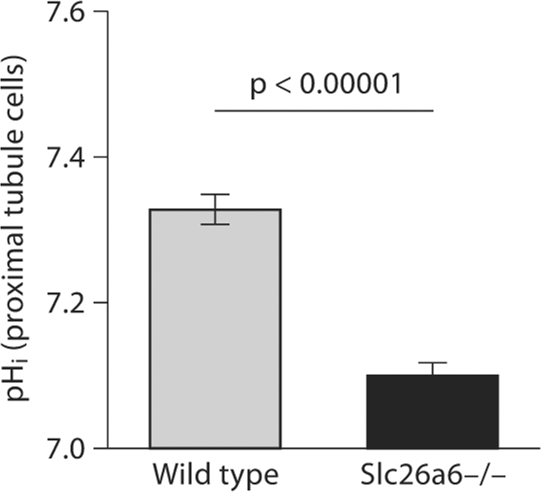
In the first series of experiments, the pHi was measured in microperfused proximal tubules in the absence of CO2/HCO3– in bath and perfusate. As demonstrated in figure 1, baseline pHi was 7.33 ± 0.02 in Slc26a6+/+ animals and 7.10 ± 0.02 in Slc26a6–/– animals, a significant reduction in mice with Slc26a6 deletion (p < 0.00001; 45 cells in 6 separate Slc26a6+/+ animals and 43 cells in 6 Slc26a6–/– animals). The reduction in baseline pHi was also evident in the presence of CO2/HCO3– in bath and perfusate (data not shown).
Fig. 1.
Baseline pHi in the kidney proximal tubule in Slc26a6+/+ and Slc26a6–/– mice; pHi was significantly decreased in the kidney proximal tubule in Slc26a6-null mice.
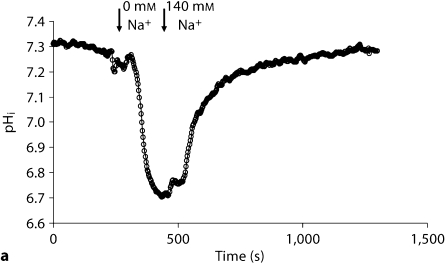
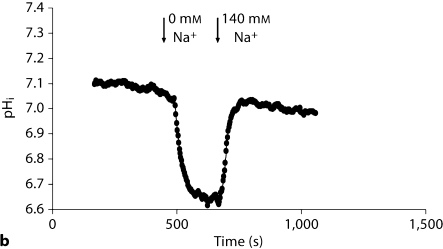
In an attempt to determine the basis of decreased pHi, we examined the activity of the apical NHE, which is predominantly mediated via NHE3, in the proximal tubule. Following the stabilization of baseline pHi, the perfusate was switched to a sodium-free solution. Representative tracings in figure 2a demonstrate rapid intracellular acidification upon switching to the sodium-free perfusate (which reflects NHE activity) in Slc26a6+/+ mice. The pHi returned to baseline levels upon switching back to the sodium-containing perfusate (fig. 2a). The representative tracings in figure 2b are from Slc26a6–/– mice and demonstrate significant blunting of cell acidification in response to sodium withdrawal. Summation of the results demonstrated significant reduction in the rate of NHE activity in Slc26a6–/– (0.26 ± 0.03 pH units/min) versus Slc26a6+/+ mice (0.49 ± 0.02 pH units/min) (p < 0.0001; 39 cells/5 Slc26a6+/+ animals and 37 cells/5 Slc26a6–/– mice). We also measured the rate of apical NHE-mediated intracellular alkalinization upon switching the perfusate to the Na-containing solution. The results showed a significant reduction in apical NHE activity in Slc26a6–/– mice (0.301 ± 0.03 pH units/min; 37 cells/5 animal tubules) versus Slc26a6+/+ mice (0.79 ± 0.07 pH units/min; 39 cells/5 animal tubules) (p < 0.001).
Fig. 2.
Representative tracings depicting apical NHE activity in isolated microperfused proximal tubules in Slc26a6+/+ (a) and Slc26a6–/– (b) mice. The rate of cell acidification was blunted in Slc26a6-null mouse.
The buffering capacity in the absence of bicarbonate was 30.8 ± 5.65 mM/pH (13 cells in 2 tubules) in Slc26a6+/+ mice versus 26.4 ± 5 mM/pH (7 cells in 2 tubules) in Slc26a6–/– mice (p > 0.05). These results indicate that the buffering capacity was comparable in the straight segment of the proximal tubule cells in wild-type and mutant mice.
The NHE-mediated EBF during the intracellular acidification – induced in response to the luminal sodium removal – was 27.45 ± 1.2 pmol/mm/min in Slc26a6+/+ and 15.01 ± 1.1 pmol/mm/min in Slc26a6–/– mice (p < 0.0001). The NHE-mediated EBF during the recovery from intracellular acidosis – induced in response to the luminal sodium addition – was 30.57 ± 3.01 pmol/mm/min in Slc26a6+/+ and 15.62 ± 1.14 pmol/mm/min in Slc26a6–/– mice (p < 0.0001). Cell volume was comparable in proximal tubule cells in Slc26a6+/+ and Slc26a6–/– mice, with values of 1.465 ± 0.02 and 1.442 ± 0.03 nl/mm in wild-type and Slc26a6 knockout mice.
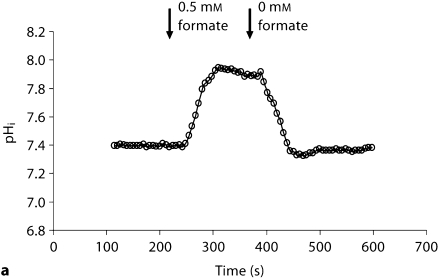
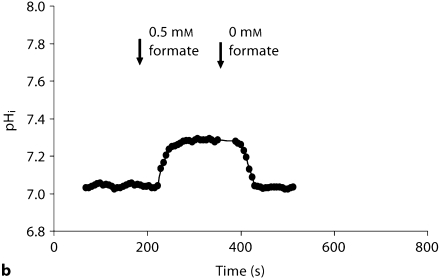
Recent studies indicate that formate can stimulate apical NHE3 and cause intracellular alkalinization in the kidney proximal tubule [24]. We next tested whether formate-induced intracellular alkalinization, mediated via NHE3, is intact in Slc26a6–/– animals. Toward this end, pHi was continuously monitored in microperfused kidney proximal tubules before and after the addition of 100 M formate to the bath. Representative tracings in figure 3 display intracellular alkalinization following the addition of formate. As indicated, the magnitude of cell alkalinization was significantly enhanced in Slc26a6+/+ mouse. Summation of the results demonstrated that Slc26a6–/– mice have blunted alkalinization in response to formate (0.16 ± 0.01 pH units, 23 cells in 5 Slc26a6–/– animals vs. 0.37 ± 0.02 pH units, 12 cells in 3 Slc26a6+/+ animals), consistent with a significant reduction in NHE3 activity in Slc26a6-null mice (p < 0.00001).
Fig. 3.
Representative tracings depicting formate-induced intracellular alkalinization in the kidney proximal tubule (NHE activity) in Slc26a6+/+ (a) and Slc26a6–/– (b) mice. The magnitude of formate-induced cell alkalinization in the proximal tubule is blunted in Slc26a6-null mice.
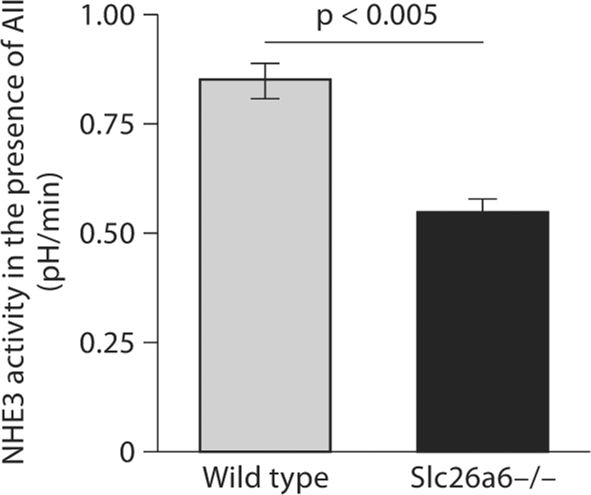
The activation of the apical NHE by angiotensin II (AII) was examined next in Slc26a6+/+ and Slc26a6–/– mice. Due to the length of the experiments needed to monitor NHE activity, and the decreased viability of proximal tubular cells with time, the effect of AII versus a diluent (vehicle) on apical NHE activity was examined in different tubules. The tubules were either exposed to AII or a vehicle and apical NHE activity was measured. For the experiments, AII at 1 nM was added to the bath, and the perfusate was switched to the sodium-free solution. The rate of intracellular acidification was determined and compared in both groups. Summation of the results in figure 4 depicts apical NHE activity in the presence of AII in Slc26a6+/+ (0.85 ± 0.04 pH units/min) and Slc26a6–/– mice (0.55 ± 0.03 pH units/min). Comparison of these results with the results of the experiments in figure 2 (see above), which were performed in the absence of AII, demonstrates that AII stimulation of the apical NHE is intact and may be more robust in Slc26a6-null mice.
Fig. 4.
AII stimulation of NHE3 activity in the kidney proximal tubules. NHE3 activity is stimulated by AII in Slc26a6+/+ and Slc26a6–/– mice (vs. no AII in fig. 3).
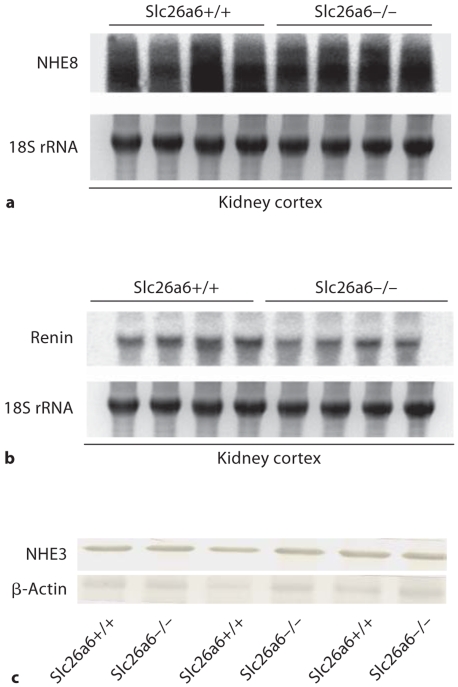
To determine whether the downregulation in apical NHE activity in Slc26a6-null mice is due to the reduction in the expression of known apical NHEs in the proximal tubule, northern hybridizations and/or western blottings were performed. Figure 5a depicts the mRNA expression of NHE8 in the kidneys of Slc26a6+/+ and Slc26a6–/– mice. NHE8 mRNA expression levels were 105 ± 16% in Slc26a6+/+ and 98 ± 8% in Slc26a6–/– animals (p > 0.05, n = 4), when the NHE8 expression levels were adjusted for RNA loading determined by 18S rRNA levels. Figure 5b displays the expression of renin mRNA in the kidneys of Slc26a6+/+ and Slc26a6–/– mice. The results demonstrated comparable renin expression levels in the kidneys of Slc26a6–/– versus Slc26a6+/+ animals (115 ± 11% in Slc26a6+/+ vs. 102 ± 8% in Slc26a6–/– mice, p > 0.05, n = 4).
Fig. 5.
Expression of NHE3, NHE8 and renin in the kidneys of Slc26a6+/+ and Slc26a6–/– mice. a NHE8 northern hybridization. b Expression of renin in the kidneys of Slc26a6+/+ and Slc26a6–/– mice. c NHE3 western blotting. Brush border membrane vesicles isolated from kidney cortices of Slc26a6+/+ and Slc26a6-null mice were blotted against NHE3 antibody. NHE3 abundance was comparable in the cortex of Slc26a6+/+ and Slc26a6-null animals.
Figure 5c shows the results of an immunoblot analysis of NHE3 in brush border membrane vesicles isolated from the kidney cortex and demonstrates comparable abundance in Slc26a6+/+ and Slc26a6–/– mice. When adjusted for protein loading by blotting with β-actin antibody, the abundance of NHE3 was estimated at 1.03 ± 0.08-fold in Slc26a6–/– versus 1.10 + 0.11-fold in Slc26a6+/+ mice (p > 0.05, n = 3).
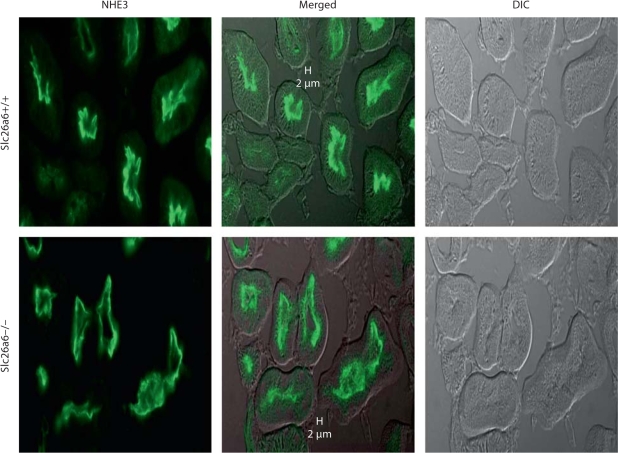
To examine whether the downregulation of apical NHE activity might be due to alteration in NHE3 distribution along the length of brush border villi, immunofluorescence labeling was performed in kidneys of Slc26a6+/+ and Slc26a6–/– animals and images were examined by confocal microscopy. As shown in figure 6, NHE3 abundance on the apical membrane villi was comparable in proximal tubules in Slc26a6+/+ and Slc26a6–/– mice.
Fig. 6.
Immunofluorescence labeling of NHE3 in the kidneys of Slc26a6+/+ and Slc26a6-null mice. DIC = Differential interference contrast (DIC). Abundance of NHE3 on the apical membrane of kidney proximal tubules was comparable in Slc26a6+/+ and Slc26a6–/– mice.
In the last series of experiments, the apical Cl–/OH– exchanger activity, measured as the rate of intracellular alkalinization upon switching to a chloride-free perfusate, was examined and found to be significantly reduced, but not abolished, in Slc26a6-null mice [0.65 ± 0.09 pH units/min (n = 35 cells; 4 animals) in Slc26a6–/– versus 0.96 ± 0.04 pH units/min (n = 30 cells; 3 animals) in Slc26a6+/+ animals; p < 0.005].
Discussion
Studies in microperfused tubules and membrane vesicles have indicated that a major fraction of filtered Cl– is reabsorbed in the proximal tubule via Cl–/base exchanges. Two well-known mechanisms of anion exchange identified in the kidney proximal tubule are Cl–/oxalate exchange and Cl–/OH–/HCO3– exchange [17,18,19,20, 30]. Slc26a6 has the ability to operate in both exchange modes [11, 13, 15, 16]. In Slc26a6-null mice, oxalate-stimulated NaCl absorption was abolished and the apical Cl–/OH–/HCO3– exchanger was significantly inhibited [20] suggesting that Slc26a6 represents the Cl-oxalate exchanger and is the major contributor to apical Cl–/OH–HCO3– exchange [20].
Parallel coordination of apical Na+/H+ exchange and Cl–/base exchange is required for the absorption of filtered NaCl in late proximal tubule, the site of reabsorption of the majority of filtered chloride [17, 18]. The present studies demonstrated that Slc26a6-null mice display reduced pHi in late proximal tubule cells (fig. 1). The reduction in cell pH was predominantly due to the downregulation of the apical NHE activity (fig. 2). The downregulation of the apical NHE was also confirmed by blunting of the formate-induced intracellular alkalinization in Slc26a6-null mice (fig. 3). Comparison of the results in the presence or absence of AII demonstrated that AII stimulation of apical NHE is intact in Slc26a6-null mice (fig. 4). The downregulation of apical NHE activity was not due to the reduced expression of NHE3 or NHE8 (fig. 5) or redistribution of NHE3 to the base of brush border villi in the kidney proximal tubule (fig. 6). It is worth mentioning that cytosolic acidification could help with the activation of the apical NHE.
Recent studies demonstrated that formate directly stimulates the apical NHE3, as shown by luminal Na-dependent, amiloride-sensitive intracellular alkalinization in kidney proximal tubule upon the addition of formate [24]. The formate stimulation of NHE3 strongly suggests that enhanced NaCl absorption in the kidney proximal tubule by formate is primarily mediated via the activation of the apical NHE, with subsequent upregulation of chloride/base exchanger [24]. In the present studies, the formate-stimulated intracellular alkalinization was significantly blunted, consistent with the downregulation of apical NHE activity in the kidney proximal tubule in Slc26a6-null mice. To avoid any direct interference by Slc26a6 deletion on formate uptake from the luminal side, formate was added only to the basolateral membrane.
The exact mechanism of apical NHE downregulation in the S3 segment of the kidney proximal tubule of Slc26a6-null mice remains speculative at present. The mRNA expression levels for NHE3 and NHE8, two apical NHEs in the proximal tubule, were not different in kidneys of Slc26a6–/– versus Slc26a6+/+ mice (fig. 5). Further, NHE3 abundance remained the same in the kidney cortex and immunofluorescence labeling demonstrated no significant alteration in its abundance at the tip of brush border villi in Slc26a6-null mice. Both NHE3 and Slc26a6 have been shown to interact with scaffolding proteins PDZK1 and NHERF through their PDZ-binding domains [31, 32]. Whether Slc26a6 associates with NHE3 through binding with PDZK1 or NHERF remains speculative. Expression of NHERF and PDKZ1 in the kidney proximal tubule of Slc26a6-null mice remained unchanged by immunofluorescent labeling (data not shown) indicating that Slc26a6 deletion does not affect the abundance of these proteins in the brush border membrane.
The renal sodium and chloride excretion remained comparable in Slc26a6+/+ and Slc26a6–/– mice [20], despite the downregulation of apical NHE activity in the S3 segment (1, 2, 3, 4), raising the possibility of either compensatory upregulation of Na+- and Cl–-dependent transporters in the distal nephron or the activation of signaling pathways that can stimulate the apical NHE in vivo. Our preliminary studies demonstrated that the expression of the apical Na+/K+/2Cl– cotransporter (BSC1) did not increase in the kidneys of Slc26a6-null mice [33].
At a cursory glance, these studies may appear in conflict with published reports on an independently generated Slc26a6-null mouse, which show comparable NHE activity with Slc26a6+/+ animals in luminal membrane vesicles isolated from the kidney cortex [34]. In response, we would like to suggest that our experiments in microperfused kidney proximal tubule are exclusively performed in the S3 segment where the majority of chloride is reabsorbed [21]. However, cortical membrane vesicles predominantly contain S2 and S1 segments of the proximal tubule, with little presence of the S3 segment.
Slc26a6 deletion decreased but did not completely abolish apical Cl–/OH– exchange or Cl–/HCO3– exchange [20]. Further, Slc26a6 deletion did not affect the Cl–/formate exchange activity in apical membranes of the kidney proximal tubule [34]. Taken together, these results support the presence of other apical chloride/base exchanger(s) in the kidney proximal tubule. The identity of this (these) other apical chloride/base exchanger(s) remains speculative. A recent study demonstrated that Slc26a7, which mediates Cl–/HCO3– exchange and anion/oxalate/sulfate exchange [10, 35], is detected on the apical membrane of kidney proximal tubule [36]. Other studies, however, have localized Slc26a7 only to the basolateral membrane of acid-secreting A-intercalated cells in the outer medullary collecting duct [35, 37, 38]. Additional studies, including examination of proximal tubule Cl–/base exchanger in Slc26a7-null mice, should clarify the localization of Slc26a7 in the kidney.
In conclusion, Slc26a6 deletion downregulates the apical NHE activity in the straight segment of the proximal tubule, via mechanisms independent of its abundance in the brush border membrane. The absence of a significant renal sodium loss in Slc26a6-null mice, despite NHE3 downregulation in the straight segment of the proximal tubule perfused in vitro, points to possible activation of signaling pathways that can stimulate the apical NHE in the proximal tubule in vivo.
Acknowledgements
These studies were supported by the National Institute of Health Grant DK 62809 and a Merit Review Award (to M.S.), an ASN Carl W. Gottschalk Award (to S.P.) and grants from Dialysis Clinic Inc. (to M.S.).
References
- 1.Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem. 1994;269:3017–3021. [PubMed] [Google Scholar]
- 2.Hastabacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander ES. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 3.Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc Natl Acad Sci USA. 1993;90:4166–4170. doi: 10.1073/pnas.90.9.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg M-L, Airola K, Holmberg C, Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 5.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(–)/HCO(3) (–) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- 6.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Gen. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 7.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl–/OH–/HCO3– exchanger in the kidney cortex. Am J Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 9.Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- 10.Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem. 2002;277:14246–14254. doi: 10.1074/jbc.M111802200. [DOI] [PubMed] [Google Scholar]
- 11.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincourt JB, Jullien D, Kossida S, Amalric F, Girard JP. Molecular cloning of SLC26A7, a novel member of the SLC26 sulfate/anion transporter family, from high endothelial venules and kidney. Genomics. 2002;79:249–256. doi: 10.1006/geno.2002.6689. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl–/HCO3– exchanger in the small intestine. Am J Physiol. 2002;282:G573–G579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 14.Vincourt JB, Jullien D, Amalric F, Girard JP. Molecular and functional characterization of SLC26A11, a sodium-independent sulfate transporter from high endothelial venules. FASEB J. 2003;17:890–892. doi: 10.1096/fj.02-0787fje. [DOI] [PubMed] [Google Scholar]
- 15.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 17.Aronson PS. The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Physiol. 1989;51:419–441. doi: 10.1146/annurev.ph.51.030189.002223. [DOI] [PubMed] [Google Scholar]
- 18.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol. 1997;273:F179–F192. doi: 10.1152/ajprenal.1997.273.2.F179. [DOI] [PubMed] [Google Scholar]
- 19.Petrovic S, Ma L, Wang Z, Soleimani M. Identification of an apical Cl–/HCO3 exchanger in rat kidney proximal tubule. Am J Physiol. 2003;285:C608–C617. doi: 10.1152/ajpcell.00084.2003. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol. 2005;288:C957–C965. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 21.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 22.Alpern RJ. Apical membrane chloride/base exchange in the rat proximal convoluted tubule. J Clin Invest. 1987;79:1026–1030. doi: 10.1172/JCI112914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum M. Effect of luminal chloride on cell pH in rabbit proximal tubule. Am J Physiol. 1988;254:F677–F683. doi: 10.1152/ajprenal.1988.254.5.F677. [DOI] [PubMed] [Google Scholar]
- 24.Petrovic S, Barone S, Weinstein AM, Soleimani M. Activation of the apical Na+/H+ exchanger NHE3 by formate: a basis of enhanced fluid and electrolyte reabsorption by formate in the kidney. Am J Physiol Renal Physiol. 2004;287:F336–F346. doi: 10.1152/ajprenal.00400.2003. [DOI] [PubMed] [Google Scholar]
- 25.Soleimani M, Bookstein C, McAteer JA, Hattabaugh YJ, Bizal GL, Musch MW, Villereal M, Rao MC, Howard RL, Chang EB. Effect of high osmolality on Na+/H+ exchange in renal proximal tubule cells. J Biol Chem. 1994;269:15613–15618. [PubMed] [Google Scholar]
- 26.Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001;60:1824–1836. doi: 10.1046/j.1523-1755.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 27.Petrovic S, Wang Z, Ma L, Seidler U, Forte JG, Shull GE, Soleimani M. Colocalization of the apical Cl–/HCO3– exchanger PAT1 and gastric H-K-ATPase in stomach parietal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1207–G1216. doi: 10.1152/ajpgi.00137.2002. [DOI] [PubMed] [Google Scholar]
- 28.Zahedi K, Wang Z, Barone S, Tehrani K, Yokota N, Petrovic S, Rabb H, Soleimani M. Identification of stathmin as a novel marker of cell proliferation in the recovery phase of acute ischemic renal failure. Am J Physiol Cell Physiol. 2004;286:C1203–C1211. doi: 10.1152/ajpcell.00432.2003. [DOI] [PubMed] [Google Scholar]
- 29.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soleimani M. Molecular physiology of the renal chloride-formate exchanger. Curr Opin Nephrol Hypertens. 2001;10:677–683. doi: 10.1097/00041552-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Thomson RB, Wang T, Thomson BR, Tarrats L, Girardi A, Mentone S, Soleimani M, Kocher O, Aronson PS. Role of PDZK1 in membrane expression of renal brush border ion exchangers. Proc Natl Acad Sci USA. 2005;102:13331–13336. doi: 10.1073/pnas.0506578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol. 2003;284:C769–C779. doi: 10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- 33.Amlal H, Wang Z, Barone S, Soleimani M. Compensatory upregulation of ion transporters in the kidney proximal tubule of Slc26a6 null mice. J Am Soc Nephrol. 2005;16:351A. [Google Scholar]
- 34.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 35.Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M. Identification of a basolateral Cl–/HCO3– exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1093–G1103. doi: 10.1152/ajpgi.00454.2002. [DOI] [PubMed] [Google Scholar]
- 36.Dudas PL, Mentone S, Greineder CF, Biemesderfer D, Aronson P. Immunolocalization of anion transporter Slc26a7 in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F937–F945. doi: 10.1152/ajprenal.00197.2004. [DOI] [PubMed] [Google Scholar]
- 37.Barone S, Amlal H, Xu J, Kujala M, Kere J, Petrovic S, Soleimani M. Differential regulation of basolateral Cl–/HCO3– exchangers SLC26A7 and AE1 in kidney outer medullary collecting duct. J Am Soc Nephrol. 2004;15:2002–2011. doi: 10.1097/01.ASN.0000135060.83250.07. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Worrell RT, Li HC, Barone SL, Petrovic S, Amlal H, Soleimani M. Chloride/bicarbonate exchanger SLC26A7 is localized in endosomes in medullary collecting duct cells and is targeted to the basolateral membrane in hypertonicity and potassium depletion. J Am Soc Nephrol. 2006;17:956–967. doi: 10.1681/ASN.2005111174. [DOI] [PMC free article] [PubMed] [Google Scholar]