Abstract
Herbivore-induced plant volatiles affect the systemic response of plants to local damage and hence represent potential plant hormones. These signals can also lead to “plant-plant communication,” a defense induction in yet undamaged plants growing close to damaged neighbors. We observed this phenomenon in the context of disease resistance. Lima bean (Phaseolus lunatus) plants in a natural population became more resistant against a bacterial pathogen, Pseudomonas syringae pv syringae, when located close to conspecific neighbors in which systemic acquired resistance to pathogens had been chemically induced with benzothiadiazole (BTH). Airborne disease resistance induction could also be triggered biologically by infection with avirulent P. syringae. Challenge inoculation after exposure to induced and noninduced plants revealed that the air coming from induced plants mainly primed resistance, since expression of PATHOGENESIS-RELATED PROTEIN2 (PR-2) was significantly stronger in exposed than in nonexposed individuals when the plants were subsequently challenged by P. syringae. Among others, the plant-derived volatile nonanal was present in the headspace of BTH-treated plants and significantly enhanced PR-2 expression in the exposed plants, resulting in reduced symptom appearance. Negative effects on growth of BTH-treated plants, which usually occur as a consequence of the high costs of direct resistance induction, were not observed in volatile organic compound-exposed plants. Volatile-mediated priming appears to be a highly attractive means for the tailoring of systemic acquired resistance against plant pathogens.
Plants respond to attack by pathogens or herbivores with extensive changes in gene expression that lead to induced resistance phenomena (Karban and Baldwin, 1997); various traits are then expressed de novo or at much higher intensities, which reduce or prevent further tissue damage. As both pathogens and herbivores can spread from the initial site of attack to other organs, such plant responses are often not restricted to the damaged tissue but are expressed systemically, in yet undamaged organs. Three plant hormones playing central roles in the long-distance signaling that underlies this systemic response to local attack are jasmonic acid (JA), ethylene, and salicylic acid (SA). SA and JA, in particular, are transported themselves or in the form of derivatives within the plant in order to elicit systemic responses (Truman et al., 2007; Wasternack, 2007; Heil and Ton, 2008).
Recent studies have revealed that long-distance signaling is not only caused by molecules that are transported in the vascular system; signals can also be volatile compounds that move in the headspace outside the plant (Heil and Ton, 2008). In particular, green-leaf volatiles and other herbivore-induced volatile organic compounds (VOCs) can mediate the systemic response of plants to local herbivore damage (Karban et al., 2006; Frost et al., 2007; Heil and Silva Bueno, 2007). Since such VOCs move freely in the air, they may also affect neighboring plants and then mediate the phenomenon of “plant-plant communication,” which has been found in taxonomically unrelated plants such as Arabidopsis (Arabidopsis thaliana), alder (Alnus glutinosa), corn (Zea mays), lima bean (Phaseolus lunatus), maple (Acer saccharum), sagebrush (Artemisia tridentata), and wild tobacco (Nicotiana attenuata; Baldwin and Schultz, 1983; Rhoades, 1983; Tscharntke et al., 2001; Engelberth et al., 2004; Heil and Kost, 2006; Karban et al., 2006; Paschold et al., 2006; Heil and Silva Bueno, 2007; Ton et al., 2007; Godard et al., 2008).
Plant-plant communication via VOCs thus appears to be a common phenomenon in herbivore resistance, and similar volatile compounds can also mediate the beneficial effects that are caused by plant growth-promoting rhizobacteria (Ryu et al., 2003, 2004b). Furthermore, exposure to VOCs such as trans-2-hexenal, cis-3-hexenal, or cis-3-hexenol enhanced resistance of Arabidopsis against the fungal pathogen Botrytis cinerea (Kishimoto et al., 2005), which indicates that VOCs may also induce disease resistance. However, the wound response, the induction of VOCs, the effects of plant growth-promoting rhizobacteria, and even the resistance to necrotrophic pathogens such as B. cinerea and Alternaria brassiccicola are mediated via JA signaling (Wasternack and Parthier, 1997; Pieterse et al., 1998; Schilmiller and Howe, 2005; Francia et al., 2007; Heil, 2008; Heil and Ton, 2008). In contrast, systemic acquired resistance (SAR) to biotrophic pathogens in many plant species is mediated by SA signaling, which increases the expression of phytoalexins and of several PATHOGENESIS-RELATED (PR) proteins (van Loon, 1997; Hammerschmidt and Smith-Becker, 1999; Durrant and Dong, 2004) and which usually is thought to act as an antagonist to JA signaling (Maleck et al., 2000; Pieterse and Dicke, 2007; Korneef and Pieterse, 2008). The volatile derivative of SA, methyl salicylate (MeSA), has been proposed as the most likely systemic signal (Park et al., 2007). In tobacco (Nicotiana tabacum), MeSA is converted back to SA, which then forms the active resistance-inducing compound (Kumar and Klessig, 2003; Forouhar et al., 2005). This mechanism might underlie the resistance induction in tobacco plants that were exposed to high MeSA concentrations (Shulaev et al., 1997). In a study on the role of MeSA as a mobile signal, Park and coworkers (2007), however, only found evidence for the vascular transport of this compound.
We used lima bean to investigate whether plant-plant signaling can also affect SAR to biotrophic bacterial pathogens. Plants were exposed to the VOCs emitted from neighbors that had been treated with the chemical SAR elicitor benzothiadiazole [BTH; benzo(1,2,3)thiadiazole-7-carbothioic acid S-methyl ester] or that had been induced biologically, and resulting changes in resistance were monitored at the phenotypic and gene expression levels. A common phenomenon involved in disease resistance is priming, which prepares the plant to respond more rapidly and/or effectively to subsequent attack (van Hulten et al., 2006; Bruce et al., 2007; Goellner and Conrath, 2008) but which comes at much lower costs than direct resistance induction (Heil and Baldwin, 2002; Walters and Boyle, 2005; Walters and Heil, 2007). Therefore, we investigated whether VOCs also can prime resistance to pathogens by first exposing plants to VOCs coming from directly induced plants and then challenging them with Pseudomonas syringae pv syringae. Finally, VOCs released from induced plants were analyzed, and the most likely candidates were evaluated for their effect on expression of the resistance marker gene PR-2 in order to understand the chemical nature of the signal.
RESULTS
Airborne Disease Resistance Induction in Nature
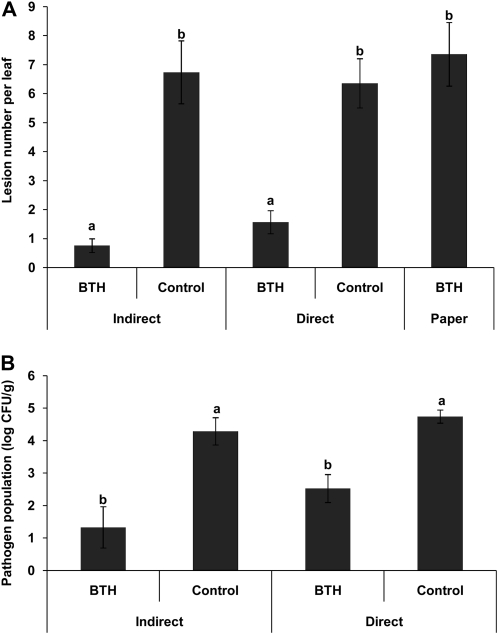
An experiment was conducted in a natural population of lima bean in the coastal area of Oaxaca, Mexico. Single shoots of the landrace line of lima bean were induced with BTH solution (direct treatment) or with water as a control, and after drying, shoots of other plants growing nearby were placed close to the treated plants. A fifth group of plants was exposed to filter paper to which BTH had been applied in order to control for putative effects of gaseous BTH components. Lesion numbers were counted 1 week after spray inoculation with a pathogenic bacterium, P. syringae strain 61, and were averaged for every shoot. Average lesion numbers per leaf were significantly affected by treatment (general linear model with treatment as a fixed factor and plant group as a random factor: F4,36 = 15.339, P < 0.001), and posthoc analysis (lsd) revealed that BTH-treated plants and plants exposed to the air from these plants had developed significantly (P < 0.001) fewer symptoms per leaf than plants in the three other groups (Fig. 1A). In a second, independent experiment that was conducted with potted plants, the treatment affected significantly (P < 0.001, Kruskal-Wallis ANOVA) the bacterial densities that developed in the leaves, and the resulting pattern confirmed the results of the field trial: bacterial densities amounted to approximately 2 × 103 colony-forming units (CFUs) both in the plants treated directly with BTH and in plants exposed to the VOCs released from those plants, whereas water-sprayed controls contained on average more than 106 CFUs and their neighbors contained on average more than 6.5 × 105 CFUs per g of fresh tissue (Fig. 1B).
Figure 1.
Airborne resistance induction in lima bean under field conditions. A, Plants treated directly with BTH (direct treatment) or exposed to the air coming from directly treated plants (indirect treatment) were compared with control plants sprayed with sterile distilled water (direct treatment) or exposed to these controls (indirect treatment). To exclude effects of volatile components of BTH, a fifth group of plants was exposed to paper strips on which BTH had been applied. B, Plants were cultivated individually in pots outside for 3 weeks. Then, 10 plants were treated with 0.5 mm BTH solution and 10 further plants were placed immediately beside the treated plants (indirect treatment). Ten further plants received a water spray as controls and were placed close to completely untreated plants. The distance between BTH-treated plants and controls was 4 m. All plants were spray inoculated with P. syringae strain 61 at 5 d after BTH treatment. Bars represent average lesion numbers per leaflet (means ± se). Sample size was n = 10 plants per treatment, and different letters indicate significant differences among treatments (P < 0.001 according to lsd).
Airborne Signaling Induces Disease Resistance
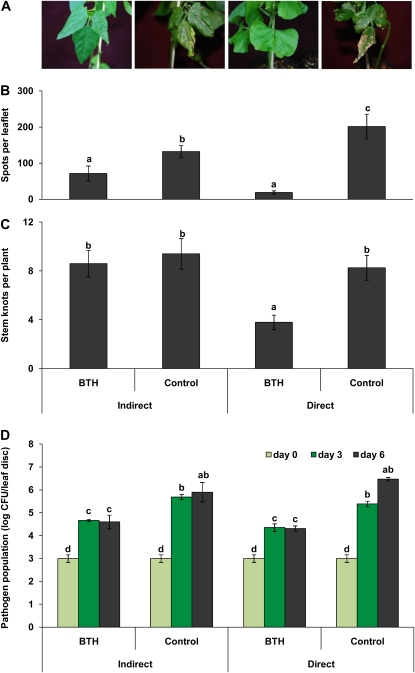
To validate and causally understand the phenomenon of airborne disease resistance induction, we used an in vivo disease assay system (see “Materials and Methods”). Untreated lima bean plants were kept in the same air with BTH-treated plants in a clear plastic box and then challenged with P. syringae strain 61. Direct BTH treatment increased plant resistance above the levels found in control plants. This became obvious from the disease severity of the infected leaves (Fig. 2A) and also when quantifying the number of bacterial spots and the development of the pathogen population per leaflet (Fig. 2, B and D). As in the field experiments, plants that had only been exposed to these plants (“indirect” treatment) were almost as resistant as the directly treated plants (Fig. 2A): the number of bacterial spots on leaves of these plants was significantly lower than on controls and only slightly, but insignificantly, higher than on leaves of directly treated plants (Fig. 2B). Both direct and indirect BTH treatments significantly reduced the development of pathogen populations: as compared with the control plants (treated with sterile distilled water), the numbers of live bacteria 3 and 6 d after pathogen challenge were significantly lower in the leaves of plants that had been treated with BTH or exposed to the air coming from these plants (Fig. 2D). When we investigated putative costs of resistance induction by quantifying the number of stem nodes after 10 d, we found a significant reduction of growth in directly BTH-treated plants, while no such effect could be observed in the indirectly treated plants (Fig. 2C).
Figure 2.
Airborne resistance induction in lima bean under in vivo conditions. Plants were either treated directly with 0.5 mm BTH (direct treatment) or exposed to the air coming from directly treated plants (indirect treatment). Control plants were sprayed with sterile distilled water (direct treatment) or exposed to these controls (indirect treatment). A, Disease symptoms caused by virulent P. syringae of directly and indirectly treated plants versus the controls. B, The quantitative evaluation of the respective disease severity (no. of bacterial spots per leaflet). C, The growth response of the plants to the different induction treatments as number of nodes that the plants had produced 10 d after pathogen challenge. D, The population of P. syringae of the respective leaf disc (diameter = 1 cm). Bars represent means ± se. Sample size was n = 5 plants per treatment, and different letters indicate significant differences among treatments (P < 0.05 according to lsd). The experiment was repeated five times with similar results. [See online article for color version of this figure.]
Plant-Derived Compounds Induce Resistance in Neighboring Plants
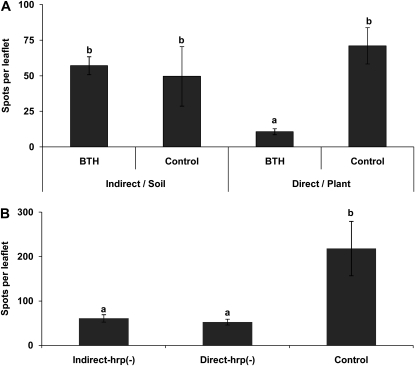
We conducted two further experiments to investigate whether volatile components of BTH rather than plant-derived compounds induced resistance in neighboring plants. In the field experiment, plants exposed to BTH-treated filter paper showed no significant reduction in lesion number as compared with controls (Fig. 1A). When we applied BTH directly onto the soil (without plants) rather than onto plants, no significant disease reduction in neighbors that were exposed to the air coming from these pots could be observed (Fig. 3A, Indirect/Soil). Second, we biologically induced plants with avirulent P. syringae strain 61-18. Plants exposed to the air from these plants [Fig. 3B, Indirect-hrp(−)] and then challenged with P. syringae showed spot numbers that were significantly lower than those of control plants and not different from those of directly induced plants (Fig. 3B).
Figure 3.
Plant-derived VOCs are the active signals. Volatile compounds of BTH do not elicit airborne resistance induction (A), while VOCs emitted by biologically induced plants do (B). A, Bacterial spot numbers per leaflet are depicted for plants to which BTH was directly applied, for the respective water-treated controls (Direct/Plant), and for plants exposed to plant-free pots where BTH or water was applied to the soil (Indirect/Soil). B, Bacterial spot numbers on plants that had been induced with the hrp(−) mutant P. syringae 61-18 prior to infection with P. syringae on plants that had been exposed to induced neighbors (Indirect) and on controls that were infected without prior treatment. Bars represent means ± se. Sample size was n = 5 plants per treatment, and different letters indicate significant differences among treatments (P < 0.05 according to lsd). The experiment was repeated three times with similar results.
VOCs-Mediated Priming of Resistance
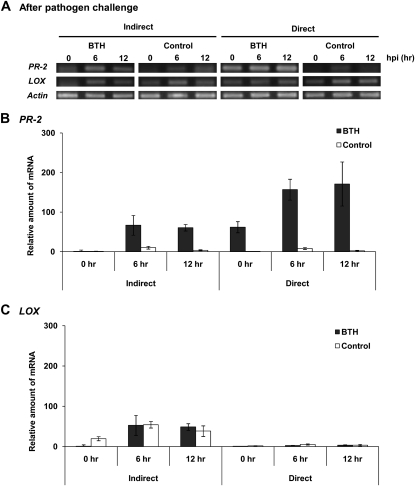
Putative priming effects were investigated by quantifying the expression of two central resistance-related genes, PR-2 and LIPOXYGENASE (LOX), in plants that were challenged with the pathogen. Direct BTH treatment induced PR-2 expression (0 h in the direct treatment; Fig. 4A) and also primed the plants, which became apparent by enhanced PR-2 expression 6 and 12 h after bacterial challenge in BTH-treated plants, in contrast to controls that had not been treated with BTH prior to challenge (Fig. 4A, 6 and 12 h in the direct treatment). Priming was observed also in the indirectly treated plants, in which PR-2 expression after 6 h was stronger than in controls (Fig. 4A). Quantitative reverse transcription (qRT)-PCR confirmed both the direct induction of PR-2 by BTH treatment (0 h) and the priming effect: the expression levels of PR-2 at 6 and 12 h after bacterial challenge were more than five times higher in plants that had earlier been exposed to the air from BTH-treated plants than in controls (Fig. 4B). By contrast, no differences in the expression of LOX (a central gene of JA signaling) were observed between BTH and control treatments (Fig. 4C).
Figure 4.
Induction and priming by direct BTH treatment and exposition to VOCs from BTH-treated plants. A, RT-PCR shows an induction of PR-2 by direct BTH treatment (0 h) and a priming effect on this gene, which was strongly expressed 6 and 12 h after P. syringae inoculation (hpi) when plants had already been treated with 0.5 mm BTH. Lima bean exposed to the directly BTH-treated plants (Indirect) also showed a priming effect (PR-2 expression 6 h after infection stronger than in controls). The housekeeping gene Actin was used to indicate equal loading. B and C, Expression levels as quantified by qRT-PCR (B) confirmed these results, while no significant responses of LOX expression could be observed (C). Bars represent means ± se. Sample size was n = 3 replications per treatment. The experiment was repeated three times with similar results.
Specific VOCs Cause Airborne Resistance Induction and Priming
We compared the VOCs released from BTH-treated plants to those released from JA-treated plants in order to identify compounds that are differentially released in response to these two defense elicitors. Among the 14 compounds that we observed regularly in the headspace of induced plants, two (nonanal and MeSA) were present at significantly higher amounts in the headspace of BTH-treated than of JA-treated plants (Table I). 2-Ethyl hexanol, which also appeared at significantly higher amounts in the headspace of BTH-treated plants, was excluded from further considerations because this compound likely represents a contamination (W. Boland, personal communication; see “Discussion”).
Table I.
VOCs in the headspace of JA-treated and BTH-treated plants
Amounts are given in nanograms released per gram leaf dry weight in 24 h (±se). Asterisks mark VOCs that after BTH treatment were released at significantly higher rates than after JA treatment (P < 0.05 according to Wilcoxon pair tests conducted for individual compounds; n = 7 plants per treatment).
| Compound | JA Treatment | BTH Treatment |
|---|---|---|
| 2-Ethyl hexanole | 0.59 ± 0.52 | 3.22 ± 1.48* |
| cis-β-Ocimene | 8.10 ± 1.67 | 0.62 ± 0.44 |
| Decanal | 0.67 ± 0.55 | 0.93 ± 0.65 |
| Linalool | 6.87 ± 1.47 | 1.35 ± 0.90 |
| Nonanal | 0.00 ± 0.00 | 0.77 ± 0.50* |
| cis-Hexenyl butyrate | 1.42 ± 0.66 | 0.55 ± 0.51 |
| MeSA | 0.45 ± 0.37 | 1.26 ± 1.00* |
| cis-Hexenyl isovalerate | 18.11 ± 3.17 | 1.05 ± 0.49 |
| Indole | 22.35 ± 2.73 | 4.32 ± 1.04 |
| cis-Hexenyl acetate | 0.66 ± 0.33 | 0.00 ± 0.00 |
| cis-Jasmone | 18.20 ± 2.89 | 0.00 ± 0.00 |
| β-Caryophyllene | 3.32 ± 1.12 | 1.36 ± 0.82 |
| trans-Geranyl acetate | 0.29 ± 0.52 | 0.00 ± 0.00 |
| Methyl jasmonate | 13.56 ± 2.11 | 1.77 ± 0.61 |
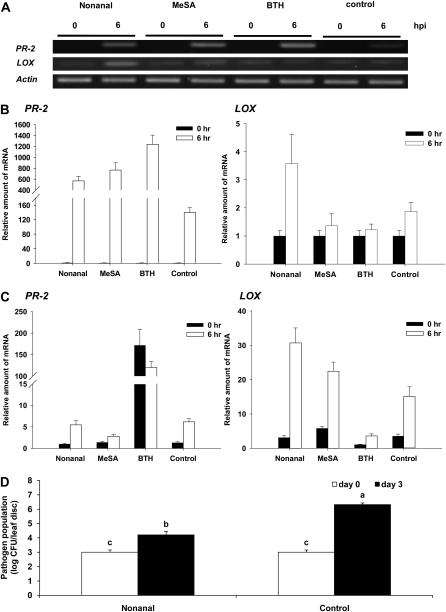
When plants were exposed to each of these two VOCs, PR-2 expression was up-regulated 6 h after exposure (Fig. 5A). This result was confirmed by qRT-PCR, which revealed strongly enhanced PR-2 expression levels after exposure to the two VOCs tested as well as after direct treatment with BTH (Fig. 5B). Nonanal, moreover, strongly induced LOX expression after 6 h (Fig. 5B) and primed the expression of PR-2, which 6 h after pathogen challenge was significantly more strongly expressed in plants that before had been exposed to nonanal (Fig. 5C). Both nonanal and MeSA also elicited greater expression of the LOX gene after 6 h as compared with controls and BTH treatments (Fig. 5C). Finally, volatile application of nonanal significantly reduced pathogen population at 3 d after pathogen challenge compared with paste control treatment (Fig. 5D).
Figure 5.
Expression of PR-2 and LOX in lima bean plants treated with individual volatiles and then challenged with pathogens. Expression rates of PR-2 and LOX were studied after exposing plants to gaseous nonanal and MeSA and compared with plants directly treated with BTH and with paste controls. A, Results of RT-PCR analyses after VOC exposition. B and C, The expression rates as quantified with qRT-PCR after the same chemical treatments (B) and pathogen challenge (C). D, The bacterial population of P. syringae of the respective leaf disc applied by nonanal in the lanolin paste and lanolin alone (control) at 0 and 3 d after pathogen challenge. Bars represent means ± se. Sample size was n = 5 plants per treatment, and different letters indicate significant differences among treatments (P < 0.05 according to lsd). The experiments were repeated three times with similar results.
DISCUSSION
Airborne induction and priming of direct and indirect plant defenses against herbivores has repeatedly been reported (Engelberth et al., 2004; Heil and Kost, 2006; Kessler et al., 2006; Frost et al., 2007; Heil and Silva Bueno, 2007; Ton et al., 2007). Herbivore resistance, however, is usually dependent on jasmonate signaling (Farmer et al., 2003; Wasternack, 2007; Heil and Ton, 2008), while SAR to biotrophic pathogens is mainly regulated via salicylate signaling (Hunt and Ryals, 1996; Durrant and Dong, 2004; Heil and Ton, 2008). We conducted this study to investigate whether VOCs that are released from SAR-expressing lima bean plants can also mediate the resistance phenotype of neighboring plants. We first observed that exposure to the air coming from plants treated with the chemical SAR inducer BTH rendered plants more resistant to subsequent challenge with the bacterial pathogen P. syringae in a natural population at the plant's center of origin in Mexico (Fig. 1, A and B). The same effect was also observed in an in vivo assay system in the laboratory (Fig. 2, A and B) and thus could be reproduced under different growing conditions. This effect became obvious both from the visual inspection of the challenged plants (Fig. 2A) and when quantifying the number of bacterial spots and the pathogen population per leaflet (Fig. 2, B and D). Thus, airborne signals indeed can enhance the resistance of lima bean to a bacterial pathogen.
Our plants had been treated with a chemical SAR elicitor, which might release volatile compounds into the gas phase. Therefore, did volatile components of the BTH, rather than plant-derived VOCs, cause the effects on neighboring plants that we observed here? We found no resistance induction in plants that had been exposed to the air released from BTH-treated filter paper or soil (Figs. 1 and 3A), whereas we indeed found airborne resistance induction caused by the air coming from plants that had been spray inoculated with P. syringae strain 61-18, which is an hrp null mutant of wild-type strain 61 and which induces SAR in lima bean (Fig. 3B). Hence, VOCs released from induced plants rather than the resistance elicitor itself appeared responsible for the observed effects, and biological SAR elicitation also can cause airborne resistance induction in neighboring plants (Fig. 3B).
An important effect that is usually associated with direct resistance induction is the growth reduction that results from the toxicity of the resistance-inducing compounds or from the fitness costs associated with resistance (Heil and Baldwin, 2002). When we assessed for the occurrence of such costs, we found no significant reduction of growth in the indirectly treated plants (Fig. 2C), although they showed an enhanced resistance when challenged (Fig. 2, A and B). This pattern is redolent of primed plants, in which a plant's defense arsenal had been sensitized rather than fully induced (Ton et al., 2007; Goellner and Conrath, 2008). Primed plants usually show no enhanced expression of phenotypic defense traits, but they respond faster or stronger to challenge inoculation than unprimed plants (van Hulten et al., 2006; Bruce et al., 2007; Goellner and Conrath, 2008). BTH is known to both induce and prime disease resistance in other plant species (Cools and Ishii, 2002; Kohler et al., 2002). In lima bean, priming was indeed observed both in the directly treated plants and in the indirectly treated plants that had only been exposed to air from BTH-treated plants: PR-2 expression in these plants 6 h after challenging them with the pathogen was stronger than in controls (Fig. 4A, indirect treatment). qRT-PCR confirmed these results: the expression levels of PR-2 at 6 and 12 h after bacterial challenge were much higher in plants that before had been exposed to the air from BTH-treated plants than in controls (Fig. 4B).
The observation that no differences in the expression of LOX (a central gene of JA signaling) occurred between BTH and control treatments (Fig. 4C) underlines the specificity of the plant response that is described in this study. Moreover, a direct involvement of gaseous BTH in the effects that we describe here could be excluded (Figs. 1 and 3). Which VOCs, then, were responsible for the responses that we observed? Green-leaf volatiles have repeatedly been reported to be active in airborne induction of herbivore resistance (Engelberth et al., 2004; Farag et al., 2005; Ruther and Kleier, 2005; Mirabella et al., 2008), and they can also enhance a plant's direct resistance to certain pathogens, particularly necrotrophic fungi (Kishimoto et al., 2005; Matsui, 2006; Shiojiri et al., 2006). Certain volatiles even might be involved in the resistance of plants to abiotic stress (Behnke et al., 2007), and BTH treatment has been reported to enhance the attractiveness of herbivore-damaged corn seedlings to parasitic wasps (Rostás and Turlings, 2008). The more common pattern, however, appears to be that JA- and SA-mediated signaling elicit very different defensive plant responses and that SA signaling suppresses JA-mediated defenses (Maleck et al., 2000; Pieterse and Dicke, 2007; Korneef and Pieterse, 2008). The high specificity of the response observed here thus made the involvement of herbivore-induced or JA-dependent VOCs less likely.
As untreated lima bean hardly elicits any volatiles (Heil, 2004; Ballhorn et al., 2008), we compared the VOCs released from BTH-treated plants with those released from JA-treated plants and then tested two of the three compounds that were present at significantly higher amounts in the headspace of BTH-treated than of JA-treated plants (2-ethyl hexanol, MeSA, and nonanal; Table I) for their resistance gene-inducing activity. All three compounds have repeatedly been reported in the context of plant pathogenesis: nonanal and 2-ethyl hexanol were found in the headspace of potato (Solanum tuberosum) tubers infected with different fungi (de Lacy Costello et al., 2001) and of various biological control bacteria (Dilantha Fernando et al., 2005), nonanal was released from whitefly-infected beans (Phaseolus vulgaris; Birkett et al., 2003), and both nonanal and 2-ethyl hexanol were reported to have in vitro antifungal (Dilantha Fernando et al., 2005) and bactericidal (Nakamura and Hatanaka, 2002) activities.
However, although 2-ethyl hexanol has repeatedly been reported in the context of pathogenesis (de Lacy Costello et al., 2001; Nakamura and Hatanaka, 2002; Dilantha Fernando et al., 2005), the compound is also a very common contaminant. In fact, no convincing study has ever reported a clear case of plant-derived 2-ethyl hexanol (W. Boland, personal communication). In our study, this VOC was found in the headspace of BTH-treated lima bean plants, but we also found small amounts of decanedioic acid, bis(2-ethylhexyl) ester, also known as Edenol 888, suggesting this industrial plasticizer as the source of the 2-ethyl hexanol. Since the 2-ethyl hexanol, moreover, was present as a racemic mixture as shown by gas chromatography on a chiral stationary phase (K. Ploss and W. Boland, personal communication), this compound must be regarded as a contaminant and was omitted in further studies.
When plants were exposed to gaseous nonanal and MeSA, PR-2 expression was up-regulated (Fig. 5, A and B), and nonanal, moreover, strongly induced LOX expression and plant resistance against P. syringae (Fig. 5, B–D). LOX and its products, the oxylipins, were associated with pathogen resistance induced by nonpathogenic pseudomonad strains and with resistance to the rust pathogen Uromyces fabae in bean (Ongena et al., 2004; Walters et al., 2006). MeSA and nonanal are, thus, the most likely plant-derived VOCs that have caused the airborne resistance effects reported here. MeSA mediated pathogen resistance of tobacco and Arabidopsis (Shulaev et al., 1997; Park et al., 2007) and induced PR-2 expression (Fig. 5, A and B) in lima bean, although it elicited no obvious priming of PR-2 in the same experimental setup (Fig. 5C). Nonanal did not significantly induce the secretion of extrafloral nectar by lima bean, an indirect defense mechanism against herbivores (Heil et al., 2008), but induced plant resistance to a bacterial pathogen (Fig. 5D). The same plant species uses different VOCs to regulate defensive responses that affect protection from different attackers.
Airborne signaling in the context of plant antiherbivore defense has been repeatedly reported and is likely to be a common phenomenon, since herbivore-induced VOCs serve multiple functions, such as the attraction of predatory arthropods (Turlings et al., 1995; De Moraes et al., 1998; Thaler, 1999; Kessler and Baldwin, 2001), the repellence of herbivores (Birkett et al., 2000; De Moraes et al., 2001), and the within-plant signaling that leads to systemic responses to local damage (Karban et al., 2006; Frost et al., 2007; Heil and Silva Bueno, 2007). In this study, we report that airborne plant-plant signaling can also prime plant resistance against a pathogenic bacterium. Lima bean plants growing adjacent to induced conspecifics were more resistant to infection by P. syringae, and nonanal was detected in the headspace of BTH-treated plants and could trigger the expression of genes that are likely to be involved in this effect. The usual costs, which result from resource allocation to resistance induction (Heil and Baldwin, 2002; Walters and Boyle, 2005) and which in our study became visible as reduced growth rates of the directly induced plants, could not be observed in the indirectly treated plants, a result that is most likely caused by the fact that VOCs primed rather than directly induced pathogen resistance. Airborne priming thus might be an attractive means of improving resistance to plant pathogens without incurring the costs that greatly compromise the benefits of direct preventative induction of resistance.
MATERIALS AND METHODS
Field Experiment
An initial experiment was conducted in a natural population of lima bean (Phaseolus lunatus) in the coastal area of the state of Oaxaca, Mexico, in December 2008. A stock solution of BTH (Syngenta; www.syngenta.com) at 0.5 mm was freshly prepared in sterile distilled water for each experiment. Ten groups comprising five plants each were treated with BTH at 0.5 mm (20 mL of solution on a shoot of approximately 1 m length with four to 12 leaves; direct treatment), tangled around these shoots after complete drying (indirect treatment), sprayed with water (control), tangled around these controls (indirect control), or tangled around a filter paper (10 cm × 1 m) on which 20 mL of BTH solution had been applied. Plants forming a group were growing within a maximal distance of 6 m, but a minimum distance of 2 m was kept between BTH-treated shoots and controls. All plants were spray inoculated with Pseudomonas syringae pv syringae strain 61 as described below 5 d after BTH treatment, and lesion numbers per leaf were counted and averaged (six to 16 leaves per shoot) for every individual shoot 1 week after inoculation. For assessing bacterial count under field conditions, plants were cultivated individually in pots outside for 3 weeks. Then, 10 plants were treated with 0.5 mm BTH solution and 10 further plants were placed immediately beside the treated plants (indirect treatment). Ten further plants received a water spray as controls and were placed close to completely untreated plants. The distance between BTH-treated plants and controls was 4 m. All plants were spray inoculated with P. syringae strain 61 as described below 5 d after BTH treatment. The youngest seven leaves were collected from every plant 5 d later and ground in water (approximately 2 mL water g−1 fresh material). The extracts were diluted 1:10, 1:100, and 1:1,000 and plated on Pseudomonas Agar F medium (Difco) with 100 μg rifampicin mL−1 to count CFUs 3 d later.
In Vivo Experiment
A second experiment similar to the one described above was conducted in the greenhouse. The seeds of lima bean (cv Jackson Wonder Bush) were surface sterilized with 6% sodium hypochlorite and then washed four times with sterile distilled water before being maintained at 25°C for 3 d until germination. The geminated seeds were then planted on soilless medium (Bunong). Plants were grown at 25°C ± 2°C under fluorescent light (12-h/12-h day/night cycle, approximately 7,000 lux light intensity) in a controlled-environment growth room. Exposure experiments under the in vivo condition were conducted in closed transparent acrylic plastic boxes (20 × 60 × 20 cm; thickness = 5 mm). A 10-mL solution of 0.5 mm BTH was sprayed on five 3-week-old lima bean plants as a direct treatment. To receive the indirect treatment, five plants of the same age were placed beside BTH-treated plants avoiding physical contact. Plants sprayed only with sterile distilled water or exposed to these plants served as controls for the direct or indirect treatment. After 1 week, all plants were spray inoculated with P. syringae strain 61 and its hrp (type III secretion system) null mutant 61-18 (Huang et al., 1988) that had been cultivated on Pseudomonas Agar F medium (Difco). For experimental use, bacteria were scraped off plates and resuspended in sterile distilled water. The bacterial suspensions were adjusted to 107 CFU mL−1 based on optical density. For assessing bacterial populations, bacteria numbers of the respective leaf discs (diameter = 1 cm) on the five leaflets were counted at 0, 3, and 6 d after pathogen challenge, similar to the field experiment.
Stem nodes were counted 10 d after pathogen challenge to quantify plant growth rates. This experiment was designed as a randomized complete block with five replications and was repeated four times independently. For biological resistance induction, we sprayed a bacterial suspension of 107 CFU mL−1 P. syringae strain 61-18 on the leaves until runoff. This strain is an hrp mutant of strain 61 that elicits SAR but no visible disease symptoms on lima bean leaves 1 week after inoculation (data not shown).
Exclusion of Direct BTH-Derived Airborne Effects
To check whether putative volatiles released from BTH itself can cause the observed effects, we applied 10 mL of 0.5 mm BTH directly onto the soil in plant-free pots and exposed lima beans to the air released from this soil.
Gas Chromatographic Profiles of Headspaces
The identification of VOCs in the headspaces of BTH-treated and JA-treated plants was conducted as described previously (Heil, 2004; Heil and Silva Bueno, 2007). In short, plants were treated with either 0.5 mm BTH or 1 mm JA solution in water, allowed to dry completely, and then bagged in PET foil (Bratenschlauch [Toppits; www.toppits.de], a material that does not emit detectable amounts of volatiles even after exposure to temperatures up to 150°C) over the next 24 h (n = 7 per treatment). The emitted VOCs were collected continuously on charcoal traps (1.5 mg of charcoal; CLSA-Filters) using a closed-loop stripping system (Donath and Boland, 1995). Organic compounds were eluted from the charcoal traps after 24 h with dichloromethane (40 μL) containing 1-bromodecane (200 ng μL−1) as a standard. Samples were then analyzed on a GC-Trace mass spectrometer (Trace GC Ultra DSQ; Thermo Electron [www.thermo.com]). The program for separation (Rtx5-MS column [Restek; www.restek.com]; 15 m × 0.25 mm, 0.25-μm coating) was 40°C initial temperature (2 min), 10°C min−1 to 200°C, then 30°C min−1 to 280°C with helium (constant flow of 1.5 mL min−1) as carrier gas. Identification of compounds was done by comparison with standard substances and with the Nist 05 library. Individual compounds (peak areas) were quantified with respect to the peak area of the internal standard.
RT-PCR and qRT-PCR
Total RNA was isolated from lima bean leaf tissues using Tri reagent (Molecular Research Center) according to the manufacturer's instructions. RT-PCR (RETROscript; Ambion) analysis was performed according to the manufacturer's instructions. RT-PCR and qRT-PCR were conducted as described previously (Ryu et al., 2004a). To detect expression levels of PR-2,LOX, and Actin genes, adequate primers were obtained from Edington et al. (1991; PR-2), Meier et al. (1993; LOX), and Maffei et al. (2006; Actin) and were as follows: PvPR-2 (forward, 5′-GCCACAAATGCCGACACTGC-3′; reverse, 5′-GGACTCACTTCATTGCCAACTGC-3′), PvLOX (forward, 5′-GTGAGAGGCGATGGAAGTGGAG-3′; reverse, 5′-TGCGAGGGTAAGGTAAGGTAGAAC-3′), and PlActin (forward, 5′-AGGCTCCTCTTAACCCCAAG-3′; reverse, 5′-GTGGGAGAGCATAACCCTCA-3′).
qRT-PCR was performed on the Chromo4 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories) by adding 10 μL of iQ SYBR Green SuperMix (Bio-Rad Laboratories), 3 μL of diluted cDNA, 10 pmol of each primer, and water (to a final volume of 20 μL) to the reaction mix. After an initial incubation at 95°C for 3 min, amplifications were performed for 45 cycles with the following cycle profile: a denaturing step at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. All experiments were conducted three times independently each time with five replications.
Defense Gene Expression and Resistance Induction by Synthetic Volatiles
The two VOCs (methyl salicylate and nonanal; purchased from Sigma-Aldrich [www.sigmaaldrich.com]) were dissolved in 2 mL of lanolin paste at 1 μg VOC μL−1 lanolin as described previously (Kost and Heil, 2006). Two portions each of the lanolin paste containing each VOC were then applied close to the two bottom leaves of a plant avoiding direct physical contact. Leaves were collected 0 and 6 h after VOC treatment and 0 and 6 h after pathogen challenge. For assessment of defense induction by gaseous nonanal, a bacterial suspension of P. syringae was applied by spraying until runoff at 1 week after chemical treatment. The bacteria numbers of P. syringae of the respective leaf disc (diameter = 1 cm) applied by nonanal in the lanolin paste and lanolin alone (control) were counted at 0 and 3 d after pathogen challenge as described above. All experiments were conducted two times independently each time with five replications.
Data Analysis
Data were subjected to ANOVA using JMP software version 4.0 (SAS Institute; www.sas.com). Significance of direct and indirect biological or chemical treatment effects was determined by the magnitude of the F value at P = 0.05. When a significant F value was obtained for treatments, separation of means was accomplished using Fisher's protected lsd at P = 0.05. Results of repeated trials of each experiment outlined above were similar. Hence, one representative trial of each experiment is reported.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers X53129, X63521, and DQ159907.
Acknowledgments
We thank Sung Jae Kim for plant care, Alan Collmer for providing P. syringae strains, Dale Walters (Scottish Agricultural College) and Jurriaan Ton (Rothamsted Research Centre) for discussions and valuable comments on earlier drafts of the manuscript, and Kerstin Ploss and Prof. Dr. Wilhelm Boland (Department of Bioorganic Chemistry, Max-Planck-Institute of Chemical Ecology) for sharing unpublished data.
This work was supported by the BioGreen21 Program (grant no. 20070401034005) of the Rural Development Administration, the 21C Frontier Microbial Genomics and Application Center Program, Korea Science and Engineering Foundation project “System development for application of genomic sequence information,” the Ministry of Education, Science, and Technology, and the Korea Research Institute of Bioscience and Biotechnology Initiative Program of South Korea and the Consejo Nacional de Ciencia y Tecnología of Mexico.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Choong-Min Ryu (cmryu@kribb.re.kr).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Ballhorn DJ, Kautz S, Lion U, Heil M (2008) Trade-offs between direct and indirect defences of lima bean (Phaseolus lunatus). J Ecol 96 971–980 [Google Scholar]
- Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221 277–279 [DOI] [PubMed] [Google Scholar]
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hasch R, Polle A, Bohlmann J, Schnitzler JP (2007) Transgenic, non-isoprene emitting poplars don't like it hot. Plant J 51 485–499 [DOI] [PubMed] [Google Scholar]
- Birkett MA, Campbell CA, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, et al (2000) New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci USA 97 9329–9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett MA, Chamberlain K, Guerrieri E, Pickett JA, Wadhams LJ, Yasuda T (2003) Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J Chem Ecol 29 1589–1600 [DOI] [PubMed] [Google Scholar]
- Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173 603–608 [Google Scholar]
- Cools HJ, Ishii H (2002) Pre-treatment of cucumber plants with acibenzolar-S-methyl systemically primes a phenylalanine ammonia lyase gene (PAL1) for enhanced expression upon attack with a pathogenic fungus. Physiol Mol Plant Pathol 61 273–280 [Google Scholar]
- de Lacy Costello BPJ, Evans P, Ewen RJ, Gunson HE, Jones PRH, Ratcliffe NM, Spencer-Phillips PTN (2001) Gas chromatography-mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol 50 489–496 [Google Scholar]
- De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393 570–573 [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410 577–580 [DOI] [PubMed] [Google Scholar]
- Dilantha Fernando WG, Ramarathnam R, Krishnamoorthy AS, Savchuk SC (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37 955–964 [Google Scholar]
- Donath J, Boland W (1995) Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry 39 785–790 [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Edington B, Lamb C, Dixon R (1991) cDNA cloning and characterization of a putative 1,3-beta-D-glucanase transcript induced by fungal elicitor in bean cell suspension cultures. Plant Mol Biol 16 81–94 [DOI] [PubMed] [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Fokar M, Zhang HA, Allen RD, Paré PW (2005) (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 220 900–909 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Alméras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6 372–378 [DOI] [PubMed] [Google Scholar]
- Forouhar F, Yang Y, Kumar D, Chen Y, Fridman E, Park SW, Chiang Y, Acton TB, Montelione GT, Pichersky E, et al (2005) Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity. Proc Natl Acad Sci USA 102 1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia D, Demaria D, Calderini O, Ferraris L, Valentino D, Arcioni S, Tamietti G, Cardinale F (2007) Wounding induces resistance to pathogens with different lifestyles in tomato: role of ethylene in cross-protection. Plant Cell Environ 30 1357–1365 [DOI] [PubMed] [Google Scholar]
- Frost C, Appel H, Carlson J, De Moraes C, Mescher M, Schultz J (2007) Within-plant signalling by volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10 490–498 [DOI] [PubMed] [Google Scholar]
- Godard KA, White R, Bohlmann J (2008) Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 69 1838–1849 [DOI] [PubMed] [Google Scholar]
- Goellner K, Conrath U (2008) Priming: it's all the world to induced disease resistance. Eur J Plant Pathol 121 233–242 [Google Scholar]
- Hammerschmidt R, Smith-Becker JA (1999) The role of salicylic acid in disease resistance. In AA Agrawal, S Tuzun, E Bent, eds, Induced Plant Defenses against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture. American Phytopathological Society Press, St. Paul, pp 37–53
- Heil M (2004) Induction of two indirect defences benefits lima bean (Phaseolus lunatus, Fabaceae) in nature. J Ecol 92 527–536 [Google Scholar]
- Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178 41–61 [DOI] [PubMed] [Google Scholar]
- Heil M, Baldwin IT (2002) Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci 7 61–67 [DOI] [PubMed] [Google Scholar]
- Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9 813–817 [DOI] [PubMed] [Google Scholar]
- Heil M, Lion U, Boland W (2008) Defence-inducing volatiles: in search for the active motif. J Chem Ecol 34 601–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Ton J (2008) Long-distance signalling in plant defence. Trends Plant Sci 13 264–272 [DOI] [PubMed] [Google Scholar]
- Huang HC, Schuurink R, Denny TP, Atkinson MM, Baker CJ, Yucel I, Hutcheson SW, Collmer A (1988) Molecular cloning of a Pseudomonas syringae pv. syringae gene cluster that enables Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J Bacteriol 170 4748–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MD, Ryals JA (1996) Systemic acquired resistance signal transduction. Crit Rev Plant Sci 15 583–606 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Karban R, Shiojiri K, Huntzinger M, McCall AC (2006) Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87 922–930 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT (2006) Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148 280–292 [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2005) Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol 46 1093–1102 [DOI] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U (2002) Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol 128 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146 839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94 619–628 [Google Scholar]
- Kumar D, Klessig DF (2003) High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proc Natl Acad Sci USA 100 16101–16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithöfer A, Arimura G, Uchtenhagen H, Bossi S, Bertea CM, Cucuzza LS, Novero M, Volpe V, Quadro S, et al (2006) Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol 140 1022–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26 403–410 [DOI] [PubMed] [Google Scholar]
- Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9 274–280 [DOI] [PubMed] [Google Scholar]
- Meier B, Shaw N, Slusarenko A (1993) Spatial and temporal accumulation of defense gene transcripts in bean (Phaseolus vulgaris) leaves in relation to bacteria-induced hypersensitive cell death. Mol Plant Microbe Interact 6 453–466 [DOI] [PubMed] [Google Scholar]
- Mirabella R, Rauwerda H, Struys EA, Jakobs C, Triantaphylides C, Haring MA, Schuurink RC (2008) The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J 53 197–213 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Hatanaka A (2002) Green-leaf-derived C6-aroma compounds with potent antibacterial action that act on both gram-negative and gram-positive bacteria. J Agric Food Chem 50 7639–7644 [DOI] [PubMed] [Google Scholar]
- Ongena M, Duby F, Rossignol F, Fauconnier ML, Dommes J, Thonart P (2004) Stimulation of the lipoxygenase pathway is associated with systemic resistance induced in bean by a nonpathogenic Pseudomonas strain. Mol Plant Microbe Interact 17 1009–1018 [DOI] [PubMed] [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318 113–116 [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT (2006) Using ‘mute’ plants to translate volatile signals. Plant J 45 275–291 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Dicke M (2007) Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci 12 564–569 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, van Wees SCM, van Pelt JA, Knoester M, Laan R, Gerrits N, Weisbeek PJ, van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades DF (1983) Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows. In PA Hedin, ed, Plant Resistance to Insects. American Chemical Society, Washington, DC, pp 55–68
- Rostás M, Turlings TCJ (2008) Induction of systemic acquired resistance in Zea mays also enhances the plant's attractiveness to parasitoids. Biol Control 46 178–186 [Google Scholar]
- Ruther J, Kleier S (2005) Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J Chem Ecol 31 2217–2222 [DOI] [PubMed] [Google Scholar]
- Ryu CM, Anand A, Kang L, Mysore KS (2004. a) Agrodrench: a novel and effective agroinoculation method for virus-induced gene silencing in roots and diverse solanaceous species. Plant J 40 322–331 [DOI] [PubMed] [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004. b) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100 4927–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Howe GA (2005) Systemic signaling in the wound response. Curr Opin Plant Biol 8 369–377 [DOI] [PubMed] [Google Scholar]
- Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J (2006) Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proc Natl Acad Sci USA 103 16672–16676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I (1997) Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385 718–721 [Google Scholar]
- Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399 686–688 [Google Scholar]
- Ton J, D'Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49 16–26 [DOI] [PubMed] [Google Scholar]
- Truman W, Bennettt MH, Kubigsteltig I, Turnbull C, Grant M (2007) Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA 104 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharntke T, Thiessen S, Dolch R, Boland W (2001) Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochem Syst Ecol 29 1025–1047 [Google Scholar]
- Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92 4169–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103 753–765 [Google Scholar]
- Walters D, Heil M (2007) Costs and trade-offs associated with induced resistance. Physiol Mol Plant Pathol 71 3–17 [Google Scholar]
- Walters DR, Boyle C (2005) Induced resistance and allocation costs: what is the impact of pathogen challenge? Physiol Mol Plant Pathol 66 40–44 [Google Scholar]
- Walters DR, Cowley T, Weber H (2006) Rapid accumulation of trihydroxy oxylipins and resistance to the bean rust pathogen Uromyces fabae following wounding in Vicia faba. Ann Bot (Lond) 97 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Parthier B (1997) Jasmonate-signalled plant gene expression. Trends Plant Sci 2 302–307 [Google Scholar]