Abstract
The purpose of the study was to evaluate ethnic differences in polysomnography measures in adolescents. Ninety-six volunteers from four ethnic groups (13 African-American, 18 Asian-American, 19 Mexican-American, and 46 Non-Hispanic White) were recruited. The subjects were in good physical and psychological health, and were asymptomatic with respect to sleep/wake complaints or sleep disorders. Polysomnography measures were collected on three consecutive nights. African-Americans manifested lower sleep efficiency, spent proportionately more time in stage 2 sleep, and had less stage 4 sleep compared to the other ethnic groups. In contrast to this, Mexican-Americans had more rapid eye movement (REM) sleep than their counterparts. The observed sleep patterns in the different ethnic groups persisted after controlling for specific demographic, clinical and psychosocial variables that are known to influence sleep measures. Gender had a differential effect on sleep patterns in the various ethnic groups. For instance, differences in non-REM sleep were more evident in African-American males, whereas increased REM sleep was most notable in Mexican-American females. At present, the clinical implications of the observed cross-ethnic differences in sleep physiology among adolescents are not clear. In previous studies, reduced sleep efficiency and stage 4 sleep, as well as increased REM sleep, were associated with psychopathology. It is not known whether the traditionally described sleep profiles, based largely on Non-Hispanic White populations, will generalize to other racial or ethnic groups. In addition to a systematic investigation of this issue, future research should attempt to identify the underlying causes for cross-ethnic variations in sleep physiology.
Keywords: Race, Sex, Sleep, Pediatric, Psychopathology, Polysomnography
1. Introduction
There is evidence of ethnic/racial disparities in the prevalence and burden of disease for a broad spectrum of physical and mental disorders. For example, the prevalence of diabetes in African-Americans and Mexican-Americans is almost double that of the rates observed in Non-Hispanic Whites, whereas Asian-Americans are more than 5 times likely to suffer from tuberculosis than the rest of the US population (Centers for Disease Control and Prevention, 2006, 2007). On the other hand, the prevalence of osteoporosis is highest among Non-Hispanic Whites (Department of Health and Human Services, 2007). Ethnic/racial differences also have been reported with regard to prevalence rates, symptom profiles and treatment response for different psychiatric disorders (Alegría et al., 2007; Breslau et al., 2006; Goodman et al., 2008; Lin, 2001). The underlying causes for such ethnic/racial disparities are not well-understood, but there is a consensus that they are likely to be multi-dimensional including socioeconomic and cultural factors, access to health care services as well as genetic/biological differences (Abe-Kim et al., 2007; Collins, 2004; Lin, 2001; Takeuchi et al., 2007). Among the latter, variations in sleep patterns might play a contributory role.
1.1. The role of sleep in physical and mental health
Sleep is a fundamental neurobehavioral state linked to critical domains of health and functioning, including attention, learning and memory, mood regulation, as well as metabolic, endocrine, immune and cardiovascular functions (Banks and Dinges, 2007; Barnard and Nolan, 2008; Smolensky et al., 2007). Prospective epidemiological studies demonstrated that sleep disruption is associated with medical conditions that are highly prevalent in our society, including cardiovascular disease, diabetes, and obesity (Knutson and Van Cauter, 2008; Miller and Cappuccio, 2007). Sleep dysregulation also is associated with a wide array of psychiatric disorders, including anxiety, mood and addictive disorders (Breslau et al., 1996; Buysse et al., 2008; Germain and Kupfer, 2008). Although there is evidence of a strong genetic influence on circadian sleep rhythm and sleep architecture, they are also highly sensitive to socio-cultural factors (Barnard and Nolan, 2008; Kimura and Winkelmann, 2007). Therefore, it is likely that ethnic/racial factors significantly impact the sleep system.
1.2. Associations among ethnicity/race, sleep and psychopathology
Given the growing interest on the relationship between ethnicity and health, and based on the evidence of sleep effects on health, it is surprising that relatively few studies exist on ethnic differences in sleep. Albeit limited, data from diverse lines of investigation, including self-report, behavioral and physiological assessments, point towards ethnic differences in certain sleep measures. For instance, studies in adults reported that ethnic-minority groups, including African-Americans and Hispanics, take longer time to fall asleep and have less efficient sleep than Non-Hispanic Whites (Durrence and Lichstein, 2006; Jean-Lous et al., 2000; Lauderdale et al., 2006; Mezick et al., 2008). Also, there is evidence that African-Americans spend proportionately less time in slow-wave or deep sleep compared with Hispanics or Non-Hispanic Whites (Mezick et al., 2008; Profant et al., 2002; Rao et al., 1999; Redline et al., 2004; Stepnowsky et al., 2003). In contrast to the ethnic differences in sleep continuity disturbances and non-rapid eye movement (NREM) sleep, few studies reported variations in rapid eye movement (REM) sleep. In one investigation, Hispanics had increased phasic REM sleep compared to African-Americans and Non-Hispanic Whites (Rao et al., 1999).
The ethnic differences in sleep regulation might be associated with inherent differences in sleep homeostasis due to the interplay of biological and socio-cultural factors (Achermann et al., 1993; Carskadon et al., 2004). Alternatively, the sleep variations might suggest differential vulnerability for specific psychiatric disorders or distinct pathophysiological mechanisms. For example, African-Americans have a lower prevalence of major depressive disorder compared with Non-Hispanic Whites (Breslau et al., 2006; Williams et al., 2008). Other studies reported that African-American patients with depression had less efficient sleep and NREM sleep changes, whereas Hispanic and Non-Hispanic White patients manifested predominantly REM sleep changes although the ethnic groups were comparable on clinical symptoms and severity of depression (Giles et al., 1998b; Poland et al., 1999).
1.3. Ethnic differences in sleep patterns among adolescents
There have been even fewer investigations focusing on ethnic differences in sleep among adolescents. Significant biological and psychosocial changes in sleep and circadian regulation occur during adolescent development (Carskadon et al., 2004; Dahl, 2008). Although sleep is particularly important during brain maturation, many adolescents have insufficient sleep, and inadequate sleep is associated with a higher frequency of emotional and behavioral problems as well as impairment in social functioning (Dahl, 2008; Roberts et al., 2008; Smaldone et al., 2007). Also, evidence suggests that most adult psychiatric disorders, including anxiety, mood, addictive and psychotic disorders, have their first onset during adolescence (Kessler et al., 2005; Rapoport et al., 2005). A systematic examination of the relationships among socio-cultural factors and behavioral and physiological indices of sleep in youngsters will be helpful in understanding the cross-ethnic differences in psychopathology, without many of the potentially confounding factors that occur in adults, such as comorbid medical and psychiatric conditions.
In terms of subjective reports of sleep patterns in different ethnic groups, the findings are mixed. For example, one study reported that African-American and Hispanic youth were more likely to sleep 6 h or less per night compared with Non-Hispanic Whites (Cornelius, 1991). In contrast to this, Roberts et al. (2000) found higher frequency of hypersomnia symptoms in African-American and Mexican-American groups. In that study, Asian-American youth had the lowest prevalence of insomnia symptoms (Roberts et al., 2000). Although there are important methodological differences among studies, the emerging theme from the survey data in youth is that, after accounting for other influential factors, sleep patterns are comparable across ethnic groups (Ebin et al., 2001; Johnson and Breslau, 2001; Roberts et al., 2006). To the best of our knowledge, there are no published reports regarding ethnic differences in sleep physiology in adolescents. Given that cross-ethnic differences exist in sleep physiology among adults and that these differences may be associated with psychopathology, it is not clear when during development these differences emerge.
In this report, polysomnography measures were compared in four ethnic groups of adolescents (namely African-American, Asian-American, Mexican-American and Non-Hispanic Whites). In addition to examining ethnic differences in sleep physiology, the effects of other demographic, clinical and psychosocial factors that potentially affect sleep were evaluated. Here, ethnic determination was based on self-identified social or cultural heritage with shared physical features. The original study was not designed to examine the relationship between ethnicity and sleep patterns. The primary focus of the study was to identify pre-morbid sleep and neuroendocrine profiles associated with the development of depressive illness during prospective follow-up. Here, only baseline sleep polysomnography data are presented.
2. Experimental/materials and methods
2.1. Participants
The participants were recruited from local pediatric clinics and schools in Los Angeles, through advertisements in local news papers and by word of mouth. The sample included 48 adolescents with no personal or family history of psychiatric illness, and 48 adolescents with no personal history of a psychiatric disorder, including depression, but were at high risk for developing depression by virtue of parental depression; at least one biological parent with a history of unipolar major depressive disorder that required treatment. All participants were medically healthy and free from alcohol or illicit drug use, as determined by physical examination, full chemistry panel, thyroid function tests, and electrocardiogram and urine drug screens. Subjects with a personal history of sleep disorder(s), or those with a family history of narcolepsy, and females with suspected pregnancy were excluded from the study. The protocol was approved by the Institutional Review Boards at the University of California at Los Angeles (UCLA) and affiliated institutions. All adolescents signed a written assent form, and parents signed an informed consent document prior to performing the research procedures.
2.2. Assessments
2.2.1. Diagnostic evaluation
Symptoms of major psychiatric disorders were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-the Present and Life-time Version (K-SADS-PL). The K-SADS-PL is a semi-structured interview designed to ascertain present and life-time history of psychiatric illness according to DSM-IV criteria (Kaufman et al., 1997). Probes and objective criteria are provided for individual symptoms at both diagnostic and sub-threshold levels. Inter-rater and test–retest reliability have been established, as well as convergent and discriminate validity (Kaufman et al., 1997). The K-SADS-PL was administered separately to the parent and the adolescent, and both were re-interviewed to resolve any discrepancies. Summary scores were tabulated based on the information obtained from both informants. The Hamilton Depression Rating Scale (HDRS), a depression severity measure (Hamilton, 1960), and Children’s Global Assessment Scale (CGAS), a global psychosocial functioning measure (Shaffer et al., 1983), also were completed. The adolescent participants completed the Beck Depression Inventory (BDI) for self-assessment of depression severity (Beck et al., 1961). The Family History-Research Diagnostic Criteria (FH-RDC), a semi-structured interview, was used for the evaluation of psychiatric disorders in family members (Andreasen et al., 1977). A parent was interviewed regarding life-time psychiatric disorders in all first-degree relatives of the adolescent subject (including the self, spouse and all offspring). The FH-RDC is sensitive for obtaining information from knowledgeable relatives (Thompson et al., 1982). Information on race/ethnicity was gathered from the adolescent and the parent, and socioeconomic status (SES) was assessed with the Hollingshead Scale (Hollingshead, 1975).
2.2.2. Information on environmental stress
In order to obtain information on persistent stress and episodic stressful life experiences during adolescence, a semi-structured interview developed in our laboratory, the UCLA Chronic and Episodic Stress Interview (Hammen et al., 1985), was modified for use with adolescents (Hammen et al., 1995). The chronic stress assessment consists of conditions that persist for 6 months or longer. In previous longitudinal studies, the magnitude of stress in pertinent domains remained stable across assessment periods (Hammen et al., 1999; Herzberg et al., 1998). In contrast, episodic life experiences are discrete, acute events with a clear period of onset and offset. Ten content areas (including family relationships, independence from the family, close friendships, romantic relationships, social life, school, work, finances, health of subject, and health of family members) were assessed. The adolescent was interviewed on the quality of relationships and performance in each domain within the past 6 months, and ratings were given for the magnitude of stress using objective criteria (1 = not at all stressful, 5 = extremely stressful).
After obtaining information on the magnitude of chronic stress in each domain, participants were probed systematically about the occurrence and timing of acute life events. Narrative summaries of each life event and the surrounding context were presented to a group of trained raters. The raters were blind to the participant’s diagnostic status and reaction to the stressor. Consensus group ratings were given for the degree of stress (1 = not at all stressful, 5 = extremely stressful) for each event, and whether the event was a positive, neutral or negative experience under the given circumstances. Only events that were considered negative were included in the analyses, and a summary score of stress impact from the negative events was tabulated. Good inter-rater reliabilities have been established for the chronic and episodic stress ratings (Hammen and Brennan, 2001).
2.2.3. Regulation of sleep–wake schedules
One week prior to the laboratory assessment, the participants were instructed to go to bed between 10:00 p.m. and 11:00 p.m., and to awake between 6:30 a.m. and 7:30 a.m. The sleep–wake schedules were monitored by daily sleep logs and wrist actigraphy.
2.2.4. Sleep protocol
Polysomnography measures were collected on 3 consecutive nights. The participants arrived in the laboratory at 8:00 p.m. Conventional electroencephalographic (EEG) electrodes were attached by 9:00 p.m., and sleep recordings were made from 10:30 p.m. (lights out) to 7:00 a.m. In order to rule out the presence of major sleep disorders, a full polysomnography was performed on the first night, including respiratory, oximetry and leg movement measurements. The International 10–20 System was used for EEG electrode placement, electromyogram (EMG), electrooculogram (EOG) and electrocardiogram. Bilateral EEG recordings were obtained from left (C3) and right (C4) central leads, referenced to the opposite mastoid, A2 and A1, as well as to a linked reference (A1 + A2). Bilateral EOG recordings were obtained, and referenced to A1 + A2 along with a submental EMG recording.
2.2.5. Scoring of sleep records
Sleep records were coded and scored “blindly” according to standard criteria (Rechtschaffen and Kales, 1968). REM latency was defined as the time between sleep onset (first minute of stage 2 or deeper sleep, followed by at least 9 min of stage 2 or deeper sleep, interrupted by no more than 1 min of waking or stage 1) and the first REM period ≥3 min in length. Other REM sleep measures, including REM activity and REM density, and additional sleep variables also were scored according to standard criteria (Kupfer et al., 1976). REM activity was scored on a scale ranging from 0 to 8 units.
2.3. Statistical methods
For all summary variables, data were examined for normality using the Shapiro–Wilk’s W statistic (Shapiro and Wilk, 1965). In cases of significantly non-normal distributions, logarithm transformations were performed to normalize the data prior to the application of statistical tests for significance. Student’s t and Pearson’s chi-square tests were used for group comparisons on demographic, clinical and psychosocial measures. Multivariate analysis of variance, with appropriate covariates (MANCOVA), was employed for comparisons on categories of polysomnography measures. Group comparisons of individual means were tested if the overall MANCOVA was significant. Regression procedures were utilized for evaluating the relative contribution of each demographic, clinical and psychosocial variable to specific sleep measures that were significant in the MANCOVA tests. One-way, repeated measures analysis of variance, with appropriate covariates, was used for examining the distribution of sleep stages across the night. The first night was considered as an adaptation night, and the mean values derived from Night 2 and Night 3 data were used. Alpha was set at .05.
3. Results
3.1. Demographic, clinical and psychosocial parameters
Demographic, clinical and psychosocial features of the four ethnic groups are outlined in Table 1. With one exception, the groups were comparable on all measures. African-Americans and Mexican-Americans came from lower socioeconomic backgrounds compared to Non-Hispanic White youth. Asian-Americans had a higher socioeconomic status than Mexican-Americans.
Table 1.
Baseline demographic and clinical parameters stratified on ethnicity.
| African-American (n = 13) | Asian-American (n = 18) | Mexican American (n = 19) | Non-Hispanic White (n = 46) | Statistic F/χ2 | p | |
|---|---|---|---|---|---|---|
| Age (years) | 14.8 ± 1.5 | 15.2 ± 1.2 | 14.8 ± 1.7 | 15.3 ± 1.5 | 0.72 | NS |
| Gender (male/female) | 6/7 | 8/10 | 7/12 | 19/27 | 0.35 | NS |
| Socioeconomic scorea | 41.3 ± 12.6a,c | 47.6 ± 12.2a,b | 41.4 ± 8.6c | 49.2 ± 7.8b | 4.37 | .006 |
| Depression in parent (%) | 7 (53.8) | 8 (44.4) | 10 (52.6) | 23 (50.0) | 0.35 | NS |
| Hamilton depression scale | 0.8 ± 1.0 | 0.4 ± 1.2 | 1.4 ± 1.5 | 1.1 ± 1.5 | 1.85 | NS |
| Beck Depression Inventory | 2.4 ± 2.5 | 1.8 ± 1.8 | 2.9 ± 3.5 | 2.9 ± 3.2 | 0.69 | NS |
| CGAS scorea | 78.5 ± 10.5 | 78.6 ± 11.2 | 80.1 ± 10.7 | 83.1 ± 9.0 | 1.38 | NS |
| Chronic stress | 18.5 ± 4.4 | 17.2 ± 4.9 | 18.2 ± 5.0 | 18.2 ± 4.4 | 0.54 | NS |
| Episodic stress | 2.7 ± 2.2 | 3.0 ± 2.5 | 2.9 ± 3.1 | 4.3 ± 3.1 | 1.90 | NS |
Data are presented in means and standard deviations, or in raw numbers.
CGAS = Children’s Global Assessment Scale.
Different subscripts denote significant differences between groups.
Higher score is associated with higher socioeconomic status or better psychosocial function.
3.2. Relationship between ethnicity and sleep measures
Mean values for the major polysomnography variables for all four groups are shown in Table 2. Since the ethnic groups differed significantly on socioeconomic status, it was entered as a covariate in the statistical analyses. Socioeconomic status did not have a significant effect on any of the sleep measures. Ethnicity significantly affected sleep continuity measures. Specifically, African-Americans had lower sleep efficiency than their counter-parts. Also, there was an effect of ethnicity on sleep architecture. African-Americans had a higher proportion of stage 2 sleep and lower proportion of stage 4 sleep than the other ethnic groups. In contrast, Mexican-Americans had a higher proportion of REM sleep than their counterparts. Ethnicity had no effect on the first REM episode, but it did affect REM sleep when all episodes were combined. Specifically, Mexican-Americans had a longer REM duration than the other groups.
Table 2.
Polysomnography variables (mean ± S.D.) in the four ethnic groups.
| African-American (n = 13) | Asian-American (n = 18) | Mexican-American (n = 19) | Non-Hispanic White (n = 46) | SES | Ethnicity | |
|---|---|---|---|---|---|---|
| Sleep continuity | 1.89 | 3.35** | ||||
| Total sleep time (min) | 456.6 ± 48.9 | 487.8 ± 32.6 | 471.3 ± 37.9 | 472.6 ± 44.6 | 1.87 | |
| Sleep latency (min) | 14.5 ± 9.2 | 13.8 ± 9.0 | 12.1 ± 5.6 | 15.2 ± 11.1 | 0.23 | |
| Sleep efficiency (%) | 92.3 ± 2.2a | 94.1 ± 1.7b | 94.4 ± 2.2b | 94.5 ± 2.6b | 3.10* | |
| Arousals | 23.1 ± 9.5 | 22.9 ± 9.6 | 19.2 ± 8.3 | 20.7 ± 8.4 | 0.83 | |
| Awake time (min) | 18.6 ± 13.7 | 19.3 ± 10.4 | 13.9 ± 8.8 | 17.7 ± 10.1 | 0.80 | |
| Sleep architecture | 0.28 | 4.26*** | ||||
| Stage 1 sleep (%) | 10.8 ± 4.7 | 9.8 ± 4.0 | 8.3 ± 2.9 | 10.1 ± 4.7 | 0.98 | |
| Stage 2 sleep (%) | 58.2 ± 7.2a | 52.8 ± 6.6b | 48.9 ± 9.3b | 52.4 ± 6.7b | 4.09** | |
| Stage 3 sleep (%) | 9.4 ± 3.1 | 12.0 ± 4.9 | 12.1 ± 6.4 | 10.3 ± 4.3 | 1.36 | |
| Stage 4 sleep (%) | 5.0 ± 5.3a | 9.5 ± 6.5b | 10.3 ± 5.4b | 10.0 ± 5.8b | 2.77* | |
| REM sleep (%) | 16.7 ± 3.1a | 16.0 ± 3.7a | 20.4 ± 3.9b | 17.3 ± 3.3a | 5.61**** | |
| REM measures | ||||||
| 1st REM episode | 0.74 | 1.25 | ||||
| REM latency (min)a | 104.1 ± 50.7 | 99.5 ± 22.8 | 103.1 ± 46.1 | 102.6 ± 35.0 | – | |
| REM activity (units) | 10.4 ± 12.2 | 16.6 ± 9.7 | 16.4 ± 10.1 | 17.3 ± 13.9 | – | |
| REM density (units/min) | 1.4 ± 0.5 | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.6 ± 0.5 | – | |
| REM duration (min) | 7.0 ± 6.7 | 9.7 ± 5.4 | 10.4 ± 5.3 | 10.1 ± 6.2 | – | |
| REM measures | ||||||
| All REM episodes combined | 0.67 | 4.27*** | ||||
| REM activity (units)a | 160.1 ± 76.1 | 176.5 ± 53.1 | 243.8 ± 101.9 | 175.3 ± 82.9 | 1.02 | |
| REM density (units/min) | 2.2 ± 1.0 | 2.3 ± 0.6 | 2.6 ± 0.8 | 2.1 ± 0.8 | 2.32 | |
| REM duration (min) | 76.4 ± 16.2a | 78.1 ± 18.7a | 94.1 ± 18.9b | 81.4 ± 20.9a | 2.73* | |
| Number of REM episodes | 4.1 ± 0.5 | 4.0 ± 0.5 | 4.4 ± 0.5 | 4.2 ± 0.6 | 1.32 | |
Analyses were performed on log-transformed data.
p ≤ .05.
p ≤ .01.
p ≤ .005.
p ≤ .001.
3.3. Unique contribution of ethnicity to the prediction of sleep measures
In order to examine the unique contribution of ethnicity to the identified polysomnography measures (namely, sleep efficiency, stage 2 sleep, stage 4 sleep, and REM sleep), linear regression analyses were conducted by including all the demographic, clinical and psychosocial variables simultaneously (see Table 3). After controlling for the other variables, ethnicity still predicted sleep efficiency. In addition to African-American youth, adolescents whose parent(s) had a history of depression also manifested lower sleep efficiency than their counterparts without parental depression. The combined factors contributed 17% variance to sleep efficiency.
Table 3.
Predictors of specific polysomnography measures (n = 96).
| Sleep efficiency β (SE) | Stage 2 sleep β (SE) | Stage 4 sleep β (SE) | Stage REM sleep β (SE) | REM duration β (SE) | |
|---|---|---|---|---|---|
| Age | −.15 (.18) | 0.89 (0.56) | −0.39 (0.42) | 0.22 (0.25) | 1.18 (1.38) |
| Sex (female) | 0.74 (0.53) | 1.37 (1.66) | 1.40 (1.25) | −1.53 (0.75)* | −12.11 (4.03)*** |
| Ethnicity (African-American) | −0.66 (0.27)* | 2.41 (0.84)*** | −1.30 (0.63)* | −1.26 (0.38)**** | −5.72 (2.03)** |
| Socioeconomic statusa | −0.00 (0.03) | 0.00 (0.08) | −0.04 (0.06) | −0.01 (0.04) | −0.09 (0.20) |
| Depression in the parent | −1.45 (0.57)* | −1.46 (1.77) | 1.89 (1.33) | 2.66 (0.80)**** | 14.25 (4.30)**** |
| Depression severity (HDRS) | −.11 (0.20) | 0.28 (0.63) | −0.14 (0.47) | 0.21 (0.29) | 1.46 (1.53) |
| Depression severity (BDI) | −0.22 (0.11) | 0.09 (0.34) | −0.30 (0.25) | 0.09 (0.15) | 0.28 (0.82) |
| Global function (CGAS)a | 0.03 (.03) | −0.14 (0.08) | 0.19 (0.06)** | 0.06 (0.04) | 0.26 (0.20) |
| Chronic stress | −0.02 (0.06) | 0.07 (0.19) | −0.13 (0.14) | −0.06 (0.09) | −0.50 (0.45) |
| Episodic stress | −0.05 (0.09) | 0.05 (0.29) | −0.48 (0.22)* | 0.05 (0.13) | 0.73 (0.70) |
SE = standard error; HDRS = Hamilton Depression Rating Scale; BDI = Beck Depression Inventory; CGAS = Children’s Global Assessment Scale.
Sleep efficiency: R2 = .17, F = 1.73, df = 10, p ≤ .10; Stage 2 sleep: R2 = .17, F = 1.68, df = 10, p ≤ .10; Stage 4 sleep = R2 = .22, F = 2.38, df = 10, p ≤ .05; Stage REM sleep: R2 = .28, F = 3.34, df = 10, p ≤ .001; REM duration: R2 = .29, F = 3.49, df = 10, p ≤ .001.
Higher score is associated with higher socioeconomic status or better psychosocial function.
p ≤ .05.
p ≤ .01.
p ≤ .005.
p ≤ .001.
Ethnicity also made a unique contribution to stage 2 sleep. None of the other variables had an effect on stage 2 sleep. Overall, the demographic, clinical and psychosocial variables contributed 17% variance to stage 2 sleep. Ethnicity contributed to stage 4 sleep as well. Adolescents with a higher level of psychosocial functioning had more stage 4 sleep than those with lower functioning. In contrast, adolescents who experienced a higher magnitude of episodic stress had a lower proportion of stage 4 sleep than those who experienced less stress. The combined factors contributed 22% variance to stage 4 sleep.
Ethnicity also made a unique contribution to proportion of REM sleep as well as the duration of REM sleep. In addition to ethnicity, gender and depression history in the parent predicted both measures of REM sleep. Specifically, females had less amount of REM sleep than males. In contrast, high-risk youth had more REM sleep than their counterparts without depression history in the parent. Together, the demographic, clinical and psychosocial measures contributed 28% variance to stage REM sleep and 29% variance to REM duration.
3.4. Interactions among ethnicity, gender and sleep physiology
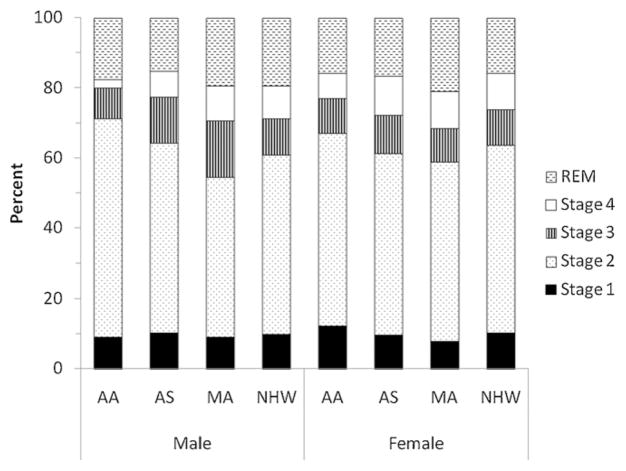
As described in Section 3.3, gender had a significant effect on the proportion of REM sleep. Post-hoc analyses were conducted to examine sleep architecture measures separately in males and females. Among males, with the exception of stage 1 sleep, ethnicity discriminated all other sleep stages (see Fig. 1). Specifically, African-Americans had a higher proportion of stage 2 sleep compared to all the other groups (F3,36 = 5.78, p ≤ .005). African-Americans also had less stage 4 sleep than Mexican-Americans and Non-Hispanic Whites (F3,36 = 3.34, p ≤ .05). Mexican-Americans had more stage 3 sleep than African-Americans and Non-Hispanic Whites (F3,36 = 3.34, p ≤ .05). Asian-Americans had less REM sleep than Mexican-Americans and Non-Hispanic Whites (F3,36 = 2.87, p ≤ .05). A different pattern emerged in females (see Fig. 1). Significant ethnic differences occurred only for REM sleep, with Mexican-Americans having a higher proportion of REM sleep than all the other groups (F3,36 = 2.87, p ≤ .05).
Fig. 1.
Sleep architecture measures stratified by gender and ethnicity (AA = African-American; AS = Asian-American; MA = Mexican-American; and NHW = Non-Hispanic White).
As described in Section 3.3, gender affected the duration of REM sleep. Because the proportion of REM sleep also varied among the ethnic groups as a function of gender, further analyses were conducted to examine the distribution of REM sleep across the night (see Fig. 2). Regardless of ethnic or gender status, the duration of REM sleep varied across the night, and the first episode was significantly shorter than the other episodes (F = 9.73, df = 3, p ≤ .0001). Also, there was an interaction between ethnicity and REM duration across the night (F = 2.75, df = 9, p ≤ .01). Although the interaction between ethnic background and gender on REM sleep across episodes was not statistically significant, there were some trends. Among males, African-Americans and Asian-Americans had considerably less REM sleep during the first three episodes in comparison with Mexican-Americans and Non-Hispanic Whites (F3,36 = 6.19, p ≤ .005). The groups were comparable on the quantity of REM sleep during the fourth episode (see Fig. 2). A different pattern emerged in females (see Fig. 2). During the first three REM periods, Mexican-Americans had more sleep than all the other groups (F3,52 = 8.88, p ≤ .0001). Non-Hispanic Whites also had considerably more REM sleep than African-Americans. During the fourth episode, Non-Hispanic Whites had less REM sleep than all the other groups (F3,52 = 3.88, p ≤ .05).
Fig. 2.
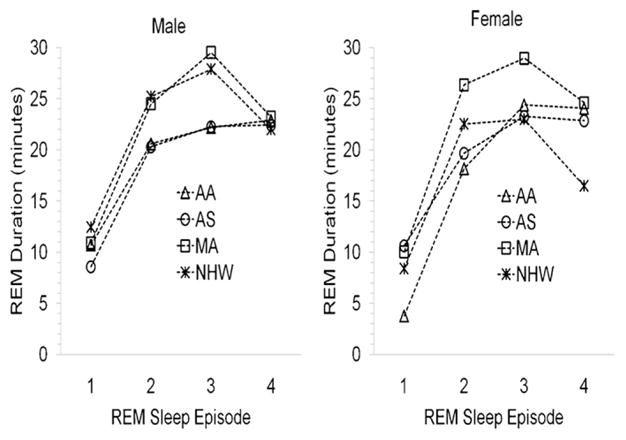
REM sleep profiles across the night stratified by gender and ethnicity (AA = African-American; AS = Asian-American; MA = Mexican-American; and NHW = Non-Hispanic White).
3.5. Interactions among ethnicity, vulnerability for depression and sleep physiology
As described in Section 3.3, depression history in the parent affected sleep efficiency and REM sleep. Post-hoc analyses did not reveal a significant interaction between ethnicity and vulnerability for depression on the sleep measures although the observed cross-ethnic differences in REM sleep were more predominant in adolescents with depression history in the parent compared to their counterparts without depression history in the parent.
4. Discussion
Comparison of polysomnography profiles of four major ethnic groups revealed generally similar patterns although some differences were observed between groups. Specifically, African-Americans had reduced sleep efficiency and stage 4 sleep, but increased stage 2 sleep, whereas Mexican-Americans manifested more REM sleep. Gender also influenced sleep patterns differentially among the ethnic groups. For instance, reduced stage 4 sleep was more predominant in African-American males, whereas increased REM sleep was most notable in Mexican-American females. These cross-ethnic differences persisted after controlling for other demographic, clinical and psychosocial factors known to affect sleep. The observed cross-ethnic differences in sleep profiles are comparable to reports in adults, suggesting that the differences emerge at least by adolescence.
These results should be considered preliminary because of the modest sample sizes, specifically in the ethnic-minority groups. Furthermore, the data on ethnicity by gender interactions, where the sample sizes were even smaller, must be interpreted with caution. Although the ethnic differences persisted after controlling for socioeconomic status and related variables, such as stress levels, other unmeasured socio-cultural factors might account for these findings. Factors that potentially affect sleep patterns in adolescents include pubertal maturation and sleep habits. Although the groups were comparable on pubertal maturation (≥Tanner Stage 3) and sleep–wake schedules were regulated before the laboratory visit, other factors, such as sleep location and context, were not assessed. For example, Stepnowsky et al. (2003) found that African-Americans had more slow-wave sleep at home compared to the hospital-setting, while the reverse was true for Non-Hispanic Whites. Information on ethnic heritage was obtained from the adolescent and parent. Hence, it is not possible to differentiate between genetic/biological and socio-cultural factors associated with inter-ethnic differences in sleep profiles; it is likely that both interplay. Moreover, the ethnic groups were not homogeneous in terms of native ethnic identification (e.g., Asian-Americans were comprised of Chinese as well as Indians). Although all participants were in good physical and psychological health at the time of assessment, a subset was at high-risk for depression based on family history. The cross-ethnic differences in sleep profiles might be associated with the vulnerability for depression, as previous studies reported co-segregation of depression and sleep markers among family members (Giles et al., 1992, 1998a). However, the ethnic groups were comparable on risk status, and ethnic effects persisted after controlling for parental depression as well as depressive symptoms and psychosocial functioning in the adolescent. Finally, the clinical significance of cross-ethnic differences in sleep profiles, including reduced sleep efficiency or stage 4 sleep, and more REM sleep, is not clear. Although these markers are associated with psychopathology traditionally (Benca et al., 1992), they are based largely on Non-Hispanic White populations and may not generalize to other ethnic/racial groups.
4.1. Relationship between sleep profiles and psychopathology
The cross-ethnic differences in sleep patterns suggest that various ethnic groups have different sleep needs that evolved as a result of interactions between genetic/biological and socio-cultural processes (Achermann et al., 1993; Carskadon et al., 2004). Alternatively, the sleep variations might suggest differential vulnerability for specific psychiatric disorders. For example, the sleep profile observed in African-Americans (particularly in males), with less efficient sleep, reduced slow-wave sleep and relatively normal REM sleep, is similar to the sleep characteristics observed in chronic insomnia (Basta et al., 2007) and in anxiety disorders (Mellman, 2006). In support of this hypothesis, in a recent study comprising a large sample of adolescent students from diverse ethnic backgrounds, African-American males reported the highest levels of physiological anxiety symptoms (McLaughlin et al., 2007). In the current study, there was no evidence of high level of anxiety symptoms, sleep complaints or stress levels in African-Americans. Nevertheless, when the physiological anxiety symptoms observed in other studies are taken in the context of residence in an inner city environment and associated stress levels, hyperarousal and chronic insomnia are likely (Basta et al., 2007).
In contrast to the NREM sleep changes in African-Americans, Mexican-Americans, females in particular, manifested increased REM sleep in the current study. Increased REM sleep is frequently associated with depression, and in the adolescent study described above, Hispanic females experienced higher levels of both depressive and anxiety symptoms than the other groups (McLaughlin et al., 2007). Epidemiological studies in adults also reported differences in the prevalence of psychiatric disorders among ethnic groups (Alegría et al., 2007; Breslau et al., 2006; Williams et al., 2008; Takeuchi et al., 2007).
Another possibility for the cross-ethnic differences in sleep profiles is that different pathophysiological mechanisms operate among groups (Basta et al., 2007). Giles et al. (1998b) found that African-American patients with depression had less sleep duration, and a higher proportion of stage 2 sleep, but lower proportion of slow-wave sleep, compared with Euro-American patients with depression. In contrast, Euro-American patients manifested shorter REM latency, more REM sleep and increased phasic activity during REM sleep. These cross-ethnic differences in sleep patterns were not accounted by demographic features or clinical profile of the depressive illness. In a separate study, African-American male patients with depression, but not females, showed evidence of reduced slow-wave sleep compared to Asian-American, Hispanic and Non-Hispanic White patients with depression (Poland et al., 1999). In addition, African-American and Asian-American patients had less REM sleep compared with Hispanic and Non-Hispanic White patients. Not only did the quantity of REM sleep differ, but also the distribution of REM sleep varied across the night among these four ethnic groups. African-Americans and Asian-Americans had considerably less REM sleep in the first three episodes compared with Hispanics and Non-Hispanic Whites, whereas the reverse was true for the fourth REM episode.
Since the sleep dysregulation in African-American patients with depression was relatively specific to NREM sleep, it is speculated that they have a weakened slow-wave sleep generator (Achermann et al., 1993). In contrast, Hispanic and Non-Hispanic White patients with depression might have increased REM sleep pressure. In addition to inherent biological differences in sleep regulation among ethnic groups, psychosocial factors also can affect sleep patterns. For example, the sleep architecture changes associated with psychosocial stress and chronic insomnia are relatively specific to NREM sleep, specifically reduction in slow-wave sleep (Basta et al., 2007), as was also observed in the current study. Similarly, increased life-stress was shown to precipitate depression in the absence of REM sleep abnormalities (Cartwright, 1983; Monroe et al., 1992). It is important to note, however, that the variability in sleep patterns among different ethnic groups is not restricted to depression. Similar patterns were observed in volunteers with no evidence of psychiatric illness, and they probably reflect ethnic differences in sleep regulation rather than an effect of depressive disorder. It is possible that the normal variations in sleep patterns among different ethnic groups become exaggerated during clinical manifestation of depression, and longitudinal studies should address this issue in the future.
4.2. Treatment implications of cross-ethnic differences in sleep patterns
Given that cross-ethnic variations in sleep profiles exist in patients with psychiatric disorders, they have potential implications for the treatment of these disorders. For example, different antidepressant drugs have distinctly different effects on sleep (Thase, 2006). Selective serotonin reuptake inhibitors strongly suppress REM sleep, and they also might worsen sleep continuity disturbances and reduce slow-wave sleep. In contrast, bupropion, nefazodone and trimipramine tend to preserve REM sleep and might improve sleep efficiency. Few drugs actually restore slow-wave sleep although some tricyclic agents and trazodone have been shown to enhance slow-wave sleep. If African-Americans and Asian-Americans have comparatively less REM sleep than Hispanics and Non-Hispanic Whites, they might be more sensitive to the effects of REM-suppressing agents. Evidence indicates that African-Americans and Asian-Americans are more sensitive to antidepressants than Non-Hispanic Whites, as demonstrated by clinical response at lower doses and a higher frequency of side effects in these groups (Lin, 2001). Ethnically sensitive treatment guidelines based on sleep profiles might reduce treatment-related sleep difficulties and enhance compliance.
In summary, polysomnography profiles of African-American, Asian-American, Mexican-American and Non-Hispanic White adolescents were, for the most part, comparable. There were some notable differences, however. African-Americans manifested reduced sleep efficiency, more stage 2 sleep and reduced stage 4 sleep that was more predominant in males. In contrast, Mexican-Americans, females in particular, had more REM sleep. The clinical significance of these cross-ethnic differences is not known. Sleep disturbances increase vulnerability for common psychiatric disorders, and many psychotropic agents also affect sleep. In order to better understand the role of sleep dysregulation in psychopathology, future research should focus on the interactions between genetic/biological and socio-cultural factors among different ethnic groups.
Acknowledgments
This work was supported, in part, by grants DA14037, DA15131, DA17804, DA17805, MH01419, MH62464 and MH68391 from the National Institutes of Health, from the National Alliance for Research on Schizophrenia and Affective Disorders, and by the Sarah M. and Charles E. Seay Endowed Chair in Child Psychiatry at UT Southwestern Medical Center. The authors also would like to thank Ms. Li-Ann Chen, MA, for technical support.
The funding agencies did not play any role in the design or conduct of the study; in collection, management, analysis or interpretation of the data; and in preparation, review or approval of the manuscript.
Footnotes
Conflict of interest
None of the authors have any financial conflicts of interest.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- Abe-Kim J, Takeuchi DT, Hong S, Zane N, Sue S, Spencer MS, Appel H, Nicado E, Alegría M. Use of mental health-related services among immigrant and US-born Asian Americans: results from the National Latino and Asian American Study. Am J Public Health. 2007;96 (1):91–98. doi: 10.2105/AJPH.2006.098541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achermann P, Dijk DJ, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31 (1–2):97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- Alegría M, Mulvaney-Day N, Torres M, Polo A, Cao Z, Canino G. Prevalence of psychiatric disorders across Latino subgroups in the United States. Am J Public Health. 2007;97 (1):68–75. doi: 10.2105/AJPH.2006.087205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Arch Gen Psychiatry. 1977;34 (10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3 (5):519–528. [PMC free article] [PubMed] [Google Scholar]
- Barnard AR, Nolan PM. When clocks go bad: neurobehavioral consequences of disrupted circadian timing. PLoS Genetics. 2008;4 (5):1–8. doi: 10.1371/journal.pgen.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and stress system. Sleep Med Clin. 2007;2 (2):279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Muck M, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4 (June):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49 (8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Breslau J, Aguilar-Gaxiola S, Kendler KS, Su M, Williams D, Kessler RC. Specifying race-ethnic differences in risk for psychiatric disorder in a US national sample. Psychol Med. 2006;36 (1):57–68. doi: 10.1017/S0033291705006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39 (6):411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gama A, Ajdacic V, Eich D, Rossler W. Prevalence, course and comorbidity of insomnia and depression in young adults. Sleep. 2008;31 (4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann NY Acad Sci. 2004;1021 (June):276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Cartwright RD. Rapid eye movement sleep characteristics during and after mood-disturbing events. Arch Gen Psychiatry. 1983;40 (2):197–201. doi: 10.1001/archpsyc.1983.01790020095009. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Highlights in minority health and disparities. 2006 http://www.cdc.gov/omhd/Highlights/2006/HMAy06AAPI.htm.
- Centers for Disease Control and Prevention. Age-adjusted prevalence of diagnosed diabetes by race/ethnicity and sex, United States, 1980–2005. 2007 http://www.cdc.gov/diabetes/statistics/prev/national/figraceethsex.htm.
- Collins FS. What we do and don’t know about ‘race’, ‘ethnicity’, genetics and health at the dawn of the genome era. Nat Gen Suppl. 2004;36 (11):S13–S15. doi: 10.1038/ng1436. [DOI] [PubMed] [Google Scholar]
- Cornelius LJ. Health habits of school-age children. J Health Care Poor Underserved. 1991;2 (3):374–395. doi: 10.1353/hpu.2010.0414. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Biological, developmental, and neurobehavioral factors relevant to adolescent driving risks. Am J Prev Med. 2008;35 (3S):S278–S284. doi: 10.1016/j.amepre.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. National Health and Nutrition Examination Survey. 2007 http://www.cdc.gov/nchs/nhanes.htm.
- Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behav Sleep Med. 2006;4 (1):29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- Ebin VJ, Sneed CD, Morisky DE, Rotheram-Borus MJ, Magnuson AM, Malotte CK. Acculturation and interrelationships between problem and health-promoting behaviors among Latino adolescents. J Adolesc Health. 2001;28 (1):62–72. doi: 10.1016/s1054-139x(00)00162-2. [DOI] [PubMed] [Google Scholar]
- Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Pharmacol Clin Exp. 2008;23 (7):571–585. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DE, Kupfer DJ, Rush AJ, Roffwarg HP. Controlled comparison of electrophysiological sleep in families of probands with unipolar depression. Am J Psychiatry. 1998a;155 (2):192–199. doi: 10.1176/ajp.155.2.192. [DOI] [PubMed] [Google Scholar]
- Giles DE, Perlis ML, Reynolds CF, Kupfer DJ. EEG sleep in African-American patients with major depression: a historical case control study. Depress Anxiety. 1998b;8 (2):58–64. [PubMed] [Google Scholar]
- Giles DE, Roffwarg HP, Dahl RE, Kupfer DJ. EEG sleep abnormalities in depressed children: a hypothesis. Psychiatry Res. 1992;41 (1):53–63. doi: 10.1016/0165-1781(92)90018-x. [DOI] [PubMed] [Google Scholar]
- Goodman A, Patel V, Leon DA. Child mental health differences amongst ethnic groups in Britain: a systematic review. BMC Public Health. 2008;8:258. doi: 10.1186/147-2458-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23 (February):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan P. Depressed adolescents of depressed and nondepressed mothers: tests of an interpersonal impairment hypothesis. J Consult Clin Psychol. 2001;69 (2):284–294. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- Hammen C, Burge D, Daley SE, Davila J, Paley B, Rudolph KD. Interpersonal attachment cognitions and prediction of symptomatic responses to interpersonal stress. J Abnorm Psychol. 1995;104 (3):436–443. [PubMed] [Google Scholar]
- Hammen C, Marks T, Mayol A, deMayo R. Depressive self-schemas, life stress, and vulnerability to depression. J Abnorm Psychol. 1985;94 (3):308–319. doi: 10.1037//0021-843x.94.3.308. [DOI] [PubMed] [Google Scholar]
- Hammen C, Rudolph K, Weisz J, Rao U, Burge D. The context of depression in clinic-referred youth: neglected areas in treatment. J Am Acad Child Adolesc Psychiatry. 1999;38 (1):64–71. doi: 10.1097/00004583-199901000-00021. [DOI] [PubMed] [Google Scholar]
- Herzberg D, Hammen C, Burge D, Daley S, Davila J, Lindberg N. Social competence as a predictor of chronic interpersonal stress. Pers Relationships. 1998;5 (2):207–218. [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven, CT: 1975. [Google Scholar]
- Jean-Lous G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47 (10):921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001;64 (1):1–7. doi: 10.1016/s0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-aged Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36 (7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62 (6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kimura M, Winkelmann J. Genetics of sleep and sleep disorders. Cell Mol Life Sci. 2007;64 (10):1216–1226. doi: 10.1007/s00018-007-6532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci. 2008;1129 (May):287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Foster G, Reich L, Thompson KS, Weiss B. EEG sleep changes as predictors in depression. Am J Psychiatry. 1976;133 (6):622–626. doi: 10.1176/ajp.133.6.622. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: The Cardia Study. Am J Epidemiol. 2006;164 (1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- Lin KM. Biological differences in depression and anxiety across races and ethnic groups. J Clin Psychiatry. 2001;62 (Suppl 13):13–19. [PubMed] [Google Scholar]
- McLaughlin KA, Hilt LM, Nolen-Hoeksema S. Racial/ethnic differences in internalizing and externalizing symptoms in adolescents. J Abnorm Child Psychol. 2007;35 (5):801–816. doi: 10.1007/s10802-007-9128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA. Sleep and anxiety disorders. Psychiatr Clin N Am. 2006;29 (4):1047–1058. doi: 10.1016/j.psc.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, hall M, Strollo PJ, Buysse DJ, Kamarck TW, Owens JF, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE Project. Psychosom Med. 2008;70 (4):410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5 (2):93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Thase ME, Simons AD. Social factors and the psychobiology of depression: relations between life stress and rapid eye movement sleep latency. J Abnorm Psychol. 1992;101 (3):528–537. doi: 10.1037//0021-843x.101.3.528. [DOI] [PubMed] [Google Scholar]
- Poland RE, Rao U, Lutchmansingh P, McCracken JT, Lesser IM, Edards C, Ott GE, Lin KM. REM sleep in depression is influenced by ethnicity. Psychiatry Res. 1999;88 (2):95–105. doi: 10.1016/s0165-1781(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol. 2002;14 (3):321–326. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- Rao U, Poland RE, Lutchmansingh P, Ott GE, McCracken JT, Lin KM. Relationship between ethnicity and sleep patterns in normal controls: implications for psychopathology and treatment. J Psychiatric Res. 1999;33 (5):419–426. doi: 10.1016/s0022-3956(99)00019-9. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MRC. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10 (5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. National Institutes of Health; Bethesda, MD: 1968. [Google Scholar]
- Redline S, Kirchner L, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164 (2):406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Chan W. Ethnic differences in symptoms of insomnia among adolescents. Sleep. 2006;29 (3):359–365. doi: 10.1093/sleep/29.3.359. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Chen IG. Ethnocultural differences in sleep complaints among adolescents. J Nerv Ment Dis. 2000;188 (4):222–229. doi: 10.1097/00005053-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Duong HT. Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12-month prospective study. J Adolesc Health. 2008;42 (3):294–302. doi: 10.1016/j.jadohealth.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia SA. Children’s Global Assessment Scale (C-GAS) Arch Gen Psychiatry. 1983;40 (11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52 (3–4):591–611. [Google Scholar]
- Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119 (Suppl 1):S29–S37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep–wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007;8 (6):668–680. doi: 10.1016/j.sleep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Stepnowsky CJ, Moore PJ, Dimsdale JE. Effect of ethnicity on sleep: complexities for epidemiologic research. Sleep. 2003;26 (3):329–332. doi: 10.1093/sleep/26.3.329. [DOI] [PubMed] [Google Scholar]
- Takeuchi DT, Zane N, Hong S, Chae DH, Gong F, Gae GC, Walton E, Sue S, Alegría M. Immigration-related factors and mental disorders among Asian Americans. Am J Public Health. 2007;97 (1):84–90. doi: 10.2105/AJPH.2006.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME. Depression and sleep: pathophysiology and treatment. Dialogues Clin Neurosci. 2006;8 (2):217–726. doi: 10.31887/DCNS.2006.8.2/mthase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WD, Orvaschel H, Prusoff BA, Kidd KK. An evaluation of the family history method for ascertaining psychiatric disorders. Arch Gen Psychiatry. 1982;39 (1):53–58. doi: 10.1001/archpsyc.1982.04290010031006. [DOI] [PubMed] [Google Scholar]
- Williams DR, González HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, Jackson JS. Prevalence and distribution of major depressive disorder in African Americans, Carribean Blacks, and Non-Hispanic Whites. Results from the National Survey of American Life. Arch Gen Psychiatry. 2008;64 (9):305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]