The number of women dying from cervical cancer in 1997 was 7% lower than in 1996 and has fallen by over 25% since 1992.1 Such rapid change must be at least partly due to cervical screening, although strong cohort effects have caused large fluctuations in cervical mortality in the past.2 We modelled mortality data, taking into account the effects of age and year of birth and looking for trends in time within four age groups to estimate the beneficial effects of cervical screening.
Subjects, methods, and results
We obtained mortality data, in 5 year age bands, from death registrations in England and Wales and calculated rates using mid-year population estimates. Mortality since 1993 was adjusted upwards by 4% because of changes in classification of cause of death.3
We modelled the data assuming that the age specific mortality is the product of a smoothly varying age effect, birth cohort effect, and age dependent period effects. Confidence intervals are approximate. Details of the statistical modelling are available from the authors on request.
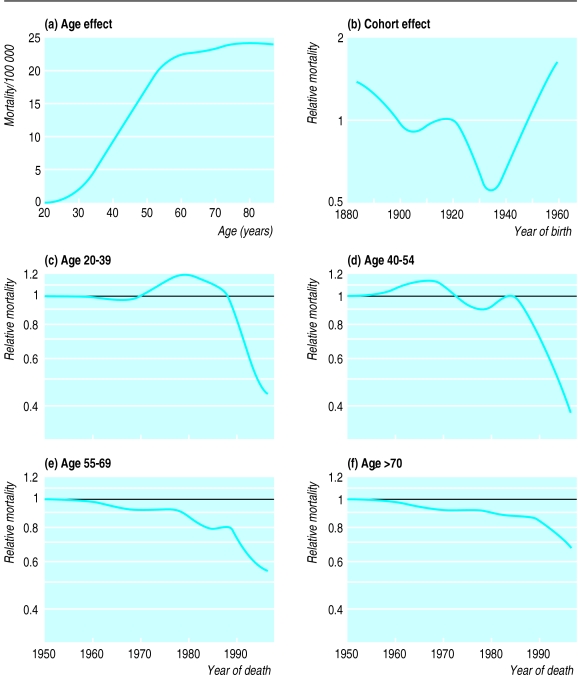
The top of the figure shows the estimated underlying mortality for cervical cancer as a function of age (a) and the multiplicative effect of year of birth on the age specific rate (b). Compared with women born in 1922, the risk for those born in 1957 is increased 1.5 times (95% confidence interval 1.2 to 1.9). The increased risk in women born since 1935 coincides with changing sexual behaviour associated with the “swinging ’60s” and the widespread use of oral contraceptives in the early 1970s.
The bottom of the figure (c-f) shows the trends in cervical cancer mortality after age and cohort effects were accounted for. No significant trends occurred in mortality before the mid-1980s, but mortality subsequently fell progressively (and significantly). The reduction in relative risk was greatest in the youngest age groups and least in those aged over 70 years.
If it is assumed that a model using only age and birth cohort effects would fit the data adequately if there had been no screening, then the estimated age and birth cohort effects can be used to predict what the death rate would have been without screening. The number of lives “saved” can be estimated from the effects of the age dependent trends on the predicted number of deaths in each age group. We estimate that there were about 1300 (1000 to 1600) fewer cervical cancer deaths in 1997 and 8250 (6900 to 9900) fewer between 1988 and 1997 as a result of screening.
Comment
Our analysis supports a beneficial effect of the national cervical screening programme (relaunched in 1988), which screens women aged 20-64. Before the relaunch screening had minimal effect on mortality. However, screening seems to have reduced cervical cancer mortality in 1997 by over 60% in those aged under 55. Although it is dangerous to attribute calendar effects to cervical screening, we know of no better explanation. This type of modelling does not permit estimation of the time lag between screening and improved mortality, but the natural course of cervical cancer and the history of cervical screening in England and Wales suggest that most of the effect on 1997 mortality is due to screening carried out between 1988 and 1995.
Our model does not constrain the calendar effects to be zero before 1980, so the small fluctuations observed between 1950 and 1987 both support the validity of the model and indicate the accuracy of our estimates. Confidence intervals may be misleading because they do not acknowledge the possibility of bias due to mismodelling.
The estimated number of lives saved by screening (1300 in 1997) is lower than some have suggested but is in keeping with our case-control based estimate of 2300 cancers prevented (95% confidence interval 1100 to 3900).4
Apology
Ideally this paper would have been published at the same time as the paper by Quinn et al showing that cervical screening in England seems to have reduced deaths from cervical cancer (3 April, pp 903-8). We apologise to Dr Sasieni and Mrs Adams that we didn’t put the two papers together and that for various reasons we have been slow in publishing this paper.
Figure.
Effect of age (a) and year of birth (b) on mortality from cervical cancer and trends in mortality after age and cohort effects were adjusted for in four age groups (c-f)
Acknowledgments
Contributors: PS designed the study, interpreted the data, wrote the paper, and is guarantor. JA analysed and interpreted the data and prepared the paper.
Footnotes
Funding: JA is funded by the National Screening Office of the NHS Cervical Screening Programme.
Competing interests: None declared.
References
- 1.Office of Population Censuses and Surveys. Deaths registered in 1997 by cause and by area of residence. London: Stationery Office; 1998. (Monitor population and health, series DH2 98/1.) [Google Scholar]
- 2.Beral V. Cancer of the cervix: a sexually transmitted infection? Lancet. 1974;i:1037–1040. doi: 10.1016/s0140-6736(74)90432-2. [DOI] [PubMed] [Google Scholar]
- 3.Rooney C, Devis T. Mortality trends by cause of death in England and Wales 1980-94: the impact of introducing automated cause coding and related changes in 1993. Pop Trends. 1996;86:29–35. [PubMed] [Google Scholar]
- 4.Sasieni PD, Cuzick J, Lynch-Farmery E National Coordinating Network for Cervical Screening Working Group. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. Br J Cancer. 1996;73:1001–1005. doi: 10.1038/bjc.1996.196. [DOI] [PMC free article] [PubMed] [Google Scholar]