Abstract
Methods for the immunological detection of Bacillus anthracis in various environmental samples and the discrimination of B. anthracis from other members of the B. cereus group are not yet well established. To generate specific discriminating antibodies, we immunized rabbits, mice, and chickens with inactivated B. anthracis spores and, additionally, immunized rabbits and mice with the tetrasaccharide β-Ant-(1→3)-α-l-Rhap-(1→3)-α-l-Rhap-(1→2)-l-Rhap. It is a constituent of the exosporium glycoprotein BclA and contains the newly discovered sugar anthrose 2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-β-d-glucose. The BclA protein is a major component of the exosporium of B. anthracis spores and is decorated by the tetrasaccharide indicated above. The anthrose-containing tetrasaccharide chain seems to be highly specific for B. anthracis, which makes it a key biomarker for the detection of these spores. The different immunizations led to anthrose-reactive polyclonal and monoclonal antibodies which were analyzed by various methods to characterize their ability to discriminate between B. anthracis and other Bacillus spp. Multiple applications, such as enzyme-linked immunosorbent assay, indirect immunofluorescence assay, and electron microscopy, revealed the specificities of the polyclonal and monoclonal antibodies generated for B. anthracis spore detection. All polyclonal antibodies were able to correctly identify the B. anthracis strains tested and showed only minimal cross-reactivities with other Bacillus strains. Moreover, the antibodies generated proved functional in a new capture assay for B. anthracis spores and could therefore be useful for the detection of spores in complex samples.
Bacillus anthracis, the causative agent of anthrax, is a rod-shaped, gram-positive bacillus. Whenever vegetative cells of B. anthracis are deprived of nutrients, spores are formed. These spores are highly resistant to any kind of environmental condition and can remain viable for years (25). Once they enter a susceptible host, like herbivores or humans, they start to germinate and multiply (24), causing a severe disease with a high mortality rate. Three distinct layers enclosing the core of the spore and housing the genome of the bacterium are the main constituents that provide protection from damage. These layers include a cortex of peptidoglycan, a proteinaceous coat, and a loosely fitting exosporium (9, 22). The B. anthracis exosporium serves as a semipermeable barrier against large, potentially harmful molecules such as antibodies and hydrolytic enzymes (8). The major component of the exosporium is the glycoprotein BclA (bacillus collagen-like protein of anthracis) (10, 27, 34), which contains multiple, collagen-like Xaa-Yaa-Gly (or XXG) repeats in its central region (34). BclA is highly immunogenic (32) and plays an important role in the association of the spore with human extracellular matrix proteins (2).
Daubenspeck and colleagues described the identification of two oligosaccharides attached to BclA, a 715-Da tetrasaccharide and a 324-Da disaccharide, and showed that multiple copies of the O-linked tetrasaccharide are attached to several sites within the central collagen-like region of BclA (6).
The tetrasaccharide β-Ant-(1→3)-α-l-Rhap-(1→3)-α-l-Rhap- (1→2)-l-Rhap contains a unique sugar residue, 2-O-methyl-4-(3-hydroxy-3-methylbutamido)-4,6-dideoxy-β-d-glucose, termed anthrose. The synthesis of the anthrose-containing tetrasaccharide, alone and in conjugation with carrier proteins, has been carried out by several groups (4, 23, 30, 39) and has also been used for vaccination experiments (23, 37). It was shown that the immunization of animals with these antigens induces antibodies that specifically bind to B. anthracis spores (23, 36, 37). However, most of these antibodies also showed cross-reactivities with other members of the B. cereus group (36).
Because of the potential use of B. anthracis as a terrorist weapon, the Centers for Disease Control and Prevention (CDC; Atlanta, GA) categorized this pathogen as a category A agent with the highest hazardous potential. When the mail system was used for biological attacks in the United States in 2001, the great efficacy of this pathogen to infect many people when it was intentionally distributed became visible (16). In addition to PCR techniques, which could be hampered by inhibitory factors, and inefficient means for the preparation of DNA from spores, the rapid immunological detection of B. anthracis from complex specimens is currently possible only when the spore concentrations are relatively high and when handheld test kits are used (12). In cases of lower concentrations, time-consuming cultivation and later identification would take too long in an emergency situation (11). Thus, there is a crucial need for a sensitive and highly specific means of identification of B. anthracis spores in environmental samples that urgently need to be tested. However, accurate detection can be problematic due to cross-reactive Bacillus spp. from the environment (7). These circumstances make it necessary to learn more about the spore components appropriate for the reliable detection of these pathogens (14). Despite this, the anthrose tetrasaccharide seems to be a promising marker for use in the development of new, specific, and sensitive detection assays.
To verify the presence of B. anthracis spores in environmental and suspect samples, we raised polyclonal and monoclonal antibodies against the anthrose tetrasaccharide and B. anthracis spores by applying different approaches. We were able to show the specificities of these antibodies for their reactions to different B. anthracis strains and their ability to distinguish between pathogenic and nonpathogenic Bacillus spp. using the tetrasaccharide for discrimination. In the capture enzyme-linked immunosorbent assay (ELISA), our antibodies showed no cross-reactivity to other members of the B. cereus group, which makes them a strong tool for the reliable and specific identification of B. anthracis spores.
MATERIALS AND METHODS
Preparation and inactivation of bacteria.
Bacterial strains (Table 1) were cultivated on appropriate nutrient media under adequate safety conditions. Except for strains obtained from strain collections, the sources of these strains were described previously (20). The sources of strains from strain collections are indicated in Table 1. To acquire highly resistant spores, cultures were stored at room temperature (RT) for 3 to 4 weeks, and colony material was inoculated in 1 ml 0.85% NaCl. For inactivation, 9 ml of 1% peracetic acid (PAA)-80% ethanol was added for 30 min at RT. The cells were centrifuged at 4,000 × g for 15 min, and the pellet was washed twice with 10 ml aqua bidest. Finally, the pellet was resuspended in 1 ml 0.85% NaCl, and the sterility was verified by cultivation of an aliquot under optimal growth conditions for 2 to 3 weeks. Afterwards, the cell count was determined by using an improved Neubauer counting chamber.
TABLE 1.
Specificity testing of capture ELISA with pc115 as the capture antibody and biotinylated pc115 as the detection antibody
| Species | Strain (reference) | OD492a |
|---|---|---|
| B. anthracis | UDIII-7 (20) | 1.913 |
| B. anthracis | CDC 1014 (20) | 1.951 |
| B. anthracis | 44/63 (20) | 1.981 |
| B. anthracis | B11/38 (20) | 1.912 |
| B. anthracis | 527 (20) | 2.011 |
| B. anthracis | B22/39 (20) | 1.983 |
| B. anthracis | Stamatin Sokol (20) | 1.972 |
| B. anthracis | ΔAmes | 1.986 |
| B. anthracis | Sterne | 1.915 |
| B. cereus | Hohenheim | 0.023 |
| B. cereus | 146 | −0.012 |
| B. cereus | NCCB 72001 (ATCC 10987) | −0.014 |
| B. cereus | DSM 31 (ATCC 14579) | ND |
| B. cereus | DSM 4490 (ATCC 11778) | 0.006 |
| B. cereus | 2617 | 0.083 |
| B. cereus | BW-B | −0.009 |
| B. cereus | DSM 31 (ATCC 14579) | 0.042 |
| B. cereus | DSM 2301 | −0.005 |
| B. cereus | DSM 345 (ATCC 11778) | 0.022 |
| B. cereus | DSM 609 (ATCC 25621) | −0.007 |
| B. cereus | A-049 | 0.321 |
| B. thuringiensis | DSM 5815 | −0.007 |
| B. thuringiensis | DSM 2046 | 0.011 |
| B. thuringiensis | Serovar konkukian strain 97-27 | 0.215 |
| B. subtilis | DSM 347 | −0.002 |
| B. subtilis | DSM 10 | −0.017 |
| B. athrophaeus | DSM 675 | −0.022 |
| B. megaterium | DSM 90 | −0.022 |
| B. megaterium | DSM 32 | 0.122 |
| B. pseudomallei | ATCC 23343 | −0.005 |
| B. mallei | ATCC 23344 | 0.025 |
| B. thailandensis | E125 | −0.013 |
| P. aeroginosa | ATCC 9027 | 0.000 |
| E. coli | K12 | 0.032 |
| Y. pseudotuberculosis | DSM 8902 | −0.006 |
| F. tularensis subsp. holarctica | LVS | −0.008 |
| F. tularensis subsp. tularensis | SchuS4 | 0.031 |
| F. philomiragia | DSM 7535 | −0.002 |
| F. novicida | ATCC 15482 | −0.006 |
The OD492 of the blank was 0.15 (SD, 0.013), and this value was subtracted from the reading for each strain. The antigen concentration for all strains was 1 × 106/ml. ND, not done.
Additionally, inactivation with paraformaldehyde (PFA) was done essentially as described for PAA inactivation by using 4% PFA in phosphate-buffered saline (PBS; pH 7.4). Cells were directly inoculated in 1 ml PFA for 1 h and centrifuged, and the pellet was resuspended in 10% PFA-0.05% glutaraldehyde for 12 to 15 h at RT. Centrifugation and washing of the cells as well as sterility testing were done as described above. No growth of bacteria was observed after both inactivation approaches.
Desalting of commercial KLH.
Keyhole limpet hemocyanin (KLH; 20 mg; product no. H 7017; Sigma-Aldrich, Taufkirchen, Germany) was dissolved in 10 mM ammonium carbonate and dialyzed against the same solvent (eight times) by using a 30,000-molecular-weight-cutoff filter Amicon Ultra Millipore centrifugal device and an Eppendorf centrifuge (4,000 rpm, 4°C). The retentate was filtered through a 0.22-mm-pore-size Millex syringe filter and lyophilized, resulting in desalted KLH (18 mg).
Conjugation of desalted KLH with the squaric acid derivative of the anthrax tetrasaccharide.
Because of the inhomogeneity and high molecular mass of KLH, it was impossible to set up the conjugation experiment and monitor the conjugation reaction by surface-enhanced laser desorption ionization-time-of-flight mass spectrometry (3, 31) to yield a conjugate with a predetermined KLH-carbohydrate ratio. Guided by the results of our recent study (13), the conjugation was set up as follows.
The desalted KLH (18 mg) was dissolved by vortexing in a borate buffer (0.5 M, pH 9.0, ∼300 ml); 1 mg of the tetrasaccharide squarate, which was prepared as described previously (29), was added; and the mixture was stirred overnight. The conjugation mixture was then desalted as described above for the commercial KLH. The retentate consisted of a soluble part and a jelly part, which were separated. Freeze-drying of the soluble part provided material (9 mg) that was dissolved in PBS and used to coat the ELISA plates, as described below. Freeze-drying of the jelly part resulted in a white solid material (8 mg) which was not examined further.
Conjugation of BSA with the squaric acid derivative of the anthrax tetrasaccharide.
The conjugation experiment was conducted with a hapten concentration of 40 mmol and was set up to yield a conjugate with a carbohydrate-protein ratio of approximately 6:1. To compensate for the hydrolysis of some of the squarate derivative during conjugation, the initial carbohydrate-bovine serum albumin (BSA) ratio was 7:1. Accordingly, BSA (30 mg, 0.00045 mM; product no. A0281; Sigma-Aldrich) was dissolved in borate buffer (0.5 M, 80 ml, pH 9.0), the tetrasaccharide (3.1 mg, 0.00315 mM) (29) was added, and the mixture was stirred for 28 h, when surface-enhanced laser desorption ionization-time-of-flight mass spectrometry showed a hapten-onto-protein loading proportion of 6.4. Borate buffer (0.05 M, pH 7.0, 1 ml) was added, and the mixture was desalted as described above. Filtration through a 0.22-mm-pore-size Millex syringe filter and freeze-drying resulted in the conjugate, which was a white solid mass (30 mg, 90%).
Immunization of animals.
All work with animals was done and registered in compliance with the German animal protection law. Rabbits (chinchilla hybrid) were immunized either with the anthrose-BSA conjugate (Fig. 1B) or with PAA-inactivated B. anthracis spores. Additionally, one chicken (White leghorn) was immunized intramuscularly with B. anthracis spores in a way similar to that in which the rabbits were immunized. The first immunization consisted of 250 μg antigen or 106 spores in 100 μl in Freund's complete adjuvant (Sigma-Aldrich). The next three booster immunizations were administered at intervals of 2 weeks (4 weeks for the chicken), and each booster immunization consisted of 125 μg antigen in Freund's incomplete adjuvant (Sigma-Aldrich). The number of spores used for the booster immunizations was the same in all cases. After the third boost, the rabbits were bled (20 ml) and serum was separated for investigation by ELISA and affinity chromatography. Eggs were collected from the chicken 10 days after each booster immunization, and immunoglobulin Y (IgY) was separated from the egg yolk, as described previously (26).
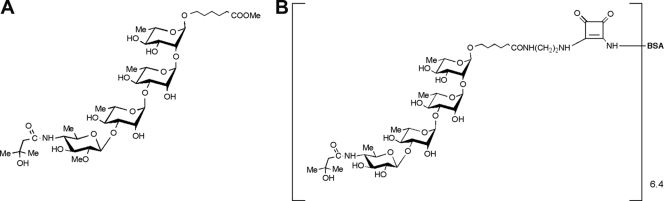
FIG. 1.
Structures of the synthetic tetrasaccharide side chain of BclA (A) and the conjugate immunogen (B) prepared from it and BSA used for immunization of the animals and the generation of antibodies. Me, methyl.
Five 8-week-old female BALB/c mice were immunized with the free anthrose tetrasaccharide (Fig. 1A), and additionally, another five mice were injected with the inactivated spores used for the rabbits and the chicken, as described above. Each subcutaneous immunization consisted of 50 μg carbohydrate in lipopeptide adjuvant (EMC Microcollections GmbH, Tuebingen, Germany) or 106 spores/ml in Freund's complete adjuvant or Freund's incomplete adjuvant and was done according to the manufacturer's instructions. The mice were immunized six times at intervals of 2 weeks. Although the antibody titer in the mice was relatively low (1:1,000) for anthrose immunization, spleen cells were used to generate monoclonal antibodies by use of the hybridoma technology. Cell fusion with the myeloma cell line P3X63Ag8 (18), cloning by limiting dilution, and the selection procedures were done as described previously (21). Screening of the culture supernatants was done by ELISA on BSA-conjugated anthrose or the soluble part of KLH-conjugated anthrose. After the subcloning and rescreening of selected hybridomas, the classes, subclasses, and light chains of the antibodies were determined with an IsoStrip kit (Roche, Indianapolis, IN), according to the manufacturer's instructions.
Affinity chromatography.
Polyclonal antianthrose rabbit serum (pc115) was first purified from BSA-reactive antibodies by the use of BSA-coupled N-hydroxysuccinimide (NHS)-activated Sepharose. In brief, 2.5 mg of BSA (Sigma-Aldrich) solubilized in 0.1 M NaHCO3-0.5 M NaCl (pH 8.3) was coupled to HiTrap NHS-activated Sepharose (Amersham/GE Healthcare, Munich, Germany) according to the manufacturer's instructions. After coupling of the BSA, 5 to 10 ml serum was incubated with the generated BSA-Sepharose for 1 h at RT to bind to the BSA antibodies. The column was washed with 30 to 50 ml PBS, and for reuse, the bound antibodies were eluted with 0.1 M glycine, pH 3.0, in 1-ml steps until the absorbance was nearly 0. Twenty microliters of 1.5 M Tris-HCl, pH 8.8, was used to neutralize the eluted antibodies. However, one step of purification was not sufficient to remove all BSA-reactive antibodies.
Following the anti-BSA purification, IgG was purified via protein G-Sepharose 4 fast flow (Amersham/GE Healthcare), according to the manufacturer's instructions. Polyclonal serum from the spore-immunized rabbit (pc114) was purified only via protein G-Sepharose, as described above.
Immunological tests. (i) Anthrose ELISA.
ELISA on anthrose-BSA or anthrose-KLH conjugates for screening was performed essentially as described elsewhere (15). In brief, flat-bottomed 96-well polystyrene plates (MaxiSorp; Nunc, Wiesbaden, Germany) were coated by passive absorption with 50 μl/well anthrose conjugate at 1.0 μg/ml in 0.1 M carbonate buffer, pH 9.6, for 2 h at 37°C or overnight (o/n) at 4°C. After the plates were washed three times with PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T), the wells were blocked with 75 μl block/dilution buffer containing 10% goat serum in PBS-T for 60 min at 37°C. After the plates were washed three times, 50 μl of primary antibodies in block/dilution buffer or undiluted culture supernatants from hybridoma cells were added to duplicate wells for 1 h at 37°C. The wells were washed three times, and 50 μl of goat anti-rabbit horseradish peroxidase (HRPO)-conjugated secondary antibody, goat anti-mouse IgG/IgM HRPO-conjugated secondary antibody, or rabbit anti-chicken HRPO-conjugated secondary antibody (all from Dianova, Hamburg, Germany) were added at 0.4 μg/ml for 1 h at 37°C. The wells were washed with 300 μl PBS-T five times, bound antibodies were detected by adding 200 μl of o-phenylendiamine (Sigma-Aldrich) as the substrate, and the reaction was stopped after 10 min by addition of 50 μl 2.5 M sulfuric acid. The optical density (OD) values at 492 nm (OD492) (reference wavelength, 620 nm) were then recorded with a Sunrise plate reader (Tecan Instruments, Crailsheim, Germany) interfaced with a computer. Samples were considered positive when they showed an OD greater than the mean for the blank plus 3 standard deviations (SDs).
(ii) Spore ELISA.
The spore ELISA was performed essentially as described elsewhere (5). In brief, flat-bottomed 96-well polystyrene plates (MaxiSorp; Nunc) were coated by passive absorption with 100 μl/well of an inactivated bacterial preparation at a concentration of 1 × 106/ml in 0.1 M NaHCO3 buffer, pH 9.6, o/n at 4°C. After the plates were washed three times with PBS-T, the wells were blocked with 100 μl 4% skim milk powder in PBS-T for 30 min at 37°C. After these washing steps, antibodies diluted in 1% skim milk powder in PBS-T were added in duplicate wells for 1 h at 37°C. Further steps were performed as described above for the anthrose ELISA.
(iii) Capture spore ELISA.
For the capture spore ELISA, 100 μl/well of polyclonal antianthrose pc115 or pc114 spore antibody (at 5 μg/ml each) was used to coat flat-bottomed 96-well polystyrene plates (MaxiSorp; Nunc) in 0.1 M NaHCO3 buffer, pH 9.6, for 2 h at 37°C or o/n at 4°C. After washing and blocking of the wells with 10% goat serum (Invitrogen, Karlsruhe, Germany) for 60 min at 37°C, plates with either pc115-biotinylated antibody (at 1.25 μg/ml in blocking buffer), pc114-biotinylated antibody (at 2.5 μg/ml in block buffer), or a pool of IgY antibodies against B. anthracis spores (H64-BA IgY; 1:750 in blocking buffer) in duplicate wells were incubated. Biotinylation of pc115 and pc114 was done with an EZ-Link sulfo-NHS-biotin labeling kit (Pierce/Perbio, Bonn, Germany), according to the manufacturer's instructions. After the plates were washed, 100 μl/well of a streptavidin-HRPO conjugate (1 μg/ml) or HRPO-labeled goat anti-chicken secondary antibody (0.4 μg/ml) (both from Dianova) was added and development occurred as described above. Samples were considered positive when they showed an OD greater than the mean for the blank (n = 6) plus 3 SDs. The coefficient of variation (CV; in percent) for all ELISAs was calculated by use of the equation (σ/μ)·100, where σ is the SD and μ is the mean.
(iv) IFA.
For the indirect immunofluorescence assay (IFA), 10 μl of PAA-inactivated bacteria (106/ml) was placed on 10-field immunofluorescence slides (BioMérieux, Marcy I'Etoile, France). The slides were dried for 1 to 2 h at RT, fixed with ice-cold methanol for 15 min or 4% PFA-PBS for 20 min, and blocked with 5% goat serum (Invitrogen) in 1% BSA-PBS for 30 min at RT. For double staining, 25 μl of polyclonal antianthrose antibody pc115 at 0.25 μg/ml and monoclonal BclA antibody EG4-4, kindly provided by J. Kearney (33), at 0.5 μg/ml was added; and the mixture was incubated for 30 min at 37°C. After the slides were washed, PBS-Alexa 594-conjugated secondary goat anti-rabbit antibody and Alexa 488-conjugated goat anti-mouse antibody (both from Molecular Probes, Karlsruhe, Germany) were added for 30 min at 37°C and the slides were then washed with PBS. Culture supernatants of mouse monoclonal antibodies were used at 1:2 to 1:5 dilutions. Secondary goat anti-mouse Alexa 488-conjugated antibody (Molecular Probes) was used for detection. A pool of polyclonal spore antibody H64-BA IgY was used at a 1:8,000 dilution, and detection by the use of goat anti-chicken Alexa 488-conjugated secondary antibody (Molecular Probes) followed. All secondary detection antibodies were used at a 1:300 dilution. Mounting was done with Prolong Gold Antifade plus 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes), according to the manufacturer's instructions, and the preparations were examined by fluorescence microscopy.
Data were acquired with an AxioCam MR camera (Zeiss) driven by Axiovision software (version 4.4; Zeiss) and were processed by using Photoshop CS3 software (Adobe Systems).
(v) Immunoelectron microscopy.
Preparations of PAA-inactivated B. anthracis spores were washed with HEPES buffer (0.05 M, pH 7.2) and adsorbed on alcian blue-coated cover slides. To prevent unspecific binding, spores were treated with blocking buffer (0.05% BSA, 0.1% gelatin in PBS) for 30 min. Subsequently, the primary antibody or undiluted antiserum was applied for 1 h at RT. Polyclonal anthrose antibody pc115 and an unspecific rabbit serum against Francisella tularensis (Becton Dickinson), which was used as a negative control, were used at a concentration of 0.7 μg/μl. Next, five washing steps with blocking buffer followed, and a goat anti-rabbit antibody conjugated with 10-nm-diameter gold (diluted 1:50 in blocking buffer; Amersham) was added for 1 h at RT. After the washing steps, a second fixation with 2.5% glutaraldehyde in 0.05% HEPES buffer was applied and the slides were rinsed with aqua bidest. The spores were then treated with an increasing ethanol concentration and infiltrated with hexamethyldisilazane (Sigma-Aldrich). The air-dried preparation was carbon coated (BAE 250; Leica) and inspected with a LEO 1530 scanning electron microscope (Zeiss) by using in-lens secondary electron and backscattered electron detectors.
RESULTS
We immunized BALB/c mice with the free anthrose tetrasaccharide in combination with a lipopeptide adjuvant previously developed for use for immunization with haptens (1). Mice were also immunized with PAA-inactivated B. anthracis spores. After the fusion of spleen cells with mouse myeloma cells, screening of the supernatants from wells with growing hybridomas was done by ELISA with a tetrasaccharide-KLH conjugate on the solid phase. Five reactive monoclonal antibodies from mice immunized with the anthrose-containing tetrasaccharide and one from mice immunized with spores were chosen for limiting dilution, and in addition to anthrose reactivity, subclones were tested for positive signals for the PAA-inactivated B. anthracis spores by ELISA (Fig. 2, right). The supernatants of all subclones showed reactivity with the anthrose-KLH conjugate as well as inactivated B. anthracis spores. The results for the three selected subclones indicated in Table 2 and Fig. 2 are representative of those for the other subclones that screened positive.
FIG. 2.
Reactivities of polyclonal (left) and monoclonal (right) anthrose and spore antibodies with the anthrose-KLH conjugate and PAA-inactivated B. anthracis CDC 1014 spores, as determined by ELISA. Coating with KLH was used as a negative control. The antibodies were used at different dilutions. ODs were measured at a wavelength of 492 nm. Values are means ± SDs of five independent experiments. The antianthrose antibodies were pc115, F6-2ID6, and F6-2IIG3; the antispore antibodies were pc114, H64-BA IgY (H64), and F7-1D3.
TABLE 2.
Overview of newly generated antibodies against anthrose
| Name of antibody | Polyclonal or monoclonal antibody | Antigen for immunization | Isotype |
|---|---|---|---|
| F6-2ID6 | Monoclonal | Anthrose tetrasaccharide | IgG2b |
| F6-2IIG3 | Monoclonal | Anthrose tetrasaccharide | IgG2b |
| F7-1D3 | Monoclonal | Inactivated B. anthracis sporesa | IgM |
| pc115 | Polyclonal | Anthrose tetrasaccharide (BSA conjugate) | IgG |
| pc114 | Polyclonal | Inactivated B. anthracis sporesa | IgG |
| H64-BA | Polyclonal | Inactivated B. anthracis sporesa | IgY |
Inactivation with PAA.
Immunization with the anthrose conjugate led to the development of specific polyclonal antibodies in rabbits. Interestingly, in addition to the generation of antibodies in mice, high-titer antianthrose antibodies were also generated in rabbits and a chicken by immunization with spores from B. anthracis, indicating that anthrose is an immunodominant component of the spore. Figure 2 shows the reactivities of polyclonal antibody pc115 in an ELISA with the anthrose-KLH conjugate and inactivated B. anthracis CDC 1014 spores compared to the reactivities of polyclonal spore antibodies pc114 and H64-BA IgY (Fig. 2, left) and the monoclonal antibodies against the free tetrasaccharide anthrose and B. anthracis spores (Fig. 2, right). KLH alone served as a negative control and raised no signals against the antibodies. BSA and the BSA-anthrose conjugate were not used to coat the ELISA plates, since immunization of the rabbit had occurred with this conjugate and such an ELISA could have false-positive results. The spore antibodies pc114, H64-BA IgY, and F7-1D3 were as reactive with the tetrasaccharide as with the spores, showing signal intensities similar to the signal intensity of the pc115 anthrose-induced antibody. This indicates a strong antibody response against anthrose when the animals were immunized with PAA-inactivated B. anthracis spores. The CV for this assay varied from 2.7% to 18.8%. Furthermore, we tested the specificity of the polyclonal antibodies for different Bacillus spp. and gram-negative strains (Table 1). For the safe handling of pathogenic B. anthracis and B. cereus strains outside a biosafety level 3 or 2 facility, we tested PAA and PFA for their abilities to inactivate such strains. In previous experiments we had already evaluated different inactivation methods that still allowed the best possible binding of antibodies. Those experiments revealed that treatment with PAA or PFA may be applied but still preserve epitope recognition by most antibodies (data not shown). Table 3 summarizes the results of the spore ELISA with the pc115, pc114, and H64-BA IgY antibodies for different strains. Positive results were given by an OD492 greater than the mean for the blank (n = 6) plus three times the SD. The pc115 and pc114 antibodies recognized spores of B. anthracis inactivated by the use of both the method with PAA and the method with PFA (data not shown), with identical results being achieved. H64-BA IgY showed stronger signals with PAA-inactivated bacteria. Moreover, all polyclonal antibodies were able to discriminate between B. anthracis and non-B. anthracis strains of the genus Bacillus. By ELISA, the pc115 anthrose antibody showed cross-reactivity with B. cereus strain 2617; the pc114 spore antibody showed cross-reactivity with B. cereus strains ATCC 10987, ATCC 14579, Hohenheim, and 2617; and the H64-BA IgY antibody also showed cross-reactivity only with B. cereus strain 2617. None of the other Bacillus spp. or gram-negative bacteria was reactive with the polyclonal antibodies.
TABLE 3.
Summary of reactivities of polyclonal anthrose and spore antibodies against PAA-inactivated Bacillus spp. and gram- negative strains by spore ELISA
| Strain | Total no. tested/no. positive for the following antibody (antigen): |
||
|---|---|---|---|
| pc115 (anthrose) | pc114 (spore) | H64-BA IgY (spore) | |
| B. anthracis | 11/11 | 11/11 | 11/11 |
| B. cereus | 12/1a | 11/4 | 9/1a |
| B. thuringiensis | 3/0 | 3/0 | 3/0 |
| Bacillus spp. | 4/0 | 4/0 | 3/0 |
| Gram-negative organisms | 11/0 | 11/0 | 10/0 |
B. cereus 2617.
To develop a specific assay for the detection of B. anthracis spores from complex probes, we determined the suitability of all polyclonal antibodies in the capture ELISA either as a coating antibody or as a detection antibody. pc115 was able to serve as the coating and detection antibody by using direct biotinylation or HRPO conjugation of the antibody. pc114 showed characteristics similar to those of pc115 and also functioned as a capture antibody as well as a detection antibody in combination with the pc115 anthrose antibody. H64-BA IgY was useful for detection with both pc115 or pc114 as the capture antibody. We tested the specificity and the sensitivity of this capture ELISA for the detection of B. anthracis spores. Table 1 provides the mean values from five independent experiments (SD = 0.013, CV = 13.1%) for testing of the specificity of the pc115 antibody as the capture antibody and the detection antibody (biotinylated). The blank used for subtraction in this assay was the no-antigen control. The cross-reactivity with one B. cereus strain seen before by the spore ELISA was not apparent by the capture ELISA, resulting in a 100% specificity for B. anthracis spores. Weak OD signals, such as those observed for B. cereus strain A-049 and the B. thuringiensis serovar konkukian strain, occurred only at high spore concentrations (≥106/ml) and were absent within one lower log stage of bacteria (data not shown). To circumvent such false-positive results, a serial dilution of a standard was always tested. Figure 3 shows the use of the above-mentioned combinations of different antibody pairs for coating and detection (shown for strain CDC 1014 in Fig. 3). The limit of detection (LOD) was calculated from the OD492 of the mean for the blank from four independent experiments plus three times the SD as the threshold (the line in Fig. 3). The CV was calculated by use of the means and SDs of the values from four independent experiments. For the pc115-biotinylated pc115 antibody pair, the LOD was >0.34 (CV = 13.1%), which corresponds to 1 × 104 to 3 × 104 spores/ml, for the pc115-biotinylated pc114 antibody pair it was >0.42 (CV = 12%), and for the pc115-H64-BA IgY antibody pair it was >0.23 (CV = 3.8%), which corresponds to 1 × 104 spores/ml (Fig. 3A). pc114 in combination with biotinylated pc115 gave an LOD of >0.25 (CV = 10.8%), and pc114 in combination with H64-BA IgY as the detector gave an LOD of >0.18 (CV = 4.4%), indicating that it was the most sensitive pair (4 × 103 spores/ml, Fig. 3B), but this pair must be investigated in more detail for its specificity. The testing of the other B. anthracis strains (Table 1) revealed no difference in the resulting LODs. The analysis of new monoclonal antibodies for use in the capture assay is still in progress.
FIG. 3.
Sensitivity of capture ELISA by the use of pc115 (A) or pc114 (B) for coating. Different concentrations of PAA-inactivated B. anthracis CDC 1014 spores were detected with biotinylated pc115 antibody (pc115-biot.), biotinylated pc114 antibody (pc114-biot.), or H64-BA IgY using the indicated concentrations and dilutions. ODs were measured at a wavelength of 492 nm. All values are means ± SDs of at least four independent experiments. Lines indicate the LODs of the polyclonal antibodies used for detection calculated by use of the mean for the blank (i.e., the no-antigen control) plus three times the SD as the threshold for positive results.
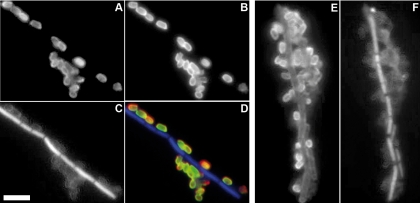
In the IFA, polyclonal antibody pc115 (Fig. 4A) stained clearly visible ring structures around the spores of inactivated B. anthracis spores. Double staining with a monoclonal BclA antibody (EG4-4; a kind gift from J. Kearney) (Fig. 4B) revealed the colocalization of BclA and anthrose on the spores (Fig. 4C). The visualization of cell nuclei by using DAPI (Fig. 4C and F) or phase-contrast microscopy (data not shown) revealed no signal on the vegetative cells by use of the pc115 antibody. In contrast, additional but weak staining of vegetative cells was observed with the pc114 (data not shown) or H64-BA IgY (Fig. 4E) spore antibody. Spore antibodies pc114 and H64-BA IgY showed only minor cross-reactivities with four and two B. cereus strains, respectively, which is comparable to the results of the ELISA presented in Table 3. In the IFA, the monoclonal antibodies showed patterns of staining of PFA-inactivated B. anthracis spores identical to those shown by polyclonal anthrose antibody pc115 (data not shown). Interestingly, by double staining with pc115, the monoclonal and polyclonal antibodies competed in their binding to the spore; some spores were stained only with the monoclonal antibody and some were stained only with the polyclonal antibody (data not shown).
FIG. 4.
Immunofluorescence staining of B. anthracis using polyclonal antibodies against anthrose and spores. Inactivated B. anthracis spores were stained with polyclonal anthrose antibody pc115 (A) and were visualized with a goat anti-rabbit Alexa 594-labeled secondary antibody. Colocalization with the BclA protein (B) was shown by using monoclonal BclA antibody EG4-4, detected with goat anti-mouse Alexa 488-labeled antibody. The overlapping of the staining of pc115 and EG4-4 appears yellow and is shown as a merged image (D). The H64-BA IgY polyclonal spore antibody (E) was visualized by using goat antichicken Alexa 488-labeled detection antibody. The presence of spores was observed by phase-contrast microscopy (data not shown), and cell nuclei were stained with DAPI (C and F). Note the staining only of spores and not of vegetative cells in the case of the anthrose and BclA antibodies. Magnification, ×100, oil immersion. Bar, 10 μm.
Additionally, we showed the reactivity of the polyclonal pc115 antibody to the anthrose exosporium component by electron microscopy, visualized by an immunogold-labeled secondary antibody that clearly localized within the exosporium structures of PAA-inactivated B. anthracis spores (Fig. 5A). The arrows in Fig. 5A indicate positively stained B. anthracis spores. Preimmune serum from rabbits immunized with anthrose-BSA (Fig. 5B) and unspecific rabbit serum (Fig. 5C) were not reactive with the B. anthracis spores. The results obtained by the use of a mixture of B. anthracis spores (uneven surface) and B. subtilis spores (smooth surface) also underlines the specificity of the anthrose antibody (Fig. 5D, arrow), since no gold particles could be observed on the surfaces of the B. subtilis spores. Bacteria were also visualized by using corresponding in-lens secondary electron detection to illustrate the exosporium structures (Fig. 5A′ to D′).
FIG. 5.
Immunoelectron micrographs (backscattered electron detection) of pc115 (A), preserum of a rabbit immunized with anthrose-BSA (B), and unspecific rabbit serum (C) on PAA-inactivated B. anthracis spores detected with gold-labeled secondary antibody. (D) Immunostaining with pc115 on a mixture containing B. anthracis spores and B. subtilis spores. (A′ to D′) In-lens secondary electron detection of the immune staining corresponding to panels A to D, respectively. Arrows, anthrose-positive B. anthracis spores. Magnification, ×150,000.
DISCUSSION
Antibodies against the tetrasaccharide anthrose are a suitable tool for use for the detection of B. anthracis spores. Different approaches lead to anthrose-reactive polyclonal and monoclonal antibodies: immunization with an anthrose-BSA conjugate, with free anthrose, and with PAA-inactivated B. anthracis spores. The antibodies described in this study were tested for their specificities and their abilities to be included in a specific and sensitive detection assay. Tamborrini et al. (36) generated antibodies against the tetrasaccharide showing cross-reactivities with other members of the B. cereus group. Our pc115 polyclonal anthrose antibody was cross-reactive with only one B. cereus strain by ELISA and IFA. This cross-reactivity was not observed by the capture ELISA. No cross-reactivities with other Bacillus strains or gram-negative bacteria suspected of being agents of bioterrorism were seen. Thus, our polyclonal anthrose antibody showed 100% specificity for B. anthracis spores. Furthermore, we were able to show the colocalization of the pc115 anthrose antibody with the anti-BclA antibody EG4-4 on the surface of B. anthracis spores by IFA, underlining the glycosylation of BclA with the tetrasaccharide and correct epitope recognition of our new antibody. The false-positive reactivity with KLH could be excluded by ELISA (Fig. 2). More studies are in progress to apply the monoclonal and polyclonal anthrose antibodies to the detection of B. anthracis in complex matrices containing interfering contaminants or carrier substances which could potentially inhibit the reaction or be cross-reactive.
Confirming the findings of other studies (38), we were able to show that the high degree of antigenicity of BclA also raised high levels of anthrose in spore-immunized animals (Fig. 2). Both polyclonal antibodies, pc114 and H64-BA IgY, as well as the monoclonal antibodies generated against inactivated B. anthracis spores, showed reactivities against the anthrose conjugates, indicating the conservation of the sugar on PAA-inactivated spores.
The generation of polyclonal and monoclonal antibodies using the tetrasaccharide anthrose as the target antigen was effected to develop highly sensitive immunological assays for the detection of B. anthracis. Such assays have not been well described before. Besides PCR-based detection, only a few immunological detection systems, such as lateral-flow immunoassays, are available, but they offer detection limits that range from only 105 to 106 spores/ml (12, 19). Unfortunately, no information on the target antigens of the antibodies used in those assays is available. With our capture ELISA with the anthrose tetrasaccharide as the target antigen, we were able to detect 1 × 104 to 5 × 104 spores/ml. Therefore, the sugar coat on the B. anthracis spore surface is an ideal indicator for spore detection. The newly generated polyclonal spore antibodies are also a useful and sensitive tool for use in combination with the anthrose antibody in the capture ELISA.
As shown by Brahmbhatt and colleagues (2), BclA acts as a shield to reduce early spore germination and decreases the association with the human extracellular matrix proteins laminin and fibronectin. In this context, anthrose may play a role in the infection of the host cell. The binding of host cells to carbohydrates is often found during the defense against a pathogen (17, 35). Anthrose could act as a key component in the attachment of the spore to the host cell surface. Similar glycosylated and highly immunogenic proteins were found in Mycobacterium tuberculosis (28).
Like Dong and colleagues (8), we found that strains of the B. cereus group, for example, B. cereus strain 2617, carried the anthrose operon in their sequences (data not shown). For strain 2617, cross-reactivities with the anthrose antibodies generated occurred by direct ELISA and IFA. They were absent by the capture ELISA, suggesting that the strain has lower levels of anthrose on the spore surface or has sugar residues structurally similar to but not identical to those of B. anthracis spores. Other strains may also contain the anthrose operon in their genomes but fail to transcribe this specific sugar residue. The sequence is perhaps not intact and is therefore not transcribed, or the strains are not able to produce anthrose but instead produce a different sugar residue as a modification, but we failed to detect such a sugar residue with our antibodies.
The methods used in this study, in which PAA and PFA were applied to inactivate spores, showed no differences in the binding behaviors of the polyclonal and monoclonal anthrose antibodies, although immunization was carried out with the native anthrose-BSA conjugate and anthrose tetrasaccharide. These methods allow the rapid inactivation of samples containing unknown agents so that potentially infectious material does not need to be handled for testing by diagnostic assays. Irradiation, which also preserves the epitope, is not applicable for use for rapid diagnosis (5). Since the inactivation of spores by the use of either PAA or PFA is based on different chemistries, they may have diverse influences on the conservation of the epitope. Comparison of the polyclonal antibodies showed no difference between the two inactivation methods. By ELISA, the monoclonal antibodies showed stronger signals with PFA-inactivated cells and reacted by IFA only when PFA was used for fixation. This indicates that the tetrasaccharide has different binding sites which could easily be destroyed or masked by the use of various inactivation or fixation methods.
We describe here the production and characterization of polyclonal and monoclonal antibodies against the exosporium tetrasaccharide anthrose. With our capture ELISA for the immunological detection of B. anthracis spores, we have a rapid and sensitive tool with a detection limit lower than the detection limits of most commercially available assays (11). The specificity and the sensitivity of the pc115 antibody in the capture ELISA in combination with polyclonal spore antibodies pc114 and H64-BA IgY make it a conclusive and reliable tool for the application and the development of new detection assays. Therefore, the basis for the introduction of the generated antibodies into other detection platforms for the easy and rapid discovery of B. anthracis by the use of complex probes has been created. The inhibitory properties of the antibodies generated here and the inhibition of the ELISA by the free tetrasaccharide, as shown previously for polyclonal spore antibodies (38), will be the subject of further studies.
Acknowledgments
We thank R. Schade and B. Diemar for immunization of the chicken and the partial preparation of IgY. We thank S. Dupke for the partial preparation and inactivation of the spores and G. Holland for the preparation of the immunoelectron microscopy staining. We also thank J. Kearney for the anti-BclA mouse monoclonal antibodies. We are grateful to W. Beyer for providing B. anthracis strains ΔAmes and Sterne and to D. Lereclus for B. thuringiensis serovar konkukian strain 97-27.
This work was partly supported by a grant from the Federal Ministry of Education and Research (project BiGRUDI).
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Bessler, W. G., and G. Jung. 1992. Synthetic lipopeptides as novel adjuvants. Res. Immunol. 143:548-553. [DOI] [PubMed] [Google Scholar]
- 2.Brahmbhatt, T. N., B. K. Janes, E. S. Stibitz, S. C. Darnell, P. Sanz, S. B. Rasmussen, and A. D. O'Brien. 2007. Bacillus anthracis exosporium protein BclA affects spore germination, interaction with extracellular matrix proteins, and hydrophobicity. Infect. Immun. 75:5233-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chernyak, A., A. Karavanov, Y. Ogawa, and P. Kovac. 2001. Conjugating oligosaccharides to proteins by squaric acid diester chemistry; rapid monitoring of the progress of conjugation, and recovery of the unused ligand. Carbohydr. Res. 330:479-486. [DOI] [PubMed] [Google Scholar]
- 4.Crich, D., and O. Vinogradova. 2007. Synthesis of the antigenic tetrasaccharide side chain from the major glycoprotein of Bacillus anthracis exosporium. J. Org. Chem. 72:6513-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang, J. L., K. Heroux, J. Kearney, A. Arasteh, M. Gostomski, and P. A. Emanuel. 2001. Bacillus spore inactivation methods affect detection assays. Appl. Environ. Microbiol. 67:3665-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daubenspeck, J. M., H. Zeng, P. Chen, S. Dong, C. T. Steichen, N. R. Krishna, D. G. Pritchard, and C. L. Turnbough, Jr. 2004. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J. Biol. Chem. 279:30945-30953. [DOI] [PubMed] [Google Scholar]
- 7.Delvecchio, V. G., J. P. Connolly, T. G. Alefantis, A. Walz, M. A. Quan, G. Patra, J. M. Ashton, J. T. Whittington, R. D. Chafin, X. Liang, P. Grewal, A. S. Khan, and C. V. Mujer. 2006. Proteomic profiling and identification of immunodominant spore antigens of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Appl. Environ. Microbiol. 72:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, S., S. A. McPherson, L. Tan, O. N. Chesnokova, C. L. Turnbough, Jr., and D. G. Pritchard. 2008. Anthrose biosynthetic operon of Bacillus anthracis. J. Bacteriol. 190:2350-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhardt, P. 1967. Cytology of Bacillus anthracis. Fed. Proc. 26:1504-1517. [PubMed] [Google Scholar]
- 10.Gerhardt, P., and E. Ribi. 1964. Ultrastructure of the exosporium enveloping spores of Bacillus cereus. J. Bacteriol. 88:1774-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hang, J., A. K. Sundaram, P. Zhu, D. R. Shelton, J. S. Karns, P. A. Martin, S. Li, P. Amstutz, and C. M. Tang. 2008. Development of a rapid and sensitive immunoassay for detection and subsequent recovery of Bacillus anthracis spores in environmental samples. J. Microbiol. Methods 73:242-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoile, R., M. Yuen, G. James, and G. L. Gilbert. 2007. Evaluation of the rapid analyte measurement platform (RAMP) for the detection of Bacillus anthracis at a crime scene. Forensic Sci. Int. 171:14. [DOI] [PubMed] [Google Scholar]
- 13.Hou, S.-J., R. Saksena, and P. Kovác. 2008. Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr. Res. 343:196-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 15.Jenzora, A., A. Jansen, H. Ranisch, M. Lierz, O. Wichmann, and R. Grunow. 2008. Seroprevalence study of Francisella tularensis among hunters in Germany. FEMS Immunol. Med. Microbiol. 53:183-189. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W. J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, and B. A. Perkins. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson, K. A. 1999. Bacterium-host protein-carbohydrate interactions and pathogenicity. Biochem. Soc. Trans. 27:471-474. [DOI] [PubMed] [Google Scholar]
- 18.Kennett, R. H., and F. Gilbert. 1979. Hybrid myelomas producing antibodies against a human neuroblastoma antigen present on fetal brain. Science 203:1120-1121. [DOI] [PubMed] [Google Scholar]
- 19.King, D., V. Luna, A. Cannons, J. Cattani, and P. Amuso. 2003. Performance assessment of three commercial assays for direct detection of Bacillus anthracis spores. J. Clin. Microbiol. 41:3454-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klee, S. R., H. Nattermann, S. Becker, M. Urban-Schriefer, T. Franz, D. Jacob, and B. Appel. 2006. Evaluation of different methods to discriminate Bacillus anthracis from other bacteria of the Bacillus cereus group. J. Appl. Microbiol. 100:673-681. [DOI] [PubMed] [Google Scholar]
- 21.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 22.Lai, E. M., N. D. Phadke, M. T. Kachman, R. Giorno, S. Vazquez, J. A. Vazquez, J. R. Maddock, and A. Driks. 2003. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J. Bacteriol. 185:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta, A. S., E. Saile, W. Zhong, T. Buskas, R. Carlson, E. Kannenberg, Y. Reed, C. P. Quinn, and G. J. Boons. 2006. Synthesis and antigenic analysis of the BclA glycoprotein oligosaccharide from the Bacillus anthracis exosporium. Chemistry (Weinheim an der Bergstrasse, Germany) 12:9136-9149. [DOI] [PubMed] [Google Scholar]
- 24.Mock, M., and A. Fouet. 2001. Anthrax. Ann. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polson, A., M. B. von Wechmar, and M. H. van Regenmortel. 1980. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 9:475-493. [DOI] [PubMed] [Google Scholar]
- 27.Redmond, C., L. W. Baillie, S. Hibbs, A. J. Moir, and A. Moir. 2004. Identification of proteins in the exosporium of Bacillus anthracis. Microbiology (Reading, England) 150:355-363. [DOI] [PubMed] [Google Scholar]
- 28.Romain, F., C. Horn, P. Pescher, A. Namane, M. Riviere, G. Puzo, O. Barzu, and G. Marchal. 1999. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect. Immun. 67:5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saksena, R., R. Adamo, and P. Kovác. 2007. Immunogens related to the synthetic tetrasaccharide side chain of the Bacillus anthracis exosporium. Bioorg. Med. Chem. 15:4283-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saksena, R., R. Adamo, and P. Kovác. 2006. Synthesis of the tetrasaccharide side chain of the major glycoprotein of the Bacillus anthracis exosporium. Bioorg. Med. Chem. Lett. 16:615-617. [DOI] [PubMed] [Google Scholar]
- 31.Saksena, R., A. Chernyak, A. Karavanov, and P. Kovác. 2003. Conjugating low molecular mass carbohydrates to proteins. 1. Monitoring the progress of conjugation. Methods Enzymol. 362:125-139. [DOI] [PubMed] [Google Scholar]
- 32.Steichen, C., P. Chen, J. F. Kearney, and C. L. Turnbough, Jr. 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185:1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swiecki, M. K., M. W. Lisanby, F. Shu, C. L. Turnbough, Jr., and J. F. Kearney. 2006. Monoclonal antibodies for Bacillus anthracis spore detection and functional analyses of spore germination and outgrowth. J. Immunol. 176:6076-6084. [DOI] [PubMed] [Google Scholar]
- 34.Sylvestre, P., E. Couture-Tosi, and M. Mock. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169-178. [DOI] [PubMed] [Google Scholar]
- 35.Szymanski, C. M., D. H. Burr, and P. Guerry. 2002. Campylobacter protein glycosylation affects host cell interactions. Infect. Immun. 70:2242-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamborrini, M., M. A. Oberli, D. B. Werz, N. Schurch, J. Frey, P. H. Seeberger, and G. Pluschke. 2009. Immuno-detection of anthrose containing tetrasaccharide in the exosporium of Bacillus anthracis and Bacillus cereus strains. J. Appl. Microbiol. 106:1618-1628. [DOI] [PubMed] [Google Scholar]
- 37.Tamborrini, M., D. B. Werz, J. Frey, G. Pluschke, and P. H. Seeberger. 2006. Anti-carbohydrate antibodies for the detection of anthrax spores. Angew. Chem. Int. Ed. 45:6581-6582. [DOI] [PubMed] [Google Scholar]
- 38.Wang, D., G. T. Carroll, N. J. Turro, J. T. Koberstein, P. Kovac, R. Saksena, R. Adamo, L. A. Herzenberg, L. A. Herzenberg, and L. Steinman. 2007. Photogenerated glycan arrays identify immunogenic sugar moieties of Bacillus anthracis exosporium. Proteomics 7:180-184. [DOI] [PubMed] [Google Scholar]
- 39.Werz, D. B., and P. H. Seeberger. 2005. Total synthesis of antigen Bacillus anthracis tetrasaccharide-creation of an anthrax vaccine candidate. Angew. Chem. Int. Ed. 44:6315-6318. [DOI] [PubMed] [Google Scholar]