Abstract
Virulence factors regulated by the CovRS/CsrRS two-component gene regulatory system contribute to the invasive diseases caused by group A Streptococcus (GAS). To determine whether the streptococcal secreted esterase (Sse), an antigen that protects against subcutaneous GAS infection, is one of these virulence factors, we investigated the phenotype of a nonpolar sse deletion mutant strain (Δsse). In addition, we examined the effects of covS mutation on sse expression. As assessed using a mouse model of subcutaneous infection, the virulence of the Δsse strain is attenuated and the overall pathology is reduced. Furthermore, GAS was detected in the blood and spleens from mice subcutaneously infected with the parental strain, whereas mice subcutaneously infected with the Δsse strain had no GAS present in their blood and spleens. The ability of the mutant to survive in the subcutis of mice appeared to be compromised. The growth of the Δsse strain in rich and chemically defined media and nonimmune human blood and sera was slower than that of the wild-type strain. Complementation restored the phenotype of the Δsse strain to that of the wild-type strain. The wild-type, Δsse, and complement strains had no detectable SpeB activity. Expression of Sse is negatively controlled by CovRS. These findings suggest that Sse is a CovRS-regulated virulence factor that is important for the virulence of GAS in subcutaneous infection and plays an important role in severe soft tissue infections and systemic dissemination of GAS from the skin.
Group A Streptococcus (GAS) is an important gram-positive human pathogen that causes both invasive and noninvasive infections. Noninvasive infections, including acute pharyngitis and superficial skin infection, result in substantial morbidity and economic loss globally (3, 30). Severe invasive GAS infections, such as necrotizing fasciitis, bacteremia, and streptococcal toxic shock syndrome, are associated with high mortality rates (28, 32, 39). Necrotizing fasciitis is characterized as a rapidly progressive infection, causing necrosis of the fascia and subcutaneous tissue that leads to systemic infection (42). Although treatment with antibiotics is effective against streptococcal throat infection, severe invasive infections are difficult to treat (42).
GAS produces an abundance of exoproteins that mediate its pathogenesis; for example, the genome of the M1 strain SF370 (8) has 123 genes that encode known or putative exoproteins. Those exoproteins that have been characterized exhibit a myriad of functions. The M protein (9), C5a peptidase (16, 40), Mac (17), and superantigens (29, 31) are involved in the evasion and interference of innate and acquired host immunity. The Lancefield T antigen and extracellular matrix-binding proteins form pili that mediate specific adhesion of GAS to pharyngeal cells, human tonsil tissue, and skin (1, 24). Streptolysin O, NAD+ glycohydrolase, streptolysin S (SLS), and the SpeB protease are cytotoxic to host cells and mediate the degradation of host molecules (10, 21, 26). Despite this extensive knowledge of the functions of these exoproteins, the functions and pathogenic roles of the majority of GAS exoproteins still remain unknown.
Similar to many other bacteria, GAS secretes a carboxylic esterase (13, 33). There is emerging evidence for a role of esterases in mediating the virulence and pathogenesis of pathogenic bacteria. A cell wall-anchored carboxylesterase has been reported to be required for the virulence of Mycobacterium tuberculosis (22). We previously reported that immunization with the secreted esterase (designated Sse for streptococcal secreted esterase) protects mice against lethal subcutaneous infection with virulent M1 and M3 strains, and this treatment inhibits GAS invasion of mouse skin tissue (20). However, whether Sse is required for GAS virulence and pathogenesis has not been established.
Many GAS virulence factors are regulated by the CovRS two-component gene regulatory system (also known as CsrRS) (7, 14, 18), including HasA, which is involved in the synthesis of the hyaluronic acid capsule, and the SpeB protease, which degrades virulence factors and other proteins. The CovR-mediated repression of SpeB is attenuated by CovS, and loss of this CovS-mediated regulation of CovR due to mutations in covS or covR leads to abrogation of SpeB production (36). Spontaneous covRS mutations that lead to upregulation of the capsule and loss of SpeB expression are significant events that contribute to the progression of invasive infections for serotype M1 GAS (4, 5, 34, 38). However, the molecular basis for this effect of covRS mutations on the progression of invasive disease is not fully understood.
This study aimed to determine whether Sse is regulated by CovRS and contributes to severe invasive infection. We found that deletion of sse reduced the severity of soft tissue infection caused by GAS and abrogated systemic GAS dissemination from the skin. In addition, we found that the levels of the sse transcript were negatively controlled by CovRS. In summary, these results suggest that Sse is a novel virulence factor that is regulated by CovRS and is required for invasive skin infection and efficient systemic dissemination of GAS from the skin.
MATERIALS AND METHODS
Bacterial strains and growth.
The GAS strains used in this study are listed in Table 1. These strains were grown in Todd-Hewitt broth supplemented with 0.2% yeast extract (THY) at 37°C in a 5%-CO2 atmosphere. Tryptose agar with 5% sheep blood and THY agar were used as solid media.
TABLE 1.
GAS strains used in this study
| Strain | Disease, source, or description | covS genotype | sse genotype | emm type | Reference |
|---|---|---|---|---|---|
| MGAS5005 | Cerebral spinal fluid | Null mutant | WT | M1 | 34 |
| Δsse mutant | MGAS5005 derivative | Null mutant | Null mutant | M1 | This study |
| Δsse-sse mutant | Nonspontaneous revertant of Δsse strain | Null mutant | WT | M1 | This study |
| MGAS2221 | Scarlet fever | WT | WT | M1 | 34 |
| 2221covSΔ1bp | Mouse-passaged derivative of MGAS2221 | Mutant | WT | M1 | 34 |
| A945a | M49 |
The strain, provided by Debra Bessen, was used as a positive control in the SpeB activity assay.
In-frame deletion of the sse gene.
The 3′and 5′ ∼1,000-bp flanking fragments of the sse fragment to be deleted were amplified from MGAS5005 chromosomal DNA using PCR and were sequentially cloned into pGRV (19) at the HindIII/HincII and HindIII sites, respectively, yielding the suicide plasmid pGRV-Δsse. This plasmid was introduced into MGAS5005 using electroporation, as described previously (19). The plasmid was inserted into the MGAS5005 genome through a homologous recombination event at one flanking fragment. The strain was selected with 150 μg/ml of spectinomycin, and it contained one copy of full-length sse and one copy of the shortened sse gene with the in-frame deletion. This strain was grown in THY without spectinomycin selection for eight passages (one passage = 0.05 to 0.7 increase in the optical density at 600 nm [OD600]) to allow the second crossover event to occur in the other flanking fragment, generating an in-frame deletion of sse (Δsse), which was spectinomycin sensitive. The culture was plated on THY agar plates after the last passage, and the colonies were spotted in parallel on THY agar plates with and without spectinomycin to identify strains that were sensitive to spectinomycin. These spectinomycin-sensitive strains were analyzed by PCR to identify Δsse strains that had a shorter sse PCR product than the wild-type strain. The region of the mutant's genomic DNA encompassing the shortened sse gene and its ∼1,100-bp flanking fragments was sequenced to confirm the in-frame deletion and to rule out spurious mutations.
Construction of plasmid pCMV-sse for complementation analysis.
A DNA fragment containing the sse gene with its own promoter and ribosome-binding site was amplified from MGAS5005 using the following primers: 5′-CGGCTGCAGTTACTATAATATTATTGCAAT-3′ and 5′-CGCCTGCAGTTAAGGAGTTTTGTTGATGGC-3′. The PCR product was cloned into pCMV (12) at the PstI site. The resulting plasmid, pCMV-sse, was introduced into the Δsse strain using electroporation, and complementation strains containing pCMV-sse (Δsse/pCMV-sse) were selected with chloramphenicol. The strains were confirmed using PCR and DNA sequence analyses.
Construction of a nonspontaneous revertant of the Δsse strain.
A nonspontaneous revertant of the Δsse strain was generated for complementation analysis of the Δsse strain. A 2,786-bp DNA fragment containing the full-length sse gene and its flanking regions was amplified with PCR using the following primers: 5′-GTCTCGAGTAAACCCATTATGGTAATA-3′ and 5′-CGTCAGGTTGACGATACTATTGAAGCCTATATTATGG-3′. The PCR product was cloned into pGRV between the XhoI and HincII sites, yielding pGRV-sse. This plasmid was introduced into the Δsse strain by electroporation. The same procedures used to generate the Δsse strain were followed to generate revertant strains (Δsse-sse). The sse gene and its flanking regions in the revertant strain were sequenced to confirm reversion and rule out spurious mutations.
Detection of Sse and SpeB.
Sse production by MGAS5005 and its isogenic strains was assessed by Western blotting, as described previously (20). Briefly, test strains were grown in protein-reduced THY, which was prepared as previously described (20), to an OD600 of 0.4, and the cultures were centrifuged to obtain the supernatant. Proteins in the culture supernatant (8 ml) were precipitated with three volumes of cold ethanol, dissolved in 0.1 ml of 8 M urea, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electrophoretically transferred onto a nitrocellulose membrane. The membrane was probed with anti-Sse mouse antiserum (20) at a dilution of 1:1,000, followed by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G at a 1:2,000 dilution. Immunoreactivity was detected by chemiluminescence using the SuperSignal West Pico kit (Pierce Biotechnology, Rockford, IL). SpeB activity in the supernatant of overnight GAS cultures was detected using the milk plate assay, as described previously (23).
Mouse infections.
Female CD-1 Swiss and immunocompetent, hairless (strain Crl:SKH1-hrBR) mice were used in animal experiments. Hairless mice were used for convenience in observing skin lesions, and CD-1 mice have been used for studies of GAS virulence by our laboratory and many other groups. The GAS strains used to infect mice were grown to mid-exponential phase, washed three times with pyrogen-free Dulbecco's phosphate-buffered saline (DPBS), and resuspended in DPBS at the desired doses. Colony counts were performed to determine the actual number of CFU used for each experiment. Three types of assays were performed: virulence, dissemination in skin, and dissemination to blood and spleen. To compare survival rates in the virulence study, groups of eight 5-week-old female outbred CD-1 Swiss mice (Charles River Laboratory) were subcutaneously infected with 0.2 ml of ∼1.4 × 108 CFU of MGAS5005 or the Δsse or Δsse-sse strain or intraperitoneally infected with 0.2 ml of ∼9 × 107 CFU of MGAS5005 or the Δsse strain. The subcutaneously infected mice were monitored twice a day for 14 days, and the intraperitoneally infected mice were monitored hourly from 9 to 24 h following infection and twice a day thereafter.
In the studies examining the dissemination in skin and from skin to blood and spleen, groups of 18 CD-1 mice were subcutaneously infected with 0.2 ml of ∼8 × 107 CFU of MGAS5005 or the Δsse strain. Six randomly selected mice from each group were sacrificed on days 2, 3, and 4 after infection to collect blood and spleens for quantification of bacterial loads and to peel off the skin around the infection site for measurement and examination of overall pathology. Each spleen was homogenized in DPBS using a Kontes pestle. The heparinized blood and spleen suspension samples were serially diluted in DPBS and plated to count the number of viable GAS CFU. In addition, groups of five immunocompetent, hairless female mice (strain Crl:SKH1-hrBR) (5 weeks old) were subcutaneously infected with 50 μl of each strain at a dose of ∼1.0 × 108 CFU to observe the appearance of the infection lesions and to measure lesion size. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee at Montana State University.
GAS growth in THY and CDM.
Cultures of MGAS5005 and the Δsse and Δsse-sse strains at the mid-exponential growth phase in THY and at an OD600 of ∼0.2 in chemically defined medium (CDM) were inoculated into THY and CDM at an OD600 of ∼0.05. Cultures were incubated at 37°C in a 5%-CO2 atmosphere. The OD600 of each culture was measured at the indicated time to obtain growth curves. CDM was prepared as described previously (37).
GAS growth in nonimmune human blood and serum and in mouse subcutis.
GAS growth in nonimmune human blood and serum was determined as previously described (19). MGAS5005, Δsse, and Δsse-sse cultures were harvested at the exponential growth phase, washed three times with DPBS, and inoculated at ∼105 CFU into 1 ml of serum or heparinized nonimmune blood from the same person. The samples were rotated end-to-end for 4 h at 37°C, and the numbers of viable GAS in the samples and inocula were determined by plating. The growth factor was defined as the ratio of CFU for each sample after a 4-h incubation over the CFU of the corresponding inoculum.
To examine the growth of the GAS strains in mouse subcutis, bacteria from each strain were subcutaneously inoculated into nine CD-1 mice in the back where the hair was shaved. The initial injection bulb was marked. At 10 min, 1 h, or 8 h after inoculation, the skin around the marked inoculation site from three mice was peeled off, cut into small pieces, and homogenized using a Kontes pestle to obtain a bacterial suspension in 0.5 ml of DPBS. Bacteria were quantified by plating the suspensions at appropriate dilutions.
Quantitative RT-PCR analysis.
Duplicate cultures of each GAS strain were grown at 37°C (5% CO2) in THY to an OD600 of 0.2 or 0.3. GAS bacteria were harvested by centrifugation and incubated at room temperature for 5 min following the addition of two volumes of RNAprotect (Qiagen, Valencia, CA) to ensure RNA integrity. Total RNA was isolated and reverse transcribed to cDNA, as described previously (11). TaqMan quantitative reverse transcription (RT)-PCR assays were performed using the ABI 7500 Fast system (Applied Biosystems Inc., Foster City, CA). Changes in levels of gene expression were compared using the ΔΔCT method with normalization to the commonly used control gene proS. All cDNA samples were assayed in triplicate, and the data represent mean values ± standard deviations.
Statistical analysis.
The survival data were analyzed using the log-rank (Mantel-Cox) test, and the sizes of skin lesions and CFU numbers in blood and spleen samples were analyzed using the two-tailed unpaired t test with Welch's correction. All analysis was conducted using the Prism software program (GraphPad Software, Inc.).
RESULTS
In-frame deletion mutant of sse gene.
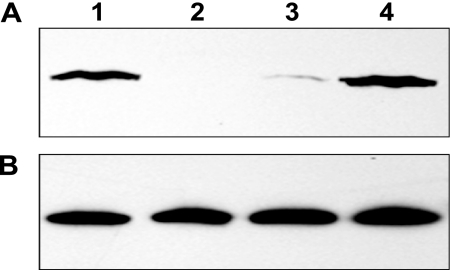
Initially, we tried to inactivate sse using an insertional inactivation strategy, as described previously (9), and obtained mutant strains that lost resistance to phagocytosis by polymorphonuclear leukocytes. These mutants could not be complemented, suggesting that there was a polar effect caused by the insertional inactivation. While we were investigating the reason for this phenotype, we used the in-frame deletion approach, which specifically deletes a stretch of codons in the target gene (25), in order to avoid polar effects. This strategy used two steps to obtain Δsse mutants. In the first step, the suicide plasmid pGRV-Δsse was integrated into the genome of MGAS5005 through homologous recombination between one flanking fragment of the sse fragment to be deleted and its homologous region in the genome. This resulted in a recombinant strain that was resistant to spectinomycin and expressed two different alleles of the sse gene: the full-length gene and the shortened version without the internal fragment. The second crossover event at the other flanking fragment occurred in the second step, resulting in the Δsse strains. The procedures were performed four times, resulting in generation of four independent Δsse mutant strains. DNA sequencing of a ∼3-kb fragment covering the shortened sse gene, flanking fragments, and ∼100 bp fragments beyond the flanking regions determined that there were no spurious mutations. All four strains produced similar phenotypes in an initial virulence test of mouse infection. We randomly selected one of these strains for full characterization. The mutant lacked a desired 621-bp fragment that encodes amino acids 55 to 261 of Sse. Western blotting analyses confirmed Sse expression in the culture supernatant of the wild-type (WT) strain but not in the Δsse strain (Fig. 1A). The levels of the secreted protein Spy0019 were similar for all strains (Fig. 1B). Thus, sse was successfully deleted using this in-frame deletion approach.
FIG. 1.
Confirmation of sse deletion and complementation. (A) Western blot demonstrating the presence or absence of Sse production in the culture supernatants of WT MGAS5005 (lane 1) and the Δsse (lane 2), Δsse/pCMV-sse (lane 3), and Δsse-sse strains (lane 4). (B) Western control blot showing similar levels of the secreted protein Spy0019 in the same samples.
Complementation of Δsse.
Although the in-frame deletion strategy does not introduce a polar effect, it is possible that a second mutation was introduced during the construction of Δsse and might be responsible for the Δsse phenotypes. To rule this out, we initially tried to complement the mutant by introducing the sse gene into the Δsse strain using pCMV (12). Surprisingly, the level of Sse produced by the complement strain was only approximately 1/10 of that produced by the WT strain (Fig. 1A, lane 3).
Instead of using another expression plasmid to resolve this problem, we followed the same procedure used for generating the Δsse strain to put sse back into the Δsse strain, yielding a nonspontaneous revertant of the Δsse strain (Δsse-sse) for use in complementation analysis. This revertant strain should be identical to the WT unless there was a second mutation introduced during generation of the Δsse strain and/or the revertant. As expected, the revertant strain secreted Sse at levels similar to those of the WT (Fig. 1A, lane 4).
The sse deletion attenuates GAS virulence in subcutaneous infection.
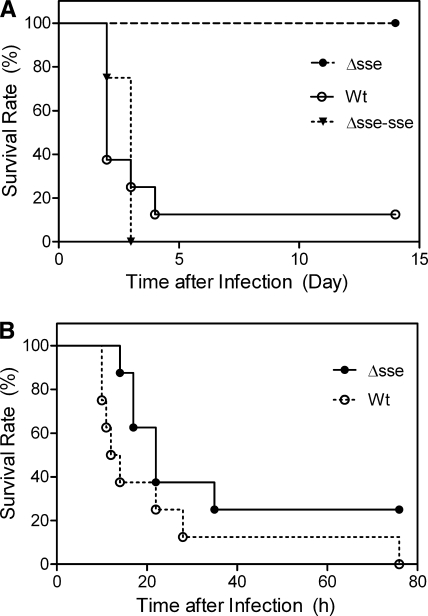
To determine whether sse is important for GAS virulence, the virulence levels of the WT, Δsse, and Δsse-sse strains were compared using mouse models of subcutaneous and intraperitoneal infections. In the subcutaneous infection, seven of the eight mice infected with the WT strain died and all eight mice infected with the Δsse-sse strain did not survive, while all mice infected with the Δsse strain survived (P values: Δsse strain versus WT, 0.004; Δsse strain versus Δsse-sse strain, 0.0002; Δsse-sse strain versus WT, 0.8899) (Fig. 2A). These results indicate that in-frame deletion of sse attenuates the virulence of GAS in the mouse model of subcutaneous infection. In the case of intraperitoneal infection, it appears that mice infected with the Δsse strain lived longer than the mice infected with the WT strain; however, the difference in survival rates was not statistically significant (P = 0.0974) (Fig. 2B).
FIG. 2.
Effects of sse deletion on GAS virulence in subcutaneous and intraperitoneal infections of mice. Groups of eight female CD-1 mice were subcutaneously infected with 1.4 × 108 CFU of MGAS5005, 1.6 × 108 CFU of the Δsse strain, or 1.5 × 108 CFU of the Δsse-sse strain (A) or intraperitoneally infected with 9.1 × 107 CFU of MGAS5005 or 9.4 × 107 CFU of the Δsse strain (B). The data represent the survival rates of the infected mice.
Effects of sse deletion on subcutaneous infection.
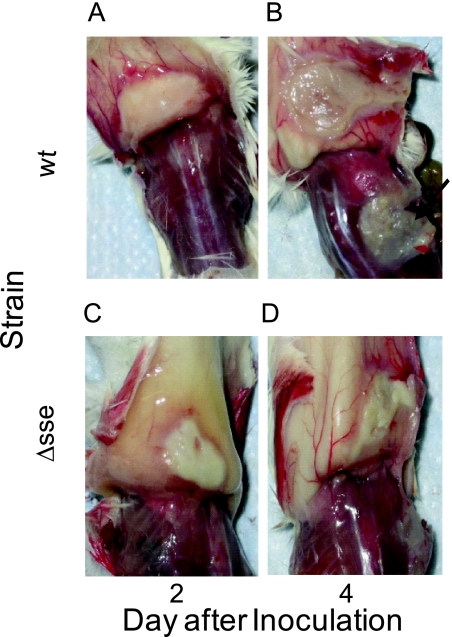
To further characterize effects of deletion of sse on subcutaneous infection, groups of CD-1 mice were infected with the WT, Δsse, or Δsse-sse strain. Six randomly selected mice from each group were sacrificed on days 2, 3, and 4 after WT and Δsse infections to collect blood and spleens for quantification of bacterial loads and to peel off the skin around the infection site for examination of overall pathology. On day 2, all the mice infected with the WT and Δsse strains showed similar levels of inflammation (Fig. 3A and C); however, the average area of the inflammation site in mice infected with the Δsse strain (103 ± 42 mm2) was significantly smaller than that in mice infected with the WT strain (259 ± 51 mm2; P = 0.0002). On day 4, the average area of the infection site in six mice infected with Δsse (104 ± 31 mm2) was again significantly smaller than that in four mice infected with the WT strain (333 ± 55 mm2; P = 0.0019). Two mice infected with the WT strain died, so the size of the infection site could not be measured. There was no significant change in lesion size in mice infected with the Δsse strain between days 2 and 4 (P = 0.4970), but there was a significant increase in the infection site area in mice infected with the WT strain from day 2 to day 4 (P = 0.0379). These results indicate that the WT strain spreads in the skin away from the injection site but the Δsse strain does not. In addition to differences in the infection area size, there was a layer of white material that had separated from the skin and stuck to the muscle underneath the infection site of mice infected with the WT strain on day 4, whereas the skin at the infection site of mice infected with the Δsse strain still looked intact on day 4 (Fig. 3B and D).
FIG. 3.
Representative images showing the difference in the subcutaneous infection of the wild-type and Δsse GAS strains. Female CD-1 mice were subcutaneously infected with 8.3 × 107 CFU of MGAS5005 (A and B) or 10.2 × 107 CFU of the Δsse strain (C and D). The images show the infection site on the inner side of the skin on days 2 and 4 after infection. The arrow indicates the white material lying on the muscle underneath the infection site.
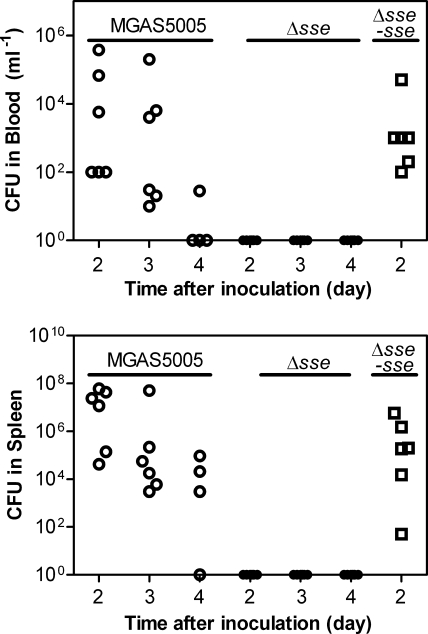
On day 2 after inoculation, the blood and spleen samples from the mice infected with the WT strain had many GAS CFU, and the GAS CFU in these samples appeared to sequentially decrease on days 3 and 4 (Fig. 4). However, there was no GAS detected on days 2, 3, and 4 in the blood and spleen samples from mice infected with the Δsse strain, whereas there were numerous GAS bacteria in the blood and spleen samples from CD-1 mice subcutaneously infected with the Δsse-sse strain (Fig. 4). These results indicate that the Δsse strain cannot disseminate from the skin into the blood and spleen.
FIG. 4.
Comparison of the systemic dissemination resulting from subcutaneously infected parental and Δsse strains. Groups of six CD-1 mice were subcutaneously infected with 8.3 × 107 CFU of MGAS5005, 10.2 × 107 CFU of the Δsse strain, or 8.6 × 107 CFU of the Δsse-sse strain. The blood and spleens were collected at 2, 3, and 4 days after infection. The data represent the viable GAS numbers in blood (top) or spleen suspension (bottom) samples.
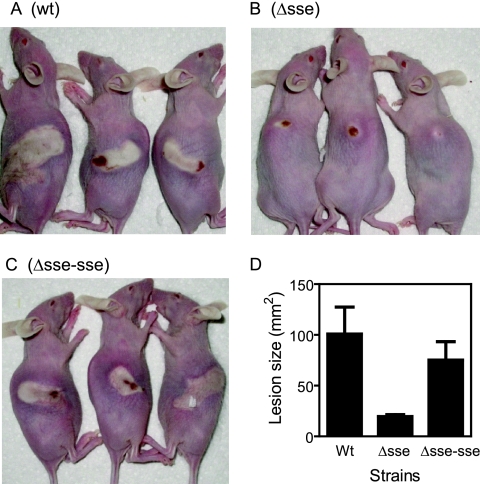
The subcutaneous infection of Crl:SKH1-hrBR mice with these strains further confirmed the contribution of Sse to GAS dissemination in the skin. On day 2 following infection, the Δsse infection lesion was small (20 ± 4 mm2) and was limited to the injection site (Fig. 5). In contrast, the WT and Δsse-sse strains caused significantly larger lesions (100 ± 53 mm2 for WT infection and 75 ± 40 mm2 for Δsse-sse infection; P values: Δsse strain versus WT, 0.0281; Δsse strain versus Δsse-sse strain, 0.0189; WT versus Δsse-sse strain, 0.2322).
FIG. 5.
Comparison of the skin lesions resulting from subcutaneously infected WT, Δsse, and Δsse-sse strains. Immunocompetent hairless female mice (strain Crl:SKH1-hrBR) were subcutaneously infected with 50 μl of 9.8 × 107 CFU of MGAS5005 (A), 9.6 × 107 CFU of the Δsse strain (B), or 9.4 × 107 CFU of the Δsse-sse strain (C). Panels A through C show representative infection lesions of three mice from each group on day two following infection. Panel D shows the mean size ± standard deviation for the lesions in each group at day 2.
Effects of deletion of sse on growth of MGAS5005.
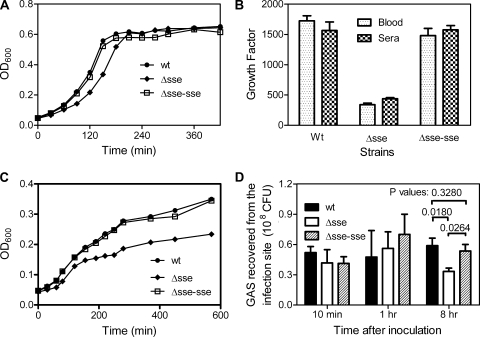
To examine whether sse deletion negatively affects the growth of GAS, the growth of the WT, Δsse, and Δsse-sse strains was compared in THY. These bacteria were incubated in THY at 37°C in a 5%-CO2 atmosphere, and the OD600 of each culture was measured over time to obtain growth curves (Fig. 6A). The growth of the Δsse strain was slightly slower than that of the WT strain during the early growth phase, and the growth of the Δsse-sse strain was restored to that of the WT strain.
FIG. 6.
Effect of the deletion of sse on GAS growth. (A and C) Growth curves of MGAS5005 and the Δsse and Δsse-sse strains in THY (A) or CDM (C). The growth of the cultures was measured as the OD600. (B) Growth factors of MGAS5005 and the Δsse and Δsse-sse strains in human blood and sera. The strains were inoculated (∼105 CFU) into 1 ml of nonimmune blood or serum and incubated for 4 h at 37°C with end-to-end rotation. Growth factor was defined as the ratio of viable CFU of GAS in each sample over the CFU of the inoculum. (D) GAS growth in vivo. Groups of CD-1 mice were subcutaneously infected with 0.2 ml of 8.2 × 107 CFU of MGAS5005, 9.0 × 107 CFU of the Δsse strain, or 8.2 × 107 CFU of the Δsse-sse strain. The skin containing the infection site was collected at 10 min, 1 h, and 8 h after infection and homogenized to quantify GAS by plating. The data represent the average number of CFU ± standard deviation for three mice for each strain at each time point. The P values were obtained using the one-tailed unpaired t test.
To test whether sse deletion reduces the growth of GAS in human blood and serum, we measured the ability of GAS strains to grow in these fluids. The mean growth factors, the ratios of GAS CFU in cultures over inocula, of the WT, Δsse, and Δsse-sse strains during a 4-h incubation were determined to be 1,724 ± 148, 338 ± 48, and 1,480 ± 208, respectively, in heparinized nonimmune human blood and 1,566 ± 197, 438 ± 26, and 1,575 ± 102, respectively, in human serum (Fig. 6B). Thus, growth of the Δsse strain in human blood and serum is significantly slower than that of the WT strain (P values: blood, 0.0021; sera, 0.0395). The growth of the Δsse-sse strain is similar to that of the WT strain, since there was no significant difference in growth between the Δsse-sse and WT strains (P values: blood, 0.0991; sera, 0.4822).
THY, blood, and serum are rich media for bacteria, whereas the subcutis is a nutrient-limited environment for bacterial pathogens. Thus, the slow growth of Δsse may be amplified or become less important in the subcutaneous environment. To examine the effect of medium conditions on GAS growth, we first analyzed the growth of the strains in chemically defined medium (CDM). The Δsse strain displayed a longer early growth phase in CDM than the WT and Δsse-sse strains (Fig. 6C). In addition, the mutant had a lower maximum OD600 than the WT and Δsse-sse strains. These results suggest that Sse does not function by providing nutrients via hydrolysis, since the ingredients in CDM are unlikely to need Sse-catalyzed hydrolysis prior to utilization. Next, we monitored the in vivo growth of these strains in mouse skin by quantifying GAS at the subcutaneous infection sites. The number of GAS recovered from the infection sites was approximately 50% of the inoculum at the 10-min time point. There was no significant change in the total CFU for the WT and Δsse-sse strains at 10 min, 1 h, and 8 h after inoculation (Fig. 6D). The CFU numbers for the Δsse strain at the 10-min and 1-h time points were similar to those of the WT and Δsse-sse strains but appeared to be significantly lower at 8 h postinoculation. Inflammation was not noticeable at the 1-h time point but was obvious at the 8-h time point. These data indicate that all of the three strains did not grow much, at least at the 1-h time point, suggesting that the slower growth of the Δsse strain observed in the rich medium might become less important in the context of subcutaneous infection. The reduction in the CFU number of the Δsse strain at the 8-h time point relative to those at the 10-min and 1-h time points apparently represents killing of the Δsse strain by the host. This compromised ability of the Δsse strain to survive in the subcutis during the host's inflammatory response suggests that the mutant may have compromised resistance to the innate defense besides its compromised growth ability.
Control of Sse by CovRS.
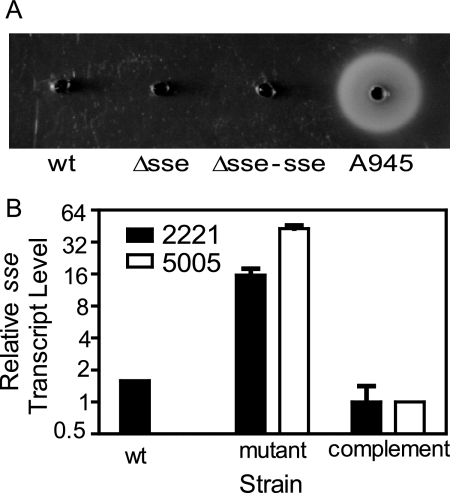
The covS gene of MGAS5005, the parental strain of the Δsse strain, has a 1-bp deletion at base 83 (34), and SpeB activity is not detectable in its culture supernatant (Fig. 7A). Since a loss of SpeB production due to certain covRS mutations is important in mediating severe invasive infection, it is possible that SpeB expression is restored in the Δsse strain, leading to attenuation of the subcutaneous infection. SpeB activity was not detected in WT, Δsse, and Δsse-sse culture supernatants but was detected in the culture supernatant of the serotype M49 strain A945, a positive control for SpeB production (Fig. 7A). In addition, the Δsse and Δsse-sse strains still maintain the covS null mutation, according to DNA sequencing. These results rule out the possibility that the phenotypes of the Δsse strain are caused by restoration of SpeB production due to sse deletion or a spurious mutation.
FIG. 7.
(A) The absence of SpeB activity in the culture supernatants of MGAS5005 and the Δsse and Δsse-sse strains, as assessed by a milk protein hydrolysis plate assay. A945, an M49 strain, is included as a positive control. (B) The CovRS system negatively regulates the transcription of sse. The data represent the relative levels of the sse transcript in the WT CovRS-expressing GAS strain MGAS2221 containing pDCBB (vector control) (Wt), isogenic covS mutant strain 2221covSΔ1bp containing pDCBB (mutant), and 2221covSΔ1bp strain containing the complementation plasmid pCovSC (complement) (2221) at the early exponential growth phase and in MGAS5005/pDCBB (mutant) and MGAS5005/pCovSC (complement) (5005) at the mid-exponential growth phase. The data are mean values ± standard deviations from duplicate cultures of each strain that were each analyzed in triplicate and normalized to the proS control gene transcript levels.
To test whether sse is regulated by the CovRS two-component regulatory system, the effect of covS mutation on sse transcription was assessed by real time RT-PCR analysis. MGAS5005 was transformed with either an empty vector (pDCBB) or a derivative containing the WT covS gene (pCovSC), and the sse transcript levels were determined from cultures grown to the exponential phase of growth in THY. Levels of the sse transcript in MGAS5005/pDCBB were 43-fold higher than those in MGAS5005/pCovSC (Fig. 7B), indicating that MGAS5005 produces high levels of Sse as a consequence of the covS mutation. To further investigate sse transcript regulation by the CovRS system, we performed real time RT-PCR on samples recovered from the clinical GAS isolate MGAS2221 (which contains WT covRS genes), an isogenic covS mutant (2221covSΔ1bp), and a derivative of 2221covSΔ1bp that was complemented with pCovSC (36). The covS mutant of MGAS2221 produced the sse transcript at approximately 10- and 16-fold greater levels than the wild-type and complemented strains, respectively (Fig. 7B). It should be noted that the MGAS5005 and MGAS2221 data were for the mid-exponential and early exponential growth phases, respectively. These results provide strong evidence that sse is negatively regulated by CovRS.
DISCUSSION
One major finding of this study is that Sse significantly contributes to the ability of the clinical isolate MGAS5005 to cause severe soft tissue infections and to efficiently disseminate from the skin into the blood and organs. Another major finding is that the transcription of sse is negatively regulated by CovRS. Our results suggest that Sse is a novel CovRS-controlled virulence factor that is important for mediating severe invasive GAS infection.
It has been established that null mutations of covRS increase the severity of experimental GAS soft tissue infections in mice (14, 18). The importance of covRS mutations in severe invasive infections is further supported by the observation that covRS mutations are selected during experimental invasive infections in mice (5, 34, 38). Even more significantly, covRS mutations may also contribute to severe invasive infections in human patients. The high virulence of a particular M3 strain appears to be associated with a Q216P mutation in CovR (27). Likewise, MGAS5005, a serotype M1 clinical isolate from the cerebral spinal fluid of a patient (36), carries a nonfunctional covS gene (34). Through the elegant studies reported by several groups over the last few years, it has become clear that the loss of SpeB expression and upregulation of hyaluronic acid capsule production as a result of covRS mutations contribute to the progression of invasive GAS infections, at least in the case of M1 GAS isolates (4, 5, 34, 38). However, the molecular basis and mechanism for how CovRS mutations promote the progression of invasive diseases have not been fully established. Here we show that CovRS-controlled Sse is important for invasive soft tissue infection and systemic dissemination from the skin for an M1 strain with a covS null mutation. Similar to its parental strain, the Δsse strain does not have detectable SpeB activity, ruling out the possibility that the phenotype of the Δsse strain is due to restoration of SpeB production. These findings suggest that Sse is a novel CovRS-controlled virulence factor that plays an important role in the progression of invasive diseases caused by CovRS/CsrRS mutations.
Previous studies indicate that CovRS directly or indirectly regulates many genes, including hasA, sls, ska, speB, and mac/mspA (7, 14, 15, 17, 34). Although the speB gene was shown to be important in the soft tissue infection of an M3 strain (21), upregulation of SpeB is not a factor in severe invasive GAS infections (2, 6). However, upregulation of the capsule and SLS (6) but not Mac/MspA (15) contributes to GAS pathogenesis and systemic dissemination in the subcutaneous infection of mice. The capsule is critical for the antiphagocytic properties of GAS, and SLS causes the lysis of host cells. Streptokinase, encoded by ska, is also important in invasive GAS infection in activating human plasminogen (35). Addition of Sse to the list of CovRS-regulated factors that are important in invasive disease progression supports the notion that multiple factors contribute to the virulence of GAS in soft tissue infections and that the functions of these factors are not redundant, i.e., all are required for maximum virulence.
Sse is unique among the known CovRS-regulated virulence factors in that this protein is required for rapid GAS growth in THY, CDM, blood, and sera. However, the mechanism of the growth alteration in the Δsse strain is unknown. CovRS may also control GAS growth in addition to the evasion of innate and acquired host immunity. This CovRS regulation would increase the GAS growth rate and decrease the GAS clearance rate to reach the same goal: establishment of GAS infection or a net increase of GAS in the host. We propose that CovRS controls GAS growth through Sse and that the rapid growth of GAS is a virulence factor. We are currently evaluating the mechanism of the effect of sse deletion on GAS growth and whether the regulation of pathogen growth is a novel virulence mechanism.
While the slower growth of the Δsse strain might have contributed to attenuated GAS dissemination and virulence in the context of subcutaneous infection, there are a few observations suggesting that the attenuation is not entirely attributable to the slower growth of the Δsse strain. First, the Δsse mutant was able to readily disseminate into the blood in the case of intraperitoneal infection but not after subcutaneous infection, suggesting that Sse is required for systemic dissemination only in the case of subcutaneous infection. Second, the number of WT and revertant bacteria at the skin infection sites did not dramatically increase at the examined time points up to 8 h following infection, suggesting that the GAS present at the infection site in the skin does not rapidly grow. However, our assay cannot rule out the possibility that GAS at the edge of the infection site could grow faster than those at the crowded center. Third, the numbers of Δsse bacteria recovered from the skin infection sites were similar at 10-min and 1-h time points but smaller at 8 h than those of the WT and revertant strains, suggesting that the Δsse strain may be more vulnerable to the host defense machinery. Further characterization of the Δsse strain is required to fully understand the basis and mechanism of the contribution of Sse to GAS virulence.
It is interesting that there was a layer of white material under the subcutaneous infection site with the WT strain but little under the infection site with the Δsse strain on day 4 after infection. The white material may be fibrinogen, which has been proposed to deposit at GAS infection sites as a result of local thrombosis for microvascular occlusion against the systemic dissemination of GAS (35). Streptokinase enhances the invasive infection by binding to plasminogen or plasmin, which presumably degrades the occlusion to allow a systemic spread of infection (35). Since streptokinase is specific for human plasminogen and unlikely to mediate fibriolysis in mice, Sse may be involved in fibriolysis in mice. Alternatively, the white material could be subcutis residues and lysed neutrophils. We are currently evaluating these possibilities. Either of these scenarios would suggest that Sse itself or in conjunction with host factors plays an active role in the invasion of skin by GAS. Sse and its homologue in Streptococcus equi (See) share 62% sequence identity. The See protein has been shown to be a nonspecific carboxylic ester hydrolase with optimal activity against acetyl esters (41). Elucidation of the exact functional mechanism of Sse will rely on identification of the in vivo substrate(s) of Sse, which we are actively pursuing.
Esterases are widely expressed by bacteria. The role of esterases in the virulence and pathogenesis of bacterial pathogens is largely unknown. Our findings on the importance of Sse in the virulence and dissemination of GAS in the context of subcutaneous infection and a recent finding (22) that the cell wall-anchored carboxylesterase of Mycobacterium tuberculosis is required for virulence indicate that the esterase class of proteins is important in bacterial pathogenesis. Further studies of the functional mechanism of Sse in GAS pathogenesis may yield generalized findings on the role of carboxylesterases in bacterial pathogenesis.
The phenotype of the Δsse strain in the context of subcutaneous infection is consistent with our previous finding that immunization of mice with Sse reduces the severity of invasive skin infection (20). Our new finding further strengthens the idea that Sse is a protective antigen against severe invasive skin infection, indicating that Sse has the potential to be used as a vaccine component for preventing necrotizing fasciitis. Inclusion of Sse in a GAS vaccine may help prevent most of the severe invasive infections involving GAS.
Acknowledgments
This work was supported in part by NIH grant P20 RR-020185 and a USDA NRI/CSREES grant, 2007-35204-18306, USDA Formula Funds, and the Montana State University Agricultural Experimental Station.
We thank Debra E. Bessen for providing the A945 strain and Mark Quinn for critical reading of the manuscript.
Editor: F. C. Fang
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Abbot, E. L., W. D. Smith, G. P. Siou, C. Chiriboga, R. J. Smith, J. A. Wilson, B. H. Hirst, and M. A. Kehoe. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell. Microbiol. 9:1822-1833. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh, C. D., H. B. Warren, V. J. Carey, and M. R. Wessels. 1998. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Investig. 102:550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685-694. [DOI] [PubMed] [Google Scholar]
- 4.Cole, J. N., J. D. McArthur, F. C. McKay, M. L. Sanderson-Smith, A. J. Cork, M. Ranson, M. Rohde, A. Itzek, H. Sun, D. Ginsburg, M. Kotb, V. Nizet, G. S. Chhatwal, and M. J. Walker. 2006. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 20:1745-1747. [DOI] [PubMed] [Google Scholar]
- 5.Engleberg, N. C., A. Heath, A. Miller, C. Rivera, and V. J. DiRita. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043-1054. [DOI] [PubMed] [Google Scholar]
- 6.Engleberg, N. C., A. Heath, K. Vardaman, and V. J. DiRita. 2004. Contribution of CsrR-regulated virulence factors to the progress and outcome of murine skin infections by Streptococcus pyogenes. Infect. Immun. 72:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federle, M. J., K. S. McIver, and J. R. Scott. 1999. A response regulator that represses transcription of several virulence operons in the group A Streptococcus. J. Bacteriol. 181:3649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, J., and M. G. Caparon. 2006. Specificity of Streptococcus pyogenes NAD+ glycohydrolase in cytolysin-mediated translocation. Mol. Microbiol. 62:1203-1214. [DOI] [PubMed] [Google Scholar]
- 11.Graham, M. R., K. Virtaneva, S. F. Porcella, W. T. Barry, B. B. Gowen, C. R. Johnson, F. A. Wright, and J. M. Musser. 2005. Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am. J. Pathol. 166:455-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanks, T. S., M. Liu, M. J. Mclure, and B. Lei. 2005. ABC transporter FtsABC of Streptococcus pyogenes mediates uptake of ferric ferrichrome. BMC Microbiol. 5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayano, S., and A. Tanaka. 1973. Extracellular esterases of group A streptococci. Infect. Immun. 7:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath, A., A. Miller, V. J. DiRita, and C. N. Engleberg. 2001. Identification of a major, CsrRS-regulated secreted protein of group A streptococcus. Microb. Pathog. 31:81-89. [DOI] [PubMed] [Google Scholar]
- 16.Ji, Y., L. MaLandsborough, A. Kondagunta, and P. P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298-1305. [DOI] [PubMed] [Google Scholar]
- 18.Levin, J. C., and M. R. Wessels. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30:209-219. [DOI] [PubMed] [Google Scholar]
- 19.Liu, M., T. S. Hanks, J. Zhang, M. J. McClure, D. W. Siemsen, J. L. Elser, M. T. Quinn, and B. Lei. 2006. Defects in ex vivo and in vivo growth and sensitivity to osmotic stress of group A Streptococcus caused by interruption of response regulator gene vicR. Microbiology 152:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, M., H. Zhu, J. Zhang, and B. Lei. 2007. Active and passive immunizations with the streptococcal esterase Sse protect mice against subcutaneous infection with group A streptococci. Infect. Immun. 75:3651-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukomski, S., C. A. Montgomery, J. Rurangirwa, R. S. Geske, J. P. Barrish, G. J. Adams, and J. M. Musser. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lun, S., and W. R. Bishai. 2007. Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. J. Biol. Chem. 282:18348-18356. [DOI] [PubMed] [Google Scholar]
- 23.Ma, Y., A. E. Bryant, D. B. Salmi, S. M. Hayes-Schroer, E. McIndoo, M. J. Aldape, and D. L. Stevens. 2006. Identification and characterization of bicistronic speB and prsA gene expression in the group A Streptococcus. J. Bacteriol. 188:7626-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manetti, A. G., C. Zingaretti, F. Falugi, S. Capo, M. Bombaci, F. Bagnoli, G. Gambellini, G. Bensi, M. Mora, A. M. Edwards, J. M. Musser, E. A. Graviss, J. L. Telford, G. Grandi, and I. Margarit. 2007. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 64:968-983. [DOI] [PubMed] [Google Scholar]
- 25.Marouni, M. J., and S. Sela. 2003. The luxS gene of Streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infect. Immun. 71:5633-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi-Akiyama, T., D. Takamatsu, M. Koyanagi, J. Zhao, K. Imanishi, and T. Uchiyama. 2005. Cytocidal effect of Streptococcus pyogenes on mouse neutrophils in vivo and the critical role of streptolysin S. J. Infect. Dis. 192:107-116. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi-Akiyama, T., T. Ikebe, H. Watanabe, T. Uchiyama, T. Kirikae, and Y. Kawamura. 2006. Use of DNA arrays to identify a mutation in the negative regulator, csrR, responsible for the high virulence of a naturally occurring type M3 group A streptococcus clinical isolate. J. Infect. Dis. 193:1677-1684. [DOI] [PubMed] [Google Scholar]
- 28.O'Loughlin, R. E., A. Roberson, P. R. Cieslak, R. Lynfield, K. Gershman, A. Craig, B. A. Albanese, M. M. Farley, N. L. Barrett, N. L. Spina, B. Beall, L. H. Harrison, A. Reingold, and C. Van Beneden. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45:853-862. [DOI] [PubMed] [Google Scholar]
- 29.Proft, T., S. L. Moffatt, C. J. Berkahn, and J. D. Fraser. 1999. Identification and characterization of novel superantigens from Streptococcus pyogenes. J. Exp. Med. 189:89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shulman, S. T. 2003. Acute streptococcal pharyngitis in pediatric medicine: current issues in diagnosis and management. Paediatr. Drugs 5(Suppl. 1):13-23. [PubMed] [Google Scholar]
- 31.Smoot, L. M., J. K. McCormick, J. C. Smoot, N. P. Hoe, I. Strickland, R. L. Cole, K. D. Barbian, C. A. Earhart, D. H. Ohlendorf, L. G. Veasy, H. R. Hill, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Characterization of two novel pyrogenic toxin superantigens made by an acute rheumatic fever clone of Streptococcus pyogenes associated with multiple disease outbreaks. Infect. Immun. 70:7095-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens, D. L. 2003. Group A streptococcal sepsis. Curr. Infect. Dis. Rep. 5:379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stock, A. H., J. Uriel, and P. Grabar. 1961. Esterase in extracellular concentrates of group A streptococci and the homologous antibody. Nature 192:434-435. [DOI] [PubMed] [Google Scholar]
- 34.Sumby, P., A. R. Whitney, E. A. Graviss, F. R. DeLeo, and J. M. Musser. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PloS Pathog. 2:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, H., U. Ringdahl, J. W. Homeister, W. P. Fay, N. C. Engleberg, A. Y. Yang, L. S. Rozek, X. Wang, U. Sjöbring, and D. Ginsburg. 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305:1283-1286. [DOI] [PubMed] [Google Scholar]
- 36.Treviño, J., N. Perez, E. Ramirez-Peña, Z. Liu, S. A. Shelburne, J. M. Musser, and P. Sumby. 2009. CovS simultaneously activates and inhibits the CovR-mediated repression of distinct subsets of group A Streptococcus virulence factor-encoding genes. Infect. Immun. 77:3141-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Rijn, I., and R. E. Kessler. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker, M. J., A. Hollands, M. L. Sanderson-Smith, J. N. Cole, J. K. Kirk, A. Henningham, J. D. McArthur, K. Dinkla, R. K. Aziz, R. G. Kansal, A. J. Simpson, J. T. Buchanan, G. S. Chhatwal, M. Kotb, and V. Nizet. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13:981-985. [DOI] [PubMed] [Google Scholar]
- 39.Ward, R. G., and M. S. Walsh. 1991. Necrotizing fasciitis: 10 years' experience in a district general hospital. Br. J. Surg. 78:488-489. [DOI] [PubMed] [Google Scholar]
- 40.Wexler, D. E., D. E. Chenoweth, and P. P. Cleary. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 82:8144-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie, G., M. Liu, H. Zhu, and B. Lei. 2008. Esterase SeE of Streptococcus equi ssp. equi is a novel nonspecific carboxylic ester hydrolase. FEMS Microbiol. Lett. 289:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, M. H., D. M. Aronoff, and N. C. Engleberg. 2005. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev. Anti-Infect. Ther. 3:279-294. [DOI] [PubMed] [Google Scholar]