Abstract
The type I interferon (IFN) cascade is critical in controlling viral replication and pathogenesis. Recognition pathways triggered by viral infection rapidly induce the type I IFN cascade, often in an IFN regulatory factor 3 (IRF-3)-dependent fashion. This dependence predicts that loss of IRF-3 would render early recognition pathways inoperative and thereby impact virus replication, but this has not been observed previously with herpes simplex virus type 1 (HSV-1) in vitro. In this study, HSV-1-infected IRF-3−/− bone marrow-derived dendritic cells (BMDCs) and macrophages supported increased HSV replication compared to control cells. In addition, IRF-3-deficient BMDCs exhibited delayed type I IFN synthesis compared to control cells. However, while IFN pretreatment of IRF-3−/− BMDCs resulted in reduced virus titers, a far greater reduction was seen after IFN treatment of wild-type cells. This suggests that even in the presence of exogenously supplied IFN, IRF-3−/− BMDCs are inherently defective in the control of HSV-1 replication. Together, these results demonstrate a critical role for IRF-3-mediated pathways in controlling HSV-1 replication in cells of the murine immune system.
Herpes simplex virus type 1 (HSV-1) is a ubiquitous human pathogen with high seroprevalence in adults (51). HSV-1 is associated with numerous human diseases ranging from the common cold sore in immunocompetent individuals to herpetic encephalitis in neonatal and immunocompromised hosts. A member of the Alphaherpesvirus family, HSV-1 exhibits two distinct phases of infection (49). Acute infection typically occurs at peripheral epithelial sites and is characterized by lytic infection and spread. In contrast, the virus shifts from lytic to latent infection in sensory neurons, which is characterized by limited gene expression and the persistence of viral genomes in a transcriptionally active state. Following certain stimuli, periodic reactivation of latency occurs and may result in shedding of infectious virus at the initial site of acute infection. Reactivation may also be associated with immunopathological diseases, most notably ocular herpetic stromal keratitis.
A role for interferons (IFNs) in controlling viral replication is well established. In recent years, viral research has focused on cellular recognition of pathogen-associated molecular patterns and subsequent IFN induction, leading to the discovery of Toll-like receptors (TLRs) and retinoic acid inducible gene 1 (RIG-I)-like sensing molecules (18). Such molecules respond to several virally derived pathogen-associated molecular patterns. These include MDA-5 and TLR-3, which recognize double-stranded RNA (13, 24); DAI and TLR-9, which recognize double-stranded DNA (14, 45); TLR-7, which recognizes single-stranded RNA (9); and RIG-I, which recognizes triphosphate and double-stranded RNA (16, 31, 53). Subsequent work identified the adaptor molecules necessary for antiviral pathway signaling, including MyD88, TRIF, and IPS-1 (19, 46, 52). Not surprisingly, numerous gene products from viruses such as hepatitis C virus, West Nile virus (WNV), influenza virus, and vaccinia virus have been identified to antagonize these pathways and serve to promote viral replication and virulence by degradation, interference, or sequestration of early recognition components (3, 11).
These newly identified recognition pathways utilize IRF-3, IRF-7, and NF-κB to induce IFN transcription through cognate binding sites on the IFN-β promoter (38). During initial induction of IFN, IRF-3, and NF-κB, which are constitutively expressed, become activated and translocate to the nucleus, where they bind the IFN-β promoter to form the IFN enhancesome (54). The initial IFN-β produced acts upon the IFN-αβ receptor in both an autocrine and paracrine manner to upregulate IFN-stimulated genes (ISGs), most notably IRF-7 (38). In concert with IRF-3, IRF-7 amplifies and facilitates expression of the full type I IFN cascade. In the absence of IRF-3, IFN-β production is reduced but the IFN-α levels remain normal, suggesting that IRF-7 activity can compensate for the loss of IRF-3 (15). In contrast, IRF-7 deficiency results in significant reduction in serum IFN levels with a corresponding increase in susceptibility to virus infection. IRF-7 was therefore dubbed “the master regulator” of type I IFN-dependent immune responses (15). IRF-7−/− mice challenged with HSV-1 showed increased mortality compared to control and IRF-3−/− mice, but no increases in virus titers were observed in IRF-3- or IRF-7-deficient cells in vitro (15). A possible explanation for this lack of phenotype in vitro is that HSV-1 may control IRF-3 activation so thoroughly that this pathway is neutralized during infection. UV-inactivated HSV-1 induces IRF-3 dimerization and activation, leading to IFN induction, suggesting that very early events in infection are responsible for triggering this cascade in the absence of viral gene expression (6, 23). ICP0, an immediate-early gene of HSV-1, interacts with IRF-3 and plays a critical role in preventing the induction of the IFN response (10, 23, 27, 28, 30, 42). Additional HSV genes such as the virion host shutoff protein, ICP34.5, and ICP27 also interfere with the activity of IRF-3 (23, 26, 48). However, the increased susceptibility of IFN receptor knockout mice to HSV-1 compared to wild-type (WT) mice suggests that despite so many genes regulating this pathway, the virus does not maintain total control over the type I IFN cascade (22, 32). In addition, numerous recognition molecules have been implicated in HSV-1 identification and the subsequent immune response (for example, TLR-3, TLR-2, TLR-9, and RIG-I), but the loss of any of these components does not result in any significant increase in viral replication in vitro (21, 35, 37, 55).
In these studies, we examined the impact of IRF-3-mediated pathways on HSV-1 replication using cells from IRF3-deficient (IRF-3−/−) mice. The absence of IRF-3 was predicted to preclude the function of early recognition pathways and thereby impact HSV-1 replication. No changes in HSV-1 replication in IRF-3-deficient mouse embryonic fibroblasts (MEFs) had been observed previously, but we reasoned that, relative to MEFs, cells of the immune system might induce more vigorous IRF-3-dependent antiviral responses, manifesting with a significant impact upon viral replication. Using IRF-3-deficient bone marrow-derived dendritic cells (BMDCs) and macrophages (BMM) we have demonstrated that IRF-3 mediated pathways are critical for control of HSV-1 replication. Moreover, control of HSV-1 replication is dependent on the type I IFN cascade in these cell types induced via IRF-3-mediated pathways.
MATERIALS AND METHODS
Cells and viruses.
Viral stocks were grown and titers were determined on Vero cells (34). HSV-1 WT strain KOS was the background strain for the present study (41).
BMDCs were generated from 6 to 8-week-old C57/BL6 (Charles River Laboratories, Wilmington, MA) or 129S6 (Taconic, Germantown, NY) mice (25, 56). Briefly, bone marrow was flushed from femurs of mice and cells were cultured as described below. For the generation of BMDCs, bone marrow was cultured in RPMI with 10% fetal calf serum, Glutamax, sodium pyruvate, nonessential amino acids, 250 U of penicillin/ml, 250 U of streptomycin/ml, and 2% granulocyte-macrophage colony-stimulating factor for 6 to 8 days at 37°C. BMDCs were then collected, counted, and divided into aliquots for infection at several multiplicities of infection (MOIs) by the addition of virus in a minimal volume of medium for 30 min at 37°C. Cells were then spun at low speed, and inocula were removed, washed, resuspended, and plated in 35-mm wells for the duration of the experiment. BMDCs were also generated from IRF-3−/− (15), IRF-7−/− (15), STAT-1−/− (29) (Taconic, Germantown, NY), and IFN-α/β/γ receptor−/− (AG129) mice (47).
BMM were cultured as described previously (56). Briefly, bone marrow was cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 5% heat-inactivated horse serum, 20% L-929 conditioned medium, 250 U of penicillin/ml, 250 U of streptomycin/ml for 7 days on non-tissue-culture-treated plates. At day 7, cells were washed with a 0.02% EDTA solution, collected, and counted. The cells were plated in 35-mm wells and rested for 3 days. The BMM were infected at MOIs of 0.01 and 1 by the addition of virus in a minimal volume of medium for 30 min at 37°C, removal of inoculum, and followed by the addition of complete medium.
IFN-β ELISA.
BMDCs were mock treated or infected at an MOI of 5 with HSV-1 and cultured in 1 ml of medium. Cultured supernatants were harvested at 3, 6, 9, and 12 h postinfection and spun at low speeds to remove cells. Supernatants were stored at −20°C before assay of IFN-β in the medium using 50 μl of harvested medium in a mouse IFN-β enzyme-linked immunosorbent assay (ELISA) as described in the kit protocol (PBL Biomedical Laboratories, Piscataway, NJ).
Antibody blockade.
MAR1-5A3, an immunoglobulin G1 (IgG1) monoclonal antibody specific to the IFN-α receptor (IFNαR; Leinco Technologies, St. Louis, MO) was utilized as described previously (39). Briefly, after infection, cells were plated in 1 ml of complete medium with 5 μg of MAR1-5A3/ml for the duration of the experiment. At specified times, cells were harvested, and the titers were determined on Vero cells under methylcellulose.
Mixing experiment.
BMDCs were collected, counted, and divided into aliquots for infection. WT and IRF-3−/− BMDCs were mixed at a ratio of 1:1 such that the cell numbers equaled those of nonmixed controls. The mixed and nonmixed populations were then immediately infected as previously described.
IFN-β pretreatment.
BMDCs were treated for 16 h with 100 U of mouse IFN-β (PBL Biomedical Laboratories, Piscataway, NJ)/ml or mock in phosphate-buffered saline. BMDCs were then collected, counted, and divided into aliquots for infection as previously described. No additional IFN-β was added after infection.
Statistics.
All statistical calculations were determined by using the Student t test and are relative to control cells unless otherwise stated.
RESULTS
Control of HSV-1 replication in BMDCs is IRF-3 dependent in vitro.
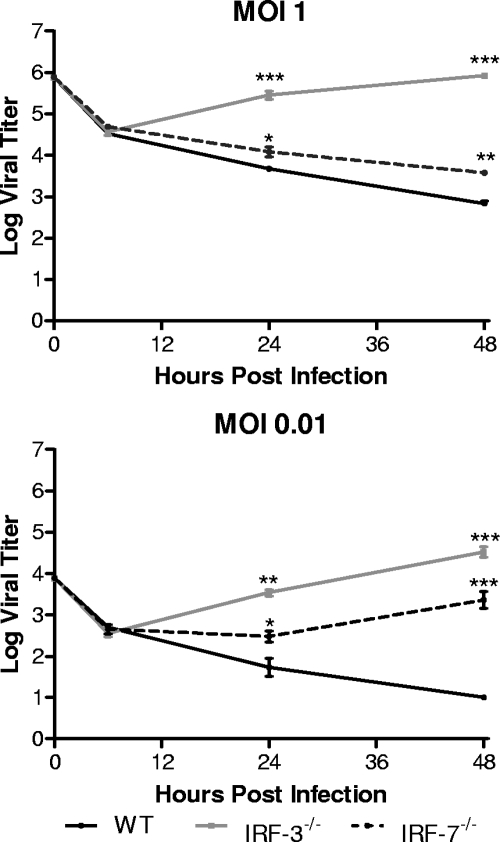
A previous study using IRF-3- and IRF-7-deficient MEFs demonstrated that the absence of either signaling molecule did not significantly alter HSV-1 replication (15). Work performed in this laboratory is in agreement with these previous observations (data not shown). BMDCs were chosen for infection in the present study due to their function as immune sentinels, their strong responses to IFN, and their critical role in controlling HSV-1 infection in vivo (17, 43, 44). IRF-3−/− BMDCs yielded at least 10 times more HSV-1 than control cells at both 24 and 48 h postinfection at each MOI tested (Fig. 1). In contrast, HSV-1-infected IRF-7−/− BMDCs did not yield any increased virus titers compared to WT control BMDCs. These results suggested that pathways for control of HSV-1 replication in BMDCs are dependent on IRF-3 but independent of IRF-7.
FIG. 1.
In vitro replication in bone marrow derived dendritic cells. Primary BMDCs were infected with WT HSV-1 at an MOI of 1 or 0.01. At indicated times postinfection, cells and supernatants were harvested and virus titers assayed on Vero cells. The results shown are the mean titers of three independent experiments. *, P < 0.05; **, P < 0.01.
BMM require IRF-3 for control of HSV-1 replication.
Primary BMM were infected in order to further assess the role of IRF-3-mediated pathways in immune cells (2, 5). These adherent BMM were also tested to exclude the possibility that the replication pattern of HSV differed between MEFs and BMDCs because of differences in adherence to plastic substrates in culture. The results, however, demonstrated that the pattern of viral replication in the IRF-3−/− BMM resembled that seen in BMDCs, with increased viral yields compared to control cells (Fig. 2). Increases of 10- to 100-fold in viral yields were demonstrated at both 24 and 48 h postinfection at MOIs of 0.01, 1, and 5 (data not shown). Interestingly, IRF-7−/− BMM also supported increased viral replication. In contrast to BMDCs, IRF-7-deficient BMM permitted a 10- to 100-fold increase in viral replication compared to controls at 24 and 48 h postinfection. This increase in virus titers was greater in magnitude at the lower MOI, but the impact of IRF-7 loss on HSV replication in BMM was less than the impact of loss of IRF-3. These data suggest a role for both IRF-3 and IRF-7 in control of HSV-1 replication in BMM. BMDCs, however, have no requirement for IRF-7 in controlling HSV-1 replication, demonstrating a difference in the innate immune response between macrophages and dendritic cells. Overall, in both cell types, IRF-3-mediated pathways are required to control HSV-1 replication in vitro.
FIG. 2.
In vitro replication in bone marrow derived macrophages. Primary BMM were infected with WT HSV-1 at an MOI of 1 or 0.01. At the indicated times postinfection, cells and supernatants were harvested, and virus titers were assayed on Vero cells. The results shown are mean titers of four independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
BMDCs lacking IFN receptors permit increased viral replication in a STAT-1-dependent manner.
Having identified a role for IRF-3-mediated pathways in controlling HSV-1 replication in BMDCs and BMM, the focus was shifted to differentiating between two non-mutually exclusive mechanisms by which IRF-3 could be controlling HSV-1 replication. First, it is possible that IRF-3−/− BMDCs have delayed or reduced type I IFN responses, disrupting the type I IFN cascade, and resulting in increased viral replication. Second, it is possible that other IRF-3-dependent processes or gene products are directly controlling HSV-1 replication. To address these possibilities, BMDCs lacking both type I and type II IFN receptors were infected with HSV-1 (Fig. 3) . These cells lack IFN binding and signaling but contain IRF-3 and thereby maintain elements of the early recognition pathway via IRF-3-dependent gene expression. The IFN receptor-deficient BMDCs permitted increased viral growth in a similar fashion to IRF-3−/− BMDCs and suggested that the type I IFN cascade was responsible for controlling HSV-1 replication (Fig. 3). Similar increases in viral replication were also seen in STAT-1−/− BMDCs. Together, these data confirm that viral replication is significantly limited in these cell types through IFN-driven STAT-1 signaling. Although these data do not completely exclude other IRF-3-dependent processes, the results strongly suggest that the increased viral yields in IRF-3−/− BMDCs and BMM are due to a delayed or defective type I IFN cascade.
FIG. 3.
In vitro replication in BMDCs lacking IFN signaling. Primary BMDCs derived from WT and IFN-α/β/γ receptor-deficient (AG129) or STAT1-deficient mice were infected with WT HSV-1 at an MOI of 1 or 0.01. At indicated times postinfection, cells and supernatants were harvested, and virus titers were assayed on Vero cells. The results shown are the mean titers of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IRF-3-deficient BMDCs have a defect in IFN-β induction compared to WT control cells.
IFN-β plays a critical role in inducing an antiviral state and controlling viral infection (18). A deficit or a delay in IFN-β induction would likely allow increased viral replication, as seen in IRF-3−/− BMDCs. In order to examine this question, IFN-β protein levels were determined by ELISA in control and IRF-3−/− BMDCs after infection with HSV-1 (Fig. 4). BMDCs were infected at an MOI of 5 to ensure uniform infection and minimize the contribution of bystander IFN. Even at this high MOI, IRF-3−/− BMDCs yielded a statistically significant increase in HSV-1 titer at 12 and 24 h postinfection. Examining IFN-β protein, IRF-3−/− BMDCs exhibited decreased and delayed IFN-β production relative to WT control BMDCs. WT BMDCs produced detectable levels of IFN-β as early as 6 h postinfection and continued to escalate at 9 and 12 h postinfection. In contrast, IRF-3-deficient BMDCs only produced measurable levels at 12 h postinfection, suggesting a defect in the initiation of IFN-β production. The IRF-3−/− BMDCs were, however, capable of producing IFN-β late in the experimental infection, thereby potentially allowing control of viral replication at these later times.
FIG. 4.
IFN-β secretion by infected BMDCs. Primary BMDCs were infected with WT HSV-1 at an MOI of 5. At indicated times postinfection, cells and supernatants were harvested. Cells were removed by low-speed centrifugation, and supernatants were assayed for IFN-β by ELISA. The results in the upper panel are mean totals from three independent experiments. Cells and supernatants were also assayed for virus titers at 6, 12, and 24 h postinfection, and virus titers were assayed on Vero cells. The results in the lower panel shown are the mean titers of three independent experiments. *, P < 0.05.
IFNαR-blocking antibody augments viral growth in WT control and IRF-3-deficient BMDCs.
To demonstrate that production of type I IFN was a primary defect, WT and IRF-3−/− BMDCs were infected and then treated with antibodies that block the IFNαR or with control IgG1 antibody (Fig. 5 and data not shown). We postulated that if the restriction of HSV-1 replication in this system was dependent on type I IFN induction, then WT and IRF-3-deficient BMDCs should yield similar virus titers in the presence of the blocking antibody. Control IgG1 had no impact on viral replication in either cell type (data not shown). In contrast, the addition of IFNαR blocking antibodies allowed both WT and IRF-3−/− BMDCs to produce higher yields of HSV-1 such that viral growth curves for these two disparate cell types were similar under these conditions (Fig. 5). It was also notable that untreated IRF-3−/− BMDC cultures yielded titers similar to those of antibody-treated BMDCs at 24 h postinfection. In contrast, by 48 h postinfection, antibody-treated IRF-3−/− BMDCs yielded 10-fold more virus than untreated cultures. Together, these data demonstrate that the type I IFN cascade is responsible for controlling HSV-1 replication in WT BMDCs and that, at late time points, IRF-3-deficient BMDCs can exert partial type I IFN-dependent control of HSV-1 replication.
FIG. 5.
IFNαR blockade in BMDCs. Primary BMDCs were infected with WT HSV-1 at an MOI of 0.01. After infection, BMDCs were plated in media containing 5 μg of IFNαR blocking antibody (MAR1-5A3)/ml for the duration of the experiment. At the indicated times postinfection, cells and supernatants were harvested, and virus titers were assayed on Vero cells. The results shown are the mean titers of three independent experiments. *, P <, 0.05; **, P < 0.01.
IFN induction from WT BMDCs fails to restore control of HSV-1 replication to IRF-3-deficient BMDCs in vitro.
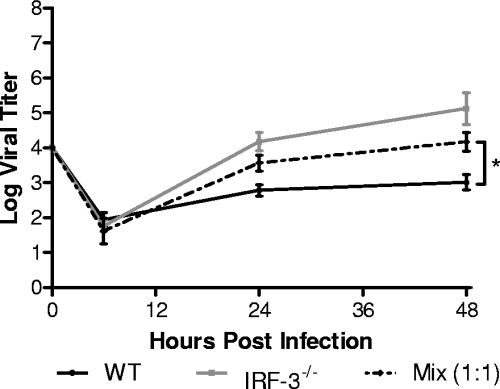
The preceding data suggested that IFN induction was defective in IRF-3-deficient BMDCs but that these cells were still capable of controlling viral infection once the type I IFN cascade had been initiated. The question arose, therefore, if the initial IFN induction and synthesis were restored, could IRF-3−/− BMDCs limit HSV-1 replication to levels seen in WT control cells? We therefore investigated whether bystander IFN, produced by WT cells, could restore control of viral replication to IRF-3−/− BMDCs by mixing them in culture at a 1:1 ratio. The mixed cell population was then infected with HSV-1 at an MOI of 0.01 and viral replication measured (Fig. 6). Viral growth kinetics under these conditions were intermediate between those observed in WT (low viral growth) and IRF-3−/− (high viral growth) BMDCs. At 48 h postinfection, the mixed BMDC population gave a 10-fold increase in viral yield over WT cells alone and a 10-fold decrease in viral yield over IRF-3−/− BMDCs alone. The results show that IRF-3−/− BMDCs are incapable of controlling viral replication even in the presence of bystander IFN induced by viral infection of WT cells. Another possibility, although less likely, is that the presence of IRF-3−/− BMDCs resulted in a reduced total type I IFN concentration, thereby permitting increased replication in WT BMDCs. In either case, HSV-1 replication in IRF-3-deficient BMDCs was not limited in the context of bystander cell-produced IFN.
FIG. 6.
In vitro replication after mixing BMDC populations. Primary WT and IRF-3−/− BMDCs were mixed at a 1:1 ratio and infected at an MOI of 0.01. At the indicated times postinfection, cells and supernatants were harvested, and virus titers were assayed on Vero cells. The results shown are the mean titers of three independent experiments. *, P < 0.05.
IRF-3-deficient BMDCs primed with IFN partially restore control of HSV-1 replication.
The results from the cell mixing experiments suggested that IRF-3−/− BMDCs were unable to respond fully to IFN production by WT cells. However, the ability to generate a delayed IFN-β response coupled with the IFN-dependent decrease in virus titers at late time points suggested that IRF-3−/− BMDCs were capable of inducing the type I IFN cascade, but with low efficiency. One possible model is that cells lacking IRF-3 are inherently slowed in their response to IFN and need additional time to properly prime in order to fully control HSV-1 replication in vitro. To test this, WT and IRF-3−/− BMDCs were pretreated overnight with IFN-β and challenged with HSV-1, and viral yields were measured (Fig. 7). IFN pretreatment of IRF-3−/− BMDCs significantly decreased HSV-1 replication compared to untreated IRF-3−/− cells with a >100-fold decrease in virus titers at 48 h postinfection. Titers observed were comparable to those in untreated WT control BMDCs. However, pretreatment of WT control cells resulted in further decreases in viral replication, to levels at, or below, the level of detection. These results together suggest that IRF-3-deficient BMDCs were capable of strongly responding to IFN, but the overall immune response in controlling HSV-1 replication was still defective compared to WT control cells.
FIG. 7.
In vitro replication after IFN-β pretreatment of BMDCs. Primary WT and IRF-3−/− BMDCs were pretreated with 100 U of mouse IFN-β/ml for 16 h. Cells were then infected with WT HSV-1 at an MOI of 0.01. At the indicated times postinfection, cells and supernatants were harvested, and virus titers were assayed on Vero cells. The results shown are the mean titers of three independent experiments. *, P < 0.05; **, P < 0.01.
DISCUSSION
Despite mice or cells lacking IFN receptors being significantly more susceptible to viral infection (22, 32), loss of IRF-3 and IRF-7 had surprisingly little impact on HSV-1 replication in vitro (15). Several groups have suggested the lack of a growth phenotype in IRF3−/− cells may be due to HSV-1 maintaining strict control over IRF-3-dependent pathways through various viral genes, including ICP0, ICP27, ICP34.5, and vhs, thereby neutralizing the impact of IRF-3-mediated pathways (6, 10, 23, 26-28, 30, 35, 42). In the present study, we have demonstrated that HSV-1 replication was controlled in an IRF-3-dependent manner in two types of immune cells. This control was dependent on type I IFN and STAT-1 signaling with a primary defect in IFN production in IRF-3−/− cells. Even in the presence of exogenously supplied IFN, however, HSV-1 replication was only partially controlled in IRF-3−/− BMDCs. Overall, the data presented here provide evidence that IRF-3-mediated pathways have a significant impact on HSV-1 replication in certain cell types.
Previous studies examining HSV-1 and IRF-3−/− used highly permissive MEFs, whereas in the present study dendritic cells and macrophages were chosen. Given the roles of dendritic cells and macrophages as sentinels of the immune system capable of controlling viral infection in vivo, it is likely that these cells induced a more vigorous immune response and were thereby less permissive to infection than MEFs (2, 5, 17, 43, 44). In the case of HSV, a virus with multiple mechanisms to subvert IFN responses, loss of IRF-3 can only manifest with increased virus titers in cells that respond strongly to IFN. This idea is supported by studies with WNV (12). Only at late time points do IRF-3−/− MEFs support nearly a 4-log increase in WNV titers compared to control cells, suggesting robust IRF-3-dependent responses in control MEFs late in infection. In contrast, examination of WNV in BMM cultures demonstrated increased viral replication in IRF-3−/− BMM immediately, as early as 24 h postinfection, and this continued through 72 h postinfection (7). A similar difference in viral replication between MEFs and immune cells was reported with mouse norovirus in the context of STAT1 deficiency (50). These data support the hypothesis that immune cells have a more vigorous antiviral response than MEFs and loss of IRF-3 on viral replication may be more accurately measured in more restrictive immune cell types. This hypothesis is especially relevant to HSV-1, which compared to WNV and mouse norovirus, has more genes for IFN regulation, produces less double-stranded RNA, and exhibits less sensitivity to type I IFN.
Not surprisingly, in the absence of IRF-3, BMDCs and BMM were unable to efficiently control HSV-1 replication (Fig. 1 and 2). Although the IFN receptors are intact in these cells, the early recognition signaling likely cannot proceed efficiently without IRF-3, leading to a delay in the type I IFN cascade. Later, once secondary rounds of infection have begun, alternate recognition pathways, most likely mediated through IRF-7, can lead to the induction of type I IFN. This recognition by a secondary pathway is supported by the observed late production of IFN-β (Fig. 4) and the concomitant decreased viral replication in IRF-3−/− BMDCs at late time points (Fig. 5). Although IRF-3 is constitutively expressed in both BMDCs and BMM, basal expression of IRF-7 varies according to cell type (33). Plasmacytoid dendritic cells (pDCs) constitutively express IRF-7, while IRF-7 expression is reduced in conventional BMDCs compared to IRF-3. BMM exhibited basal expression of both IRF-3 and IRF-7, potentially explaining increased replication in IRF-7−/− BMM but not in IRF-7−/− BMDCs (8). These studies observed a parallel trend in WNV replication in IRF-7−/− BMM and BMDCs, as seen here with HSV-1. However, in both cell types, the presence of IRF-7 cannot compensate for the loss of IRF-3-mediated pathways. These results suggest that IRF-3-mediated pathways provide the major pathway of control of HSV-1 replication.
Together, our results demonstrate that the early recognition response through IRF-3-mediated pathways controls HSV-1 replication in BMDCs and leads to the following model (Fig. 8). Upon virus entry, an as-yet-undetermined sensor recognizes HSV-1 and triggers a signaling cascade that activates IRF-3. IRF-3 activation leads to production of IRF-3-dependent gene products, type I IFN, and an ensuing type I IFN cascade, resulting in control of the HSV-1 infection. In the absence of the type I IFN cascade, achieved by knockout (Fig. 3) or receptor blockade (Fig. 5), BMDCs are unable to control viral replication. Similarly, ablating IRF-3 and the early recognition response results in increased viral replication due to delayed and reduced IFN-β production (Fig. 1, 2, and 4). Exogenous IFN provided by bystander cells (Fig. 6) or pretreatment (Fig. 7) partially restores control of HSV-1 replication in IRF-3−/− BMDCs, and yet these cells remain defective in their control of HSV-1 replication compared to treated WT cells.
FIG. 8.
Model for continued defect in IRF-3-deficient BMDCs. Postattachment, HSV-1 infection is recognized through an unknown sensor mechanism that leads to activation of IRF-3. The early recognition pathway mediates the production of type I IFN- and IRF-3-dependent ISGs, leading to the control of HSV-1 replication via the type I IFN cascade. However, pretreatment with IFN does not restore HSV-1 replication in IRF-3−/− BMDCs to WT levels. The continued defect is potentially due to three, nonexclusive mechanisms outlined in white squares: defective IFN amplification, defective antiviral trigger signaling, and IRF-3-dependent gene synergy with the antiviral response. One or more of these mechanisms leads to continued defect in the control of HSV-1 replication in IRF-3−/− BMDCs compared to WT BMDCs after IFN treatment.
Several non-mutually exclusive possibilities exist to explain this persistent defect in the ability of IRF-3−/− BMDCs to control HSV-1 replication (Fig. 8). One possibility is a defective autocrine and paracrine IFN amplification response. While WT BMDCs quickly respond to IFN through STAT-1 and IRF-3 signaling pathways, IRF-3−/− BMDCs can only respond through STAT-1-dependent, but IRF-3-independent pathways. The absence of IRF-3 thereby severely decreases or ablates the expression of several gene products, including IFN-β, IFN-α4, and IFN-α5 (1), resulting in less robust IFN signaling. A second possibility is that IRF-3-dependent ISGs synergize with type I IFN receptor-dependent ISGs and control HSV-1 replication but fail to be produced robustly in IRF-3−/− BMDCs. A third possibility is that virus recognition may be required to augment the ongoing immune response. IFN primed IRF-3−/− BMDCs may produce IFN effectors, but a lack of viral recognition signaling results in a delayed effector response from ISGs. IRF-3−/− BMDCs may therefore require HSV-1 recognition signaling through a secondary pathway before fully committing to a complete IFN effector response, and this delay could result in the observed increased viral replication compared to WT controls.
Together, these data demonstrate that immune cells lacking IRF-3 are inherently defective in the control of HSV infection. These data, however, conflict with previously published in vivo data following intravenous (i.v.) infection (15). A possible explanation is that after i.v. infection, IFN was being produced by pDCs. pDCs, typically found in the lymph nodes away from the site of infection, are a major producer of type I IFN, and they rely on TLR-9 and IRF-7 pathways to induce IFN in response to HSV-1 (4, 20, 40). After i.v. infection, therefore, pDC production of type I IFN likely overcomes the IFN deficit and thereby is able to control HSV-1 replication in the absence of IRF-3. Previous in vitro studies in MEFs suggested a role for HSV-1 gene components in interfering with and neutralizing the activity of IRF-3 (10, 23, 26-28, 30, 36, 42, 48). In the cell types used in the present study, heightened immune responses likely reduced the efficacy of one or more viral immunoregulatory components or presented too great of a challenge for the viral activities to counter. Therefore, the efficacy of HSV-1 genes in antagonism of IRF-3 likely depends on the overall capacity of the infected cell to mount an immune response to the incoming virus. Ongoing experiments in our laboratory seek to determine the precise molecules responsible for HSV-1 recognition. As mentioned previously, several candidates in the early recognition pathways have been implicated (21, 35, 37, 55), and cells lacking these components are currently being tested for their ability to control viral replication in BMDCs. Furthermore, in vivo studies in IRF-3 deficient animals are currently under way in order to examine HSV-1 replication and pathogenesis in peripheral and neuronal tissues.
Acknowledgments
This study was supported by NIH grants to D.A.L. (EY 09083) and the Department of Ophthalmology and Visual Sciences (P30EY02687) from the National Eye Institute. V.D.M. was also supported by an NIH Research Training Grant in the Visual Sciences (T32 EY013360-09). Support from Research to Prevent Blindness to the Department and a Senior Scientific Investigator Award to D.A.L. are gratefully acknowledged.
IRF-3−/−, IRF-7−/− mice were generously provided by T. Tanaguchi, and AG129 mice were generously provided by R. Zinkernagel. We especially thank Marco Colonna and Steve McCartney for providing granulocyte-macrophage colony-stimulating factor and for helpful insight into BMDC cultures. We also thank M. Diamond for helpful feedback and provision of mice and members of the Leib lab for helpful discussions.
Footnotes
Published ahead of print on 16 September 2009.
REFERENCES
- 1.Andersen, J., S. VanScoy, T. F. Cheng, D. Gomez, and N. C. Reich. 2008. IRF-3-dependent and augmented target genes during viral infection. Genes Immun. 9:168-175. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, D., S. Mrzyk, N. van Rooijen, K. P. Steuhl, and A. Heiligenhaus. 2000. Macrophage-depletion influences the course of murine HSV-1 keratitis. Curr. Eye Res. 20:45-53. [PubMed] [Google Scholar]
- 3.Bowie, A. G., and L. Unterholzner. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 8:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, H., T. M. Tumpey, H. F. Staats, N. van Rooijen, J. E. Oakes, and R. N. Lausch. 2000. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Investig. Ophthalmol. Vis. Sci. 41:1402-1409. [PubMed] [Google Scholar]
- 6.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daffis, S., M. A. Samuel, B. C. Keller, M. Gale, Jr., and M. S. Diamond. 2007. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 3:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daffis, S., M. A. Samuel, M. S. Suthar, B. C. Keller, M. Gale, Jr., and M. S. Diamond. 2008. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J. Virol. 82:8465-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529. [DOI] [PubMed] [Google Scholar]
- 10.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredericksen, B. L., M. Smith, M. G. Katze, P. Y. Shi, and M. Gale, Jr. 2004. The host response to West Nile Virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 78:7737-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 15.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772. [DOI] [PubMed] [Google Scholar]
- 16.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 17.Kassim, S. H., N. K. Rajasagi, X. Zhao, R. Chervenak, and S. R. Jennings. 2006. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J. Virol. 80:3985-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 19.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 20.Krug, A. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433. [DOI] [PubMed] [Google Scholar]
- 21.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 22.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto, M., S. Kikkawa, M. Kohase, K. Miyake, and T. Seya. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293:1364-1369. [DOI] [PubMed] [Google Scholar]
- 25.McCartney, S. A., L. B. Thackray, L. Gitlin, S. Gilfillan, H. W. Virgin, and M. Colonna. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog. 4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melchjorsen, J., J. Siren, I. Julkunen, S. R. Paludan, and S. Matikainen. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-κB and IRF-3. J. Gen. Virol. 87:1099-1108. [DOI] [PubMed] [Google Scholar]
- 27.Melroe, G. T., N. A. DeLuca, and D. M. Knipe. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411-8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melroe, G. T., L. Silva, P. A. Schaffer, and D. M. Knipe. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360:305-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meraz, M. A., J. M. White, K. C. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riley, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 30.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myong, S., S. Cui, P. V. Cornish, A. Kirchhofer, M. U. Gack, J. U. Jung, K. P. Hopfner, and T. Ha. 2009. Cytosolic viral sensor RIG-I is a 5′-triphosphate-dependent translocase on double-stranded RNA. Science 323:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasieka, T. J., B. Lu, S. D. Crosby, K. M. Wylie, L. A. Morrison, D. E. Alexander, V. D. Menachery, and D. A. Leib. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 82:5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash, A., E. Smith, C. K. Lee, and D. E. Levy. 2005. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J. Biol. Chem. 280:18651-18657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rader, K. A., C. E. Ackland-Berglund, J. K. Miller, J. S. Pepose, and D. A. Leib. 1993. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 74(Pt. 9):1859-1869. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen, S. B., S. B. Jensen, C. Nielsen, E. Quartin, H. Kato, Z. J. Chen, R. H. Silverman, S. Akira, and S. R. Paludan. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 90:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saira, K., Y. Zhou, and C. Jones. 2007. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces degradation of interferon response factor 3 and, consequently, inhibits beta interferon promoter activity. J. Virol. 81:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato, A., M. M. Linehan, and A. Iwasaki. 2006. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. USA 103:17343-17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, M. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan, K. C., K. S. Lai, G. P. Dunn, A. T. Bruce, M. S. Diamond, J. D. Heutel, C. Dungo-Arthur, J. A. Carrero, J. M. White, P. J. Hertzog, and R. D. Schreiber. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res. 26:804-819. [DOI] [PubMed] [Google Scholar]
- 40.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 41.Smith, K. O. 1964. Relationship between the envelope and the infectivity of herpes simplex virus. Proc. Soc. Exp. Biol. Med. 115:814-816. [DOI] [PubMed] [Google Scholar]
- 42.Sobol, P. T., and K. L. Mossman. 2006. ICP0 prevents RNase L-independent rRNA cleavage in herpes simplex virus type 1-infected cells. J. Virol. 80:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprecher, E., and Y. Becker. 1987. Herpes simplex virus type 1 pathogenicity in footpad and ear skin of mice depends on Langerhans cell density, mouse genetics, and virus strain. J. Virol. 61:2515-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprecher, E., and Y. Becker. 1986. Skin Langerhans cells play an essential role in the defense against HSV-1 infection. Arch. Virol. 91:341-349. [DOI] [PubMed] [Google Scholar]
- 45.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270:155-167. [DOI] [PubMed] [Google Scholar]
- 47.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitley, R. J., (ed.). 1996. Herpes simplex viruses. Lippincott-Raven Publishers, Philadelphia, PA.
- 50.Wobus, C. E., S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964-973. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 53.Yoneyama, M. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5:730. [DOI] [PubMed] [Google Scholar]
- 54.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, S.-Y., E. Jouanguy, S. Ugolini, A. Smahi, G. Elain, P. Romero, D. Segal, V. Sancho-Shimizu, L. Lorenzo, A. Puel, C. Picard, A. Chapgier, S. Plancoulaine, M. Titeux, C. Cognet, H. von Bernuth, C.-L. Ku, A. Casrouge, X.-X. Zhang, L. Barreiro, J. Leonard, C. Hamilton, P. Lebon, B. Heron, L. Vallee, L. Quintana-Murci, A. Hovnanian, F. Rozenberg, E. Vivier, F. Geissmann, M. Tardieu, L. Abel, and J.-L. Casanova. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522-1527. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, X., R. Goncalves, and D. M. Mosser. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 14:14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]