Abstract
The maintenance of epithelial cell function requires the establishment and continuous renewal of differentiated apical and basolateral plasma membrane domains with distinct lipid and protein compositions. Newly synthesized proteins destined for either surface domain are processed along the biosynthetic pathway and segregated into distinct subsets of transport carriers emanating from the trans-Golgi network. Recent studies have illuminated additional complexities in the subsequent delivery of these proteins to the cell surface. In particular, multiple routes to the apical and basolateral cell surfaces have been uncovered, and many of these involve indirect passage through endocytic compartments. This review summarizes our current understanding of these routes and discusses open issues that remain to be clarified.
Keywords: apical, AP-1B, basolateral, biosynthetic delivery, endosome, kidney, lipid raft, MDCK, polarized traffic
Virtually all newly synthesized glycoproteins destined for endocytic organelles or the cell surface are cotranslationally inserted in the endoplasmic reticulum membrane, where addition of N-glycans occurs. Properly folded and assembled proteins are exported to the Golgi complex where, in addition to other posttranslational modifications, N-linked glycans are remodeled and O-linked glycosylation is initiated and extended. The distal compartment of the Golgi, termed the trans-Golgi network (TGN) has long been considered to be the primary sorting station for newly synthesized proteins destined for delivery to endosomes, lysosomes, secretory granules and the cell surface.
Protein sorting in polarized cells poses an additional constraint in that newly synthesized cargo must be eventually delivered to the appropriate subdomain of the plasma membrane. The conventional model for polarized biosynthetic trafficking has been that apical and basolateral proteins are sorted in the TGN into post-Golgi vesicles that fuse directly with the plasma membrane. Evidence for this was supported by early live cell imaging studies in which the apical and basolateral cargo proteins were observed to be sorted into distinct carriers that emanated from the TGN and were delivered to the plasma membrane without apparently detouring through endosomes (1,2). In the past several years, this relatively simple model has been challenged by the observation that biosynthetic cargo traverses intermediate compartments en route from the TGN to the plasma membrane. Moreover, recent studies implicating a role for epithelial-specific adaptor protein (AP) complexes and for endocytic compartments in biosynthetic membrane traffic suggest that key differences exist in post-Golgi sorting mechanisms between polarized and non-polarized cells. Additional distinctions in the development and organization of plasma membrane domains in cells grown as planar monolayers versus those grown in 3D cultures are also beginning to emerge. These observations have led to the speculation that sorting of some proteins is not confined to the Golgi complex but instead may occur at multiple locations along the biosynthetic pathway. These studies and their impact on our current appreciation of biosynthetic sorting mechanisms are discussed in more detail below.
Post-Golgi Sorting of Biosynthetic Cargo in Non-polarized Epithelial Cells
Accumulated data over the past few decades has cemented the idea that biosynthetic and endocytic pathways intersect in non-polarized cells. Endocytosed toxins such as cholera are known to undergo retrograde transport, albeit inefficiently, as far back as the endoplasmic reticulum, from where they enter the cytosol to exert their toxic effects (3). Conversely, it has been demonstrated that some biosynthetic cargos access endocytic compartments before surface delivery. For example, newly synthesized transferrin receptor (TfR) and asialoglycoprotein receptor H1 were shown to pass through endosomes en route from the TGN to the plasma membrane in HEp.2 and Madin-Darby canine kidney (MDCK) cells, respectively (4–6). Similarly, Lock et al. observed using live cell imaging that E-cadherin traffics through Rab11-positive recycling endosomes in HeLa and MDCK cells (7). Not all newly synthesized proteins take a route through recycling endosomes, as GPI-anchored proteins are excluded from this pathway (4). In the most comprehensive of these studies, Ang et al. investigated the significance and extent of endosomal transit of the basolateral marker vesicular stomatitis virus glycoprotein (VSV G) (8). In these experiments, YFP-tagged VSV G was staged in the TGN of MDCK cells stably expressing the human TfR, and the cells were imaged after warming in the presence of fluorescently labeled human transferrin (Tf). Although initially segregated, a fraction of YFP-VSV G released from the TGN rapidly appeared in Tf-positive structures that presumably represent recycling endosomes. These findings were supported by immunoisolation experiments demonstrating the recovery of labeled Tf in YFP-VSV G containing compartments (8). Furthermore, delivery of VSV G to the cell surface was dramatically inhibited when recycling endosomes containing horseradish-peroxidase (HRP) conjugated to Tf were functionally inactivated using diaminobenzidine and H2O2, suggesting that passage through this compartment is a required step in surface delivery of VSV G (8). Similarly, in a recent study Cancino et al. found that basolateral cargos VSV G and TfR moved from the TGN into recycling endosomes during biosynthetic delivery in partially polarized Fischer rat thyroid cells that were grown on coverslips and analyzed 1 day after reaching confluency (9).
Biosynthetic Sorting Pathways in Polarized Epithelial Cells
More recent studies have extended these findings to polarized MDCK cells cultured on permeable supports for at least 3–4 days after reaching confluency. Based on these studies, it is increasingly clear that multiple pathways exist from the Golgi complex to the apical and basolateral cell surfaces. In addition, polarized cells extend a single primary cilium as a third membrane compartment. Experiments in these cells present significant challenges, in part because the endocytic pathway is more complex in polarized versus non-polarized cells. Whereas nonpolarized cells have a uniform population of early endosomes, polarized cells have distinct apical and basolateral early endosomes (BEE) (10). Moreover, nonpolarized cells contain a single, juxtanuclear recycling compartment that is identified morphologically by the presence of TfR and the small G protein Rab11. In contrast, polarized cells contain at least two functionally distinct recycling endosomes. TfR in polarized cells passes through the common recycling endosome (CRE), a compartment that receives cargo internalized from apical and BEE and is Rab11-negative (11–13). Rab11 in polarized cells is localized instead to the apical recycling endosome (ARE), a collection of subapical tubular membranes, which receives cargo transcytosed from the basolateral surface as well as a subset of apically recycling proteins (11,14). There is some debate whether the ARE represents a discrete endosomal compartment or is alternatively a subdomain of the CRE (15); in any event, Rab11 and TfR are clearly segregated in polarized MDCK cells (Figure 1). The localization of Rab10 is also different in polarized versus non-polarized MDCK cells: whereas Rab10 in non-polarized cells colocalizes with Golgi/TGN markers giantin and furin (16), in polarized MDCK cells Rab10 colocalizes with internalized Tf in CRE (but not with IgA in ARE) (17). The sections below highlight recent studies aimed at addressing the itinerary of biosynthetic cargos destined for the different plasma membrane domains in polarized MDCK cells.
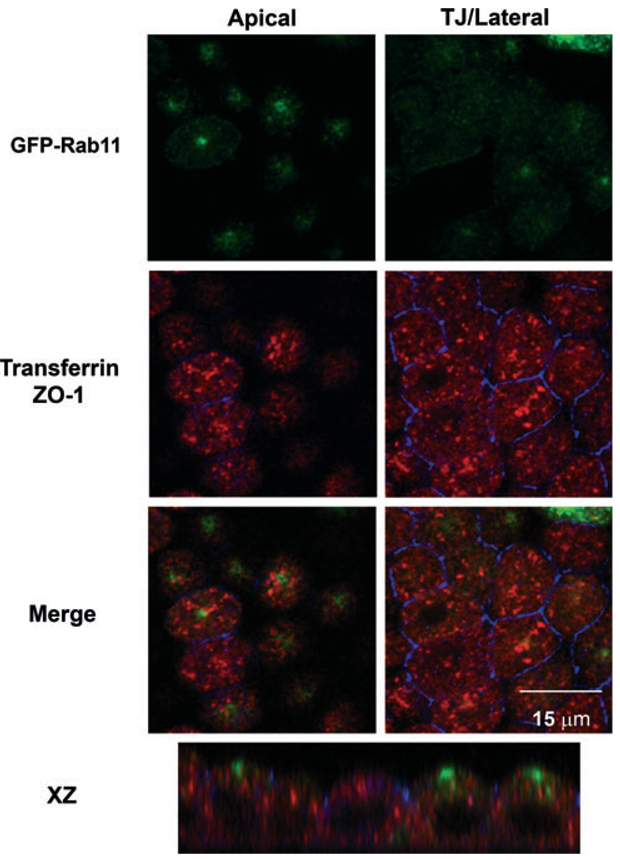
Figure 1. Distribution of recycling endosomes in polarized MDCK cells.
Filter-grown MDCK cells stably expressing GFP-Rab11 (green) were incubated with basolaterally added canine Tf for 30 min, then fixed and processed for indirect immunofluorescence with antibodies against canine Tf (in red) and the tight junction marker ZO-1 (in blue). Individual and merged confocal sections taken just beneath the apical surface and at the level of the tight junction/lateral border are shown. An xz section is shown in the bottom panel. Note the segregation of Rab11 and transferrin, which mark the apical recycling and common recycling endosomes, respectively.
Biosynthetic Sorting of Basolateral Cargo
Similar to results in non-polarized cells, surface delivery of many basolateral proteins involves intermediate transit through endocytic compartments (Figure 2). However, for some cargos the routes to the surface apparently changes with the reorganization of the endosomal system as cells polarize. This complication has led to some as yet unresolved controversies in the field.
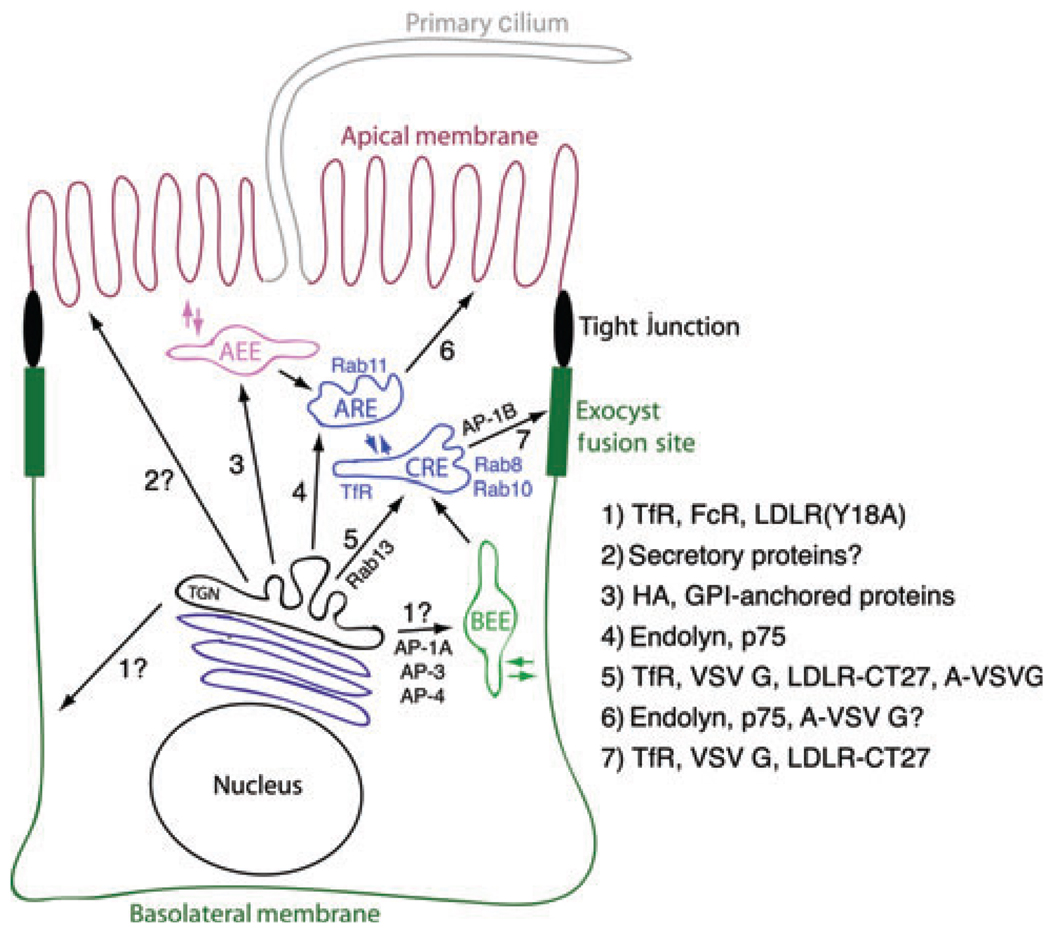
Figure 2. Biosynthetic trafficking routes in polarized kidney cells.
The model depicts the multiple pathways used by biosynthetic cargo proteins to reach their final destinations from the TGN. Arrows indicate the paths from one organelle to the next. Specific cargo proteins that are thought to utilize each route are noted on the right. Direct routes to the apical and basolateral surface as well as a pathway via the basolateral early endosomes (BEE; pathways 1 and 2) are predicted but have not been experimentally verified. AEE, apical early endosome; ARE, apical recycling endosome; CRE, common recycling endosome. Trafficking pathways to the primary cilium are not well defined and are omitted in this model, as are the trafficking routes used by proteins not destined for the plasma membrane. Refer the text for additional details.
Basolateral sorting information typically consists of either tyrosine-based (YxxØ, FxNPxY) or dileucine-based short peptide motifs encoded in the cytoplasmic tails of transmembrane receptors. Fc receptors contain dileucine-based basolateral sorting signals, TfR and VSV G contain YxxØ motifs, and the low-density lipoprotein receptor (LDLR) contains both YxxØ and FxNPxY motifs. Frequently, these peptide motifs are recognized by heterotetrameric cytosolic clathrin AP complexes, which interact directly with the sorting signals and trigger cargo incorporation into nascent vesicles. Columnar epithelial cells express five different classes of AP complexes: AP-1A, AP-1B, AP-2, AP-3 and AP-4. AP-1A, AP-2, AP-3 and AP-4 are ubiquitously expressed. AP-2 facilitates endocytosis from the plasma membrane and AP-1A, AP-3 and AP-4 select cargos at the TGN or endosomes [reviewed in (18)]. In contrast, AP-1B is epithelial cell-specific, and not expressed in other polarized cells such as neurons or hepatocytes (19). AP-1A and AP-1B are closely homologous and share in common β and γ large subunits as well as the small σ1 subunit. The only difference is the incorporation of the medium subunits μ1A or μ1B (20). Despite this close homology, AP-1A and AP-1B form distinct vesicle populations have largely non-overlapping functions (20–22).
AP-1B is localized in CRE and is required for basolateral sorting of biosynthetic and endocytic cargos in polarized columnar epithelial cells (22,23). Biosynthetic cargos that are sorted via AP-1B travel from the TGN into CRE before delivery to the basolateral surface. This pathway is regulated by Rab13 and can be inhibited by overexpression of Rab13 mutant proteins (24). Cargos following this route include VSV G and a truncated version of the LDLR (LDLR-CT27) that contains only the FxNPxY motif in its cytoplasmic tail (12,25). Additionally, TfR utilizes this trafficking route under some conditions (see below). It should be noted, however, that in addition to interacting with μ1B/AP-1B, VSV G was also shown to interact with μ4/AP-4 and δ/AP-3 (25,26). Therefore, biosynthetic trafficking of VSV G may occur via alternative routes when CRE are not functional. For example, inactivation of the CRE localized v-SNARE cellubrevin/VAMP-3 leads to a dispersal of TfR localization and a loss of AP-1B staining in CRE, but has no effect on VSV G sorting to the basolateral membrane (25). The same study showed, however, that inactivation of VAMP-3 leads to apical missorting of LDLR-CT27 in the biosynthetic and endocytic pathways, and endocytic missorting of TfR (25).
In CRE, cargos destined for the basolateral membrane are incorporated into AP-1B vesicles. These AP-1B vesicles associate with a vesicle-tethering complex, the exocyst, for tethering to the basolateral membrane (22). Furthermore, AP-1B and the v-SNARE VAMP-3 colocalize in clathrin-coated vesicles as shown by electron microscopy (25). VAMP-3 facilitates vesicle fusion with the plasma membrane by forming SNARE pairs with the basolaterally localized t-SNARE syntaxin 4 (25). The trafficking pathway involving AP-1B is regulated by the small G proteins Cdc42, RalA and Rab8 (16,27), and involves the actin motor myosin VI (28). Another regulator of the AP-1B pathway might be Rab10. In polarized cells overexpression of the activated mutant of Rab10 and Rab10Q68L, leads to apical missorting of VSV G (16). In partially polarized cells grown on coverslips proteins with YSTI or FTLS sorting signals are also missorted to the apical membrane in the presence of Rab10Q68L (16). At present it is not known whether the cargos analyzed in partially polarized cells travel directly to the basolateral membrane or whether they transit through CRE.
Indirect trafficking of proteins destined for the basolateral membrane was initially observed in an early study examining the biosynthetic route of polymeric immunoglobulin receptor (pIgR) expressed in filter-grown MDCK cells. This study concluded that pIgR passes through ARE/CRE and BEE en route to the basolateral surface (29). This finding was based on the presence of newly synthesized protein in compartments accessible to apically internalized HRP-conjugated wheat germ agglutinin (WGA) or to basolaterally internalized soluble HRP. Newly synthesized TfR was also found to access endocytic compartments including CRE before basolateral delivery in these studies (29). This result was challenged by more recent experiments examining TfR delivery pathways in MDCK cells that were knocked down for μ1B of AP-1B, which concluded that newly synthesized TfR traffics directly to the basolateral surface in polarized cells (12). In partially polarized epithelial cells that were analyzed about 1 day after reaching confluency, however, μ1B knockdown leads to apical missorting of TfR and injection of anti-AP-1B antibodies disrupted surface delivery of TfR indicating a transit of TfR through recycling endosomes (9,12). Perhaps TfR can use two different pathways to the basolateral membrane; acute inhibition of one may completely reroute the receptor into the remaining pathway. In contrast to TfR, VSV G traffics through CRE independent of the polarity state of the cells (8,9,12).
Some basolateral proteins avoid the CRE and may traffic instead through BEE. These cargos possibly include pIgR, TfR and a mutant version of LDLR in which the FxNPxY signal is inactivated [LDLR(Y18A)]. The remaining sorting signal in LDLR(Y18A) interacts with μ2, μ3 and μ4 (25). Moreover, the YxxØ motif in the cytoplasmic tail of TfR was shown to interact with the μ subunits of all known AP complexes including AP-1A, AP-1B and AP-4 (25,30), and pIgR interacts with AP-1A as well (25,30). In agreement with this observation that basolateral cargos can interact with multiple clathrin adaptors, a recent study suggests that clathrin plays a broad role in sorting of cargo from the TGN directly to the basolateral membrane (31). Here, direct sorting was analyzed using video microscopy in non-polarized MDCK cells after knockdown or acute crosslinking of clathrin chains. However, due to the rapid entry of cargos released from the TGN into endosomes (within minutes), this study could not formally exclude a sorting through BEE or CRE. Therefore, basolateral cargo may be selected by clathrin adaptors such as AP-1A, AP-3 or AP-4 at the TGN and sorted into BEE from which they may cycle to the basolateral membrane. In agreement with this hypothesis, AP-4 was suggested to play a role in basolateral sorting of LDLR, TfR and mannose-6-phosphate receptor (32). Cargo with tyrosine-based sorting signals that are not efficiently selected by TGN adaptors may be delivered instead into CRE.
To date, sorting via AP-1B is the only pathway known from CRE to the basolateral surface. In contrast, there seem to be many pathways from the TGN to the basolateral surface that do not involve CRE. In addition to pathways from the TGN involving conventional adaptor complexes, non-conventional adaptors and sorting signals also exist. For example, PDZ-interacting domains were recently shown to play a role in sorting from the TGN to the basolateral membrane (33). Moreover, Naked2 is necessary for basolateral sorting of TGFα to the basolateral membrane in specialized carriers (34,35).
Finally the sorting of E-cadherin to the basolateral membrane seems to depend on complex interactions of its cytoplasmic tail with multiple binding partners and passage through diverse endosomal population. In a recent study, newly synthesized E-cadherin was shown by video microscopy and colocalization experiments to travel into TfR-positive CRE in non-polarized and polarized MDCK cells (36). Intriguingly, the same study showed that overexpression of dominant-negative Rab11 (Rab11S25N) resulted in apical missorting of E-cadherin indicating an involvement of ARE in basolateral sorting of E-cadherin (36). Exit of E-cadherin from the TGN was shown to depend on its direct interaction with ankyrin-G in partially polarized human bronchial epithelial cells (37). The same study also showed that ankyrin-G’s binding partner β-2-spectrin was involved in this process (37). Furthermore, E-cadherin also directly interacts with the phosphatidylinositol phosphate kinase PIPKIγ661 (38). Interestingly, PIPKIγ661 was shown to directly bind to AP-1B and functional PIPKIγ661 and AP-1B are both necessary for efficient trafficking of E-cadherin to the basolateral membrane in polarized epithelial cells (38). A provocative model for E-cadherin trafficking would be that ankyrin-G/β-2-spectrin mediates E-cadherin exit from the TGN, and that E-cadherin interacts with PIPKIγ661/AP-1B after entering CRE. Subsequent sorting to the basolateral membrane may then involve ARE.
Biosynthetic Sorting of Apical Cargo
In contrast to basolateral sorting signals, which are largely peptide-based cytosolic motifs, apical targeting information on proteins is extraordinarily diverse. Cytoplasmic tail sequences that target newly synthesized proteins to the apical surface have been reported for some proteins, including the multiligand receptor megalin and an increasing array of polytopic proteins (39–41). Both N- and O-linked glycans [e.g. on endolyn and the 75 kDa neurotrophin receptor (p75), respectively] have also been described as sorting motifs for a number of proteins [reviewed in (42)]. A role for galectin-3 has been proposed for the apical sorting of some proteins whose targeting is dependent on glycosylation (43). Additionally, apical sorting information has also been identified within the membrane spanning domains of some proteins, including influenza hemagglutinin (HA) and neuraminidase (44,45). Glycosylphosphatidylinositol (GPI)-linkages have also been demonstrated to play a role in apical sorting in some epithelial cell lines, and recent studies show that oligomerization of these proteins is a crucial step in their apical sorting (46). It has been suggested that the association of influenza HA and GPI-anchored proteins in glycolipid-enriched microdomains or ‘lipid rafts’ is important for their sorting. Association with lipid rafts is generally assumed based on the insolubility of a significant fraction of a given protein in cold Triton X-100 and/or its migration/flotation along with detergent-insoluble membranes in Optiprep gradients. Indeed there is a striking correspondence between protein association with detergent resistant membranes isolated using these approaches and lipid raft-dependent functions (47). However, lipid raft association of influenza HA can be uncoupled from apical delivery (48) and the presence of a GPI-anchor does not in and of itself impart apical targeting of a protein (49). Regardless of these uncertainties, it is becoming evident that proteins associated with lipid rafts are handled differently from ‘raft-independent’ proteins along the biosynthetic pathway (see below).
A challenge in the field has been to determine how proteins with distinct signals are selectively recognized and sorted into apically destined transport carriers along the biosynthetic pathway. Indeed, over the past several years, it has become clear that apical proteins are directed into different populations of transport carriers emanating from the TGN, and moreover, that these carriers take divergent routes to the apical membrane (Figure 2).
During export from the TGN, proteins are packaged into vesicular and tubular cargo carriers. Given the diversity in apical targeting signals, sorting and packaging of individual classes of proteins into transport carriers likely occurs via different mechanisms. Indeed, there is growing evidence that suggests the existence of multiple pathways from the TGN to the apical surface. To date, these pathways have been delineated primarily by comparing the transport of raft-associated versus raft-independent apical markers. Using various approaches, several groups have observed differentially regulated surface delivery of raft-associated and raft-independent apical proteins.
Using live cell imaging, Jacob et al. followed the TGN export of fluorescently tagged sucrase-isomaltase (SI), which associates with lipid rafts, and the nonraft-associated apical marker lactase-phlorizin hydrolase (LPH). These two proteins initially exited the TGN together in large carriers that subsequently gave rise to smaller vesicles that preferentially contained either of the two cargos (50). Subsequent studies demonstrated that post-TGN trafficking of SI but not LPH was actin-dependent, though both required microtubules for efficient surface delivery (51). Proteomic analysis of immunoisolated vesicles enriched in SI identified several proteins (annexin 2, the motor protein myosin I, and its regulator, α-kinase 1 or ALPK1) that were absent from vesicles containing the nonraft marker LPH (52). SiRNA-mediated knockdown of ALPKI in polarized Caco-2 cells inhibited apical delivery of SI, but surprisingly, the effect on LPH was not examined (52).
Similarly, differential effects of modulating cellular phosphatidylinositol levels and perturbing actin-dependent processes on apical surface transport kinetics of the nonraft-associated p75 neurotrophin receptor and the raft-associated marker influenza HA have been observed (53). In these studies, increased PI(4,5)P2 levels stimulated by overexpression of phosphatidylinositol 5-kinase were shown to stimulate HA delivery via a process dependent on Neuronal Wiskott-Aldrich syndrome protein (N-WASP) mediated activation of the Arp2/3 complex that appears to involve the formation of actin comets. These comets may facilitate the propulsion of HA-containing vesicles through the actin-rich terminal web that underlies the apical membrane (53). A recent report has also suggested a selective role for actin dynamics modulated by LIMK1 and cofilin in the TGN export of fluorescently tagged p75 but not a raft-associated apical protein or a basolateral marker (54). Thus, actin polymerization mediated by different effectors may drive the formation of distinct transport carriers enriched in different classes of apically destined proteins.
Recent studies have begun to examine the itinerary taken by newly synthesized apically destined proteins in polarized kidney cells. Whereas experiments in MDCK cells grown on plastic rather than permeable supports suggested that apical and basolateral proteins traverse a common endocytic compartment (presumably the CRE), differences between apical and basolateral delivery routes have begun to emerge from studies in polarized cells. Moreover, and consistent with the studies in non-polarized cells, it appears that apical proteins with distinct sorting signals take distinct routes to the cell surface. A consistent observation in these studies is the differential itinerary of raft-associated and raft-independent proteins. However a consensus regarding the compartments through which these two classes of proteins pass has yet to emerge. In part, this is due to inconsistencies in the definitions of endocytic compartments, limitations of the experimental approaches used and technical considerations such as the growth conditions of the cells.
Several studies have concluded that GPI-anchored proteins pass through endocytic compartments before arriving at the apical surface. Quantitative live cell imaging of YFP-GPI released from the TGN after a low-temperature block revealed that whereas TGN export was the rate limiting step in apical delivery, YFP-GPI accumulated transiently in an unidentified subapical compartment before reaching the apical membrane (55). Similarly, biochemical studies on another raft-associated protein, influenza HA, found that apical delivery of this protein was inhibited by inactivation of compartments accessible to apically internalized HRP-WGA (56). Under the internalization conditions used, HRP-WGA efficiently entered EEA1-positive early endosomes but was excluded from the Rab11-positive ARE (56). This pathway may be regulated by Rab14, as expression of Rab14 dominant-negative constructs in MDCK cells resulted in the mislocalization of apical raft-associated but not raft-independent proteins (57).
In contrast, proteins sorted by glycosylation-dependent signals appear to traverse the ARE before reaching the cell surface. Apical delivery of endolyn (56) and p75 (K. Cresawn and O. Weisz, unpublished observation) but not HA was inhibited upon expression of a dominant-negative mutant of myosin Vb, which disrupts cargo exit from the ARE (58). Moreover, a fraction of newly synthesized endolyn released from a low-temperature block colocalized with Rab11 in a subapical region (58). These studies are generally consistent with the observation by Polischuk et al. that p75 takes a divergent route to the apical surface from YFP-GPI (59). TGN to apical membrane trafficking of p75 in polarized (but not in non-polarized) MDCK cells was inhibited by disrupting the function of kinesin KIF5B, a plus-end directed microtubule motor (60), suggesting that carriers containing this protein are ferried on microtubule tracks. In contrast, apical delivery of a GPI-anchored protein was unaffected by these manipulations (60). Subsequent fusion of p75-containing carriers with the apical membrane is apparently dependent on syntaxin 3, as microinjection of antibodies or nocodazole-mediated redistribution of this protein disrupted p75 delivery to the apical surface (61). Syntaxin 3 also plays a role in apical delivery of the raft-associated cargo HA as the addition of anti-syntaxin 3 antibodies to semi-permeabilized MDCK cells inhibited apical delivery of HA (62). In addition, overexpression of syntaxin 3 inhibited apical transport of signal-less pIgR and GPI-anchored pIgR (63).
Other studies suggest that a pathway for newly synthesized proteins also exists from the CRE to the apical surface. In support of this, surface delivery of an apically targeted variant of VSV G is sensitive to expression of Rab13 mutants, which disrupt trafficking from the TGN to CRE (24). However, the apical sorting determinant in this protein has not been defined. It is assumed that this protein is sorted to the apical membrane due to the fusion with green fluorescence protein (GFP) which masks the basolateral sorting motif so that it is no longer able to interact with adaptor complexes (64). Similarly, an AP-1 motif is also preserved in an apically directed pIgR mutant that may also use this route (29). It remains to be shown whether endogenous apical proteins may also travel through CRE during biosynthetic delivery.
The route that a protein takes to the apical surface appears to be dependent on its targeting signal. For example, conversion of endolyn to a GPI-anchored protein preserves its apical targeting, but polarized surface delivery is no longer dependent on the N-glycosylation of the protein (65). Importantly, surface delivery of this mutant protein, unlike wild-type endolyn, is inhibited by inactivation of HRP-WGA-accessible compartments (56). Conversely, apical delivery of an HA mutant that does not associate with lipid rafts becomes unaffected by ablation of HRP-WGA-accessible compartments (56). Thus, altering the apical sorting information in a protein can change the route it takes to the surface. This brings up the interesting possibility that proteins with redundant apical targeting information might toggle between delivery pathways in response to physiological cues. However, direct evidence for this possibility is lacking.
In a variation on this theme, a recent report found that both raft-associated and raft-independent proteins associated sequentially with Rab4, Rab8 and Rab11 positive compartments, and were subsequently sequestered into distinct vesicle populations (66). These conclusions were based on colocalization of apical cargo with these Rab proteins after release from a 20°C block, as well as on effects of siRNA-mediated Rab knockdown on apical delivery kinetics. A significant limitation of these experiments is the use of stable cell lines for these immunofluorescence studies, which precludes the differentiation between newly synthesized versus recycling proteins. Nevertheless, the conclusions of this study are generally consistent with the idea that apical proteins segregate into distinct carriers based on their raft association properties.
While these studies have shed new light on apical trafficking routes, there are still many unresolved issues. For example, what pathways are used by polytopic and other proteins targeted by signals in their cytoplasmic tails? In hepatocytes at least, polytopic proteins are known to take a unique route to the apical canalicular membrane (67). Additionally, a direct pathway to the apical surface in MDCK cells has not yet been described. Admittedly, identification of such a pathway is a challenge, as the evidence relies primarily on negative data. A priori, one might expect that some secreted proteins might be targeted directly to the apical surface without intersecting endosomes, as fluid phase proteins internalized from the apical surface are largely routed to lysosomes or transcytosed to the basolateral domain (68).
Traffic to the Cilium
In addition to the apical and basolateral surface, polarized epithelial cells must selectively deliver proteins to the primary cilium. Mechanisms that direct membrane trafficking into the cilium are just starting to emerge. One requisite for cilia outgrowth seems to be an established polarity, and the knock down of many proteins such as FAPP2 and other apical transport proteins including annexin 13, galectin 3 and syntaxin 13 results in defects in ciliogenesis (69,70). Conversely, acute deciliation leads to loss of polarity (71). Trafficking to the cilia itself is regulated by a conserved multiprotein complex the BBSome (72). The BBSome interacts with the Rab8 exchange factor Rabin-8 (72), and functional Rab8 is necessary for cilium outgrowth (72,73). Studies in zebrafish showed that the cilia-localized coiled-coil protein Elipsa binds to Rab8 via rabaptin5 and therefore may anchor the intraflagellar transport (IFT) particles to Rab8-positive membranes (74). In addition, the Par3/6 complex as well as the exocyst complex localize to both the lateral membrane below the tight junctions and the base of the cilium (75–77); and Par3 and the exocyst subunit Sec10 have been shown to be essential for cilium outgrowth (75,78). Although the involvement of Rab8 and the exocyst in both basolateral sorting and ciliogenesis are well established, it is not at all clear how they distinguish between both pathways. Perhaps the cilium grows out after cells are polarized because this process depends on functional ARE/CRE to discriminate basolateral from ciliary targeting. Moreover, regulators that are specific for sorting into the cilium also exist. For example, the small G protein Arf4 binds to the ciliary targeting signal VxPx in rhodopsin and orchestrates the assembly of a ciliary targeting complex also containing Rab11, the Rab11 effector FIP3 and the Arf GTPase-activating protein ASAP1 for sorting of rhodopsin to the cilium in photoreceptors (79,80). While it remains to be shown if the same complex is involved in targeting proteins to the cilium in non-retinal cells, the ciliary protein polycystin-2 has a very similar targeting signal (RVxP) (81).
Summary and Open Questions
There is increasing awareness of the complexity of polarized biosynthetic trafficking routes. There are clearly multiple pathways to both apical and basolateral cell surfaces and many of these involve transient passage through endocytic compartments. Moreover, cells need to identify which proteins to send to the primary cilium. Cargo sorting into the different pathways to the surface is dependent on the dominant sorting signal. While recent studies clearly advance our understanding of how polarized sorting and delivery is accomplished, they also raise many new questions that remain to be addressed.
The most obvious of these are: why there are so many pathways, and why many of these pathways are indirect? While no consensus has yet emerged, there are several intriguing possibilities. It should be noted that there is also no direct retrograde pathway from the cell surface to the TGN during endocytosis from both apical and basolateral membranes. Perhaps retrieval and reuse of the sorting machinery may necessitate a movement through endosomes to maintain compartment integrity. Moreover, segregating proteins into distinct pathways that are independently regulated may enable selective surface delivery of subsets of functionally related proteins in response to different physiological cues. Alternatively or in addition, combining newly synthesized and recycling proteins into a common depot from which they can be selected for exocytosis, intracellular retention or degradation. Thus, intersection of the biosynthetic and endocytic/recycling pathways may enable finer modulation of the cell surface density of ion transporters or other proteins required to maintain polarized cell function. Finally, use of indirect trafficking routes may provide additional quality control checkpoints to reroute misfolded or missorted cargo.
Acknowledgments
We apologize to investigators whose studies could not be cited in this review for lack of space and thank Geri Kreitzer for helpful discussions and Jim Goldenring for MDCK cells expressing GFP-rab11. Studies on polarized membrane traffic in the Fölsch and Weisz laboratories are supported by NIH R01 grants GM070736 (to H.F.) and DK54407 and DK064613 (to OA.W.). P.E.M. is funded by individual NRSA F32DK082109. Technical support was provided by the morphology core of the Pittsburgh Center for Kidney Research (P30 DK079307).
References
- 1.Keller P, Toomre D, Diaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nature Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- 2.Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinnapen DJ, Chinnapen H, Saslowsky D, Lencer WI. Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol Lett. 2007;266:129–137. doi: 10.1111/j.1574-6968.2006.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- 5.Laird V, Spiess M. A novel assay to demonstrate an intersection of the exocytic and endocytic pathways at early endosomes. Exp Cell Res. 2000;260:340–345. doi: 10.1006/excr.2000.5006. [DOI] [PubMed] [Google Scholar]
- 6.Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci U S A. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E, Gonzalez A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol Biol Cell. 2007;18:4872–4884. doi: 10.1091/mbc.E07-06-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheff DR, Kroschewski R, Mellman I. Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol Biol Cell. 2002;13:262–275. doi: 10.1091/mbc.01-07-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 12.Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci U S A. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson A, Nessler R, Wisco D, Anderson E, Winckler B, Sheff D. Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell. 2007;18:2687–2697. doi: 10.1091/mbc.E05-09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuck S, Gerl MJ, Ang A, Manninen A, Keller P, Mellman I, Simons K. Rab10 is Involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 17.Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct. 2003;28:419–429. doi: 10.1247/csf.28.419. [DOI] [PubMed] [Google Scholar]
- 19.Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- 20.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 21.Folsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 24.Nokes RL, Fields IC, Collins RN, Folsch H. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J Cell Biol. 2008;182:845–853. doi: 10.1083/jcb.200802176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Folsch H. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol. 2007;177:477–488. doi: 10.1083/jcb.200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura N, Plutner H, Hahn K, Balch WE. The δ subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface. Proc Natl Acad Sci U S A. 2002;99:6755–6760. doi: 10.1073/pnas.092150699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folsch H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005;15:222–228. doi: 10.1016/j.tcb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol. 2007;177:103–114. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized Madin-Darby canine kidney cells. J Biol Chem. 2000;275:15207–15219. doi: 10.1074/jbc.275.20.15207. [DOI] [PubMed] [Google Scholar]
- 30.Orzech E, Schlessinger K, Weiss A, Okamoto CT, Aroeti B. Interactions of the AP-1 Golgi adaptor with the polymeric immunoglobulin receptor and their possible role in mediating brefeldin A-sensitive basolateral targeting from the trans-Golgi network. J Biol Chem. 1999;274:2201–2215. doi: 10.1074/jbc.274.4.2201. [DOI] [PubMed] [Google Scholar]
- 31.Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 33.Maday S, Anderson E, Chang HC, Shorter J, Satoh A, Sfakianos J, Folsch H, Anderson JM, Walther Z, Mellman I. A PDZ-binding motif controls basolateral targeting of syndecan-1 along the biosynthetic pathway in polarized epithelial cells. Traffic. 2008;9:1915–1924. doi: 10.1111/j.1600-0854.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Hao M, Cao Z, Ding W, Graves-Deal R, Hu J, Piston DW, Coffey RJ. Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-alpha containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells. Mol Biol Cell. 2007;18:3081–3093. doi: 10.1091/mbc.E07-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Franklin JL, Graves-Deal R, Jerome WG, Cao Z, Coffey RJ. Myristoylated Naked2 escorts transforming growth factor alpha to the basolateral plasma membrane of polarized epithelial cells. Proc Natl Acad Sci U S A. 2004;101:5571–5576. doi: 10.1073/pnas.0401294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–C556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- 37.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem. 2007;282:26552–26561. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 38.Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Type Igamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J Cell Biol. 2007;176:343–353. doi: 10.1083/jcb.200606023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodson CA, Ambrogi IG, Scott RO, Mohler PJ, Milgram SL. Polarized apical sorting of guanylyl cyclase C is specified by a cytosolic signal. Traffic. 2006;7:456–464. doi: 10.1111/j.1600-0854.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 41.Takeda T, Yamazaki H, Farquhar MG. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol. 2003;284:C1105–C1113. doi: 10.1152/ajpcell.00514.2002. [DOI] [PubMed] [Google Scholar]
- 42.Potter BA, Hughey RP, Weisz OA. Role of N- and O-glycans in polarized biosynthetic sorting. Am J Physiol Cell Physiol. 2006;290:C1–C10. doi: 10.1152/ajpcell.00333.2005. [DOI] [PubMed] [Google Scholar]
- 43.Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R. Requirement for galectin-3 in apical protein sorting. Curr Biol. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 44.Lin S, Naim HY, Rodriguez AC, Roth MG. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol. 1998;142:51–57. doi: 10.1083/jcb.142.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kundu A, Avalos RT, Sanderson CM, Nayak DP. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, Zurzolo C. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J Cell Biol. 2004;167:699–709. doi: 10.1083/jcb.200407094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 48.Tall RD, Alonso MA, Roth MG. Features of influenza HA required for apical sorting differ from those required for association with DRMs or MAL. Traffic. 2003;4:838–849. doi: 10.1046/j.1398-9219.2003.0138.x. [DOI] [PubMed] [Google Scholar]
- 49.Paladino S, Sarnataro D, Zurzolo C. Detergent-resistant membrane microdomains and apical sorting of GPI-anchored proteins in polarized epithelial cells. Int J Med Microbiol. 2002;291:439–445. doi: 10.1078/1438-4221-00151. [DOI] [PubMed] [Google Scholar]
- 50.Jacob R, Naim H. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol. 2001;11:1444–1450. doi: 10.1016/s0960-9822(01)00446-8. [DOI] [PubMed] [Google Scholar]
- 51.Jacob R, Heine M, Alfalah M, Naim HY. Distinct cytoskeletal tracks direct individual vesicle populations to the apical membrane of epithelial cells. Curr Biol. 2003;13:607–612. doi: 10.1016/s0960-9822(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 52.Fehrenbacher KL, Boldogh IR, Pon LA. Taking the A-train: actin-based force generators and organelle targeting. Trends Cell Biol. 2003;13:472–477. doi: 10.1016/s0962-8924(03)00174-0. [DOI] [PubMed] [Google Scholar]
- 53.Guerriero CJ, Weixel KM, Bruns JR, Weisz OA. Phosphatidylinositol 5-kinase stimulates apical biosynthetic delivery via an Arp2/3-dependent mechanism. J Biol Chem. 2006;281:15376–15384. doi: 10.1074/jbc.M601239200. [DOI] [PubMed] [Google Scholar]
- 54.Salvarezza SB, Deborde S, Schreiner R, Campagne F, Kessels MM, Qualmann B, Caceres A, Kreitzer G, Rodriguez-Boulan E. LIM kinase 1 and cofilin regulate actin filament population required for dynamin-dependent apical carrier fission from the trans-Golgi network. Mol Biol Cell. 2009;20:438–451. doi: 10.1091/mbc.E08-08-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hua W, Sheff D, Toomre D, Mellman I. Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging. J Cell Biol. 2006;172:1035–1044. doi: 10.1083/jcb.200512012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cresawn KO, Potter BA, Oztan A, Guerriero CJ, Ihrke G, Goldenring JR, Apodaca G, Weisz OA. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitt KN, Hernandez-Deviez D, Ballantyne SD, Spiliotis ET, Casanova JE, Wilson JM. Rab14 regulates apical targeting in polarized epithelial cells. Traffic. 2008;9:1218–1231. doi: 10.1111/j.1600-0854.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bahler M, Goldenring JR. Myosin Vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- 60.Jaulin F, Xue X, Rodriguez-Boulan E, Kreitzer G. Polarization-dependent selective transport to the apical membrane by KIF5B in MDCK cells. Dev Cell. 2007;13:511–522. doi: 10.1016/j.devcel.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kreitzer G, Schmoranzer J, Low SH, Li X, Gan Y, Weimbs T, Simon SM, Rodriguez-Boulan E. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- 62.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci U S A. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, Weimbs T. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol. 1998;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toomre D, Keller P, White J, Olivo JC, Simons K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J Cell Sci. 1999;112(Pt 1):21–33. doi: 10.1242/jcs.112.1.21. [DOI] [PubMed] [Google Scholar]
- 65.Potter BA, Ihrke G, Bruns JR, Weixel KM, Weisz OA. Specific N-glycans direct apical delivery of transmembrane, but not soluble or glycosylphosphatidylinositol-anchored forms of endolyn in Madin-Darby canine kidney cells. Mol Biol Cell. 2004;15:1407–1416. doi: 10.1091/mbc.E03-08-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cramm-Behrens CI, Dienst M, Jacob R. Apical cargo traverses endosomal compartments on the passage to the cell surface. Traffic. 2008;9:2206–2220. doi: 10.1111/j.1600-0854.2008.00829.x. [DOI] [PubMed] [Google Scholar]
- 67.Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J Biol Chem. 2000;275:15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- 68.Bomsel M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torkko JM, Manninen A, Schuck S, Simons K. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci. 2008;121:1193–1203. doi: 10.1242/jcs.015495. [DOI] [PubMed] [Google Scholar]
- 71.Overgaard CE, Sanzone KM, Spiczka KS, Sheff DR, Sandra A, Yeaman C. Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol Biol Cell. 2009;20:102–113. doi: 10.1091/mbc.E08-07-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 73.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 75.Sfakianos J, Togawa A, Maday S, Hull M, Pypaert M, Cantley L, Toomre D, Mellman I. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol. 2007;179:1133–1140. doi: 10.1083/jcb.200709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 77.Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, Burrow CR, Lipschutz JH. The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun. 2004;319:138–143. doi: 10.1016/j.bbrc.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 78.Zuo X, Guo W, Lipschutz JH. The Exocyst Protein Sec10 Is Necessary for Primary Ciliogenesis and Cystogenesis In Vitro. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-07-0772. PMID: 19297529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci U S A. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]