Abstract
Hematopoietic stem cell (HSC) homeostasis depends on the balance between self renewal and lineage commitment, but what regulates this decision is not well understood. Using loss-of-function approaches in mice, we found that glycogen synthase kinase–3 (Gsk3) plays a pivotal role in controlling the decision between self renewal and differentiation of HSCs. Disruption of Gsk3 in BM transiently expanded phenotypic HSCs in a β-catenin–dependent manner, consistent with a role for Wnt signaling in HSC homeostasis. However, in assays of long-term HSC function, disruption of Gsk3 progressively depleted HSCs through activation of mammalian target of rapamycin (mTOR). This long-term HSC depletion was prevented by mTOR inhibition and exacerbated by β-catenin knockout. Thus, GSK-3 regulated both Wnt and mTOR signaling in mouse HSCs, with these pathways promoting HSC self renewal and lineage commitment, respectively, such that inhibition of Gsk3 in the presence of rapamycin expanded the HSC pool in vivo. These findings identify unexpected functions for GSK-3 in mouse HSC homeostasis, suggest a therapeutic approach to expand HSCs in vivo using currently available medications that target GSK-3 and mTOR, and provide a compelling explanation for the clinically prevalent hematopoietic effects observed in individuals prescribed the GSK-3 inhibitor lithium.
Introduction
Stem cells possess the unique ability to self renew and differentiate into a diverse range of specialized cell types. HSCs have provided an important window into stem cell biology as well as a valuable clinical tool for treatment of hematopoietic malignancies and other disorders. However, the complex signaling network regulating the balance between HSC self renewal and differentiation is still not well understood.
One important regulator of HSC homeostasis is suggested by the highly prevalent clinical finding that therapeutic lithium increases circulating HSCs (as CD34+ cells; ref. 1) and peripheral blood counts (2–4) in greater than 90% of patients taking lithium and the laboratory findings that lithium also increases transplantable HSCs in mice (2, 3). Because lithium directly inhibits glycogen synthase kinase–3 (GSK-3; ref. 5), activating critical signaling pathways such as the Wnt and PI3K/PTEN/Akt pathways (6, 7), these clinical and laboratory observations implicate GSK-3 as an important regulator of HSC homeostasis (6, 8, 9). Support for this hypothesis comes from pharmacological studies showing that HSCs and hematopoietic progenitor cells (HPCs) are increased, and hematopoietic repopulation is enhanced, when BM transplant recipient mice are treated with alternative GSK-3 inhibitors (10–12). Furthermore, mouse ES cells treated with GSK-3 inhibitors maintain pluripotency (13, 14), and mouse ES cells lacking Gsk3a and Gsk3b maintain expression of markers of pluripotency under conditions that induce control ES cells to differentiate (15). These observations suggest a negative role for GSK-3 in ESC renewal. However, to our knowledge, Gsk3 loss of function in HSCs has not previously been performed, and the downstream pathways regulated by GSK-3 in HSCs have not yet been established.
Canonical Wnt signaling, which inhibits GSK-3 and thereby stabilizes β-catenin, plays a central role in the self renewal of diverse stem cell populations (13, 15–19). A role for Wnt signaling in hematopoiesis is supported by observations that Wnt ligands enhance proliferation of HSCs ex vivo (20–22) and that Wnt antagonists inhibit HSC proliferation and reconstitution (23, 24). In addition, overexpression of stabilized β-catenin promotes HSC self renewal and proliferation ex vivo under certain conditions (20–23, 25), and conditional deletion of β-catenin using vav-cre impairs HSC function in competitive repopulation assays (26). Furthermore, long-term reconstituting capacity in serial transplants is impaired in HSCs recovered from fetal liver of Wnt3a KO embryos (20–23, 25, 27) or from adults overexpressing the Wnt inhibitor Dkk in the hematopoietic niche (28), which suggests that Wnt signaling is required to maintain the long-term repopulating activity of HSCs.
However, there are conflicting reports on the requirement for Wnt/β-catenin signaling in basal hematopoiesis: conditional disruption of β-catenin and γ-catenin/plakoglobin in adult HSCs does not affect their ability to self renew and reconstitute hematopoietic lineages (24, 29, 30). In addition, although overexpression of stabilized β-catenin increases immunophenotypic HSCs, this is associated with a loss of repopulating activity and hematopoietic failure in vivo (31, 32), findings that appear incompatible with a positive role for β-catenin in hematopoiesis. A general conclusion from these apparently conflicting reports is that the role of Wnt signaling in hematopoiesis is complex and context dependent (18, 19). However, although the β-catenin loss-of-function studies suggest that canonical Wnt signaling is not essential for basal hematopoiesis in adults, they do not rule out a possible role for the Wnt/β-catenin pathway under nonbasal conditions and are still compatible with gain-of-function experiments in which the pathway is activated.
GSK-3 is also inhibited by Akt/PKB, which in turn requires the activity of PI3K and is antagonized by phosphatase and tensin homolog (PTEN), a PI3 phosphatase. Loss of Pten transiently increases HSCs, which is followed by progressive HSC depletion, increased lineage commitment resembling myeloproliferative disorder, and acute leukemia (33, 34). This expansion and subsequent depletion in Pten KO HSCs is mediated through mammalian target of rapamycin (mTOR), as the phenotype is reversed by treatment with rapamycin (33), and a similar HSC phenotype is observed with KO of tuberous sclerosis complex 1 (Tsc1), a negative regulator of mTOR (35, 36). As GSK-3 is an indirect target of PTEN and antagonizes mTOR through phosphorylation of Tsc2 (37), inhibition of GSK-3 could mimic the hematopoietic phenotype of Pten and Tsc1 KOs.
Currently available pharmacological data from humans and mice suggest that GSK-3 is an important regulator of HSC homeostasis, but the pathways regulated by GSK-3 in HSCs have not been defined. Furthermore, Gsk3 loss of function in HSCs/HPCs has not previously been reported for either Gsk3a or Gsk3b, and this is an essential step to defining the role of Gsk3 within HSCs. In the present study, we found that knockdown of Gsk3a/b (hereafter, Gsk3 is used to refer to both genes) initially expanded the HSC-enriched pool of lineage–sca-1+c-kit+ (LSK) cells, similar to the effects of lithium and other GSK-3 inhibitors, and showed that this required endogenous β-catenin function. However, in assays of long-term stem cell function, Gsk3-deficient HSCs were progressively depleted, revealing an unexpected positive role for GSK-3 in the maintenance of HSC self renewal. Furthermore, our data suggest that GSK-3 functions downstream of PTEN to antagonize mTOR signaling in phenotypic HSCs (HSC-enriched LSK population) in addition to its role in antagonizing Wnt/β-catenin signaling. Based on these observations, we conclude that Gsk3 loss of function coupled with inhibition of mTOR expands phenotypic HSCs in vivo. These findings point to a critical role for GSK-3 in regulating the decision between self renewal and differentiation in HSCs.
Results
Gsk3 loss of function in hematopoietic cells.
Therapeutic lithium increases the number of circulating CD34+ stem cells (1) in humans, increases peripheral blood counts, especially neutrophils, in a high percentage of treated patients, and enhances stem and progenitor cell numbers in rodents (2–4, 9, 38, 39). However, since these early studies were performed, immunophenotypic markers of HSCs and HPCs have become available (40). We therefore used flow cytometry (FCM) on BM from lithium-treated mice (Supplemental Results; supplemental material available online with this article; doi: 10.1172/JCI40572DS1) and found an increase in immunophenotypic HSCs/HPCs, as detected by LSK markers (ref. 40 and Supplemental Figure 1A) or by detection of the SLAM marker immunophenotype (CD150+CD48–) characteristic of HSCs (ref. 41 and data not shown). The selective GSK-3 inhibitor 6-bromo-indirubin 3′-oxime (6BIO) also increased the number of LSK cells after 2 weeks (Supplemental Figure 1C), consistent with recent reports using 6BIO (11) or the GSK-3 inhibitor CHIR-911 (10). We also observed a parallel increase in BM cellularity after treatment with either lithium or 6BIO, with no observable change in BM architecture (Supplemental Figure 1B and data not shown). Lithium, 6BIO, AR-A014418, and other structurally distinct GSK-3 inhibitors also increased hematopoietic colony formation ex vivo (Supplemental Figure 1D).
The parallel effects of lithium and alternative GSK-3 inhibitors support the hypothesis that GSK-3 is a significant target of lithium in HSCs/HPCs and suggest a critical function for GSK-3 in hematopoiesis. However, results based on systemically delivered inhibitors do not address whether GSK-3 functions cell autonomously in HSCs/HPCs; in addition, off-target effects remain a formal possibility. As Gsk3 loss of function has not previously been reported in HSCs, we tested depletion of Gsk3 in BM cells using RNAi and conventional Gsk3b KO (42). Two distinct shRNAs that target sequences conserved in both Gsk3a and Gsk3b were cloned into a lentivirus that also expresses GFP (43). Donor BM cells were infected with control or shRNA constructs (Gsk3-rnai-C2 and Gsk3-rnai-C4) and transplanted into lethally irradiated primary recipients. Peripheral blood was sampled at 4-week intervals for 20 weeks to confirm engraftment. After 8 weeks, a majority of peripheral blood cells in both Gsk3-rnai and control vector transplants were derived from GFP+ donor cells, including T cells (CD4+ and CD8+), B cells (B220+), myeloid cells (Gr1+CD11b+), and erythroid cells (TER119+), indicating successful engraftment and reconstitution (Figure 1A and data not shown). The contribution to the mature myeloid lineage, as marked by Gr1+CD11b+ cells, was increased at 8 and 12 weeks in hosts receiving Gsk3-rnai BM (Figure 1B), similar to known effects of lithium treatment (2–4, 9, 38, 39). GSK-3α and GSK-3β protein levels remained low in BM harvested from primary recipients 4 months after transplantation with both Gsk3-rnai vectors but not with control lentivirus (Figure 1C). Furthermore, β-catenin protein levels were elevated in Gsk3-depleted BM cells harvested from primary recipients of Gsk3-rnai cells (Figure 1C), consistent with reduced GSK-3–dependent phosphorylation and activation of Wnt signaling. Because subsequent results with the Gsk3-rnai-C2 and Gsk3-rnai-C4 constructs were similar, only data using the Gsk3-rnai-C2 construct are shown.
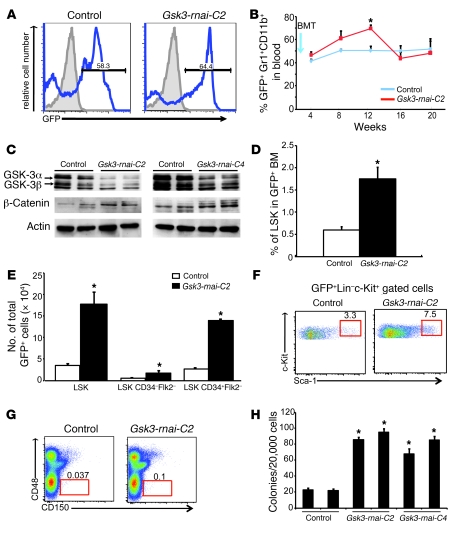
Figure 1. Gsk3 depletion expands HSCs and HPCs in primary transplants.
(A) Irradiated mice were reconstituted with BM after transduction with lentivirus with or without Gsk3-rnai. Peripheral blood was examined 20 weeks after transplantation; numbers within histograms indicate percent GFP+ cells. Shown is 1 representative of 5 similar experiments; similar results were obtained with Gsk3-rnai-C4. (B) GFP+ myeloid cells (Gr1+CD11b+) in peripheral blood for 10 control and 9 Gsk3-rnai-C2 recipients after BM transplantation (BMT; arrow). (C) Immunoblots for GSK-3α/β and β-catenin in BM from primary recipients 16 weeks after transplantation. Data represent independent replicates from 6 control and 6 Gsk3-rnai recipients. (D) Percent GFP+ LSK cells in control and Gsk3-rnai-C2 primary recipients. (E) Absolute number of GFP+ LSK, LSK CD34–Flk2–, and LSK CD34+Flk2– cells. (F) Representative FCM showing GFP+ cells in the HSC-containing LSK fraction (red gate) for control and Gsk3-rnai-C2 primary recipients. (G) Representative FCM using SLAM markers; the difference between control and Gsk3-rnai was significant (P < 0.05). Numbers in F and G indicate percent cells within gates. (H) Colony formation using GFP+ cells plated in methylcellulose with cytokines and scored for CFU-C (see Supplemental Figure 1). Data represent mean colonies per well performed in duplicate groups for 5 mice per construct repeated in 3 separate experiments. *P < 0.05 versus respective control value.
Importantly, both percentage and number of immunophenotypic HSCs/HPCs increased greater than 4-fold in Gsk3-rnai–transduced cells compared with control cells, as assessed by the increase in GFP+ LSK cells (Figure 1, D–F) as well as CD150+CD48– cells (Figure 1G). Multiparametric FCM analysis of CD34 and flk-2 showed an increase in immunophenotypic short-term HSCs (ST-HSCs) and long-term HSCs (LT-HSCs; Figure 1E). The total cellularity of the BM was only marginally increased (Supplemental Figure 2B), in contrast to the effect of the systemically delivered GSK-3 inhibitors (Supplemental Figure 1B). Furthermore, BM harvested at 4 months from primary transplants of Gsk3-rnai–transduced BM yielded greater than 4-fold more colonies in methylcellulose culture than did control BM (Figure 1H).
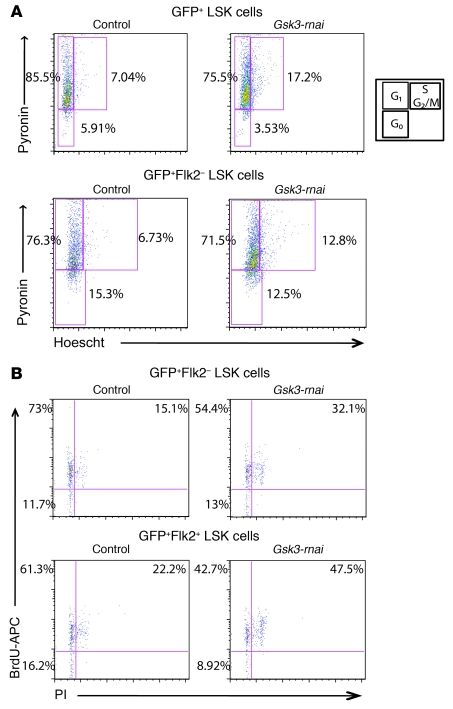
To explore the mechanism by which Gsk3 depletion expands the size of the phenotypic HSC/HPC pool, we examined the cell cycle and survival status of GFP-marked LSK cells. GFP+ LSK or GFP+ LSK Flk2– cells were purified from control or Gsk3-rnai–transduced BM after 4–5 months in primary recipients and stained with pyronin and Hoechst followed by FCM (44). Compared with controls, approximately 2-fold more Gsk3-depleted cells had entered the S/M/G2 phases of the cell cycle in both the GFP+ LSK and GFP+ LSK Flk2– populations (Figure 2A), demonstrating increased cycling of Gsk3-deficient LSK cells. Transplant recipients were also fed BrdU for 7 days prior to BM harvest. Incorporation of BrdU coupled with analysis of propidium iodide (PI) staining confirmed that Gsk3 depletion increased the percentage of LSK cells in S/M/G2 more than 2-fold (Figure 2B). Annexin V staining in Gsk3-deficient LSK cells was not significantly different from that of controls (Supplemental Figure 2A), indicating that the increase in LSK cells is not caused by a change in the rate of cell death. These data indicate that loss of Gsk3 results in accelerated cell cycle progression within the LSK cell population.
Figure 2. Gsk3 knockdown increases cycling of the HSC-enriched LSK cell population.
(A) To assess cell cycle status of the HSC-enriched LSK population, sorted GFP+ LSK and GFP+ LSK Flk2– cells from primary recipients of control and Gsk3-rnai 4 months after BM transplantation were stained with Hoechst and Pyronin and analyzed by FCM. Representative FACS data are shown for control versus Gsk3-rnai-C2. (B) At 4 months after BM transplantation, primary recipients of control and Gsk3-rnai were fed BrdU in the drinking water for 7 days. Sorted GFP+ LSK Flk2+ and Flk2– cells were stained with BrdU-APC antibody and PI to analyze BrdU incorporation. Representative FACS data are shown for control versus Gsk3-rnai-C2. Similar results were obtained by BrdU versus 7-AAD staining (BD). Percent cells are shown for the indicated gates and quadrants. In A and B, lower left gate represents G0, upper left represents G1, and upper right represents S, G2, and M phases of the cell cycle, as shown in the diagram in A.
Functional HSCs are reduced in Gsk3-deficient BM.
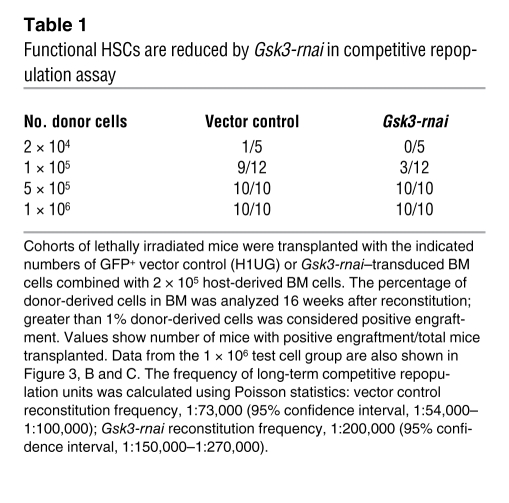
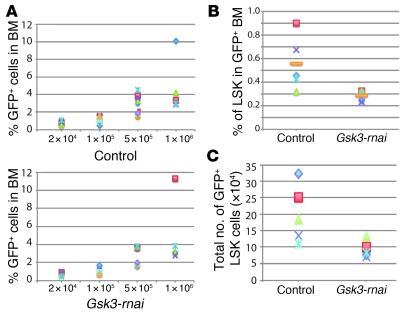
Because inhibition of GSK-3 activity or expression increased immunophenotypic HSCs and HPCs and increased functional HPCs within the LSK cell population, we measured competitive repopulating units (45) as a functional test of HSCs in Gsk3-depleted versus control BM (Table 1 and Figure 3). Control and Gsk3-rnai–infected BM was transplanted to irradiated recipients and harvested after 4 months. Sorted GFP+ cells were mixed at dilutions from 2 × 104 to 1 × 106 cells with a constant number (2 × 105) of GFP– recipient cells and transplanted to lethally irradiated recipient mice. After another 4 months, BM was harvested, and chimerism was analyzed as the percentage of GFP+ cells. Despite the increase in phenotypic HSCs and HPCs observed in primary transplants, Gsk3-deficient cells were less efficient in competitive reconstitution than were control cells (Table 1 and Figure 3, B and C), with approximately 3-fold fewer functional HSCs in the Gsk3-rnai group.
Table 1 .
Functional HSCs are reduced by Gsk3-rnai in competitive repopulation assay
Figure 3. Gsk3-depleted HSCs are functionally deficient.
(A) Limiting dilution experiments were performed with 4 doses (x axis) of GFP+ test BM from control and Gsk3-rnai primary recipients (4 donors per group) combined with a fixed 2 × 105 unlabeled competing cells transplanted into groups of at least 5 recipients per dose. Chimerism at 4 months after transplantation for each dose is represented as the percentage of GFP+ cells in BM for control and Gsk3-rnai. (B) Percent donor-derived immunophenotypic HSCs/HPCs (as GFP+ LSK cells) in the 1 × 106 test cell group 4 months after transplantation. (C) Absolute number of donor-derived immunophenotypic HSCs/HPCs (as GFP+ LSK cells) in the 1 × 106 test cell group 4 months after transplantation.
As a further test of LT-HSC function, we performed serial, noncompetitive transplantation to secondary and tertiary lethally irradiated hosts. BM was harvested from primary recipients after 4 months, and 2 × 105 sorted GFP+ cells were transplanted into lethally irradiated secondary recipients. Of recipients of Gsk3-depleted cells, 50% died within 1 month of transplantation, whereas 100% of vector control BM recipients survived (Figure 4A). All control recipients and surviving recipients of Gsk3-rnai–infected cells showed long-term multilineage reconstitution with high levels of GFP+ donor cells contributing to multiple peripheral blood lineages (data not shown) and BM. However, in contrast to primary recipients, BM harvested at 4 months from surviving Gsk3-rnai secondary recipients did not show an increase in GFP+ LSK cells (Figure 4B) or CD150+CD48– cells (data not shown), despite the higher number of GFP+ LSK CD150+CD48– cells originally present in the Gsk3-rnai donor BM (Figure 1, D and E). Reduction in GSK-3α and GSK-3β protein was confirmed by Western blot of GFP+ BM cells harvested from secondary recipients after 4 months (data not shown).
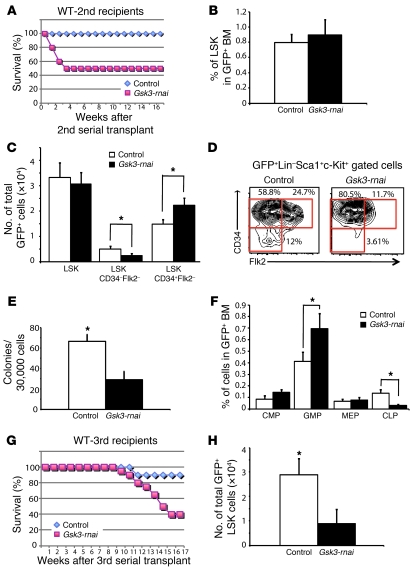
Figure 4. Gsk3 knockdown depletes HSCs in serial BM transplants.
(A) Noncompetitive serial transplants were performed by transplanting 2 × 105 sorted GFP+ cells from primary recipients of control or Gsk3-rnai transduced BM into lethally irradiated recipients (10 mice per group). Survival of secondary recipients receiving control or Gsk3-rnai BM is shown as a Kaplan-Meier plot. (B) Percent HSC-containing LSK fraction in control and Gsk3-rnai secondary recipients. (C) Absolute number of GFP+ LSK, LSK CD34–Flk2–, and LSK CD34+Flk2– cells in control and Gsk3-rnai secondary recipients. (D) Representative FCM data, presented as the distribution of CD34–Flk2–, CD34+Flk2–, and CD34+Flk2–, which immunophenotypically correspond to LT-HSCs, ST-HSCs, and MPPs in the LSK population, from control and Gsk3-rnai secondary recipients. Percent cells are shown for the indicated gates. (E) Colony formation assay with sorted GFP+ cells from control and Gsk3-rnai secondary recipient BM was performed and scored as in Figure 1 using GFP+ BM from 5 control and 5 Gsk3-rnai mice. (F) The frequencies of common myeloid progenitor (CMP), granulocyte-monocyte progenitor (GMP), and megakaryocyte-erythroid progenitor (MEP) cells were measured by detection of CD16/32 and CD34 expression in the lineage–sca-1–c-kit+ gated population. The common lymphoid progenitor (CLP) fraction was measured as CD127+ cells in the lineage–sca-1loc-kitlo gate. (G) Lethally irradiated mice were reconstituted with 4 × 105 sorted GFP+ BM cells from secondary recipients of vector or Gsk3-rnai transduced BM. The Kaplan-Meier survival curve shows the survival of tertiary recipients of BM from control or Gsk3-rnai mice. (H) Absolute number of immunophenotypic HSCs/HPCs, as LSK cells, in control and Gsk3-rnai tertiary recipients. *P < 0.05.
Analysis of CD34 and flk-2 further showed that immunophenotypic LT-HSCs were decreased in Gsk3-rnai secondary recipients, and the increase in ST-HSCs (LSK Flk2+CD34+) was attenuated (approximately 2-fold; Figure 4, C and D) compared with that in primary recipients (Figure 1E). Similarly, colony formation was reduced 2-fold in secondary recipients of Gsk3-rnai compared with vector control (Figure 4E). Furthermore, the proportion of granulocyte-monocyte progenitor cells in the GFP+ population increased, while the percentage of common lymphoid progenitor cells decreased significantly (Figure 4F). These observations suggest that Gsk3 is required for the maintenance of LT-HSCs and that prolonged loss of GSK-3 activity may promote exit of HSCs from the stem cell pool. To extend this analysis, we transplanted GFP+ cells recovered from secondary recipients to lethally irradiated tertiary recipients; 12 of 20 mice receiving Gsk3-depleted BM died within 4 months (Figure 4G). LSK cells were reduced 3- to 4-fold in Gsk3-depleted BM in surviving tertiary recipients (Figure 4H), and this marked reduction in HSCs was confirmed by the reduced level of CD150+CD48– cells (data not shown). These data indicate that loss of Gsk3 leads to a progressive decline in HSC function and/or number in the course of long-term serial transplantation. This apparent positive function of Gsk3 in HSC maintenance was further supported by serial transplantation of HSCs from Gsk3b KO mice (see below).
Role of Wnt/β-catenin signaling in response to Gsk3 knockdown.
Gsk3 loss-of-function mutations result in stabilization of β-catenin protein and constitutive activation of Wnt signaling (15, 46). Thus, the effects of GSK-3 inhibitors or Gsk3 depletion in HSCs/HPCs could be mediated by activation of downstream Wnt signaling. Wnt signaling is active in HSCs under basal conditions (10, 11, 23, 28) and is further activated in HSCs isolated from lithium-treated Bat-gal (47) reporter mice (data not shown). Furthermore, β-catenin protein levels were persistently elevated in Gsk3-rnai–infected BM cells (Figure 1C), which suggests that downstream Wnt signaling is activated in these Gsk3-depleted cells.
Although the requirement for Wnt signaling in basal hematopoiesis remains controversial, activation of Wnt signaling can enhance HSC self renewal and HPC function in vivo and ex vivo (10, 11, 20–23, 25). We therefore tested whether β-catenin is required for the effects of Gsk3 depletion on hematopoiesis using a conditional β-catenin loss-of-function allele (β-cateninfl/fl) crossed to interferon-inducible Mx-cre mice (29). Cre was induced in Mx-cre;β-cateninfl/fl mice with polyinosine-polycytidine (polyI:polyC), and BM was harvested and infected with control or Gsk3-rnai lentivirus. After infection, cells were transplanted to lethally irradiated hosts, and after 4 months, BM from these primary recipients was harvested and analyzed by FCM. Depletion of Gsk3 increased the number of GFP+ LSK cells (as well as CD150+CD48– cells) derived from WT hosts, and loss of β-catenin blocked this effect in primary recipients (Figure 5A). In addition, β-cateninfl/fl conditional KO (CKO) blocked the increase in colony formation observed in Gsk3-rnai;β-catenin+/+ BM (compare Supplemental Figure 3 and Figure 1H), demonstrating that β-catenin is required for the effect of Gsk3 depletion in primary transplanted HSCs/HPCs.
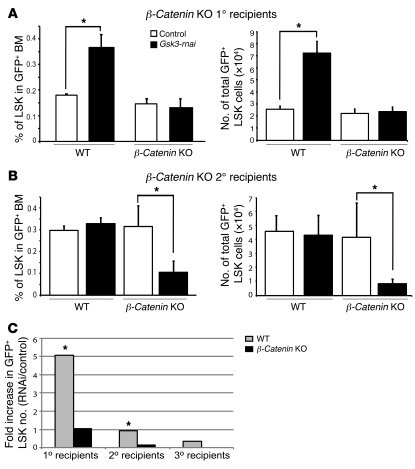
Figure 5. β-catenin is required for the increase in HSCs/HPCs induced by Gsk3-rnai.
(A) BM cells were harvested from Mx-Cre;β-cateninfl/fl mice with or without injection of polyI:polyC for 14 days, transduced with control or Gsk3-rnai carrying lentivirus, and transplanted into lethally irradiated recipient mice. After 4 months, percentage and absolute number of HSC-containing LSK fraction were compared among the 4 groups. (B) BM cells were harvested at 4 months from primary recipients of WT and Mx-Cre;β-cateninfl/fl mice transduced with vector control or Gsk3-rnai lentivirus (from primary recipient mice in A) and transplanted into lethally irradiated secondary hosts. After 4 months, percentage and absolute number of HSC-containing LSK fraction were compared among the 4 groups. (C) Summary of serial transplantation data in WT versus β-catenin CKO mice. Shown is fold change in GFP+ LSK cells in recipients of Gsk3-depleted BM normalized to vector control, for otherwise WT primary, secondary, and tertiary recipients as well as for primary and secondary β-catenin CKO recipients. Survival in tertiary recipients of Gsk3/β-catenin–deficient BM was too low for statistical significance. *P < 0.05.
BM from Gsk3-rnai, WT, and β-catenin CKO primary recipients was also transplanted to secondary lethally irradiated recipients. As with Gsk3-rnai;β-catenin+/+ BM, transplantation of Gsk3-rnai;β-cateninfl/fl CKO BM resulted in reduced survival. After 4 months, BM was harvested, and LSK cells were measured within the GFP+ population. Loss of β-catenin resulted in a 3-fold reduction in LSK cells after secondary transplantation of Gsk3-depleted BM (Figure 5B). BM from each group of secondary recipients was also transplanted to tertiary recipients, but the survival of Gsk3-rnai;β-cateninfl/fl recipients at 4 months was too low for analysis (data not shown). The serial BM from WT and β-catenin CKO transplantation data (summarized in Figure 5C) demonstrate that β-catenin contributes to HSC maintenance throughout successive transplants of Gsk3-deficient BM cells. These data, taken together with previously published observations from others (11, 20–28), support a positive role for the Wnt/β-catenin pathway in HSC maintenance in the context of reduced GSK-3 activity, but also suggest that an additional GSK-3–regulated pathway plays a distinct and possibly antagonistic role in HSC maintenance.
mTOR inhibition expands HSCs in Gsk3-deficient BM.
Although GSK-3 regulates multiple pathways, the initial expansion and subsequent depletion of phenotypic HSCs — as well as the reduction in functional HSCs — observed with Gsk3 knockdown was strikingly similar to the hematopoietic phenotype previously reported for Pten KO mice (33, 34). PTEN is a negative regulator of the PI3K/Akt pathway and maintains GSK-3 activity by antagonizing Akt-dependent phosphorylation of GSK-3. Pten CKO within HSCs activates the mTOR pathway, and the transient expansion and subsequent depletion of HSCs in Pten KO cells can be rescued by treatment of mice with the mTOR inhibitor rapamycin. However, the mechanism for PTEN suppression of mTOR activity has not been fully elucidated. GSK-3 was recently shown to inhibit mTOR signaling through phosphorylation of Tsc2, which, together with Tsc1, antagonizes mTOR signaling (37). Furthermore, deletion of Tsc1 increases proliferation and reduces self renewal of HSCs, similar to loss of Pten (35, 36) and reduced Gsk3 expression (present study). Thus, we hypothesized that the reduction in HSCs in Gsk3-depleted BM is caused by activation of mTOR. To test this hypothesis, we used Western blotting and FCM to examine phosphorylation of ribosomal protein S6, a marker for activation of mTOR, in Gsk3-deficient and control BM. S6 phosphorylation was modestly increased in primary recipients of Gsk3-rnai BM (data not shown) and more demonstrably increased in secondary recipients (Figure 6, A and B). S6 phosphorylation was not affected by deletion of β-catenin in either primary or secondary recipients.
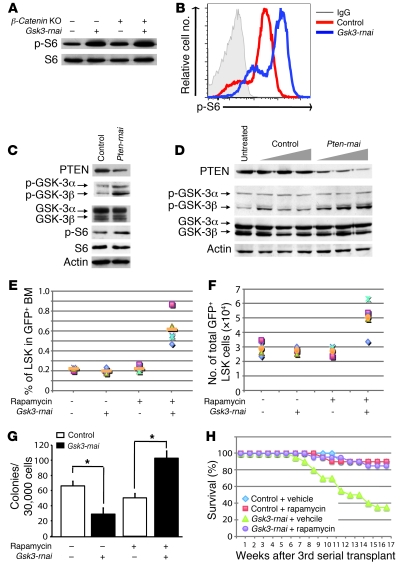
Figure 6. Increased HSCs/HPCs in rapamycin-treated recipients of Gsk3-depleted BM.
(A) BM was harvested from Mx-Cre;β-cateninfl/fl mice treated with or without polyI:polyC, transduced with control or Gsk3-rnai lentivirus, and transplanted into irradiated recipients. After 4 months, GFP+ cells were sorted (pooled from 5 mice per group), and phospho–ribosomal protein S6 (p-S6) was detected in cell lysates by immunoblot. (B) Flow cytometric detection of phospho–ribosomal protein S6 with BM cells in A. (C) Irradiated mice were reconstituted with BM transduced with control vector or Pten-rnai. After 4 months, phospho–GSK-3 and phospho–ribosomal protein S6 were assessed in GFP+ cells by immunoblot. (D) NIH3T3 cells were infected for 3 days with control or Pten-rnai lentivirus, and phospho–GSK-3α/β in control and Pten-depleted cells was detected by immunoblot. (E) Noncompetitive serial transplants. GFP+ cells (2 × 106) from primary recipients of control or Gsk3-rnai were transplanted into 10 irradiated recipients per group. After 1.5–2 months, secondary recipients were injected with rapamycin or vehicle every other day for 8 weeks. Percent GFP+ LSK cells was compared among the 4 groups. (F) Absolute number of GFP+ LSK cells as in E. (G) Colony formation with sorted GFP+ cells from E. (H) Kaplan-Meier plot showing survival of tertiary recipients transplanted with BM from control- and rapamycin-treated secondary recipients in E–G. Shown is control vector BM from secondary recipients treated with vehicle or rapamycin and Gsk3-rnai–infected BM from secondary recipients treated with vehicle or rapamycin transplanted to lethally irradiated tertiary recipients. *P < 0.05.
These observations suggest that GSK-3 is a downstream target of PTEN-regulated pathways in hematopoietic cells. To test this directly, we reduced expression of Pten in BM cells by lentivirally mediated RNAi and harvested BM from primary hosts after 4 months; the inhibitory phosphorylation of GSK-3β (serine-9) increased (Figure 6C), consistent with reduced PTEN activity and increased Akt-mediated GSK-3β phosphorylation. Knockdown of Pten in NIH-3T3 cells also increased phosphorylation of GSK-3β (Figure 6D). These data indicate that GSK-3β is indeed a downstream target of PI3K/PTEN regulation in hematopoietic cells.
We then treated secondary recipients of Gsk3-rnai and control BM with rapamycin, as this prevents HSC depletion in Pten KOs (33). Rapamycin restored the LSK population in secondary recipients of Gsk3-depleted BM, with a 3- to 4-fold increase in LSK cells compared with vehicle-injected controls (Figure 6, E and F), which suggests that Gsk3 depletion increases the HSC population above baseline, perhaps through activation of Wnt/β-catenin signaling in the absence of the antagonistic influence of mTOR signaling in HSCs. Colony formation also increased 2- to 3-fold in BM harvested from rapamycin-treated Gsk3-rnai recipients (Figure 6G), further demonstrating that rapamycin rescues the effect of Gsk3-rnai in secondary recipients. Furthermore, rapamycin treatment also rescued survival of tertiary recipients of Gsk3-depleted BM (Figure 6H), which indicates that inhibition of mTOR preserves LT-HSCs in Gsk3-depleted BM. However, it will be important in future work to measure the number of functional HSCs after treatment with rapamycin using competitive repopulation assays.
Gsk3b is required for maintenance of the HSC-enriched LSK cell population.
Surprisingly, reduced expression of Pten had little effect on phosphorylation of GSK-3α (Figure 6D), raising the possibility that GSK-3β is a specific target of PI3K/PTEN regulation in HSCs. Although Gsk3a and Gsk3b are structurally similar and are clearly redundant in Wnt signaling (15), the 2 genes are not redundant in all contexts (42, 48). To test the specific role of Gsk3b in HSC maintenance, we performed transplants with hematopoietic cells derived from Gsk3b–/– embryos, which express WT levels of Gsk3a. Homozygous loss of Gsk3b in mice is lethal between 15 and 18 days of gestation (42). We therefore harvested fetal liver cells from E17.5 WT, Gsk3b+/–, and Gsk3b–/– embryos and transplanted them into lethally irradiated adult mice. After 4 months, recipients of WT, Gsk3b+/–, and Gsk3b–/– BM showed long-term multilineage reconstitution derived from donor cells (Figure 7, A and B). However, there was no significant increase in the percentage or absolute number of LSK or CD150+CD48– cells in primary hosts receiving Gsk3b–/– fetal liver cells (Figure 7C). This lack of increase in HSCs in primary recipients with selective loss of Gsk3b is in contrast to RNAi-mediated knockdown of both Gsk3a and Gsk3b; because the increase in HSCs after Gsk3 depletion required β-catenin, this observation is consistent with the well-established redundant roles for Gsk3a and Gsk3b in Wnt signaling (15). However, in secondary transplant recipients, Gsk3b–/– donor cells demonstrated a 3- to 4-fold reduction in LSK cells compared with WT cells (Figure 7D), which suggests that Gsk3b regulates HSC homeostasis through a Wnt/β-catenin–independent pathway that becomes evident in secondary transplants and is not compensated for by Gsk3a. Although the respective roles of Gsk3a and Gsk3b may differ in fetal versus adult HSCs, these observations, taken together with the selective phosphorylation of GSK-3β when Pten expression is reduced in hematopoietic cells (or in fibroblasts), suggest that GSK-3β is a selective target of PTEN-regulated pathways and is required for the maintenance of HSC self renewal.
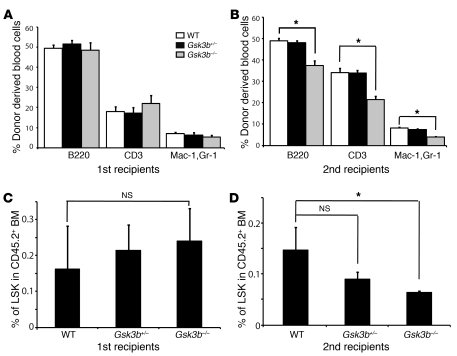
Figure 7. Gsk3b KO depletes HSCs in serial BM transplants.
Noncompetitive serial transplants were performed by transplanting 4 × 105 fetal liver cells (CD45.2) from E17.5 WT, Gsk3b+/–, and Gsk3b–/– embryos into lethally irradiated recipient mice (CD45.1). (A and B) Reconstitution of peripheral blood, including B cells (B220+), T cells (CD3+), and myeloid cells (Mac-1+GR-1+), in primary (A) and secondary (B) recipients of WT, Gsk3b+/–, and Gsk3b–/– fetal liver cells. Secondary transplants were performed after 16 weeks of engraftment by pooling BM from 3–4 reconstituted primary recipients to transplant 4 × 105 whole BM cells into lethally irradiated CD45.1 secondary recipients (10 hosts per genotype). (C and D) Percentage of LSK cells in CD45.2+ BM was compared among recipients of WT, Gsk3b+/–, and Gsk3b–/– fetal liver cells in primary (C) and secondary (D) hosts. *P < 0.05.
Discussion
The data presented here demonstrate an essential role for Gsk3 in the maintenance of LT-HSCs. Gsk3 loss of function phenocopies Pten and Tsc1 mutations in HSCs/HPCs, supporting the hypothesis that GSK-3 functions downstream of PTEN to suppress mTOR-dependent HSC activation and lineage commitment. However, inhibition of GSK-3 also stabilizes β-catenin within HSCs/HPCs to induce a β-catenin–dependent increase in phenotypic HSCs, and β-catenin KO accelerates the loss of LT-HSCs in Gsk3-depleted BM, consistent with prior reports with activators of Wnt signaling (20–23, 25, 28). Thus, Gsk3 functions in at least 2 apparently opposing processes within HSCs/HPCs (Figure 8). These observations indicate that Gsk3 plays an essential role in regulating the balance between self renewal and lineage commitment in HSCs. These observations also support the hypothesis that the highly prevalent effects of lithium on hematopoiesis in bipolar patients are mediated by inhibition of GSK-3 and suggest a therapeutic approach, using currently approved GSK-3 and mTOR inhibitors, to expand HSCs in vivo.
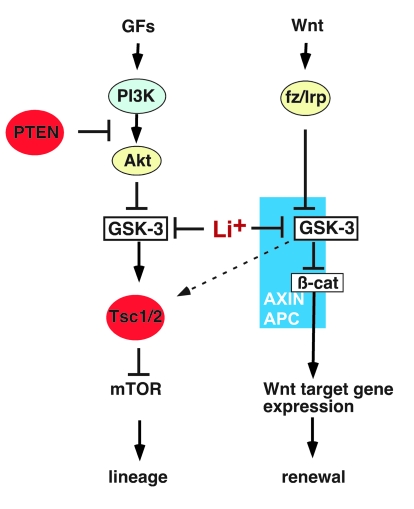
Figure 8. GSK-3 functions in 2 major pathways to regulate HSC self renewal and lineage commitment.
Inhibition of GSK-3 activates Wnt and mTOR signaling. In the canonical Wnt pathway, GSK-3 and β-catenin bind to the Axin complex, along with APC. GSK-3 phosphorylates β-catenin, targeting it for rapid destruction. Wnt binding to the Fz/Lrp receptor complex causes inhibition of GSK-3, which in turn stabilizes β-catenin and activates Wnt target genes that promote progenitor proliferation and self renewal. In PI3K/PTEN-regulated pathways, growth factors (GFs) bind to surface receptors and activate PI3K, leading to activation of Akt, whereas PTEN inhibits activation of Akt. Once activated, Akt phosphorylates and inhibits GSK-3. GSK-3 phosphorylates Tsc2, inhibiting the mTOR pathway. Thus, inhibition of GSK-3 activates mTOR and promotes proliferation and exit from the LT-HSC pool. Inhibition of GSK-3 thus activates distinct downstream signaling pathways that have opposing functions in HSC renewal and differentiation.
Pten KO in HSCs/HPCs leads to activation and subsequent depletion of HSCs, increased lineage commitment resembling myeloproliferative disorder, and leukemia (33, 34), and this is prevented by the mTOR inhibitor rapamycin, which suggests that PTEN-mediated suppression of mTOR is required for maintenance of quiescent HSCs. Similarly, deletion of the mTOR inhibitor Tsc1 shifts HSCs from a quiescent to a proliferative state and reduces HSC self renewal (35, 36). We show that reduced expression of Gsk3, either through RNAi or by homozygous Gsk3b KO, yielded a similar hematopoietic phenotype. This Gsk3 phenotype was also reversed by rapamycin, and GSK-3β phosphorylation increased in Pten-depleted BM, which suggests that GSK-3β functions downstream of PTEN to antagonize mTOR activation and maintain stem cell self renewal. In support of this, GSK-3 has previously been shown to antagonize mTOR activation in HEK293T cells by phosphorylating Tsc2 (37). Interestingly, that work showed that Wnts could activate mTOR by inhibiting GSK-3, suggesting a bifurcation of the canonical Wnt pathway that could activate distinct and potentially opposing processes (Figure 8). Furthermore, very recent work suggests that Wnt signaling through mTOR may also cause epidermal stem cell exhaustion, and this can also be prevented by rapamycin (49). Although it is not yet known whether Wnts activate mTOR in HSCs/HPCs, activation of both mTOR- and β-catenin–dependent processes could explain some of the conflicting reports on Wnt effects in hematopoiesis (18, 19), if differing experimental conditions bias the effects toward either mTOR- or β-catenin–dependent responses.
Although the Gsk3-depletion phenotype we observed was similar to the Pten KO, important differences should also be noted. Conditional deletion of Pten (or Tsc1) leads to rapid HSC exhaustion, whereas the reduction in HSCs observed with Gsk3-rnai became evident more slowly, through serial transplants and competitive repopulation assays. Indeed, the initial expansion in phenotypic HSCs was observed 4 months after primary transplant; the number of phenotypic HSCs declined to control levels in secondary recipients, and only fell below control levels in tertiary transplants (Figure 5C). We propose that the delay is because the activation of HSCs and their subsequent exit from the HSC pool are balanced by enhanced Wnt signaling, which would slow the rate of HSC activation and depletion (Figure 8); this idea is supported by the more rapid decline in phenotypic HSCs in Gsk3-rnai;β-catenin KO BM. Pten deletion also leads to leukemia in a substantial fraction of animals, which we have not observed so far with the Gsk3-rnai–transplanted mice. However, PTEN regulates multiple downstream effectors in addition to GSK-3, and modulation of these pathways could contribute to the Pten HSC phenotype independently of GSK-3 function. It would be interesting to test whether the acute leukemia observed in Pten KO mice is blocked in mice expressing nonphosphorylatable mutants of Gsk3.
We found that β-catenin was required for the initial increase in phenotypic HSCs/HPCs in response to Gsk3 inhibition and for the maintenance of Gsk3-depleted HSCs in long-term transplant assays. These observations are consistent with previous studies showing that activation of canonical Wnt signaling can promote HSC self renewal and proliferation ex vivo (20–23, 25, 28). Although basal hematopoiesis was unaffected when β-catenin was deleted in adult BM, consistent with previous reports (29, 30), inhibition of Gsk3 can be considered a Wnt gain of function. The issue of whether canonical Wnt signaling is required for basal HSC homeostasis remains controversial (18, 19). A recent report showed that canonical Wnt signaling can function in HSCs in the absence of β-catenin (24), which suggests that loss of β-catenin does not necessarily block all Wnt signaling. In addition, conditional deletion of β-catenin with cre recombinase driven by the vav promoter impairs LT-HSC self renewal (26). As vav expression begins in utero, whereas Mx-cre was used to delete β-catenin in adult BM (present study and refs. 29, 30), a reasonable explanation for these differences is that the role of Wnt/β-catenin signaling in basal hematopoiesis depends on the developmental context. In support of an early requirement for Wnt signaling in developing HSCs, long-term reconstituting capacity in serial transplants is impaired in HSCs recovered from fetal liver of Wnt3a KO embryos (27).
Lithium’s effects on hematopoiesis have been known for decades and affect more than 90% of patients taking it, yet to our knowledge, the mechanism of lithium action in this setting had not previously been defined. Plausible targets of lithium in addition to GSK-3 include inositol monophosphatase, which may indirectly regulate inositol trisphosphate signaling, and related phosphomonoesterases that play important roles in cell metabolism (50, 51). Therefore, a priori, it should not be considered obvious that GSK-3 is the biologically relevant target of lithium in HSCs, and it is essential to validate GSK-3 as the target in this setting. In support of this hypothesis, structurally diverse GSK-3 inhibitors mimicked lithium effects on the HSC pool and on progenitor cells (Supplemental Figure 1 and refs. 10–12). Importantly, we show here, for the first time to our knowledge, that depletion of Gsk3a and Gsk3b mimics lithium action in HSCs and HPCs as well as more differentiated myeloid cells. Although these observations suggest that targeting GSK-3 may be a fruitful approach to treating hypoproliferative hematopoietic disorders, the reduction in LT-HSCs with Gsk3 loss of function suggests this should be approached with caution. Lithium has, in fact, been tested in clinical trials to enhance hematopoietic recovery after myelosuppressive chemotherapy, but this approach has not seen wide use, perhaps in part because of the risk of lithium side effects in already critically ill patients, but also because of limited success in restoring hematopoiesis in patients with reduced numbers of HSCs. It is also possible that the β-catenin–dependent increase in HSCs in response to lithium is offset by activation of mTOR and exit from the HSC pool, which would be consistent with the increase in more differentiated hematopoietic cells (especially those of myeloid lineage) commonly observed with lithium. In this case, we suggest that the combination of lithium and rapamycin, both now in wide clinical use, might achieve a more marked and durable increase in HSCs.
In summary, we have provided evidence for dual functions of GSK-3 within hematopoietic cells. GSK-3 antagonizes the canonical Wnt pathway, and we showed here that inhibition of GSK-3 activated the pathway to enhance HSC self renewal. This response to GSK-3 inhibition required β-catenin, as phenotypic HSCs were reduced in β-catenin CKO BM in both primary and secondary transplant recipients of Gsk3-depleted BM. GSK-3 also antagonizes mTOR signaling, and we showed that inhibition of Gsk3 either by RNAi or by conventional gene KO activated mTOR (similar to Pten or Tsc1 KOs) and led to activation of HSCs, with an initial expansion of LSK cells followed by dramatic depletion of HSCs (as assessed by long-term reconstitution assays). That this was successfully prevented by treatment with rapamycin raises the intriguing possibility that the combination of lithium and rapamycin could be used to expand HSCs either in vivo or ex vivo in HSC transplants and in the therapy of hypoproliferative hematologic diseases.
Methods
Mice.
C57BL/6 WT (CD45.2), CD45.1 congenic (The Jackson Laboratory), and Mx-cre;β-cateninfl/fl mice were bred in house in a pathogen-free mouse facility of the University of Pennsylvania. Transplant recipients were female mice 10–12 weeks old. All work with mice was done according to a protocol reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
FCM and HSC isolation.
BM cells were flushed from the long bones (tibias and femurs) of mice with Hank’s buffered salt solution without calcium or magnesium, supplemented with 2% heat-inactivated calf serum. For detection of LSK Flk2 CD34 cells, whole BM cells were incubated with Biotin-conjugated monoclonal antibodies to lineage markers, including B220 (6B2), CD4 (GK1.5), CD8 (53-6.7), Gr-1 (8C5), Mac-1 (M1/70), Ter119, and IL-7R (A7R34), in addition to PE Cy5.5–conjugated anti–Sca-1 (Ly6A/E; D7), allophycocyanin–Alexa Fluor 750–conjugated anti–c-kit (ACK2), PE-conjugated anti-Flk2 (Ly-72/A2F10), and Alexa Fluor 647–conjugated anti-CD34. Biotin-conjugated lineage markers were detected using streptavidin-conjugated PE-Texas Red. Nonviable cells were excluded from sorting and analyses using the viability dye DAPI (1 g/ml). Cells were sorted with a FACSAria (BD) or MoFlo (Cytomation) automated cell sorter. Analysis was performed on LSR II or FACSCalibur flow cytometer (BD). Data were analyzed using FlowJo software (Tree Star).
Constructs and lentiviruses.
shRNAs sequences were designed using software from the Broad Institute (http://www.broad.mit.edu/genome_bio/trc/publicSearchForHairpinsForm.php), which identified 5 potential shRNA sequences in Gsk3 and PTEN. The shRNAs were cloned into the H1UG1 lentivirus (43), a 4-component, replication-incompetent system derived from FG12 (52), which uses the human U1 promoter to drive shRNA expression and the human Ubiquitin-C promoter to drive expression of GFP for lineage tracing. H1UG1 was provided by A. Gewirtz (University of Pennsylvania School of Medicine). High-titer lentiviral supernatant was produced by transient transfection of 293T cells and was tested in NIH3T3 cells.
BM transduction and transplantation.
Lentiviral transduction of 5-FU–treated BM cells and transplantation into lethally irradiated (10 Gy) recipients was performed as described previously (53).
Cell cycle analysis and BrdU incorporation.
Sorted GFP+ LSK and GFP+ LSK Flk2– cells from primary recipients of Gsk3-rnai or vector control for 4 months were incubated, as described previously (44), with 5 μg/ml Hoechst 33342 (Invitrogen) in HBSS containing 20 mM HEPES, 5 mM glucose, and 10% FBS at 37°C for 45 minutes, then incubated for 45 minutes with 1 μg/ml Pyronin (Sigma-Aldrich), and analyzed with an LSRII flow cytometer (BD Biosciences). For BrdU labeling, primary recipients of Gsk3-rnai or vector control were fed 0.5 mg/ml BrdU in the drinking water for the last 7 days of a 4-month transplant, and GFP+ LSK Flk2+ and GFP+ LSK Flk2– cells were sorted. BrdU incorporation was determined by FACS analysis using APC-conjugated antibodies specific to BrdU and PI according to the manufacturer’s protocol (BD Biosciences).
Long-term noncompetitive repopulation assay.
Adult recipient mice were irradiated with a Cs-137 Irradiator in 2 equal doses of 5 Gy separated by at least 2 hours. Cells were injected into the retroorbital venous sinus of anesthetized recipients. Each secondary recipient mouse received 2 × 105 sorted GFP+ BM cells from primary recipients of Gsk3-rnai or vector control, and each tertiary recipient mouse received 4 × 105 sorted GFP+ BM cells from secondary recipients. Beginning 4 weeks after transplantation and continuing for at least 16 weeks, blood was collected from the tail veins of recipient mice, red blood cells were lysed by ammonium chloride–potassium (Ack) buffer, and the remaining cells were stained with directly conjugated antibodies to B220 (6B2), Mac-1 (M1/70), CD4 (L3T4), CD8 (Ly-3), and Gr-1 (8C5) to monitor engraftment by FCM.
Long-term competitive repopulation assay.
Sorted GFP+ cells from primary recipients of Gsk3-rnai or vector control (tester) were transplanted into lethally irradiated B6 recipients together with 2 × 105 competitor B6 BM cells (CD45.2+; refs. 44, 45, 54). In limiting dilution analyses, decreasing numbers of tester GFP+ cells were used (i.e., 1 × 106, 5 × 105, 1 × 105, and 2 × 104). Beginning 4 weeks after transplantation and up to 16 weeks, blood was collected from the tail veins of recipient mice, red blood cells were lysed in Ack buffer, and the remaining cells were stained with directly conjugated antibodies to B220 (6B2), Mac-1 (M1/70), CD4 (L3T4), CD8 (Ly-3), and Gr-1 (8C5) to monitor engraftment by FCM. Blood and BM were analyzed 16 weeks after transplantation. The number of competitive repopulation units was calculated with L-Calc software (StemCell Technologies; refs. 44, 54).
Methylcellulose culture.
Sorted GFP+ BM cells were plated in individual wells of 6-well plates (Corning) containing 550 μl 1.0% methylcellulose (Stem Cell Technologies) as previously described (10, 34). The methylcellulose was supplemented with 1% penicillin/streptomycin (Gibco; Invitrogen), 50 ng/ml SCF, 10 ng/ml IL-3, 10 ng/ml IL-6, and 3 U/ml erythropoietin. Colonies were incubated at 37°C in humidified incubators at 5% CO2. Colony formation was scored by counting all colonies of greater than 30 viable cells after 10–14 days of culture.
Administration of polyI:polyC and rapamycin.
As described previously (29), polyI:polyC (Sigma-Aldrich) was resuspended in Dulbecco PBS at 2 mg/ml. Mice received 25 μg/g polyI:polyC every other day for 2 weeks. Rapamycin (LC Laboratories) was dissolved in absolute ethanol at 10 mg/ml and diluted in 5% Tween-80 (Sigma-Aldrich) and 5% PEG-400 (Hampton Research) before injection and was administered by intraperitoneal injection at 4 mg/kg rapamycin in 200 μl total volume/injection every other day for 8 weeks.
Statistics.
All data are mean ± SD. Statistical significance was determined by a 2-tailed Student’s t test, and a P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Celeste Simon, Morrie Birnbaum, Nancy Speck, Steve Liebhaber, Hui-Chuan Huang, Anthony Chi, Avinash Bhandoola, and Sean Morrison for insightful comments and discussion. We thank Anthony Chi and Avinash Bhandoola for help in FCM analysis of BAT-Gal mice, Alan Gewirtz for the H1UG vector, Jasmine Smith and Jake Kushner for the tip on dosing 6BIO, and all the members of Klein lab for help and discussions. We thank William DeMuth and the Clinical High-Speed Cell Separation Facility of the Abramson Family Cancer Research Institute for technical support. This study is supported by grants from the NIH (1RO1MH58324), the American Federation for Aging Research, and the Institute for Regenerative Medicine at the University of Pennsylvania. J. Huang is supported by a Leukemia and Lymphoma Society Career Development Award.
Footnotes
Conflict of interest: S.G. Emerson owns stock in Aastrom Biosciences. The authors have declared that no other conflict of interest exists.
Citation for this article: J. Clin. Invest. 119:3519–3529 (2009). doi:10.1172/JCI40572.
Yi Zhang’s present address is: Department of Internal Medicine (Hematology-Oncology), University of Michigan, Ann Arbor, Michigan, USA.
Stephen G. Emerson’s present address is: Office of the President, Haverford College, Haverford, Pennsylvania, USA.
References
- 1.Ballin A., Lehman D., Sirota P., Litvinjuk U., Meytes D. Increased number of peripheral blood CD34+ cells in lithium-treated patients. Br. J. Haematol. 1998;100:219–221. doi: 10.1046/j.1365-2141.1998.00537.x. [DOI] [PubMed] [Google Scholar]
- 2.Boggs D.R., Joyce R.A. The Hematopoietic Effects of Lithium. Semin. Hematol. 1983;20:129–138. [PubMed] [Google Scholar]
- 3.Joyce R.A. Sequential effects of lithium on haematopoiesis. Br. J. Haematol. 1984;56:307–321. doi: 10.1111/j.1365-2141.1984.tb03958.x. [DOI] [PubMed] [Google Scholar]
- 4.Ricci P., et al. Haematological effects of lithium carbonate: a study in 56 psychiatric patients. Haematologica. 1981;66:627–633. [PubMed] [Google Scholar]
- 5.Klein P.S., Melton D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedgepeth C., et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 7.Stambolic V., Ruel L., Woodgett J. Lithium inhibits glycogen synthase kinase–3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 8.Phiel C.J., Klein P.S. Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 9.Focosi D., Azzara A., Kast R.E., Carulli G., Petrini M. Lithium and hematology: established and proposed uses. J. Leukoc. Biol. 2009;85:20–28. doi: 10.1189/jlb.0608388. [DOI] [PubMed] [Google Scholar]
- 10.Trowbridge J.J., Xenocostas A., Moon R.T., Bhatia M. Glycogen synthase kinase–3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat. Med. 2006;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- 11.Goessling W., et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes T., et al. Glycogen synthase kinase–3beta inhibition preserves hematopoietic stem cell activity and inhibits leukemic cell growth. Stem Cells. 2008;26:1288–1297. doi: 10.1634/stemcells.2007-0600. [DOI] [PubMed] [Google Scholar]
- 13.Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3–specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 14.Ying Q.L., et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doble B.W., Patel S., Wood G.A., Kockeritz L.K., Woodgett J.R. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staal F.J., Burgering B.M., van de Wetering M., Clevers H.C. Tcf-1–mediated transcription in T lymphocytes: differential role for glycogen synthase kinase–3 in fibroblasts and T cells. Int. Immunol. 1999;11:317–323. doi: 10.1093/intimm/11.3.317. [DOI] [PubMed] [Google Scholar]
- 17.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 18.Staal F.J., Sen J.M. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur. J. Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra S., Kincade P.W. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4:27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin T.W., Solar G.P., Ziegler F.C., Liem L., Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- 21.Van Den Berg D.J., Sharma A.K., Bruno E., Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- 22.Willert K., et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 23.Reya T., et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 24.Jeannet G., et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 25.Baba Y., et al. Constitutively active beta-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J. Immunol. 2006;177:2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C., et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luis T.C., et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 28.Fleming H.E., et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobas M., et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch U., et al. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 31.Scheller M., et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat. Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 32.Kirstetter P., Anderson K., Porse B.T., Jacobsen S.E., Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz O.H., et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 35.Chen C., Liu Y., Liu R., Ikenoue T., Guan K.L., Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan B., et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoki K., et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 38.Gallicchio V.S., Messino M.J., Hulette B.C., Hughes N.K. Lithium and hematopoiesis: effective experimental use of lithium as an agent to improve bone marrow transplantation. J. Med. 1992;23:195–216. [PubMed] [Google Scholar]
- 39.Gallicchio V.S., Chen M.G. Modulation of murine pluripotential stem cell proliferation in vivo by lithium carbonate. Blood. 1980;56:1150–1152. [PubMed] [Google Scholar]
- 40.Purton L.E., Scadden D.T. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Kiel M.J., Yilmaz O.H., Iwashita T., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Hoeflich K.P., et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF- kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 43.Balint K., et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J. Clin. Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersenev A., Wu C., Balcerek J., Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J. Clin Invest. 2008;118:2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szilvassy S.J., et al. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc. Natl. Acad. Sci. U. S. A. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegfried E., Chou T.B., Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/S0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 47.Maretto S., et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacAulay K., et al. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007;6:329–337. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Castilho R.M., Squarize C.H., Chodosh L.A., Williams B.O., Gutkind J.S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.York J.D., Guo S., Odom A.R., Spiegelberg B.D., Stolz L.E. An expanded view of inositol signaling. Adv. Enzyme Regul. 2001;41:57–71. doi: 10.1016/S0065-2571(00)00025-X. [DOI] [PubMed] [Google Scholar]
- 51.Gurvich N., Klein P.S. Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol. Ther. 2002;96:45–66. doi: 10.1016/S0163-7258(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 52.Qin X.F., An D.S., Chen I.S., Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. U. S. A. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pui J.C., et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/S1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 54.Maillard I., et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.