Abstract
We recently developed a novel targeting Sindbis virus envelope pseudotyped lentiviral vector, 2.2ZZ, which acquires specific transduction capacity by antibody conjugation and binding with specific antigens on the surface of targeted cells. Here we characterize the virological properties of this vector by examining its targeting to CD4 antigen. Our results show that entry is dependent on CD4 cell surface density and occurs via the clathrin-mediated endocytic pathway. These findings provide insight into the mechanism of infection by a new viral vector with combined properties of Sindbis virus and lentiviruses and infectivity conferred by monoclonal antibody-ligand interactions.
Effective gene therapy in clinical settings will require the targeting of such therapies to specific tissues and organs while maintaining stable gene expression. We describe a novel targeting vector which acquires specific transduction capacity by antibody conjugation and binding to specific antigens on the surface of targeted cells. Our lab modified the fusion protein of Sindbis virus envelope, E2, by inserting an Fc-binding portion, the ZZ domain, of protein A (17, 19, 21). The lentiviral vector pseudotyped by this modified Sindbis envelope, which we designated 2.2ZZ vector, binds to and enters cells bearing specific cell surface antigens only when conjugated with the appropriate monoclonal antibody (17, 21). The 2.2ZZ vector has been used to target human leukocyte antigen (HLA) class I, CD4, CD19, CD20, CD34, CD45, CD146, P glycoprotein of melanoma cells, and prostate stem cell antigen successfully (10, 14-17, 20, 21). Wang and coworkers adapted an early form of 2.2ZZ, M168 (17), to generate a modified envelope with membrane-bound antibodies and used it to successfully target CD20 on B cells (27).
Here, we characterized the virological properties of this newly generated 2.2ZZ targeting vector, one that bears some properties of Sindbis virus and some properties of lentiviruses and also possesses certain novel properties of infectivity conferred by monoclonal antibody-ligand interactions, by studying the effect of surface receptor concentration on its transduction and its endocytic pathways. Native Sindbis virus exploits the clathrin-mediated pathway to enter cells (6, 8), whereas human immunodeficiency virus (HIV) fuses directly with the plasma membrane (25), although recent evidence suggests that HIV enters cell via endocytosis (13). Our goal was to ascertain the pathway of viral entry for this chimeric virus. We therefore examined 2.2ZZ vector targeting to CD4 antigen, since it is one of the best-characterized cell surface molecules with regard to its clathrin-mediated internalization and signaling pathway (22). We have previously demonstrated the viral specificity by targeting transduction to HLtat/CD4 cells and peripheral blood mononuclear cells via anti-CD4 antibodies (14). In this study, we first tested three antibodies which target different epitopes of CD4: the anti-CDR2-like region of CD4 in domain I (Leu3a) (12), the anti-CDR2-like region of CD4 in domain III (OKT4) (12), and domain II of CD4 (BL4) (2). Each antibody directs similar transduction efficiencies of the 2.2ZZ vector (data not shown). We selected the BL4 antibody for further studies of mechanics of viral transduction.
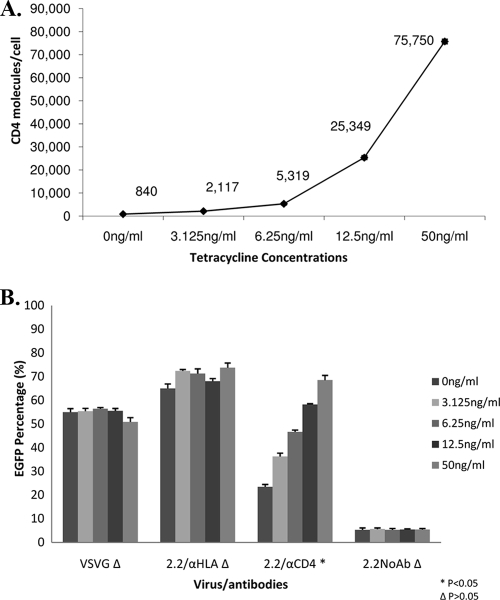
We examined whether surface receptor concentrations have an effect on vector transduction by using a 293 Affinofile cell line that inducibly expresses human CD4 molecules under the control of tetracycline (7, 9). Transduction by vesicular stomatitis virus G (VSV-G) pseudotypes and transduction of 2.2ZZ directed by antibodies to HLA class I showed no significant differences in transduction levels among cells expressing different numbers of CD4 molecules (P > 0.05) (Fig. 1B). On the other hand, enhanced transduction was observed when anti-CD4 antibodies were used, and that enhancement correlated with the increase of CD4 molecules present on cell surfaces (Fig. 1A). These findings show that on this cell line higher receptor concentration results in increased transduction by the 2.2ZZ vector.
FIG. 1.
Higher density of CD4 molecules on cell surfaces led to increased transduction of 2.2ZZ. (A) 293 Affinofile cells (1 × 105) carrying the tetracycline-inducible CD4 expression system were treated with different concentrations of tetracycline (0, 3.125, 6.25, 12.5, and 50 ng/ml) for 8 h. The cells were stained with anti-CD4 antibodies conjugated with PE. The number of CD4 molecules/cell was determined by normalizing the mean fluorescence of the cells to that of commercial PE beads. (B) After 8 h of induction with tetracycline, cells were transduced by 20 ng (HIV-1 p24) VSV-G pseudotyped lentiviral vectors, 2.2ZZ vectors with 0.4 μg anti-HLA or anti-CD4 antibodies, and 2.2ZZ vectors in the absence of antibodies, all for a period of 2 h. Three days postinfection, transductions were monitored by EGFP expression. P values represent significances of differences among cells treated with different concentrations of tetracycline.
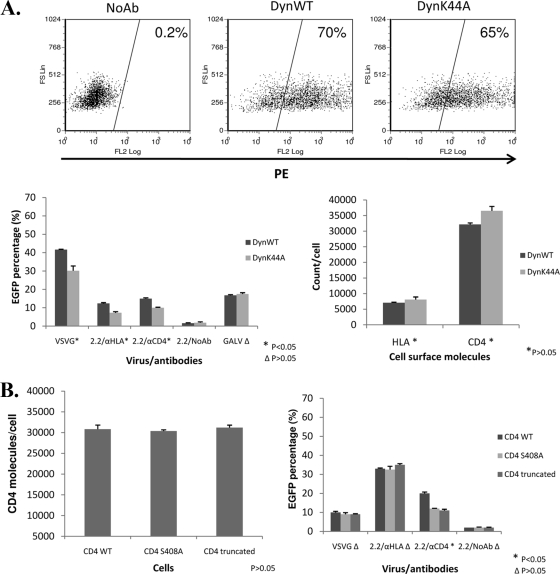
To determine whether the endocytic pathway is required for entry of 2.2ZZ, we first utilized a dominant-negative mutant of dynamin (dynK44A) to block the endocytic pathway (5). The dynamin wild type (dynWT) was used as a control. Both plasmids were transiently transfected into the 293T cells stably expressing CD4 molecules (293T/CD4 cells) to acquire at least 65% of dynamin expression (Fig. 2A). We then assessed transduction efficiency of 2.2ZZ vectors with anti-CD4 antibodies in the dynWT- and dynK44A-transfected cells. VSV-G pseudotypes served as a positive control, since VSV enters cells via the clathrin-mediated pathway (6, 26). Gibbon ape leukemia virus (GALV) pseudotypes served as a negative control, since the fusion of GALV occurs at the plasma membrane (18). For VSV-G pseudotypes and 2.2ZZ vectors with anti-HLA or anti-CD4 antibodies, the transduction efficiency was reduced in the dynK44A-transfected cells compared to the dynWT-transfected cells (P < 0.05) (Fig. 2A). No difference in transduction by GALV pseudotypes was observed in the dynWT- and dynK44A-transfected cells (P > 0.05). Since the surface levels of HLA and CD4 are not significantly different between the dynWT- and dynK44A-transfected cells (P > 0.05) (Fig. 2A), the difference in transduction efficiency does not result from differences in expression levels of the surface receptors. These data show that overexpression of dynK44A suppressed the transduction of 2.2ZZ using the CD4 molecule for entry, indicating that endocytosis likely plays a role in the entry of the 2.2ZZ vector.
FIG. 2.
Transduction of 2.2ZZ in 293T/CD4 cells was blocked by the dominant-negative mutant of dynamin and decreased in the CD4 mutant cells. (A) (Top) Three micrograms of hemagglutinin-tagged wild-type dynamin or dominant-negative mutant of dynamin was transfected into 1.2 × 106 293T cells stably expressing CD4 molecules by FuGENE (Roche). Cells (1 × 105) were stained with antihemagglutinin antibodies 24 h posttransfection. (Bottom left) Forty-eight hours posttransfection, 1 × 105 cells were transduced by 20 ng (HIV-1 p24) VSV-G pseudotyped lentiviral vectors, 2.2ZZ vectors with 0.4 μg anti-HLA or anti-CD4 antibodies, 2.2ZZ vectors in the absence of antibodies, and GALV pseudotyped lentiviral vectors for 2 h. Three days postinfection, transductions were monitored by EGFP expression. P values represent significances of differences between dynWT- and dynK44A-transfected cells. (Bottom right) Staining of the surface HLA and CD4 molecules. The number of HLA or CD4 molecules/cell was determined by normalizing the mean fluorescence of the cells to that of commercial PE beads. P values represent significances of differences between dynWT- and dynK44A-transfected cells. (B) (Left) Staining of the surface CD4 molecules on 293T cells stably expressing wild-type CD4, Ser408A CD4, and truncated CD4. The number of CD4 molecules/cell was determined by normalizing the mean fluorescence of the cells to that of commercial PE beads. P value represents significance of difference among cells expressing wild-type CD4, Ser408 CD4, and truncated CD4. (Right) 293T cells (1 × 105) stably expressing wild-type CD4, Ser408A CD4, and truncated CD4 were transduced by 20 ng (HIV-1 p24) VSV-G pseudotyped lentiviral vectors, 2.2ZZ vectors with 0.4 μg anti-HLA or anti-CD4 antibodies, and 2.2ZZ vectors in the absence of antibodies for 2 h. Three days postinfection, transductions were monitored by EGFP expression. P values represent significances of differences among cells expressing wild-type CD4, Ser408 CD4, and truncated CD4.
We also tested the role of endocytosis in the entry of 2.2ZZ using CD4 genes that differ in endocytosis properties. Mutation or truncation of the cytoplasmic tail results in mutants of CD4 that internalize at reduced rates (1). While wild-type CD4 molecules internalize at a rate of 5%/min, the mutant CD4 molecules internalize at a rate that is fourfold lower (24). We generated 293T cell lines that stably express wild-type CD4, CD4 with a mutation at Ser408, and cytoplasmic tail-truncated CD4. Staining by phycoerythrin (PE)-conjugated antibodies (clone S3.5; Caltag) of domain I of CD4 showed similar levels of CD4 molecules on the surfaces of cells (P > 0.05) (Fig. 2B). VSV-G pseudotypes and 2.2ZZ vectors with anti-CD4 antibodies were used to transduce cells expressing either wild-type or mutant CD4. For VSV-G pseudotypes and 2.2ZZ vectors with anti-HLA antibodies, transduction efficiency was not significantly different between cells expressing wild-type and those expressing mutant CD4 (P > 0.05) (Fig. 2B). However, transduction efficiency by the 2.2ZZ vector with anti-CD4 antibodies was significantly reduced (P < 0.05) in cells expressing mutant CD4 (Fig. 2B). These data suggest that 2.2ZZ targeted to CD4 on the cell surface enters those cells via endocytosis of the CD4 molecules.
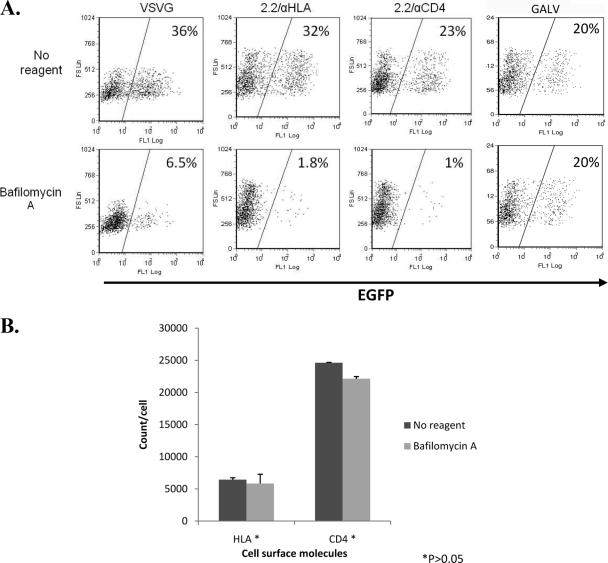
The most common endocytic pathway through which viruses enter cells is the clathrin-mediated pathway, the classic low-pH-dependent pathway. To determine whether the clathrin pathway is required for the 2.2ZZ vector, as it is for native Sindbis virus, we first blocked the clathrin pathway by neutralizing the endosome environment using 125 nM bafilomycin A (23) for 30 min. The 293T/CD4 cells were treated with bafilomycin A 30 minutes before, and again during, transduction. VSV-G pseudotypes, 2.2ZZ vectors with anti-HLA or anti-CD4 antibodies, and GALV pseudotypes were examined for transduction efficiency. Transductions by VSV-G pseudotypes and 2.2ZZ vectors with anti-HLA or anti-CD4 antibodies were all blocked in cells treated with bafilomycin A (Fig. 3A), while transductions by GALV pseudotypes were not affected. Since the surface levels of HLA and CD4 are not significantly different between the non-reagent-treated and bafilomycin A-treated cells (P > 0.05) (Fig. 3B), the difference in transduction efficiency does not result from differences in expression levels of the surface receptors. These results are consistent with utilization of the endocytic pathway by native VSV as well as the HLA and CD4 molecules and further indicate that the entry of 2.2ZZ vector is via clathrin-mediated endocytosis.
FIG. 3.
Acidification inhibitors blocked transduction of 2.2ZZ. (A) 293T cells (1 × 105) stably expressing CD4 molecules were pretreated with 125 nM bafilomycin A for 30 min. Cells were then transduced by 20 ng (HIV-1 p24) VSV-G pseudotyped lentiviral vectors, 2.2ZZ vector with 0.4 μg anti-HLA or anti-CD4 antibodies, and GALV pseudotyped lentiviral vectors for 2 h in the presence of bafilomycin A. Two hours later, viruses were removed and cells were washed once with 1× phosphate-buffered saline and cultured in medium for 3 days. Transductions were monitored by EGFP expression. (B) Staining of the surface HLA and CD4 molecules. The number of HLA or CD4 molecules/cell was determined by normalizing the mean fluorescence of the cells to that of commercial PE beads. P values represent significances of differences between non-reagent-treated and bafilomycin A-treated cells.
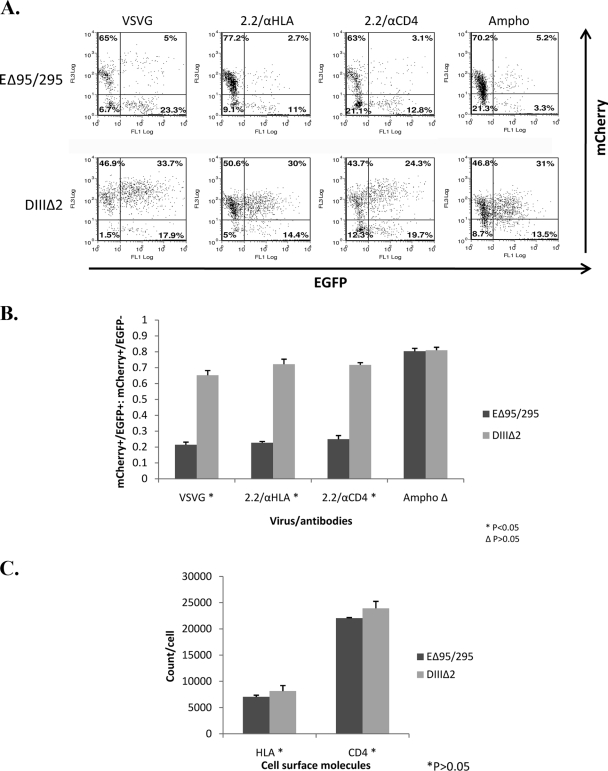
Besides using acidification inhibitors, we applied a more direct means that utilized a dominant-negative mutant of Eps15, EΔ95/295, to demonstrate that infectivity occurs via the clathrin-mediated pathway (3, 4). Eps15 is a protein that binds to the AP-2 adapter required for internalization by clathrin-coated pits. The dominant-negative form of Eps15 inhibits endocytosis by clathrin-coated pits by competing with the endogenous Eps15 for AP-2 (3). The 293T/CD4 cells were transiently transfected with EΔ95/295. Another mutant, DIIIΔ2 (3, 4), which lacks the AP-2 binding domain, was used as a negative control. These two plasmids, EΔ95/295 and DIIIΔ2, contain the enhanced green fluorescent protein (EGFP), and so transfection efficiency could be monitored by measuring the EGFP+ cells. Lentiviral vectors (FU11mCherry) that express mCherry protein instead of EGFP were used to generate the VSV-G pseudotypes and 2.2ZZ vectors. The 293T/CD4 cells overexpressing the EGFP-tagged EΔ95/295 and DIIIΔ2 proteins were transduced by VSV-G pseudotypes and 2.2ZZ vectors conjugated with anti-HLA or anti-CD4 antibodies. Amphotropic murine retrovirus fuses at the plasma membrane (11); therefore, lentiviral vectors pseudotyped by amphotropic murine retroviral envelope were included as a negative control. Infectivity was assessed by measuring the mCherry+ cells. Our results showed that transductions by VSV-G pseudotypes and 2.2ZZ vectors with anti-HLA or anti-CD4 antibodies were markedly inhibited within the EGFP+ cell populations (measured by ratio of mCherry+/EGFP+ to mCherry+/EGFP− cells) overexpressing the EΔ95/295 protein compared to cells overexpressing the DIIIΔ2 protein (P < 0.05) (Fig. 4A and B). Transductions by amphotropic pseudotypes were not significantly affected by the clathrin pathway inhibitor, EΔ95/295. Since the surface levels of HLA and CD4 are not significantly different between the EΔ95/295- and DIIIΔ2-transfected cells (Fig. 4C), the difference in transduction efficiency does not result from differences in expression levels of the surface receptors. These results confirm that 2.2ZZ targeting to the CD4 molecule enters cells via the clathrin-mediated pathway.
FIG. 4.
Transduction of 2.2ZZ in 293T/CD4 cells was blocked by dominant-negative mutant of Eps15. (A) Three micrograms of EGFP-fused dominant-negative mutant of Eps15 (EΔ95/295) and DIIIΔ2, which lacks the AP-2 binding domain, was transfected into 1.2 × 106 293T cells stably expressing CD4 molecules by FuGENE. Forty-eight hours posttransfection, 1 × 105 cells were transduced by 100 ng (HIV-1 p24) VSV-G pseudotyped lentiviral vectors or 2.2ZZ vector with 0.4 μg anti-HLA or anti-CD4 antibodies for 2 hours and 5 × 104 cells were transduced by 4 μl 100× concentrated amphotropic retroviral envelope pseudotyped lentiviral vectors for 6 h. Three days postinfection, transfections were monitored by EGFP expression and transductions were monitored by mCherry expression. (B) Calculations of the ratios of mCherry+/EGFP+ to mCherry+/EGFP− cells. P values represent significances of differences between EΔ95/295- and DIIIΔ2-transfected cells. (C) Staining of the surface HLA and CD4 molecules. The number of HLA or CD4 molecules/cell was determined by normalizing the mean fluorescence of the cells to that of commercial PE beads. P values represent significances of differences between EΔ95/295- and DIIIΔ2-transfected cells.
Our results provide insight into the mechanism of infection by our targeting vectors. The efficiency of transduction increases with greater CD4 receptor density and higher rates of endocytosis. Thus, the properties of any given receptor will be critical for the future application of targeting to specific cells for laboratory or clinical purposes.
These results have implications for the evolution of viral entry processes. The clathrin-dependent pathway for endocytosis utilized by ZZ virus entry when directed to CD4 as a receptor is the same pathway utilized by native Sindbis virus envelope, which utilizes heparin sulfate and laminin as receptors. Thus, redirection of viral tropism to utilize completely different binding receptors still maintains the same fundamental pathway for entry. Using antibodies to redirect viral infectivity through different cell surface molecules can be considered to reflect a natural evolutionary process whereby viruses acquire the ability to utilize different cell surface receptors. In nature, this evolution probably occurs through genetic variation rather than through bridging molecules such as antibodies; however, regardless of the mechanism, the first step in viral infection is the acquisition of binding to new cell surface receptors. Our results suggest that the mechanics of subsequent steps of internalization and fusion are conserved and therefore occur independently of the initial ligand-receptor interaction. Thus, at least in the case of Sindbis virus, docking of envelope to the receptor and subsequent internalization and fusion events are likely to have evolved independently.
Acknowledgments
We thank D. S. An (UCLA) for the FU11mCherry vector. We thank A. Benmerah for the EΔ95/295 and DIIIΔ2 plasmids. We thank E. Bayrd and R. Lee for editorial assistance with the manuscript. Flow cytometry was performed in the Flow Cytometry Core Facility at the UCLA AIDS Institute.
This work was supported by U.S. National Institutes of Health grant AI069350.
M.L. designed and performed the studies, analyzed data, and wrote the manuscript. K.M. and N.P. contributed vital reagents, helped with troubleshooting, participated in experimental design and discussion, and reviewed the manuscript. M.K. helped with the experiments using amphotropic virus. B.L. contributed to the tetracycline-inducible 293 cell line. I.S.Y.C. supervised the study.
We declare no competing financial interests.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Bedinger, P., A. Moriarty, R. C. von Borstel II, N. J. Donovan, K. S. Steimer, and D. R. Littman. 1988. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature 334:162-165. [DOI] [PubMed] [Google Scholar]
- 2.Benkirane, M., M. Hirn, D. Carriere, and C. Devaux. 1995. Functional epitope analysis of the human CD4 molecule: antibodies that inhibit human immunodeficiency virus type 1 gene expression bind to the immunoglobulin CDR3-like region of CD4. J. Virol. 69:6898-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan, D. P., and B. M. Sefton. 1978. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell 15:985-992. [DOI] [PubMed] [Google Scholar]
- 7.Johnston, S. H., M. A. Lobritz, S. Nguyen, K. Lassen, S. Delair, F. Posta, Y. J. Bryson, E. J. Arts, T. Chou, and B. Lee. 2009. A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J. Virol. 83:11016-11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kielian, M. 1995. Membrane fusion and the alphavirus life cycle. Adv. Virus Res. 45:113-151. [DOI] [PubMed] [Google Scholar]
- 9.Lassen, K. G., M. A. Lobritz, J. R. Bailey, S. Johnston, S. Nguyen, B. Lee, T. Chou, R. F. Siliciano, M. Markowitz, and E. J. Arts. 2009. Elite suppressor-derived HIV-1 envelope glycoproteins exhibit reduced entry efficiency and kinetics. PLoS Pathog. 5:e1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang, M., N. Pariente, K. Morizono, and I. S. Chen. 2009. Targeted transduction of CD34+ hematopoietic progenitor cells in nonpurified human mobilized peripheral blood mononuclear cells. J. Gene Med. 11:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71:767-773. [DOI] [PubMed] [Google Scholar]
- 12.Merkenschlager, M., D. Buck, P. C. Beverley, and Q. J. Sattentau. 1990. Functional epitope analysis of the human CD4 molecule. The MHC class II-dependent activation of resting T cells is inhibited by monoclonal antibodies to CD4 regardless whether or not they recognize epitopes involved in the binding of MHC class II or HIV gp120. J. Immunol. 145:2839-2845. [PubMed] [Google Scholar]
- 13.Miyauchi, K., Y. Kim, O. Latinovic, V. Morozov, and G. B. Melikyan. 2009. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morizono, K., G. Bristol, Y. M. Xie, S. K. Kung, and I. S. Chen. 2001. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 75:8016-8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morizono, K., and I. S. Chen. 2005. Targeted gene delivery by intravenous injection of retroviral vectors. Cell Cycle 4:854-856. [DOI] [PubMed] [Google Scholar]
- 16.Morizono, K., G. E. Ringpis, N. Pariente, Y. Xie, and I. S. Chen. 2006. Transient low pH treatment enhances infection of lentiviral vector pseudotypes with a targeting Sindbis envelope. Virology 355:71-81. [DOI] [PubMed] [Google Scholar]
- 17.Morizono, K., Y. Xie, G. E. Ringpis, M. Johnson, H. Nassanian, B. Lee, L. Wu, and I. S. Chen. 2005. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 11:346-352. [DOI] [PubMed] [Google Scholar]
- 18.O'Hara, B., S. V. Johann, H. P. Klinger, D. G. Blair, H. Rubinson, K. J. Dunn, P. Sass, S. M. Vitek, and T. Robins. 1990. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1:119-127. [PubMed] [Google Scholar]
- 19.Ohno, K., K. Sawai, Y. Iijima, B. Levin, and D. Meruelo. 1997. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15:763-767. [DOI] [PubMed] [Google Scholar]
- 20.Pariente, N., S. H. Mao, K. Morizono, and I. S. Chen. 2008. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J. Gene Med. 10:242-248. [DOI] [PubMed] [Google Scholar]
- 21.Pariente, N., K. Morizono, M. S. Virk, F. A. Petrigliano, R. E. Reiter, J. R. Lieberman, and I. S. Chen. 2007. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol. Ther. 15:1973-1981. [DOI] [PubMed] [Google Scholar]
- 22.Pelchen-Matthews, A., J. E. Armes, and M. Marsh. 1989. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 8:3641-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez, L., and L. Carrasco. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitcher, C., S. Honing, A. Fingerhut, K. Bowers, and M. Marsh. 1999. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol. Biol. Cell 10:677-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659-668. [DOI] [PubMed] [Google Scholar]
- 26.Sun, X., V. K. Yau, B. J. Briggs, and G. R. Whittaker. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 338:53-60. [DOI] [PubMed] [Google Scholar]
- 27.Yang, L., L. Bailey, D. Baltimore, and P. Wang. 2006. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA 103:11479-11484. [DOI] [PMC free article] [PubMed] [Google Scholar]