Abstract
Objective
To determine whether breast feeding protects infants against pneumonia and whether the protection varies with age.
Design
Nested case-control study.
Setting
Pelotas, southern Brazil.
Subjects
Cases were 152 infants aged 28-364 days who had been admitted to hospital for pneumonia. Controls were 2391 cases in a population based case-control study.
Main outcome measure
Odds ratio of admission for pneumonia according to type of milk consumed (breast milk alone, breast and formula milk, or formula milk and other fluids only), use of fluid supplements apart from formula milk, and use of solid supplements.
Results
Infants who were not being breast fed were 17 times more likely than those being breast fed without formula milk to be admitted to hospital for pneumonia (95% confidence interval 7.7 to 36.0). This relative risk was 61 (19.0 to 195.5) for children under 3 months old, decreasing to 10 (2.8 to 36.2) thereafter. Supplementation with solids was associated with a relative risk of 13.4 (7.6 to 23.5) for all infants and 175 (21.8 to 1405.1) for those under 3 months old.
Conclusion
Breast feeding protects young children against pneumonia, especially in the first months of life. These results may be used for targeting intervention campaigns at the most vulnerable age groups.
Key messages
Pneumonia is the leading cause of death in children under 5 years old across the world
In Brazil infants who were not breast fed were 17 times more likely than those receiving breast milk alone to be admitted for pneumonia
The relative risk of admission was 61 for children under 3 months of age, decreasing to 10 thereafter
Supplementation with solids was associated with a relative risk of 13.4 for all infants
Mothers must be encouraged to breast feed very young infants and be advised of the right time to introduce supplementary foods
Introduction
Pneumonia is the leading cause of death in children under 5 years old worldwide,1,2 and breast milk is the most important food in the first year of life.3
Several studies in less developed countries have assessed the effect of breast feeding on the risk of developing acute lower respiratory infections, particularly pneumonia.4 Most of these studies show a protective effect of breast milk on pneumonia, but causality has not yet been shown.4 In addition, whether this protection changes with age, as has been shown for diarrhoea,5 is not known.
We performed a nested case-control study in southern Brazil to assess whether breast feeding protects young children against pneumonia and whether this protection varies with age.
Participants and methods
Study population
Throughout 1993 all women who lived in urban areas and had their babies in Pelotas, southern Brazil, were interviewed soon after delivery in the city’s hospital. Over 99% of all births in this city take place in such hospitals.6 A systematic sample of 655 newborn infants was selected for home visiting at 1 and 3 months of age according to date and time of birth. In the first month the mothers of 99.1% (5256) of the children were interviewed and in the third month 98.3% (5214). These infants were also visited at the age of 6 months. As additional funds were obtained for data collection, the sampling fraction was increased to 1144, including the 655 visited at 1 and 3 months of age. These samples represent 12.3% (655) of all children from the original cohort in the first and third months and 21.6% (1144) in the sixth.
Defining cases
Cases of pneumonia were identified through daily visits to the city’s hospitals. Children who were born in 1993 and had been admitted to a hospital when aged 28-364 days were considered for inclusion in the study. Two independent referees (paediatricians) reviewed all the available information on each child from hospital’s records. Pneumonia was diagnosed from the presence of all clinical signs (difficult or rapid breathing, chest indrawing), presence of rales, and—whenever available—results of laboratory and radiological tests. Whenever the two referees disagreed, a third senior referee established the final diagnosis.
Defining controls
The control group was made up of children taking part in the cohort study. For cases aged 28-89 days, controls were infants at the first home visit, who were aged about 30 days. For cases aged 90-179 days, controls were infants at the second home visit, and for cases aged 180-364 days, controls were infants at the third visit.
For each interview all available controls were used. The study was therefore ratified for age but not matched at the individual level. A child who became a case at, say, 9 months old should have been a control at an earlier age. This characterises this study as a case base, or inclusive, design.7
Questionnaire
The mothers of cases were interviewed at home soon after the infant had been discharged from hospital using the same questionnaire as was used for the mothers of controls. Information on diet was collected for cases at the age of their corresponding controls at the home visit. For example, for a case aged 45 days dietary information was obtained for the exact age of 30 days. Three variables were studied.
Type of milk consumed—breast milk alone, breast and formula milk, or other fluids alone (water, teas, juices, formula milk, or any other liquid supplement except breast milk; this group was considered to be completely weaned)
Use of fluid supplements—whether infants received water, teas, juices, or any other liquid supplements excluding formula milk
Use of solid and semisolid supplements.
Social class was based on family income, parental schooling, the occupation of the head of household (person with highest salary). This resulted in the following categories: bourgeois and new small bourgeois (professionals and owners of large businesses), small traditional bourgeois (owners of small businesses and shopkeepers), atypical proletariat (non-manual workers in regular employment), typical proletariat (manual workers in regular employment), and subproletariat (unemployed and casual workers).8 Family income was defined as the total amount received by all people who lived in the one home during the previous month. This total was converted into the number of minimum wages.
Sample size
The sample size studied was sufficient for detecting an odds ratio of 2.0 for exposures present in 25% of the control children, with an α error of 0.05 and a power of 80%.9 An additional 40% was added to adjust for confounding variables and to compensate for possible refusals.10 According to this estimate, the final sample size should have at least 143 cases and 572 controls (four controls per case).
Statistical analysis
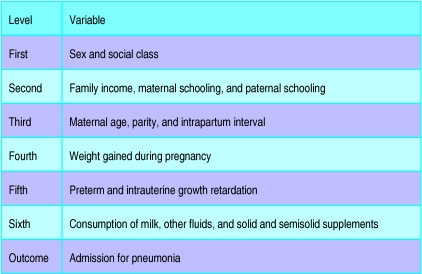
We measured odds ratios with 95% confidence intervals and used the χ2 test for contingency tables in analyses.11 We adjusted analyses using unconditional multiple logistic regression according to a previously determined hierarchical framework (figure). In this model some variables are assumed to mediate their effects through other variables as well as directly. The outcome variable was admission for pneumonia. The significance level for the inclusion of each variable in the model was measured by the likelihood ratio test. The final model included all variables that had a P value up to 0.10 after adjustment for variables in the same and higher levels of the framework. In addition, each ordinal variable—for example, family income group—was evaluated for linear tendency. When this association was significant and did not deviate from linearity, the variable was included in the model as a linear component. When missing values were less than 5% of all cases, they were recorded as the mode. Data on social class were missing in 7% of interviews,12 and we therefore created a separate category for missing values for this variable. All data were analysed with spss for Windows13 and Egret.14
Results
Of the 5304 infants in the original cohort, 152 (2.9%) were admitted to a hospital with pneumonia in the postneonatal period.
Among 250 variables tested, only social class, family income, and maternal schooling, age, parity, and weight gained during pregnancy were associated with outcome (table 1).
Table 1.
Unconditional multiple logistic regression model for risk of postneonatal pneumonia
| Variable | Odds ratio (95% CI) |
|---|---|
| Model 1: sex | |
| Male | 1.16 (0.84 to 1.60) |
| Female | 1.00 |
| P value | 0.36 |
| Model 2: model 1+social class* | |
| Bourgeois and new small bourgeois | 0.20 (0.27 to 1.44) |
| Small traditional bourgeois | 1.49 (0.89 to 2.47) |
| Atypical proletariat | 1.00 |
| Typical proletariat | 1.78 (1.14 to 2.76) |
| Subproletariat | 3.50 (2.15 to 5.70) |
| P value for linear trend | <0.001 |
| Model 3: model 2+maternal schooling+family income | |
| Maternal schooling (years): | |
| 0 | 2.70 (0.98 to 7.45) |
| 1-4 | 3.24 (1.73 to 6.09) |
| 5-8 | 1.97 (1.07 to 3.61) |
| ⩾9 | 1.00 |
| P value for linear trend | <0.001 |
| Model 4: model 3+maternal age+parity | |
| Maternal age (years): | |
| <20 | 1.98 (1.12 to 3.51) |
| 20-24 | 1.32 (0.82 to 2.13) |
| 25-29 | 1.00 |
| 30-34 | 0.96 (0.56 to 1.63) |
| ⩾35 | 0.75 (0.40 to 1.42) |
| P value for linear trend | 0.08 |
| Parity: | |
| 0 | 1.00 |
| 1 | 1.05 (0.64 to 1.74) |
| 2 | 1.53 (0.87 to 2.68) |
| ⩾3 | 2.86 (1.64 to 4.99) |
| P value for linear trend | <0.01 |
| Model 5: model 4+weight gained during pregnancy | |
| Weight gained during pregnancy | |
| <10 kg | 1.38 (1.00 to 1.92) |
| ⩾10 kg | 1.00 |
| P value | 0.05 |
Bourgeois and new small bourgeois are equivalent to professionals and owners of large businesses, small traditional bourgeois to owners of small businesses and shopkeepers, atypical proletariat to non-manual workers in regular employment, typical proletariat to manual workers in regular employment, and subproletariat to unemployed and casual workers.
Table 2 shows the frequency distributions of cases and controls according to these variables. The fact that there were more controls aged 6-11 months was due to the sampling scheme used in the cohort study. This does not affect the analyses since all information on feeding was referred to the exact age at the start of each age range.7
Table 2.
Numbers (percentages) of cases of pneumonia and controls according to main risk factors
| Risk factor | Cases (n=152) | Controls (n=2391) |
|---|---|---|
| Sex: | ||
| Male | 81 (53) | 1197 (50) |
| Female | 71 (47) | 1194 (50) |
| Age (days): | ||
| 30-89 | 47 (31) | 649 (27) |
| 90-179 | 62 (41) | 644 (27) |
| 180-364 | 43 (28) | 1098 (46) |
| Social class*: | ||
| Bourgeois (all categories) | 26 (17) | 499 (21) |
| Atypical proletariat | 39 (26) | 1034 (43) |
| Typical proletariat | 42 (27) | 518 (22) |
| Subproletariat | 30 (20) | 174 (7) |
| Family income (No of minimum wages per month†): | ||
| ⩽1 | 34 (22) | 415 (17) |
| 1.1-3 | 72 (47) | 1103 (46) |
| >3 | 46 (30) | 873 (36) |
| Maternal schooling (years): | ||
| 0 | 6 (4) | 63 (3) |
| 1-4 | 67 (44) | 557 (23) |
| 5-8 | 65 (43) | 1153 (48) |
| ⩾9 | 14 (9) | 618 (26) |
| Maternal age (years): | ||
| <20 | 35 (23) | 358 (15) |
| 20-24 | 41 (27) | 719 (30) |
| 25-29 | 35 (23) | 633 (26) |
| 30-34 | 26 (17) | 438 (18) |
| ⩾35 | 15 (10) | 243 (10) |
| Parity: | ||
| 0 | 42 (28) | 849 (35) |
| 1 | 30 (20) | 663 (28) |
| 2 | 26 (17) | 465 (19) |
| ⩾3 | 54 (35) | 414 (17) |
| Weight gained during pregnancy: | ||
| <10 kg | 82 (54) | 1584 (66) |
| ⩾10 kg | 70 (46) | 807 (34) |
Bourgeois (all categories) includes professionals, owners of large businesses, owners of small businesses, and shopkeepers; atypical proletariat is equivalent to non-manual workers in regular employment, typical proletariat to manual workers in regular employment, and subproletariat to unemployed and casual workers.
Around $100 in 1993.
Table 3 shows that the relative risk of admission for pneumonia for infants receiving breast and formula milk or other fluids alone was 3.8 and 16.7 respectively in comparison with infants who were exclusively breast fed. When infants who received fluid supplements were compared with those who did not the risk disappeared after adjusted analysis. Infants receiving solid and semisolid supplements had a relative risk of 8.5 of being admitted in comparison with those who did not receive such supplements.
Table 3.
Odds ratios for developing pneumonia according to type of food given
| Variable | Cases (n=152) | Controls (n=2391) | Odds ratio (95% CI)*
|
|
|---|---|---|---|---|
| Crude | Adjusted† | |||
| Type of milk consumed | ||||
| Breast milk alone | 9 | 779 | 1.0 | 1.0 |
| Breast and formula milk | 23 | 563 | 4.5 (2.1 to 9.9) | 3.8 (1.7 to 8.9) |
| Other fluids alone (completely weaned) | 120 | 1049 | 19.0 (9.3 to 38.7) | 16.7 (7.7 to 36.0) |
| P value | <0.001 | <0.001 | ||
| Fluid supplementation | ||||
| Fluids given | 149 | 2230 | 4.5 (1.4 to 14.5) | 1.3 (0.3 to 4.9) |
| Fluids not given | 3 | 161 | 1.0 | 1.0 |
| P value | <0.001 | 0.73 | ||
| Solid and semisolid supplementation | ||||
| Supplements given | 97 | 1226 | 13.4 (7.6 to 23.5) | 8.5 (4.7 to 15.4) |
| Supplements not given | 55 | 1165 | 1.0 | 1.0 |
| P value | <0.001 | <0.001 | ||
Stratified by age groups of 1-2.9, 3-5.9, and 6-11.9 months.
For sex, social class, family income, and maternal schooling, age, parity, and weight gained during pregnancy. In addition, each feeding variable was controlled for the other two.
Table 4 shows that after adjustment infants receiving breast and formula milk at the age of 1-2.9 months were 2.9 times more likely to be admitted for pneumonia than were those who received breast milk alone. The relative risk for infants who were completely weaned was 61.1. From age 3-5.9 months these relative risks were 3.4 and to 10.1 respectively. From age 6-11.9 months the odds ratios were 3.7 and 9.2 respectively. The interaction between age and the type of milk consumed was significant (P<0.001).
Table 4.
Odds ratios for developing pneumonia according to type of food given stratified for age
| Cases (n=152) | Controls (n=2391) | Odds ratio (95% CI)
|
||
|---|---|---|---|---|
| Crude | Adjusted* | |||
| Age 1-2.9 months | ||||
| Type of milk consumed: | ||||
| Breast milk alone | 5 | 392 | 1.0 | 1.0 |
| Breast and formula milk | 7 | 169 | 3.2 (1.0 to 10.4) | 2.9 (0.8 to 10.5) |
| Other fluids alone (completely weaned) | 35 | 88 | 31.2 (11.9 to 81.9) | 61.1 (19.0 to 195.5) |
| P value | <0.001 | <0.001 | ||
| Age 3-5.9 months | ||||
| Type of milk consumed: | ||||
| Breast milk alone | 3 | 212 | 1.0 | 1.0 |
| Breast and formula milk | 11 | 164 | 4.7 (1.3 to 17.2) | 3.4 (0.9 to 13.5) |
| Other fluids alone (completely weaned) | 48 | 268 | 12.5 (3.8 to 40.8) | 10.1 (2.8 to 36.2) |
| P value | <0.001 | <0.001 | ||
| Age 6-11.9 months | ||||
| Type of milk consumed: | ||||
| Breast milk alone | 1 | 175 | 1.0 | 1.0 |
| Breast and formula milk | 5 | 230 | 3.8 (0.4 to 32.9) | 3.7 (0.4 to 33.8) |
| Other fluids alone (completely weaned) | 37 | 693 | 9.3 (1.3 to 68.6) | 9.2 (1.2 to 69.7) |
| P value | <0.001 | <0.01 | ||
For sex, social class, family income, and maternal schooling, age, parity, and weight gained during pregnancy. In addition, each feeding variable was controlled for other two.
The crude analysis showed that the risk associated with the intake of supplementary foods in the first months was 175 for children aged 1-2.9 months, 9.1 for children aged 3-5.9 months, and 0.7 for children aged 6-11.9 months. The odds ratio of pneumonia admission for all children who received supplementary food was 13.4.
Discussion
Methodological limitations
Case-control studies may be affected by several biases.4,15–18 Reverse causality bias—that is, repeated respiratory illnesses leading to a change in breastfeeding pattern—was avoided by regarding as still breastfed infants who had stopped breast feeding because of a respiratory infection up to two months before admission. Another possibility is recall bias, since mothers of cases in a given age range (1-2.9, 3-5.9, and 6-11.9 months) were asked to provide retrospective information on feeding patterns at the beginning of that interval, while mothers of control children were interviewed within a few days of that date. To assess how this could affect the estimates of relative risk we analysed the reported feeding patterns of 32 infants who had been both a case and a control. For 26 infants the type of milk consumed was the same in both interviews (three were receiving breast milk alone, five were receiving breast and formula milk, and 18 were completely weaned). The kappa index was 0.81, reflecting good concordance. Of the six mothers whose information was discordant, five overestimated and one underestimated the intake of breast milk. With this adjustment the odds ratio for breast and formula milk increased from 3.5 to 5.6 and that for formula milk decreased from 9.9 to 6.9 (table 5). Therefore, recall bias may have reduced the estimate of risk for children receiving both breast and formula milk and increased the risk for infants who had been completely weaned. However, our main conclusions remain unchanged. Berkson paradox was controlled for during adjusted analysis,19 and limitation related to diagnostic criteria was reduced by using referees.12
Table 5.
Simulation to assess effect of misclassification on odds ratios for being admitted for pneumonia for all children
| Type of milk consumed | Cases
|
Controls (n=2391) | Odds ratio
|
||
|---|---|---|---|---|---|
| Original (n=152) | Adjusted (n=152) | Original | Adjusted* | ||
| Breast milk alone | 9 | 10.6 | 779 | 1.0 | 1.0 |
| Breast and formula milk | 23 | 43.2 | 563 | 3.5 | 5.6 |
| Other fluids alone (completely weaned) | 120 | 98.2 | 1049 | 9.9 | 6.9 |
For sex, social class, family income, and maternal schooling, age, parity, and weight gained during pregnancy.
Previous studies
Recent publications have emphasised the need for using standard definitions of feeding patterns to allow comparison between studies.20 In our sample few infants were exclusively breast fed—20% in the first month and 1.6% at three months—because formula milk and herbal teas are widely used.21 The low rate of exclusive breast feeding precludes the use of such infants as the baseline category with the lowest expected risk. We therefore used three different variables (type of milk consumed, intake of fluid supplements, and intake of solid and semisolid supplements) to characterise feeding patterns. With this approach the dose-response effect of the type of milk consumed could be assessed—most studies treat breast feeding as a dichotomous variable4,18—and the effects of milk, fluids, and other foods could be separated.
Several studies from less developed countries show that the risk of acquiring an acute lower respiratory infection or pneumonia is 1.5-4 times greater among infants who are not breast fed.4,18,22–27 In our study the risk of admission for pneumonia was 17 times greater among infants who were not being breast fed. Even for children who received both maternal and formula milk, the risk was about four times greater than that for children who received breast milk alone. This marked dose-response effect, along with the biological plausibility of a link between breast feeding and pneumonia, is strongly supportive of a causal association.4,18,22
A Peruvian study investigated whether the protection of breast feeding against respiratory infections changed with age,24 but it was controlled for few confounding factors. In Brazil the interaction observed was not significant.22 Further research from Brazil,23 Argentina,25 India,26 and China27 did not report interactions between age and breast feeding. In our study the protective effect of breast milk was markedly stronger among young infants than at later ages. This finding is biologically plausible since the immature immune system of young infants is likely to render as even more important the protection afforded by breast milk.3
Conclusions
The relative risks of pneumonia associated with the introduction of supplementary foods also varied markedly with age. To our knowledge, this interaction had not been previously described in the literature.
This study shows that breast feeding protects infants against pneumonia and that this proctection varies considerably according to infant age. These findings reinforce the need for targeting breastfeeding promotion efforts at the mothers of very young infants and for recommending the timely introduction of supplementary foods.
Figure.
Hierarchical framework for multiple logistic regression
Acknowledgments
We particularly thank Alexander M Walker, Department of Epidemiology, Harvard School of Public Health, and Saul S Morris, Department of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine for their critical reading of the manuscript and their generous comments. We thank the three independent referees who diagnosed pneumonia from hospital records: Drs Elaine P Albernaz, Luciani M Oliveira, and Ricardo Halpern.
Editorial by Latham
Footnotes
Funding: This study was supported by the European Community, World Health Organisation, and Fundação de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS), Brazil.
Competing interests: None declared.
References
- 1.Pio A, Leowski J, Dam HG. The magnitude of the problem of acute respiratory infections. In: Douglas RM, Kerby-Eaton E, editors. Acute respiratory infections in childhood. Proceedings of an international workshop, Sydney, August 1984. Adelaide: University of Adelaide; 1985. pp. 100–103. [Google Scholar]
- 2.Schwartz B, Lipman H, Lob-Levyt J, Gove S. The aetiology of acute lower respiratory infections among young children in developing countries. Geneva: World Health Organisation; 1994. [Google Scholar]
- 3.Jelliffe DB, Jelliffe EPF. Human milk in the modern world. Oxford: Oxford University Press; 1978. pp. 84–96. [Google Scholar]
- 4.Victora CG, Kirkwood B, Ashworth A, Black RE, Rogers S, Sazawal S, et al. Potential interventions for the prevention of childhood pneumonia in developing countries: improving nutrition. Am J Clin Nutr (in press). [DOI] [PubMed]
- 5.Feachem RG, Koblinski MA. Interventions for the control of diarrhoea diseases among young children: promotion of breast-feeding. Bull World Health Organ. 1984;62:271–291. [PMC free article] [PubMed] [Google Scholar]
- 6.Victora CG, Barros FC, Halpern R, Menezes AMB, Horta BL, Tomasi E, et al. Longitudinal study of mother and child population in an urban region of southern Brazil, 1993. Methodological aspects and preliminary results. Revista de Saúde Pública. 1996;30:34–45. doi: 10.1590/s0034-89101996000100005. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues L, Kirkwood BR. Case-control design in the study of common diseases: updates on the demise of the rare disease assumption and the choice of sampling scheme for controls. Int J Epidemiol. 1990;13:87–93. doi: 10.1093/ije/19.1.205. [DOI] [PubMed] [Google Scholar]
- 8.Bronfman M, Lombardi C, Facchini LA, Victora CG, Barros FC, Beria JU, et al. The operation of the concept of social class in epidemiological studies. Revista de Saúde Pública. 1988;22:253–265. doi: 10.1590/s0034-89101988000400001. [DOI] [PubMed] [Google Scholar]
- 9.Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, et al. Epi Info, version 6: a word processing, database, and statistics program for epidemiology on microcomputers. Atlanta, GA: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 10.Smith PG, Day NE. The design of case-control studies: the influence of confounding and interactions effects. Int J Epidemiol. 1984;3:356–365. doi: 10.1093/ije/13.3.356. [DOI] [PubMed] [Google Scholar]
- 11.Rossner B. Fundamentals of biostatistics. 4th ed. Boston, MA: Duxbury Press; 1995. Hypothesis testing: categorical data; pp. 345–442. [Google Scholar]
- 12.César JA, Victora CG, Santos IS, Barros FC, Albernaz EP, Oliveira LM, et al. Hospitalisation due to pneumonia: the influence of socio-economic and pregnancy factors in a cohort of children in southern Brazil. Revista de Saúde Pública. 1997;31:53–61. doi: 10.1590/s0034-89101997000100008. [DOI] [PubMed] [Google Scholar]
- 13.Norussis NJ. Chicago, IL: SPSS; 1993. Statistical package for social sciences for Windows. [Google Scholar]
- 14.Epidemiological graphics, estimation and testing package—EGRET. Washington, DC: Statistics and Research Corporation; 1988. [Google Scholar]
- 15.Victora CG. Case-control studies in maternal child health. In: Boerma JT, editor. Measurement of maternal child mortality, morbidity and health care: interdisciplinay approaches. Liège: International Union for the Scientific Study of Population; 1992. pp. 85–108. [Google Scholar]
- 16.Victora CG. Case-control studies of the influence of breastfeeding on child morbidity and mortality: methodological issues. In: Atkinson SA, Hanson LA, Chandra RK, editors. Breastfeeding, nutrition, infection and infant growth in developed and emerging countries. St Johns, Newfoundland: ARTS Biomedical Publishers and Distributors; 1990. pp. 405–418. [Google Scholar]
- 17.Schlesselmann JJ. Case-control studies. New York: Oxford University Press; 1982. pp. 27–68. [Google Scholar]
- 18.Morris SS. Risk factors for acute lower respiratory tract infections. Results from five recently completed case-control studies. London: Maternal and Child Epidemiology Unit, London School of Hygiene Tropical Medicine; 1995. pp. 2–12. [Google Scholar]
- 19.Victora CG, Huttly SR, Fuchs SC, Olinto MTA. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 20.Labbok M, Krasovec K. Toward consistency in breastfeeding definitions. Studies in Family Planning. 1990;21:226–230. [PubMed] [Google Scholar]
- 21.Barros FC, Victora CG, Vaughan JP. Breastfeeding and socioeconomic status in Southern Brazil. Acta Paediat Scand. 1986;75:558–562. doi: 10.1111/j.1651-2227.1986.tb10250.x. [DOI] [PubMed] [Google Scholar]
- 22.Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombardi C, Teixeira AMB, et al. Evidence for a strong protective effect of breast-feeding against infant death due to infectious diseases in Brazil. Lancet. 1987;ii:319–322. doi: 10.1016/s0140-6736(87)90902-0. [DOI] [PubMed] [Google Scholar]
- 23.Victora CG, Fuchs SC, Flores JA, Fonseca W, Kirkwood B. Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics. 1994;93:977–985. [PubMed] [Google Scholar]
- 24.Brown KH, Black RE, Romana GL, Kanashiro HC. Infant-feeding practices and their relationship with diarrhoea and other diseases in Huascar (Lima), Peru. Pediatrics. 1989;83:31–40. [PubMed] [Google Scholar]
- 25.Cerqueiro MC, Murthag P, Halac A, Avila M, Weissembacher M. Epidemiology of acute respiratory tract infection in children. Rev Infect Dis. 1990;12:1021–1028. doi: 10.1093/clinids/12.supplement_8.s1021. [DOI] [PubMed] [Google Scholar]
- 26.Elleasted-Sayed J, Coodin FJ, Dilling LA, Haworth JC. Breastfeeding against infections in Indian Infants. Can Med Assoc J. 1979;120:295–298. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Shunzhang Y, Li W. Artificial feeding and hospitalization in the first 18 months of life. Pediatrics. 1988;81:58–62. [PubMed] [Google Scholar]