Squamous epithelium of the skin is generated by a highly regulated series of events that occurs within ‘epidermal proliferating units’ (EPUs) thought to be associated with a single stem cell.1 Upon commitment to the keratinocyte pathway, cells enter into the ‘transit amplifying’ compartment, located in the basal layer, and undergo several rounds of mitosis.2 These cells then undergo a proliferation-differentiation switch and gradually approach the skin surface as they differentiate. Even subtle alterations in the rates of cell division, cell death or transit time could lead to pathologic conditions. The regulatory pathways that control these cell fate decisions would seem among the likeliest to contribute to malignancy.
However, Nature is full of surprises, and an article by Chen and colleagues in the current issue highlights yet another one. A molecule that normally lives in the upper, post-mitotic cell layers of the skin, and which has no major function in these developmental processes, is aberrantly expressed in the basal layer of neoplastic skin lesions and has other properties consistent with an oncogene important in skin tumor progression. Kruppel-like factor 4 (KLF4, also called GKLF) is a zinc finger transcription factor that is dispensable for embryogenesis.3 It is expressed in the post-mitotic, differentiating cells of a variety of epithelia including the epidermis.3–6 Although mechanisms remain poorly understood, KLF4 can function as a transforming oncogene in vitro,6,7 and can likewise cooperate with just three other genes (c-MYC, Sox2 and Oct3/4) to convert adult fibroblasts into pluripotent embryonic stem cells.8
In spite of its negligible role in skin morphogenesis, KLF4 mRNA and protein are upregulated early and often in squamous cell carcinoma (SCC) progression.6,9 In fact, KLF4 is expressed in the early, potentially reversible lesion termed actinic keratosis (AK). In these premalignant lesions, KLF4 is expressed in all cell layers, including the basal layer where it is normally not detected. Previously, we used the term ‘maturation-independent’ to describe SCC and AK lesions in which KLF4 protein is not restricted to the post-mitotic, differentiating compartment.9 This failed regulation represents a potential early event in SCC tumor progression. Chen and colleagues now confirm these studies using a distinct KLF4 antibody for immunostaining assays, and report complementary studies such as Western blot and RNA analysis. They show that maturation-independent expression of KLF4, especially nuclear expression, is associated with an aggressive skin cancer phenotype, and even metastasis. Taken together, the published data support a model in which SCC results when a cancer precursor cell acquires properties normally segregated among three distinct epithelial compartments: the self-renewal property of stem cells, the proliferative capacity of transit amplifying cells, and the transforming activity of oncogenes such as KLF4 that are normally restricted to the post-mitotic, differentiating compartment.
KLF4 appears sufficient to recapitulate several aspects of SCC tumor progression. Previously, we engineered transgenic mice to conditionally express KLF4 in the proliferation-competent basal cell layer.10 These mice develop lesions over a period of days to weeks that include hyperplasia, dysplasia and SCC-like tumors. Unlike the latent, focal tumors that are observed in most models of cancer, these lesions evolved rapidly—within days of induction. Lesions involved the majority of the skin surface in a relatively uniform, diffuse fashion, suggesting that other genetic changes may not be required for this rapid-onset phenotype. The resulting tumors shared molecular and genetic similarities to human SCC, including reliance upon p53 inactivation. Compared to this simple model, human SCCs develop over a prolonged interval and are likely to contain additional genetic and epigenetic changes.
Similar to mouse models of SCC, in human SCCs the role of p53 appears prominent.11 Morphologically normal human skin, especially from sun-exposed areas, contains frequent ‘p53 clones’.1 These small clusters of 60–3000 keratinocytes are identified by their increased staining of p53 protein, and ~50% of the clones contain a mutated p53 gene.1,12 p53 clones are often shaped like an inverted cone, as might be expected if they derive from a single stem cell, and show evidence of spreading beyond the architectural boundaries of a normal EPU. Otherwise, p53 clones are morphologically similar to normal cells. Separately, genetic studies in mice indicate strong links between p53 deficiency and progression to cutaneous SCC, with p53 functioning somewhat like a cutaneous SCC ‘gatekeeper’.13–15 These results point to p53 alteration as an early step, or perhaps the first step, in the genesis of cutaneous SCC. The subsequent steps that may convert a p53 clone into a morphologically-distinctive, neoplastic lesion such as AK remain poorly understood. While a role for an oncogene in this conversion seems likely, frequent mutation of Ras or other known oncogenes has not been reported.
The ability of KLF4 to induce diffuse SCC-like skin lesions within a short timeframe, and the strict dependence upon p53 status, point to KLF4 upregulation as a likely step that cooperates with p53 alteration in human SCC tumorigenesis. Normally, KLF4 and p53 appear to be mutually antagonistic. KLF4 can directly suppress p53 expression.7 Conversely, p53 suppresses KLF4-induced dysplasia if the size of the skin patch containing deregulated KLF4 is too small.10 For example, uniform expression of KLF4 across the skin is sufficient to initiate dysplasia even in p53 WT animals, but ‘patchy’ or mosaic KLF4 expression (obtained by use of an X chromosome-linked transgene) yielded no skin phenotype unless p53 was altered by loss of either one or both copies. This straightforward mouse genetic experiment suggests a model in which p53 alteration (e.g., mutation and/or allelic loss) would render an EPU competent to expand in response to KLF4 expression in basal cells. Thus, the p53 clones that exist in human skin represent an ideal setting in which deregulation of KLF4 could promote tumor formation.
As shown for p53 in other tumor types,16,17 p53 is haplo-insufficient for suppression of KLF4-induced skin tumors.10 There is increased cancer susceptibility even though one wild type p53 gene remains. For p53, this haplo-insufficiency could be related to its function as a multimer in equilibrium with monomer, as small-fold changes in monomer concentration would result in larger-fold changes in the active homotetramer.18 Rowland and colleagues showed that KLF4 can directly lower p53 protein concentration by interacting with the p53 promoter and suppressing p53 transcription. 7 Since many p53 clones in the skin appear to contain copies of wild type p53,1,12 suppression by KLF4 may be functionally important and could contribute to p53 haploinsufficiency.
How does p53 block the transforming activity of KLF4 in vivo? Although KLF4 can directly suppress p53 expression, it is unknown whether p53 signaling may likewise affect activity of the KLF4 protein. Mosaic expression of basal KLF4 shows that the required patch size is smaller in p53-deficient skin.10 This permissive effect may indicate competition between adjacent EPUs, with cell arrest/death signals predominating when the patch size is small and p53 is wild type. The timing of onset of maturation-independent KLF4 expression can now be mapped by analysis of earlier lesions. It will be interesting to see whether reversible actinic keratosis lesions or the p53 clones of 60–3000 cells that are frequent in sun-exposed skin show KLF4 deregulation.
It is currently unclear how KLF4 expression is suppressed in normal, proliferating squamous epithelial cells. Not only KLF4 protein, but also the mRNA is low or absent in the normal basal cells of the skin and oral mucosa.4,6 While a role for transcriptional control seems likely, results that we obtained in KLF4 transgenic mice suggest that posttranscriptional mechanisms suppress the mRNA in dividing cells (see Fig. 1).9 In addition, Chen and colleagues showed that the KLF4 protein is actively targeted for degradation in dividing cells.19 Given the potential for KLF4 to induce malignant transformation and to promote pluripotency when co-expressed with genes such as c-MYC, it is perhaps not surprising that multiple mechanisms operate to prevent high-level KLF4 expression in dividing cells. Each of these mechanisms somehow gets bypassed during tumor progression, leading to maturation-independent expression.
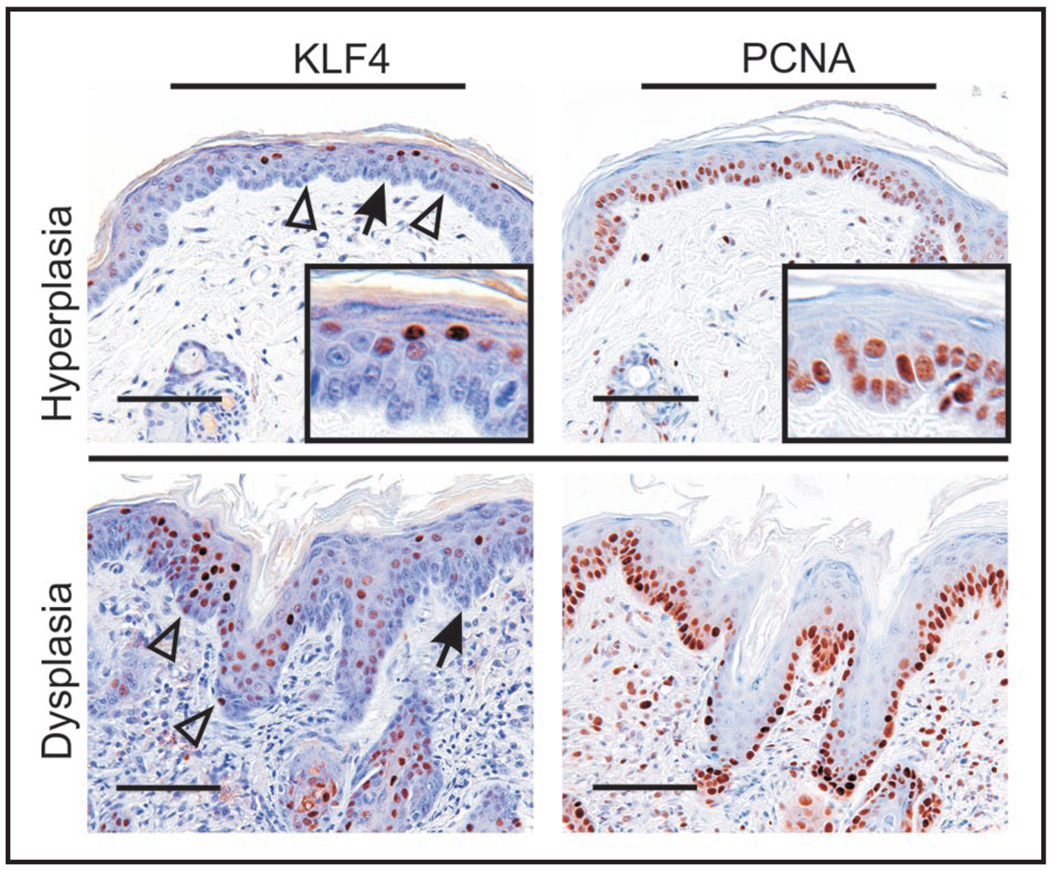
Figure 1.
A KLF4 transgenic mouse model of SCC shows progression to maturation-independnet, constitutive nuclear staining of KLF4, as seen in human skin tumors (Chen, Y.J. et al., this issue). KLF4 was induced in the skin of transgenic mice by administration of doxycycline for 14 days, using a keratin 14 (K14) promoter-dependent expression strategy10. Skin sections were analyzed by immunostaining, showing that all the epidermal cell layers had become K14-positive. Even though KLF4 transcription is driven by doxycycline in all K14-positive cell layers, only low level KLF4 is detected in the basal, PCNA-positive, epidermal cells of hyperplastic skin (upper panels, see open arrowheads). This result suggests that restriction of KLF4 expression to post-mitotic cells is, at least in part, a post-transcriptional effect. This restriction is lost during tumor progression, as dysplastic skin shows frequent basal expresssion of nuclear KLF4 (lower panels). Insets show the cell-type specific staining at increased magnification. Arrows indicate the demo-epidermal junction, and open arrowheads indicate examples of KLF4-positive, basal keratinocytes. Adapted from reference 10. Scale bars 100µ (upper panels) or 50µ (lower panles).
One attractive possibility is that KLF4 induces the cancer stem cell phenotype in the skin, just as it induces the stem cell phenotype in fibroblasts.8 Basal expression of KLF4 could promote expansion of stem cells within an EPU, leading to a clonal outgrowth of a tumor. If so, then molecularly targeted therapies aimed at KLF4 could effectively target cancer stem cells. These same therapies may reverse lesions that show maturation independent expression and nuclear localization of KLF4.
KLF4 deficient mice develop normally in utero but subsequently die in the early postpartum period due to dehydration, a consequence of failure to form an adequate skin permeability barrier.3 The specific stage of skin development at which KLF4 executes this function has not been identified experimentally. KLF4 can have dramatic effects at even low levels of expression, and might exert its barrier effect prior to onset of the high level expression seen in post-mitotic cells. Also unknown at the present time is whether KLF4 is normally required strictly for formation of the skin permeability barrier during development, or whether it has a more general role in maintenance of the barrier in postpartum animals or in adults. This question is ultimately important for understanding potential toxicities in patients undergoing KLF4-directed therapies.
References
- 1.Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, Leffell DJ, Tarone RE, Brash DE. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 3.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 4.Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 5.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster KW, Ren S, Louro ID, Lobo Ruppert SM, McKie Bell P, Grizzle W, Hayes MR, Broker TR, Chow LT, Ruppert JM. Oncogene expression cloning by retroviral transduction of adenovirus E1a-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zinc finger protein GKLF. Cell Growth Differ. 1999;10:423–434. [PubMed] [Google Scholar]
- 7.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Huang CC, Liu Z, Li X, Bailey SK, Nail CD, Foster KW, Frost AR, Ruppert JM, Lobo Ruppert SM. KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia. Cancer Biology and Therapy. 2005;4:1401–1408. doi: 10.4161/cbt.4.12.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster KW, Liu Z, Nail CD, Li X, Fitzgerald TJ, Bailey SK, Frost AR, Louro ID, Townes TM, Paterson AJ, Kudlow JE, Lobo Ruppert SM, Ruppert JM. Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24:1491–1500. doi: 10.1038/sj.onc.1208307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brash DE. Roles of the transcription factor p53 in keratinocyte carcinomas. Br J Dermatol. 2006;154:8–10. doi: 10.1111/j.1365-2133.2006.07230.x. [DOI] [PubMed] [Google Scholar]
- 12.Backvall H, Stromberg S, Gustafsson A, Asplund A, Sivertsson A, Lundeberg J, Ponten F. Mutation spectra of epidermal p53 clones adjacent to basal cell carcinoma and squamous cell carcinoma. Exp Dermatol. 2004;13:643–650. doi: 10.1111/j.0906-6705.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 13.Donehower LA. The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol. 1996;7:269–278. doi: 10.1006/scbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- 14.Kinzler KW, Vogelstein B. Cancer-susceptibility genes: gatekeepers and caretakers. Nature. 1997;386:761. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 15.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 16.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 17.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 18.Okorokov AL, Sherman MB, Plisson C, Grinkevich V, Sigmundsson K, Selivanova G, Milner J, Orlova EV. The structure of p53 tumour suppressor protein reveals the basis for its functional plasticity. EMBO J. 2006;25:5191–5200. doi: 10.1038/sj.emboj.7601382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZY, Wang X, Zhou Y, Offner G, Tseng CC. Destabilization of Kruppel-like factor 4 protein in response to serum stimulation involves the ubiquitin-proteasome pathway. Cancer Res. 2005;65:10394–10400. doi: 10.1158/0008-5472.CAN-05-2059. [DOI] [PubMed] [Google Scholar]