Abstract
The T-cell costimulatory receptors, CD28 and the inducible costimulator (ICOS), are required for the generation of follicular B helper T cells (TFH) and germinal center (GC) reaction. A common signal transducer used by CD28 and ICOS is the phosphoinositide 3-kinase (PI3K). Although it is known that CD28-mediated PI3K activation is dispensable for GC reaction, the role of ICOS-driven PI3K signaling has not been defined. We show here that knock-in mice that selectively lost the ability to activate PI3K through ICOS had severe defects in TFH generation, GC reaction, antibody class switch, and antibody affinity maturation. In preactivated CD4+ T cells, ICOS delivered a potent PI3K signal that was critical for the induction of the key TFH cytokines, IL-21 and IL-4. Under the same settings, CD28 was unable to activate PI3K but supported a robust secondary expansion of T cells. Thus, our results demonstrate a nonredundant function of ICOS-PI3K pathway in the generation of TFH and suggest that CD28 and ICOS play differential roles during a multistep process of TFH differentiation.
Keywords: CD28, follicular B helper T-cell, germinal center, ICOS, PI3K
Follicular B helper T cells (TFH) are a subset of CD4+ T cells that facilitates germinal center (GC) reaction, B cell proliferation, and B cell differentiation (1). TFH cells have an ability to migrate into B cell area using chemokine receptor CXCR5, and they abundantly express costimulatory molecules such as ICOS, PD-1, and CD40L. TFH cells can arise in the absence of factors that mediate Th1, Th2, or Th17 differentiation, but depend on Bcl-6 (2–5). TFH cells express a high level of IL-21, which provides a robust stimulus for proliferation and differentiation of B cells (6, 7). IL-21 also plays an indispensible role in the generation of TFH cells, probably by enhancing Bcl-6 expression (3). Recent studies also revealed an exquisite regulation of IL-4 transcription and translation that allows highly targeted secretion of IL-4 by TFH cells while they form conjugates with cognate B cells (8). Thus, IL-21 and IL-4 appear to be crucial for differentiation and/or function of TFH cells.
ICOS is a CD28 family costimulatory receptor that is expressed in recently activated or antigen-experienced T cells (9, 10). By binding to ICOS ligand (ICOS-L) expressed on antigen presenting cells (APCs), ICOS delivers costimulatory signals that augment T-cell proliferation and expression of an array of cytokines including IL-4, IL-10, and IL-21 (10–12). Both in mice and humans, interruption of ICOS-ICOS-L interaction leads to impaired GC reaction, Ab class switch, and affinity maturation (13–16). Recent findings suggested that these defects in humoral immune responses in ICOS-deficiency are due to the lack of TFH cells (17–19). Conversely, dysregulated overexpression of ICOS in sanroque mice causes a lupus-like autoimmune disease that is associated with an increased number of TFH cells, spontaneous GC reaction, and augmented IL-21 production (20–22).
The prototype T-cell costimulator CD28 is also required for GC reaction, humoral immunity, and generation of TFH cells (23, 24). It is puzzling that the generation of TFH requires both CD28 and ICOS, although the two costimulators have a seemingly redundant function in activating PI3K (25, 26). Whether CD28-mediated PI3K pathway plays significant roles in T-cell proliferation, cytokine production, and survival has been a matter of hot debate (27). Recent data from knock-in mice showed that CD28-mediated PI3K pathways do not have any obvious nonredundant role in T-cell functions and humoral immune responses (28).
To address the role of ICOS-mediated PI3K signal transduction pathways in the context of the overall ICOS function, we generated a knock-in mouse strain in which the cytoplasmic tail of ICOS cannot recruit PI3K. Here, we show that the generation of TFH cells critically depends on the PI3K signaling initiated by ICOS. Consequently, GC reaction, Ab class switch, and affinity maturation are drastically diminished in the knock-in mice. We find evidence that in preactivated CD4+ T cells, expression of IL-21 and IL-4 is heavily dependent on PI3K and that the dominant activator of PI3K in this context is ICOS, not CD28.
Results
Normal Inducible Expression Pattern of ICOS-YF with Altered Signaling Capacities.
We generated knock-in mice, termed ICOS-YF hereafter, possessing a tyrosine-to-phenylalanine mutation at amino acid residue 181 in the cytoplasmic tail of ICOS, a mutation known to abrogate ICOS-mediated PI3K recruitment (29) (details in SI Text and Fig. S1). We compared littermates of ICOS-WT (+/+) and ICOS-YF (yf/yf) mice along with nonlittermate ICOS-KO (-/-) mice that have <2 weeks of age difference. All of these mice have been backcrossed five generations into C57BL/6.
Since tyrosine residues in the cytoplasmic tails of membrane proteins are often involved in protein trafficking and recycling, we tested whether ICOS-YF maintained its expression pattern on the cell surface. As shown in Fig. S2A, WT and YF-mutant ICOS displayed an identical inducible expression pattern. Thus, the tyrosine-to-phenylalanine mutation does not alter the expression pattern of ICOS, and all of the phenotypic outcomes should be attributable to the altered signaling capacities of the mutant ICOS.
In vitro binding assays using GST fusion proteins have shown that the Tyr 181 residue of ICOS is critical for recruiting PI3K (29). Consistently, anti-ICOS immunoprecipitates from WT CD4+ T-cell blasts contained the regulatory subunit of PI3K, p85α (Fig. S2B, WT). There was a basal level of p85α associated with ICOS that increased upon ligation of TCR or ICOS. The TCR-independent ICOS-mediated p85α recruitment may reflect a potential antigen-independent function of ICOS on cytoskeletal rearrangement of T cells (30). However, the amount of p85α was maximal when the T cells were activated by a combination of anti-CD3 and anti-ICOS mAb. Importantly, the ICOS-p85α interaction was abrogated when the Tyr 181 was mutated to phenylalanine (Fig. S2B, YF).
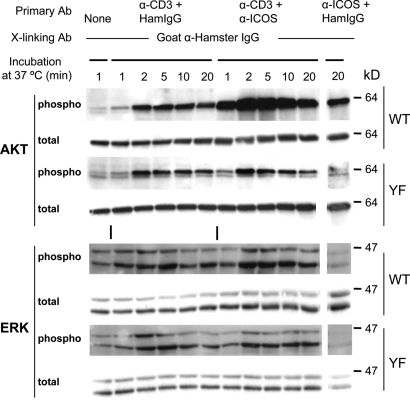
It has been shown that ligation of ICOS strongly enhances TCR-mediated activation of AKT and, to some extent, MAPKs (ERK, JNK, and p38) (25, 26). We examined these signal transduction events in primary T-cell blasts derived from WT or ICOS-YF mice. In keeping with the PI3K activation, ICOS engagement dramatically augmented TCR-mediated AKT activation as judged by the increase phosphorylation of AKT at Ser-473 in WT T cells (Fig. 1, AKT, WT). The ability of ICOS to enhance TCR-mediated AKT activation was completely abrogated in ICOS-YF T cells (Fig. 1, AKT, YF). ERK phosphorylation was moderately enhanced by ICOS ligation in WT but not in ICOS-YF. This is consistent with the observations that PI3K can activate Ras-MAPK pathway (31, 32). ICOS did not augment phosphorylation of JNK and p38 in primary CD4+ blasts under our experimental settings (Fig. S3). As shown by others (25, 26), CD28-costimulation strongly enhanced JNK activation with a moderate level of AKT phosphorylation (Fig. S4).
Fig. 1.
Defective AKT and ERK activation by ICOS-YF. CD4+ T blasts were stimulated with antibodies against CD3 and/or ICOS, and the activation of AKT or MAPKs was measured by immunoblotting using phospho-specific antibodies. A representive of three independent experiments is shown.
It was shown that ligation of ICOS can facilitate Ca2+ mobilization when TCR signal is suboptimal, possibly through PI3K (25, 29). As shown in Fig. S5, both WT and Y181F mutant ICOS were able to augment TCR-mediated Ca2+ flux in CD4+ T blasts. Thus, ICOS can augment TCR-mediated Ca2+ flux in a PI3K-independent manner.
Collectively, the Y181F mutation selectively disrupts PI3K-dependent signaling pathways, AKT and ERK, without affecting Ca2+ signaling.
Reduced Basal Serum Ig Levels in ICOS-YF Mice.
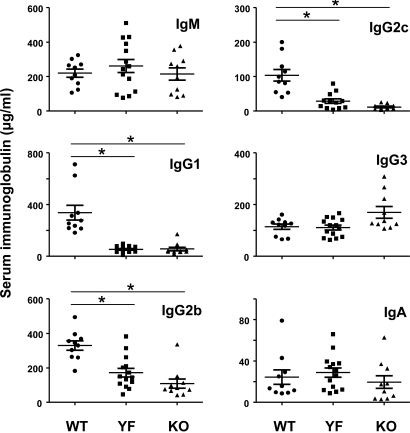
One of the hallmarks of ICOS-deficient mice or humans is a reduction of class-switched immunoglobulins in serum, a reflection of defective GC reaction (13–16). Thus, we quantified the basal serum Ig levels by ELISA from 2-month-old mice of WT, YF, and KO mice (Fig. 2). As previously documented, ICOS-KO mice displayed up to 10-fold reduction in serum concentrations of IgG1, IgG2b, and IgG2c without any difference in IgM compared with WT control. Remarkably, ICOS-YF mice had an identical serum IgG1 level as that of ICOS-KO. Serum IgG2b and IgG2c concentrations in ICOS-YF mice were also reduced to levels close to those of ICOS-KO mice. These results suggest that the ICOS function in supporting Ig class switch critically relies on signaling mechanisms dependent on the Tyr 181.
Fig. 2.
ICOS-YF and KO mice have reduced levels of class-switched immunoglobulins in serum. Sera were obtained from nonimmunized mice of ICOS WT, YF, and KO mice at 8 weeks of age. Concentrations of IgM, G1, G2a, G2c, G3, and A were determined by isotype specific ELISA. Each data point represents a serum Ig level of an individual mouse (n = 10 WT, 14 YF, and 10 KO). *, P < 0.01.
Defective GC Reaction in Peyer's Patches of ICOS Mutants.
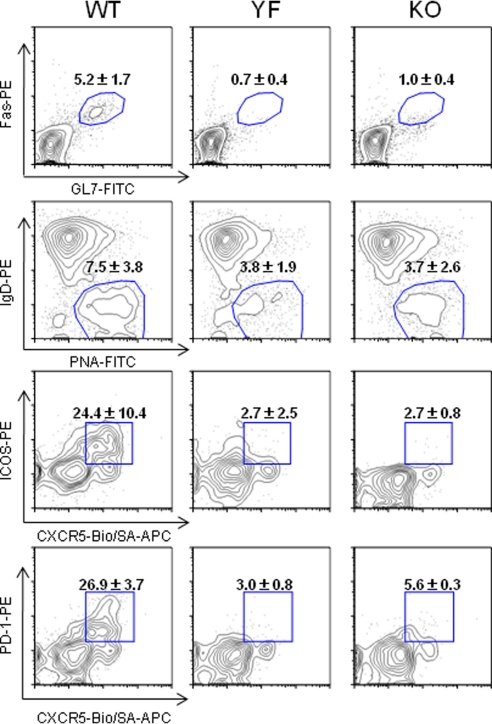
Peyer's patches (PPs) are part of gut-associated immune tissue in which ongoing humoral immune responses against the intestinal microflora are taking place. It has been shown that, in ICOS-KO mice, the number of PPs is normal, but the size and cellularity of PPs are dramatically reduced, and the active GCs are not detected (33). However, the basis of these defects has been unknown. We chose to analyze the PPs of ICOS mutant mice to gain insights into the cellular basis of GC defects. ICOS-YF mice had a normal number of PPs, as do ICOS-KO mice (Fig. S6A). However, the total cellularity of PP was substantially reduced in ICOS-YF mice to a level similar to that of ICOS-KO mice (Fig. S6B). Accordingly, the GC area was greatly reduced in both ICOS-YF and KO mice (Fig. S6C). Flow cytometric analysis revealed that there is a drastic reduction in the percentage of GC B cells over the total B cells (Fig. 3Top). Importantly, the percentages of TFH cells over the total CD4+ T cells in the PPs of ICOS-YF and KO were reduced by 5- to 9-fold compared with that of WT mice (Fig. 3 Bottom). Consistent with the GC defects, the content of secreted IgA in the feces was dramatically reduced in YF and KO mice (Fig. S6D). This is in contrast to the normal serum IgA levels in ICOS mutant mice (Fig. 2), suggesting that the serum IgA level is mainly controlled by T-independent mechanisms (34). Thus, the Tyr 181 motif of ICOS plays a critical role in TFH differentiation and GC reaction in the PP.
Fig. 3.
ICOS-YF as well as KO mice have severely impaired humoral immune responses in the PP. GC B cells and TFH cells in the PP were analyzed by flow cytometry. The (Top) two were gated on B220+ B cells and the (Bottom) two on CD4+ T cells. Numbers represent mean ± SD of data pooled from four mice per genotype.
Defective Humoral Immunity in ICOS Mutants.
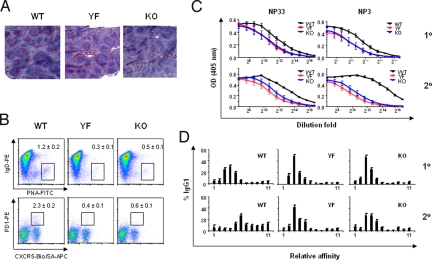
It has been shown that ICOS-KO mice have impaired GC reaction, Ab class switch, and affinity maturation upon immunization (13–15). We immunized mice with alum-precipitated NP-CGG to examine the role of ICOS-PI3K pathway in humoral immune responses. Both ICOS-YF and KO mice had severely impaired GC reaction along with reduced TFH cells in the spleen (Fig. 4 A and B). Anti-NP IgG1 antibody titers in serum were substantially reduced in both ICOS-YF and KO mice (Fig. 4C). The difference in anti-NP IgG1 titer was more pronounced in high-affinity Ab (Fig. 4C, NP3) as opposed to the total Ab (Fig. 4C, NP33). It was also clear that the difference in high-affinity Ab titers became bigger upon secondary immunization (Fig. 4C, NP3 1 ° vs. 2 °). We assessed affinity maturation process more precisely by measuring anti-NP antibodies after a differential washing step using NaSCN solutions during ELISA (SI Text). As depicted in Fig. 4D, a shift from lower to higher affinity anti-NP IgG1 was readily seen in WT mice upon secondary immunization, but this was not observed in ICOS-YF and KO mice. Collectively, these data demonstrate that ICOS-YF mice have severely impaired GC reaction, TFH generation, Ab class switch, and affinity maturation.
Fig. 4.
Impaired Ab responses in ICOS-YF and ICOS-KO mice upon immunization. (A) Defective GC reaction. Cryosections of spleens from mice immunized with NP16-CGG/alum 12 days before were stained with PNA. (B) Decreased GC B cells and TFH cells in ICOS-YF and KO mice. Mice were immunized with NP16-CGG/alum, and the splenocytes were analyzed 12 days later. Percentages represent mean ± SD of data from three mice per genotype. A representative of two independent experiments. (C) Impaired class switch. Sera were prepared from immunized mice at day 11 (1°) or day 7 (2°) postinjection, and antigen-specific IgG1 were measured by ELISA using NP33-BSA vs. NP3-BSA. Numbers represent mean ± SD of data from six mice per genotype. A representative of two independent experiments. (D) Defective Ab affinity maturation. Mice were immunized at day 0 and boost injected at day 30. Serum samples were prepared at day 11 after primary injection or day 7 after secondary injection, and the anti-NP IgG1 was measured after differential washes with increasing concentrations of NaSCN solution. Each histogram represents mean ± SD of data from six mice per genotype. A representative of two independent experiments.
ICOS Promotes Expression of IL-21 and IL-4 in a PI3K-Dependent Manner.
The lack of CXCR5+CD4+ TFH cells during humoral immune reaction in ICOS mutant mice prompted us to examine if ICOS is directly involved in upregulation of CXCR5. When T cells were activated by soluble anti-CD3 Ab in the presence of APCs, there was no difference in the expression levels of OX40 and CXCR5 on CD4+ T cells (Fig. S7A). This result is consistent with the notion that CXCR5 is mainly induced by OX40, whose expression is enhanced by CD28 costimulation (35). Thus, ICOS is not required for the induction of CXCR5, and the lack of CXCR5+CD4+ cells in ICOS mutants probably reflects a failed TFH differentiation program.
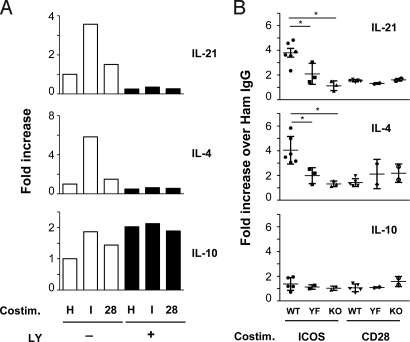
Next, we sought to examine the impact of ICOS costimulation on cytokine gene expression in the CD4+ T cells. We activated highly purified CD4+ T cells for 2 days in vitro using antibodies against CD3 and CD28, rested them 1 day in the absence of stimuli, and then restimulated the cells with a combination of TCR and costimulatory signals. This regimen allowed us to use CD4+ T cells with maximal surface ICOS expression within a time frame when primed CD4+ T cells migrate to B cell follicles in secondary lymphoid organs (day 3 postimmunization) (36). Under these conditions, ICOS played a dominant role over CD28 in augmentation of IL-21 and IL-4 expression (Fig. 5A). Further, pharmacological inhibition of PI3K activity during the restimulation period negated all of the costimulatory impacts of ICOS on IL-21 and IL-4 expression. In contrast, IL-10 expression was marginally increased by ICOS and CD28, but largely unaffected by PI3K inhibition. Consistent with results from PI3K inhibition experiments, ICOS-mediated upregulation of IL-21 and IL-4 was abrogated in ICOS-YF T cells to levels close to those of ICOS-KO T cells (Fig. 5B). In parallel, the differences in cytokine induction capacity of ICOS vs. CD28 in the primed CD4+ T cells well correlated with their abilities to activate PI3K, a potent AKT phosphorylation by ICOS but not by CD28 (Fig. S7B). Despite the weak costimulatory activity in cytokine expression, CD28 had a major impact on the secondary expansion of primed CD4+ T cells, whereas ICOS played minor contribution (Fig. S7C). Interestingly, the ICOS-mediated proliferation is disrupted in ICOS-YF T cells, suggesting that the ICOS-PI3K pathway may have an additional role in the secondary expansion of primed CD4+ T cells. Taken together, these data show that ICOS-PI3K signaling plays a dominant role in augmenting expression of IL-21 and IL-4 during secondary activation of CD4+ T cells and that CD28 is not able to substitute ICOS.
Fig. 5.
ICOS-YF and ICOS-KO CD4+ T cells have defects in IL-21 and IL-4 expression. (A) ICOS induces IL-21 and IL-4 in a PI3K-dependent manner. Preactivated CD4+ T cells were restimulated with anti-CD3 plus hamster IgG (H), anti-ICOS (I), or anti-CD28 (28) followed by goat anti-hamster IgG for 6 h without or with LY 294002. The cytokine mRNA levels in the restimulated cells were analyzed by qPCR. A representative of three independent experiments. (B) Abrogation of IL-21 and IL-4 induction in ICOS-YF T cells. Cytokine gene expression was analyzed in WT, YF, and KO T cells as described in A. Each data point represents fold increase over hamster IgG control. Data pooled from six independent experiments (n = 5–6 WT, 2–3 YF, and 2–3 KO). *, P < 0.02.
Discussion
In this study we show that ICOS potently activates PI3K in synergy with the TCR. When its ability to activate PI3K is selectively abrogated, ICOS cannot support the generation of TFH cells, GC reaction, Ab class switch, and affinity maturation. We found a nonredundant role of ICOS-PI3K pathway in upregulation of IL-21 and IL-4, key cytokines crucial for TFH generation and function.
It has been shown that in murine Th2 clones, ICOS is constitutively bound to PI3K, and ICOS ligation further increases PI3K recruitment (37). We confirmed these results in activated CD4+ T blasts. The finding that ligation of ICOS without TCR stimulation can activate PI3K signaling cascades explains TCR-independent, yet PI3K-dependent ICOS function in cytoskeletal rearrangement that may have a potential role in T-cell adherence and migration (30). However, it has been shown that interruption of ICOS function does not affect T-cell trafficking itself in vivo (38, 39). Regardless, it is clear that co-ligation of the TCR and ICOS gives rise to a maximal PI3K signaling. Biochemical and imaging data have indicated that ICOS is in complex with TCR complexes, and it gets recruited into the immunological synapses, supporting the view that ICOS probably functions in conjunction with the TCR (40–42).
The mechanism by which ICOS enhances TCR-mediated Ca2+ mobilization is not clear. It was proposed that the ICOS-mediated PI3K pathway can enhance PLCγ1 function through ITK leading to sustained Ca2+ flux (29). Since ICOS-YF T cells have intact Ca2+ flux, we conclude that ICOS can mediate Ca2+ flux through a yet unknown mechanism, but clearly in a PI3K-independent manner. Although the overall defects in humoral immunity in ICOS-YF mice are very close to those of ICOS-KO mice, we observed marginally higher levels of serum IgG2b and IgG2c in ICOS-YF mice compared to ICOS-KO mice (Fig. 2). Also, preactivated ICOS-YF CD4+ T cells produced slightly higher levels of IL-21 and IL-4 compared to ICOS-KO counterparts (Fig. 5B). The intact capacity of ICOS-YF to augment TCR-mediated Ca2+ flux may explain these residual T-cell functions.
Our finding that PI3K plays a key role in ICOS-mediated TFH cells is very relevant to the results that inactivation of the p110δ isoform of PI3K leads to impaired humoral immunity and reduced size of PPs in the gut (32). It will be interesting to see whether a lack of TFH underlies these phenotypes and whether the p110δ isoform is the PI3K under the control of ICOS.
Similar to ICOS, CD28 can activate PI3K through its Tyr-based motif (YMNM) in the cytoplasmic tail (27). Why is CD28 unable to compensate the lack of ICOS-PI3K signaling pathway? Our data show that it is due to an intrinsic weak capacity of CD28 to activate PI3K compared with that of ICOS. It has been shown that the membrane proximal segment containing the YMFM motif of the ICOS tail is much stronger than its CD28 counterpart (containing the YMNM motif) in the ability to activate PI3K when chimeric receptors were compared in transfected human CD4+ T cells (25). The same observation was made in murine CD4+ T cells upon ligation of endogenous CD28 and ICOS (26). Particularly, in CD4+ T cells that rested for 1 day after a 2-day stimulation, CD28 did not evoke any PI3K activity above the TCR stimulation, whereas it could still strongly enhance secondary expansion of T cells (Fig. S7 B and C). Recent results from CD28 knock-in mice reinforced the notion that CD28-mediated PI3K does not play any nonredundant function in T-cell proliferation, cytokine production, and survival; it is, rather, signals emanating from the carboxy terminal proline-rich motif that play more important roles (28).
Deficiency of either CD28 or ICOS results in defective TFH and humoral immunity, suggesting distinct roles for the two costimulators (13–15, 23, 24, 35). What differential roles do they play during the process of TFH generation? Based on our results and the data in literature, we propose a model in which CD28 and ICOS support TFH cell differentiation in rather specialized manners. During the first 2–3 days of antigenic exposure, CD4+ T cells interact with dendritic cells in the T-cell zone of secondary lymphoid organs (36). At this stage, antigen-specific T cells rapidly proliferate producing IL-2, upregulate CXCR5 through OX40, and induce ICOS. Since these processes are known to be dependent on CD28 costimulation (35, 43, 44), CD28-deficiency may heavily compromise TFH differentiation at this stage. The primed CD4+ T cells then migrate toward B cell follicles and interact with cognate B cells. During this T:B interaction, T cells make helper cytokines such as IL-21 and IL-4. IL-21 may play a key role in facilitating full differentiation of TFH cells (3, 12), whereas IL-4 and IL-21 induce B cell proliferation and differentiation (6). Our data show that ICOS provides a unique PI3K-mediated signal to enhance IL-21 and IL-4 at this stage, and CD28 cannot substitute ICOS. Therefore, ICOS-deficiency is likely to block this later stage of TFH differentiation. This model is consistent with the finding that a transient activation of CD28 at the early phase of immunization is sufficient for GC formation (45). On the other hand, T cells primed in B cell-deficient mice showed normal expansion, but failed to induce Ab class switch during in vitro coculture with B cells, suggesting an incomplete helper T differentiation in the absence of B cells (46).
In sum, we demonstrated here that the function of ICOS in supporting TFH generation and humoral immunity critically relies on the evolutionarily conserved tyrosine-based signaling motif in its cytoplasmic tail. We provided evidence that ICOS can induce key TFH cytokines by activating PI3K through this signaling motif, probably when primed T cells contact with B cells to complete the TFH differentiation program.
Materials and Methods
Animals.
ICOS-YF knock-in mice were generated in 129 background and then backcrossed into C57BL/6 background. ICOS-KO mice have been described (13). The animals were housed in the Institut de Recherches Cliniques de Montreal (IRCM) Animal Facility under specific pathogen-free conditions. Animal experiments were performed according to animal use protocols approved by the IRCM Animal Care Committee.
Antibodies and Cytokines.
Details of the reagents are described in SI Text.
In Vitro T-Cell Culture.
All T cells were cultured in RPMI1640 medium (1 × 106 cells/mL) supplemented with 10% FBS, glutamine, penicillin, streptomycin, β-mercaptoethanol. Total LN cells were activated by soluble anti-CD3 (1 μg/mL). For preparation of CD4+ T blasts for biochemical analyses and Ca2+ flux experiments, CD4+ T cells were positively selected (>95%) from single cells suspensions of spleen and LN using CD4 selection kit (Stem Cell Technologies) and then activated by culturing with plate-bound anti-CD3 (3 μg/mL) plus soluble anti-CD28 (1 μg/mL) for 2 days and expanded in media containing recombinant IL-2 (100 μg/mL) for 3 days. For experiments described in Fig. 5 and Fig. S7 B and C, splenic CD4+ T cells were negatively selected (>90%) using the MACS CD4+ T-cell isolation kit (Myltenyi). The CD4+ T cells were stimulated for 2 days as described above, except that 10 ng/mL IL-6 were added to enhance IL-21 expression (47). Subsequently, the activated cells were collected, washed once in complete medium, and rested for 1 day in complete medium without IL-2 at 1 × 106 cells/mL in 6-well plates (2 mL/well) to avoid overcrowding.
Acute T-Cell Activation, Lysis, and Immunoprecipitaton.
The CD4+ T-cell blasts were harvested and stimulated by combinations of anti-CD3 (1 μg/mL), anti-ICOS (2 μg/mL), and control hamster IgG. The bound antibodies were cross-linked by goat anti-hamster IgG (20 μg/mL) at 37 °C. After washing, cells were lysed in lysis buffer (1% Nonidet P-40, 20 mM Tris pH 7.4, 137 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM PMSF, and 0.1 mM sodium orthovanadate) for 20 min on ice. After clearance of cell debris, lysates were boiled in Laemmli sample buffer for immunoblot analysis. For immunoprecipitation of ICOS-associated proteins, the lysates were incubated with anti-ICOS antibody (2 μg/mL) for 1 h at 4 °C, and the immune complexes were recovered by a mixture of protein G-agarose beads (Thermo Scientific) and protein A-agarose beads (Pierce).
T-Cell Restimulation Assays.
Negatively selected CD4+ T cells were stimulated for 2 days and rested for 1 day in media alone. For cytokine qPCR, 5 million CD4+ T blasts were restimulated for 6 h in 400 μL media containing Ab cocktails: Anti-CD3 (1 μg/mL) plus either hamster IgG, anti-ICOS, or anti-CD28 (2 μg/mL each), followed by goat anti-hamster IgG (20 μg/mL). For PI3K inhibition experiments, cells were pretreated for 1 h with LY 294002 (50 μM; Calbiochem) and then stimulated with the Abs in the continued presence of the inhibitor. RNA was isolated using the TRIzol reagent (Invitrogen). cDNA was prepared from the extracted RNA using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). HGPRT, IL-21, IL-4, and IL-10 TaqMan primers and probes were from Applied Biosystems. Quantitative real-time PCR was performed by using a TaqMan 7300/7500 system and software (Applied Biosystems). Fold expression was calculated using the ΔΔCT method using HGPRT as a reference gene. For proliferation assays, cells were restimulated in U-bottom 96 wells (1 × 105 cells/well) with the Abs for 24 h. For the last 8 h of incubation, 3H-thymidine was added at 1 μCi/well.
Ca2+ Flux, Immunization of Mice, and ELISA.
Details are described in the SI Text.
Statistical Analysis.
The significance of the data were tested by Student's t test.
Supplementary Material
Acknowledgments.
This work was supported by grants from the Canadian Institutes for Health Research (to W.-K.S. and T.W.M.) and Vascular System Research Center Grant and Regional Research Center Program from Korea Science and Engineering Foundation (to J.C.). W.-K.S. is a recipient of a New Investigator Award from the Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911573106/DCSupplemental.
References
- 1.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 2.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:357–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: Predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 7.Dullaers M, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutloff A, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 11.Lohning M, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Tafuri A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 14.McAdam AJ, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 15.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 16.Grimbacher B, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 17.Bossaller L, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 18.Akiba H, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 19.Hu YL, et al. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 20.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 21.Yu D, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 22.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- 24.Linterman MA, et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Parry RV, et al. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-x(L), and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 26.Arimura Y, et al. A co-stimulatory molecule on activated T cells, H4/ICOS, delivers specific signals in T(h) cells and regulates their responses. Int Immunol. 2002;14:555–566. doi: 10.1093/intimm/dxf022. [DOI] [PubMed] [Google Scholar]
- 27.Parry RV, Riley JL, Ward SG. Signalling to suit function: Tailoring phosphoinositide 3-kinase during T-cell activation. Trends Immunol. 2007;28:161–168. doi: 10.1016/j.it.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Dodson LF, et al. Targeted knockin mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol Cell Biol. 2009;29:3710–3721. doi: 10.1128/MCB.01869-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurieva RI, et al. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J Immunol. 2007;179:1096–1103. doi: 10.4049/jimmunol.179.2.1096. [DOI] [PubMed] [Google Scholar]
- 30.Franko JL, Levine AD. Antigen-independent adhesion and cell spreading by inducible costimulator engagement inhibits T cell migration in a PI-3K-dependent manner. J Leukoc Biol. 2009;85:526–538. doi: 10.1189/jlb.0808505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells V, Downward J, Mallucci L. Functional inhibition of PI3K by the betaGBP molecule suppresses Ras-MAPK signalling to block cell proliferation. Oncogene. 2007;26:7709–7714. doi: 10.1038/sj.onc.1210580. [DOI] [PubMed] [Google Scholar]
- 32.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 33.Iiyama R, et al. The role of inducible co-stimulator (ICOS)/B7-related protein-1 (B7RP-1) interaction in the functional development of Peyer's patches. Immunol Lett. 2003;88:63–70. doi: 10.1016/s0165-2478(03)00054-3. [DOI] [PubMed] [Google Scholar]
- 34.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker LS, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garside P, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 37.Feito MJ, et al. Mechanisms of H4/ICOS costimulation: Effects on proximal TCR signals and MAP kinase pathways. Eur J Immunol. 2003;33:204–214. doi: 10.1002/immu.200390023. [DOI] [PubMed] [Google Scholar]
- 38.Smith KM, et al. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J Immunol. 2003;170:2310–2315. doi: 10.4049/jimmunol.170.5.2310. [DOI] [PubMed] [Google Scholar]
- 39.Odegard JM, et al. ICOS controls effector function but not trafficking receptor expression of kidney-infiltrating effector T cells in murine lupus. J Immunol. 2009;182:4076–4084. doi: 10.4049/jimmunol.0800758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redoglia V, et al. Characterization of H4: A mouse T lymphocyte activation molecule functionally associated with the CD3/T cell receptor. Eur J Immunol. 1996;26:2781–2789. doi: 10.1002/eji.1830261134. [DOI] [PubMed] [Google Scholar]
- 41.Buonfiglio D, et al. Characterization of a novel human surface molecule selectively expressed by mature thymocytes, activated T cells and subsets of T cell lymphomas. Eur J Immunol. 1999;29:2863–2874. doi: 10.1002/(SICI)1521-4141(199909)29:09<2863::AID-IMMU2863>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 42.Fos C, et al. ICOS ligation recruits the p50α PI3K regulatory subunit to the immunological synapse. J Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 44.McAdam AJ, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 45.Walker LS, et al. Established T cell-driven germinal center B cell proliferation is independent of CD28 signaling but is tightly regulated through CTLA-4. J Immunol. 2003;170:91–98. doi: 10.4049/jimmunol.170.1.91. [DOI] [PubMed] [Google Scholar]
- 46.Macaulay AE, DeKruyff RH, Umetsu DT. Antigen-primed T cells from B cell-deficient JHD mice fail to provide B cell help. J Immunol. 1998;160:1694–1700. [PubMed] [Google Scholar]
- 47.Dienz O, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.