Abstract
Oxidative mechanisms during nuclear sclerosis of the lens are poorly understood, in particular metal-catalyzed oxidation. The lysyl oxidation product adipic semialdehyde (allysine, ALL) and its oxidized end-product 2-aminoadipic acid (2-AAA) were determined as a function of age and presence of diabetes. Surprisingly, whereas both ALL and 2-AAA increased with age and strongly correlated with cataract grade and protein absorbance at 350 nm, only ALL formation but not 2-AAA was increased by diabetes. To clarify the mechanism of oxidation, rabbit lenses were treated with hyperbaric oxygen (HBO) for 48 h, and proteins were analyzed by gas and liquid chromatography mass spectrometry for ALL, 2-AAA, and multiple glycation products. Upon exposure to HBO, rabbit lenses were swollen, and nuclei were yellow. Protein-bound ALL increased 8-fold in the nuclear protein fractions versus controls. A dramatic increase in methyl-glyoxal hydroimidazolone and carboxyethyl-lysine but no increase of 2-AAA occurred, suggesting more drastic conditions are needed to oxidize ALL into 2-AAA. Indeed the latter formed only upon depletion of glutathione and was catalyzed by H2O2. Neither carboxymethyl-lysine nor glyoxal hydroimidazolone, two markers of glyco-/lipoxidation, nor markers of lenticular glycemia (fructose-lysine, glucospane) were elevated by HBO, excluding significant lipid peroxidation and glucose involvement. The findings strongly implicate dicarbonyl/metal catalyzed oxidation of lysine to allysine, whereby low GSH combined with ascorbate-derived H2O2 likely contributes toward 2-AAA formation, since virtually no 2-AAA formed in the presence of methylglyoxal instead of ascorbate. An important translational conclusion is that chelating agents might help delay nuclear sclerosis.
Introduction
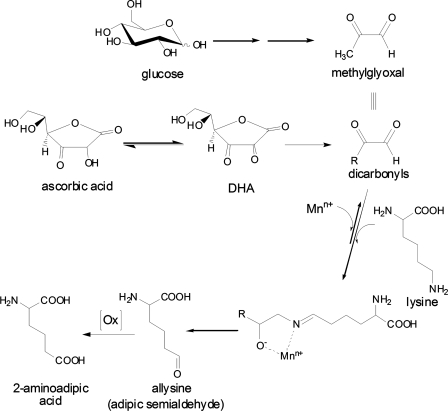
The aging human lens accumulates a number of postsynthetic protein modifications that are thought to predispose lens crystallins toward aggregation and cataractogenesis. These include glycation by sugars, ascorbic acid, and oxoaldehydes, 3-hydroxy-kynurenination, oxidation, and photooxidation, deamidation, deamination, and protein truncation (reviewed in Ref. 1). Among the oxidative mechanisms, those involving formation of protein disulfides and mixed disulfides with glutathione or cysteine are well understood (2). Similarly, the presence of methionine sulfoxide in human lens crystallins has been known for several years (3, 4). In recent years, however, considerable interest has focused on the formation of so-called protein carbonyls as markers of oxidative stress in biology. Stadtman and co-workers (5) demonstrated that proteins incubated in the presence of redox active metals, such as Cu(II) and Fe(III), with hydrogen peroxide or ascorbic acid, underwent metal-catalyzed oxidation (MCO),3 whereby histidine, proline, lysine, and arginine are preferentially oxidized. Lysine residues are oxidized into allysine (adipic semialdehyde) (Fig. 1), while proline and arginine residues into glutamic semialdehyde (6, 7). These “protein carbonyls” have been immunologically detected as markers of oxidative stress with the 2,4-dinitrophenylhydrazine assay (DNP) in a large variety of system, including diabetic rat lens (8).
FIGURE 1.
The two-step oxidation of lysyl residues in proteins exposed to catalytic metals. In step 1, oxidative deamination assisted by an oxoaldehyde derived from either methylglyoxal or ascorbic acid leads to the formation of “protein carbonyls,” one of which is allysine (aminoadipic semialdehyde) that is assayed in this work as 6-hydroxynorleucine upon sodium borohydride reduction. In step 2, it is proposed that H2O2 from either ascorbate oxidation or oxygenase enzyme catalyzes allysine oxidation into the stable end-product 2-aminoadipic acid.
Garland was the first to hypothesize that MCO could occur in the lens and contribute to cataract formation because “all the ingredients necessary to promote this form of oxidative process” were present in the human lens, including Cu- and Fe-ions, ascorbic acid, and oxygen, even though the latter is present at low levels(9). This hypothesis was confirmed in subsequent studies illustrating lenticular presence of redox active metals (10), protein carbonyls (11), and specific forms of protein backbone damage that are known to require oxidative metals to form, such as the hydroxylated forms of phenylalanine, tyrosine, valine, and leucine (12).
However, while chemical evidence for metal-catalyzed oxidation was established based on the presence of hydroxylated amino acids and protein carbonyls, many questions remain concerning the presence, nature, and mechanism of formation for protein carbonyls in the lens. First and foremost protein carbonyls can originate from the glycation products fructose-lysine and its enolized 5,6-dideoxy form (13), and thus the DNP assay cannot differentiate between oxidized lysine residues (“allysine”) and intermediate glycation adducts. Second, multiple pathways for lysyl oxidation are theoretically possible, including MCO catalyzed by ascorbate as proposed by Stadtman and co-workers (7), or by methylglyoxal as proposed by Suyama (14), myeloperoxidase (15), or lysyl oxidase (16). Some of these might be amenable to chelation therapy, while others might not.
What prompted the present study was our recent discovery that the protein carbonyl allysine (adipic semialdehyde) can become further oxidized into 2-aminoadipic acid (2-AAA). In human skin, this thermodynamically stable oxidation end-product of lysine, accumulated to a much larger extent than its precursor measured as 6-hydroxynorleucine (17) (Fig. 1). This finding implied that some oxidative mechanism was active in the extracellular matrix and raised the question whether intracellular long-lived proteins, such as the crystallins were also subject to lysyl oxidation and 2-AAA formation.
To address these questions, we have used gas chromatography-mass spectrometry (GC/MS) to determine allysine and 2-AAA formation in human water-soluble and insoluble human lens crystallins as a function of age, degree of lens pigmentation, and presence of diabetes. We have then performed mechanistic studies on the role of oxygen levels for MCO using the hyperbaric oxygen/rabbit lens model and determined its relationship to other pathways of carbonyl and oxidant stress in the lens. Finally, to understand the role of glutathione (GSH) as an endogenous inhibitor of carbonyl and oxidative stress in the lens, we cultured intact mouse lenses in the presence of ascorbic acid but under depletion of GSH using buthionine sulfoximine.
EXPERIMENTAL PROCEDURES
Chemicals
Standards of l-2-aminoadipic acid and d8-lysine (dl-[3,3,4,4,5,5,6,6-2H8]lysine), d3-methionine ([methyl-2H3]l-methionine), and d4-methionine (dl-[3,3,4,4-2H4]methionine) were purchased from Sigma-Aldrich; [guanidine-15N2]l-arginine was purchased from Cambridge Isotope Laboratories (Andover, MA), CEL (Nϵ-carboxyethyl-lysine), and d4-CML (Nϵ-carboxymethyl-lysine-4,4,5,5-d4) were from Drs. Susan Thorpe and John Baynes (Department of Chemistry and Biochemistry, University of South Carolina, Columbia, SC); CML was from Dr. Marcus Glomb (Institute of Food Chemistry, Technical University of Berlin, Berlin, Germany); fructosyl-lysine (FL)(18) and 13C6-FL, d2-13C1- CEL, G-H1(19), d2-G-H1, MG-H1(19), d3-MG-H1, d3-2-aminoadipic acid and d4-methioninesulfoxide (d4-MetSOX) (20) were prepared in our laboratory either following previous reports or by new developed methods (see supplemental materials). All synthesized standards were confirmed by MS, 1H-NMR, 13CNMR. 6-Hydroxynorleucine and d3-6-hydroxynorleucine were synthesized as described in a previous study (17). All other chemicals were purchased from Sigma.
Human Lens Samples
A total of 54 human lenses was obtained from the National Disease Research Interchange (NDRI) (Philadelphia, PA). Categorization is by gender and race, diagnosis of diabetes, and primary cause of death based on information provided by NDRI. All lenses were classified according to the degree of pigmentation into Pirie grade I (n = 20), II (n = 16), III (n = 9), IV (n = 0) as in the past (21). Additionally, UV absorbance at λ360 nm of lens soluble and insoluble proteolytic digest was also recorded. Lenses were stored at −80 °C until use.
Preparation of Lens Proteins
Human lenses were decapsulated and homogenized in 5 mm Chelex-100-treated sodium phosphate buffer (pH 7.4) with protease inhibitors added (Roche) (2.0 ml per lens) in a Con-Torqueglass homogenizer (Eberbach, Ann Arbor, MI) for 3 min on ice. The homogenate was centrifuged at 20,000 × g for 30 min at 4 °C. The supernatant and pellet were separated. The pellet was suspended in 2.0 ml of ice-cold buffer and recentrifuged. The two supernatant fractions were pooled as the water-soluble fraction (WS). The WS fraction was further dialyzed against water for 48 h with a change of water after 24 h. The dialysate was lyophilized. The pellet (water-insoluble fraction (WI)) was further washed with 2.0 ml of water, and the supernatant was discarded after centrifugation. Both the WS and WI fractions were delipidated by treatment with 5.0 ml of chloroform/methanol, 2:1 (v/v), with shaking for 24 h and centrifugation at 3500 × g for 30 min. The pellet was then taken into 5.0 ml of diethyl ether, allowed to stand for 10 min, and centrifuged at 3500 × g for 30 min. The residue was lyophilized.
Borohydride Reduction and Acid Hydrolysis
Approximately 2 mg of each of WS and WI samples were weighed and placed into a 12 × 75 mm borosilicate glass test tube followed by reduction with 0.1 m sodium borohydride to allow quantitation of 2-aminoadipic semialdehyde as 6-hydroxynorleucine as previously described (17) (Fig. 1). After dialysis (for WS) or wash (WI), the sample was placed into a 13 × 100 mm borosilicate glass tube fitted with a PTFE (polytetrafluoroethylene)-faced, rubber-lined screw cap. Each sample was acid hydrolyzed for 18 h with 2 ml of 6 m HCl as detailed elsewhere (17). To minimize discoloration and artifact formation during acid hydrolysis, the concentration of tissue to acid was maintained at ∼1 mg/ml. In addition, the acid was degassed under vacuum followed by purging with argon by bubbling for at least 20 min. The acid was immediately pipetted into each tube followed by thoroughly purging the tube with argon before sealing with the screw cap.
AGEs Determination by GS/MS and LC-MS/MS
Amounts of 6-hydroxynorleucine, 2-aminoadipic acid, carboxymethyl-lysine (CML), and carboxyethyl-lysine (CEL) were determined in acid hydrolysates of processed lens samples, and derivatized as their trifluoroacetyl methyl esters by selected ion monitoring gas chromatography (GC)/MS as previously described (17). Amounts of FL, MetSOX, G-H1, and MG-H1 in enzymatically digested samples (see below) were measured by electron spray positive ionization-mass spectrometric multiple reaction monitoring (ESI+ MRM) by using LC-MS/MS system composed of a 2690 Separation module with a Quattro Ultima triple quadrupole mass spectrometry detector (Water-Micromass, Manchester, UK) following the previously published procedure by Thornalley et al. (22). Analytes released by self-digestion of proteases in assay blanks were subtracted from analytic estimates.
Rabbit Lens Hyperbaric Treatment and Protein Preparation
New Zealand White rabbit lenses (n = 6 per group) were treated with 50 atm 100% O2 or normal atm air in HEPES TC-199 buffer for 48 h as described previously (23). After hyperbaric O2 treatment, each rabbit lens was frozen immediately and dissected into cortex (C) and nuclear (N) fractions. Both cortex and nuclear fractions were processed the same as for human lenses described above. The WS and WI fractions were either acid hydrolyzed or enzymatically digested for the determination of AGEs as described above.
Mouse Lens Organ Culture
2-month-old C57BL/6 mice fresh lenses were used for organ culture. In 12-well plates, three lenses in each well, three well in each group were cultured in M199 medium with 2 mm ascorbic acid with or without the presence of 10 μm l-buthionine-sulfoximine (BSO) and dimethylfumarate (DMF). The medium was refreshed every 12 h. The total culture was 72 h. The lenses were briefly washed with cold PBS and homogenized in 10% trichloroacetic acid. The precipitate was washed twice with 500 μl of ethyl-ether and air dried for 10 min. The total protein extract was digested for LC-MS analysis.
Lens Protein Enzymatic Digestion
3 mg of lens protein were used for enzymatic digestion. Briefly, the lens protein was reconstituted in an Eppendorf tube with 500 μl of 5.0 mm argon exchanged, chelex-treated phosphate buffer, pH 7.0, and 35 μl of protease K (10 mg/ml in argon-exchanged water, Roche Applied Science), at 37 °C for 24 h with rotation, with 3 μl of chloroform/toluene, 1:1 (v/v) to prevent bacterial growth. Peptidase (Sigma P7500) (3.6 mg/100 mg protein) was added followed by protease K digestion for another 24 h at 37 °C, then 48 h digestion with Pronase (Sigma P5147) by adding Pronase (3.6 mg/100 mg protein) every 24 h, followed by aminopeptidase M (Roche Applied Science) (1 unit/100 mg protein) at 37 °C for 24 h under the same conditions as mentioned above. Corresponding enzyme blanks incubated without added protein were used as background controls.
The overall percentage of digestion for each sample was determined by the ninhydrin reaction by dividing total leucine equivalents assayed for each digest by the same upon acid hydrolysis. For the results below, protein concentration was approximated by assuming that every 10 μmol of leucine equivalent is equal to 1 mg of protein.
In Vitro Incubation
Bovine lens protein water soluble extract (BLPS, 10 mg) each in 5 ml of PBS buffer containing 5 μm CuCl2 was incubated with 5 mm methylglyoxal (MGO) or ascorbic acid (ASA) with or without 1 mm catalase for 7 days and added fresh every 24 h. BLPS alone incubated in the same buffer served as control. 100 μl of incubation mixture was taken every 24 h for the H2O2 assay. The H2O2 was determined by using the Amplex Red hydrogen peroxide assay kit (Invitrogen) based on the manufacturer's protocol. After incubation, protein was dialysed against PBS for 48 h, and 3 mg of protein were reduced and hydrolyzed as above for GC/MS analysis. Another incubation using poly-l-lysine (20 mg) (∼15,000 Da, Sigma) in PBS buffer containing 5 mm CuCl2 was carried out at 37 °C for 7 days to produce allysine. The incubation mixture was dialyzed against chelex-treated PBS plus 1 mm DTPA for 48 h and then without DTPA. Samples A and B were preincubated with 10 mm N-acetyl-cysteine for 1 h at room temperature. Samples A and C were incubated with 100 μm H2O2 up to 6 h. B was incubated with 100 mm NaBH4 (control) for up to 6 h. At 0, 30, 120, 360 min time points, one-fourth of the fraction was taken and quickly dialyzed against cold PBS for 24 h. A and C were then reduced with NaBH4 and processed for 6-hydroxynorleucine and 2-AAA determination.
Statistical Methods
Regression analysis, Spearman's correlations, and the Mann-Whitney Test were computed using SPSS software REF testing for homogeneity of variance was done using either the F-test or the Burr-Foster Q-Test as previously described. Data were transformed with either the square root or log y transformations. Significance was considered at p < 0.05.
RESULTS
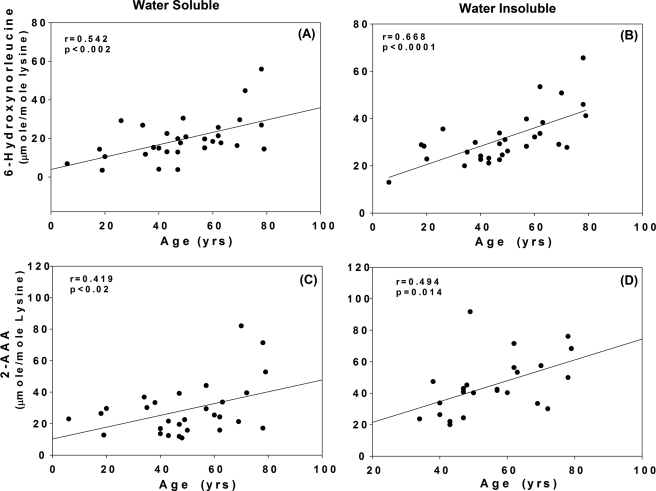
The relationship between lysine, allysine, and 2-AAA is depicted in Fig. 1. Relevant to the results presented below is the anticipated role of H2O2 for the conversion of allysine/2-aminoadipic semialdehyde (measured as 6-hydroxy-norleucine) into 2-AAA. Allysine and 2-aminoadipic acid were determined simultaneously by GC/MS in protein fractions from human lenses obtained at autopsy. An age-related linear increase in both oxidation products was observed, whereby mean allysine levels increased over the human life span 5- and 3-fold from 4 to 30 μmol/mol lysine (p < 0.002) and 12 to 50 μmol/mol lysine (p < 0.0001) in the water-soluble and -insoluble fractions, respectively (Fig. 2, top panels). A similar accumulation of 2-AAA was noted with the increase highest in the water-insoluble fraction (Fig. 2, bottom panel). Overall, almost twice as much 2-AAA accumulated than allysine.
FIGURE 2.
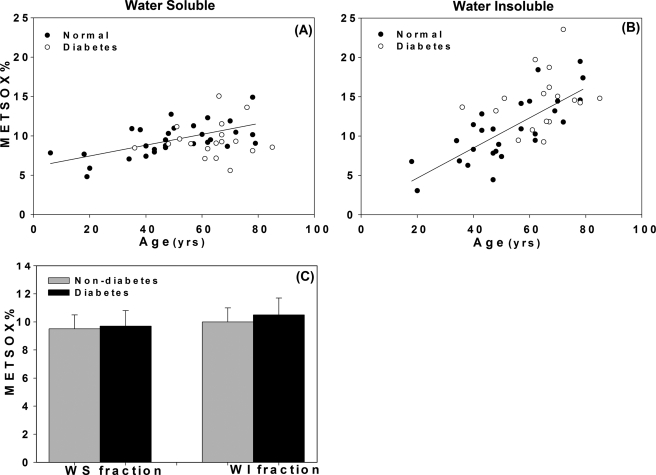
Levels of 6-hydroxynorleucine and 2-aminoadipic acid versus chronological age in WS (panels A and C) and WI (panels B and D) lens protein fractions from non-diabetic individuals. Regression line equations where x is age and y is parameter. A and B, 6-hydroxynorleucine (A): y = 0.32x + 3.91, n = 30, r = 0.54, p < 0.002; (B): y = 0.38x + 12.81, n = 30, r = 0.67, p < 0.0001; C and D, 2-aminoadipic acid, (C): y = 0.37x + 10.25, n = 30, r = 0.42, p < 0.02 (D): y = 0.66x + 8.25, n = 23, r = 0.49, p < 0.01. Bars represent means ± S.D.
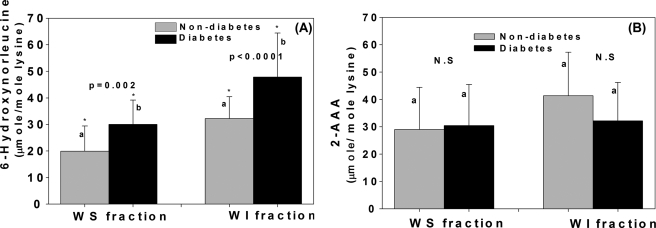
Most revealing were the effects of diabetes on these oxidation products. Diabetes markedly increased allysine in both water-soluble (p = 0.002) and -insoluble fractions (p < 0.0001) but had no effect on 2-AAA (Fig. 3, A and B). The latter even tended to be lower in the water-insoluble fraction (Fig. 3B).
FIGURE 3.
Effects of diabetes on levels of 6-hydroxynorleucine. (WS: p = 0.002; WI; p < 0.0001) (A) and 2-aminoadipic acid (WS: n.s; WI: n.s) (B) in WS and WI fractions upon adjustment to age 50 years. Student's t test was used to compare two groups, A p value below 0.05 was considered significant. Bars represent means ± S.D.
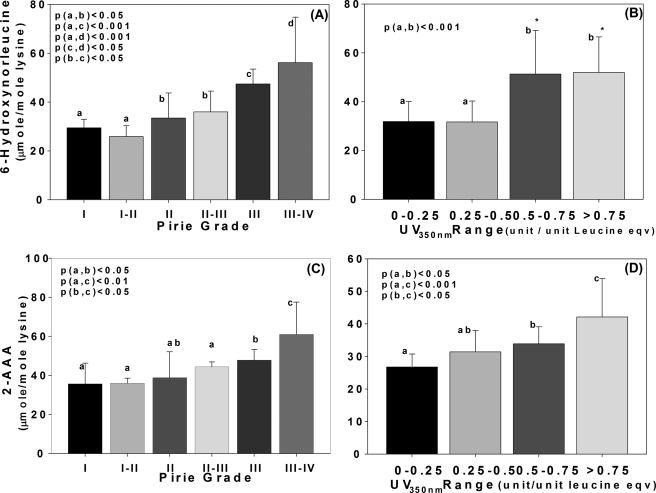
Levels were then examined in the water-insoluble fraction as a function of lens pigmentation expressed either as Pirie cataract grade (24) (Fig. 4, top panels) or protein-bound absorbance at 350 nm (Fig. 4, bottom panels). When expressed as a function of Pirie grade, there was a continuous increase in both allysine and 2-AAA with the highest levels found in Pirie III-IV lenses (p < 0.05 to <0.001). When expressed in terms of absorbance at 350 nm, a similar pattern was noted for 2-AAA, although there was a significant threshold (p < 0.05) for allysine in crystallins absorbing above 0.5 units/leucine equivalent.
FIGURE 4.
Levels of 6-hydroxynorleucine and 2-aminoadipic acid versus Pirie classification and UV absorbance at 350 nm after age adjustment to 50 years. Data are shown for water-insoluble proteins only. Panels A and C, Pirie cataract grade comprising four groups of discoloration degree. Panels B and D, comparison with UV absorbance units at 350 nm categorized from 0–0.25, 0.25–0.5, 0.5–0.75, and >0.75. 6-Hydroxynorleucine (A and B) and 2-aminoadipic acid (C and D) level was age adjusted to 50 years. The one-way ANOVA was used to compare the data of all groups, and a p value below 0.05 was considered as significant. Bars represent means ± S.D.
The surprising lack of increase in 2-AAA in the diabetic lens suggested that H2O2, a candidate oxidant for the conversion of allysine into 2-AAA, was not increased in the diabetic lens. To verify this, we determined the levels of methionine sulfoxide, which would also be oxidized by H2O2. The data showed no elevation of methionine oxidation in diabetic lens compared with non-diabetic individuals (Fig. 5), confirming that neither H2O2 nor some other oxidant mechanism was increased in the diabetic lens.
FIGURE 5.
Plots of methionine sulfoxide versus chronological age and presence of diabetes (A and B) and age adjustment to 50 years (C) in WS and WI fractions. For each graph, regression line for non-diabetic individuals is shown. Regression line equations where x is age and y is parameter: A, y = 0.068x + 6.08, n = 30, r = 0.62, p < 0.0001; B, y = 0.19x + 0.82, n = 30, r = 0.77, p < 0.0001; (E): Student's t test was used to compare two groups, p value below 0.05 was considered significant. Methionine sulfoxide was not elevated by diabetes (p = N.S.). Bars represent means ± S.D.
To understand the role of oxygen tension in the formation of allysine and 2-AAA, rabbits were exposed to hyperbaric oxygen for 48 h, as previously described (23). Lenses were processed with the same protocol as for human lenses and separated into cortical and nuclear water-soluble and -insoluble fractions. A massive, 2–6-fold increase in allysine was noted in both protein fractions (Fig. 6, upper panels). Surprisingly, however, none of these “protein carbonyls” were further oxidized into 2-AAA in the process (Fig. 6, bottom panels), i.e. whatever 2-AAA was there, it was already present before the hyperbaric treatment.
FIGURE 6.
Rabbit lens 6-hydroxynorleucine and 2-aminoadipic acid level before and after HBO in WS and WI fractions of lens cortex and nucleus. A and B, 6-hydroxynorleucine (WS: p < 0.0001(cortex and nucleus versus control; WI: p < 0.0001 (cortex and nucleus versus control)). C and D, 2-AAA (WS and WI: n.s (cortex and nucleus versus control)). The Student's t test was used to compare the control (Ctr) and HBO. 2-AAA levels were not elevated by HBO (p = N.S.). Bars represent means ± S.D.
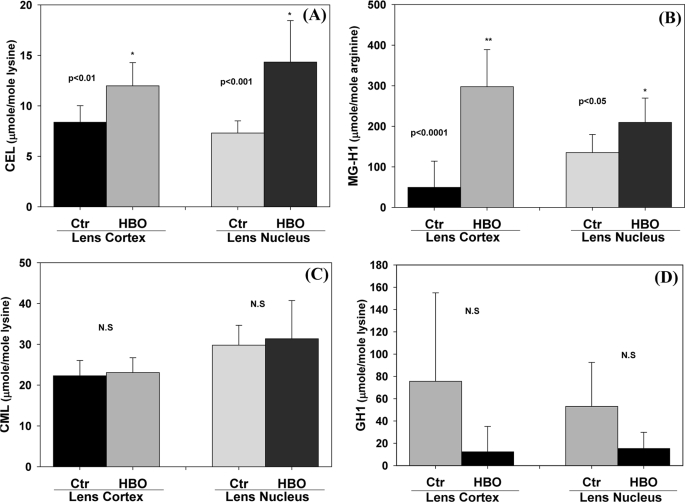
To differentiate between metal-catalyzed mechanisms involving only redox active metal (i.e. “pure” Stadtman MCO), versus the “Suyama pathway,” which involves methylglyoxal-copper/iron assisted MCO of lysine (Fig. 1), we determined the methylglyoxal-dependent AGEs carboxyethyl-lysine (CEL) and methyglyoxal hydroimidazolone (MG-H1), as well as the glycoxidation/lipoxidation products CML and G-H1. Results showed that the methylglyoxal modifications CEL and MG-H1 were significantly elevated in all fractions and parts of the lens, whereas both CML and G-H1 were unchanged, the latter tending to be lowered by treatment (Fig. 7). This clearly shows that methylglyoxal is increased, while glycoxidation/lipoxidation is not. Methionine sulfoxide in these lenses was also unchanged (data not shown). This again demonstrates that oxidant, and thus H2O2 was not increased even with 48 h of hyperbaric O2 treatment. How these results differ from previous similar studies (23) will be discussed below.
FIGURE 7.
Rabbit lens insoluble fraction AGEs level before and after hyperbaric oxygen treatment. For details see the legend for Fig. 6. A, CEL (p < 0.01 (cortex versus ctr); p < 0.001,(nucleus versus ctr)); B, MG-H1 (p < 0.0001 (cortex versus ctr); p < 0.05 (nucleus versus ctr)); C, CML (n.s (cortex and nucleus versus ctr)); D, G-H1 (n.s (cortex and nucleus versus ctr)) were determined by LC-MS. The Student's t test was used to compare the Ctr and HBO. CML and G-H1 were not elevated by HBO. Bars represent means ± S.D.
The high resistance of protein carbonyls and methionine toward further oxidation in diabetes and hyperbaric O2 treatment suggested to us the hypothesis that glutathione would prevent the oxidant from attacking the proteins. To test this hypothesis, we cultured for 72 h intact mouse lenses in the presence of ascorbic acid without or with buthionine sulfoximine to inhibit GSH synthesis (25). As a result (Fig. 8), there was a massive increase in the methylglyoxal-derived AGEs CEL and MG-H1, including a tripling of 2-AAA levels, indicative of increased metal-catalyzed damage to lysine residues. Evidence of active oxidation was reflected in the fact that methionine sulfoxide was 150% (Fig. 8, inset). Interestingly, the protein carbonyls (6-hydroxynorleucine) were very low, likely reflecting rapid and quantitative conversion of the semialdehyde into the more stable end-product 2-AAA.
FIGURE 8.
Mouse lens organ culture in M199 medium containing ascorbic acid with or without GSH depletion induced by BSO. Mouse lens proteins after 72 h culture in M199 medium containing 2 mm ascorbic acid with or without BSO were extracted, and AGEs levels were determined by LC/MS. A, CEL, FL, G-H1, MG-H1; inset: MetSOX, CML; B, 2-AAA and 6-hydroxynorleucine. The Student's t test was used to compare two groups. * indicates comparison is significant at p < 0.001. Bars represent means ± S.D.
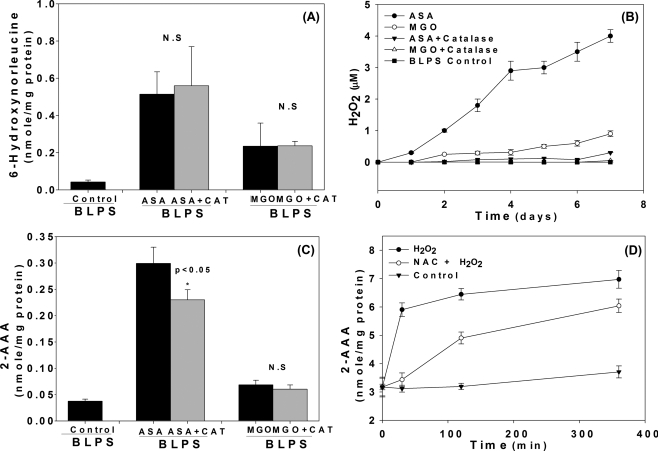
To evaluate the role of H2O2 amid several possible mechanisms for allysine and 2-AAA formation, we incubated bovine lens crystallins with either ascorbic acid (5 mm) or methylglyoxal (MGO, 5 mm) with or without catalase. The results in Fig. 9A show that allysine, as expected, formed both from ASA and MGO, and that catalase only partially suppressed 2-AAA from ASA (Fig. 9C) even though H2O2 formation was nearly totally suppressed by catalase (Fig. 9B). Moreover, there was minimal 2-AAA formation in the presence of MGO, strongly suggesting that H2O2 is not necessary for allysine formation. However, addition of H2O2 to oxidized poly-l-lysine rapidly induced 2-AAA formation (Fig. 9B) that slowed down but was not suppressed by 10 mm N-acetyl-lysine (Fig. 9D).
FIGURE 9.
MCO in vitro. BLPS was incubated with MGO or ASA with or without catalase (CAT) for 7 days. A and C, 6-hydroxynorleucine and 2-AAA levels were determined by GC/MS; B, the H2O2 was determined daily during incubation measured by Amplex-Red assay. D, oxidized poly-l-lysine was incubated with H2O2 with or without N-acetyl-cysteine (NAC).
DISCUSSION
The above data unequivocally establish the fact that protein carbonyls stemming from metal-catalyzed oxidation are present in the aging human lens and that these modifications are increased in the presence of diabetes. Thus they confirm the previous report by Boscia (11) in which the authors used the DNP assay and UV spectroscopy to quantitate total DNP-reactive carbonyls.
The Boscia study revealed an age-related increase from 45–75 years from 1 to 2 nmol/mg human lens proteins that was further catalyzed by diabetes, senile cataract, and myopia. In contrast, our levels of allysine (adipic semialdehyde measured as 6-hydroxynorleucine) reached only up to 0.15 nmol/mg collagen in old age, i.e. about 6–10× less. This is in part explained by the fact that allysine is only one of the two protein carbonyls described by Requena and Stadtman and co-workers (7), the other one being glutamyl semialdehyde, i.e. the oxidation product of arginine and proline. These latter studies showed that the glutamyl semialdehyde is formed in quantities 1.5–4× larger than adipic semialdehyde (allysine) upon MCO. It is thus likely that substantial amounts of glutamic semialdehyde also occur in the lens. This assumption would leave a gap of about 0.5–1.0 nmol/mg protein between our study and the Boscia study, which we believe is best explained based on nonspecific binding of DNP to glycated residues that are also increased in diabetes. Thus, additional studies will be needed to precisely assess the extent of protein carbonyl formation caused by MCO.
The mechanistic studies with rabbit lenses exposed to hyperbaric oxygen confirm the critical role of oxygen in the formation of allysine. Furthermore, the dramatic elevation of methylglyoxal AGE in these lenses provides strong support for the Suyama proposition of a combined attack of methylglyoxal and catalytic metal onto lysine residues (Fig. 1). At the same time, this finding raises the intriguing question of whether “pure” MCO involving Mn+/H2O2, i.e. without oxoaldehyde, is a significant mechanism in vivo. Indeed, Stadtman and co-workers (26) often facilitated MCO using ascorbic acid, which is present in the lens in mm concentrations and has been unequivocally shown to be a precursor of ascorbylation products in the lens. However, one question that still remains to be addressed is the extent to which methylglyoxal comes from glycolysis versus ascorbate oxidation during hyperbaric O2 treatment.
In this study, we have not investigated the nature of the metal responsible for the oxidation. Both Cu2+ and Fe3+ have been proposed by Suyama and Stadman (5, 14). Padgaonkar et al. (23) found that O2-induced effects on rabbit lens nuclear supernatants could be prevented by a copper chelator, but not by an iron chelator. In a previous study in our laboratory, we demonstrated the presence of redox active copper in human lenses and showed that the activity was increased in lenses with severe cataracts (10). Mean endogenous copper content in pooled young, old, and cataract fractions increased from 0.016 to 0.026 nmol/mg protein, respectively, in WS (p < 0.05) and WIS (p < 0.001) fractions, and Cu(II) binding was 20–30% increased in cataractous versus old and young lenses in WS (p < 0.01) and WIS (p < 0.001) fractions. Also, levels of CML, and pentosidine were markedly elevated in WS and WIS fractions from cataractous versus old or young crystallins (20% to several folds, p < 0.05 to p < 0.001). In a separate unpublished experiment, protein-bound Fe was not elevated. These data together with those from Fu et al. (12) clearly support the existence of catalytically active metals in the lens, although the relative roles and source of copper versus iron need to be defined.
The most intriguing finding is the uncoupling between the oxidation of lysine residues into allysine, and the total suppression of further oxidation to the stable end-product 2-aminoadipic acid in diabetes. In skin, however, diabetes induces 2-AAA accumulation to a much larger extent than allysine (17), suggesting either a dramatic increase in some proxidant species or an absence of defense mechanisms. Surprisingly, while capillary H2O2 production is increased in diabetes (27), levels are 100 times lower in serum (about 1 μm (28)) than the lens (about 100 μm (29)). However, vascular GSH is very low, i.e. 1 μm (30) while very high in the lens GSH (3–5 mm). This was a clue that GSH must be playing a crucial role in the inhibition of the further oxidation of allysine into 2-AAA, as demonstrated by the large increase in 2-AAA in lenses cultured in the presence of buthionine sulfoximine.
There are two potential mechanisms by which GSH may inhibit allysine oxidation., i.e. one in which the oxidant species (e.g.H2O2) is detoxified by GSH peroxidase (31) and the other a mechanism in which GSH ties up the carbonyl group of allysine by forming a hemi-mercaptal (32), preventing it thus from further oxidation. Whatever the mechanism, the most striking finding in our study was that it took severe depletion of GSH from the lens to induce both allysine and methionine oxidation.
The above results on the relationship between GSH depletion, allysine, and methionine oxidation to 2-AAA and MetSOX, respectively, need to be reconciled with previous experiments in which cultured rabbit lenses were subjected to HBO for similar duration and found to be profoundly depleted in GSH after only 12 h (23). Why then was neither 2-AAA nor MetSOX increased under such conditions in our experiments? One possibility is that the more easily oxidizable crystallin SH groups were oxidized first (33), as reflected by rapid formation of protein disulfides in these lenses. Another possibility is that GSH depletion is not entirely sufficient unless ascorbic acid is present as in the mouse experiments that were conducted in the presence of 2 mm ascorbic acid. Finally, the existence of methionine sulfoxide reductase in the lens may have partially reverted the oxidation of methionine (34). This, however, would not have explained the lack of allysine oxidation.
Finally, our results and hypothesis are tilted toward a role for H2O2 as the oxidant species. In the extravascular space, such as skin collagen, there is little doubt that other mechanisms besides H2O2 need to be considered, such as peroxinitrite, lipid peroxides, hypochlorous acid, and free radicals. In the lens however, there is no evidence as yet for 3-nitrotyrosine and chloramines, suggesting ONOO- and hypochlorous acid should not play a role, although in vitro studies have been carried out (35). Also, there is no evidence for the presence of myeloperoxidase in the lens (36). Nevertheless, carbonyls and especially methionine residues, while most easily oxidized by H2O2 or possibly other oxidants, could conceivably be oxidized via different and less selective reactions that are in competition with cysteine oxidation and the cell defense (antioxidants) and repair systems. Contrary to that, lysine oxidation into allysine goes through lysine-selective Suyama pathway and other less selective MCO systems.
In summary, the data presented in this report suggest metal-catalyzed oxidation of lysines occurs in the aging human lens, most likely by the Suyama mechanism involving Cu2+, methyglyoxal, and H2O2 produced from ascorbic acid (Fig. 1). The latter appears to be the most likely, but not exclusive prooxidant for methionine and allysine oxidation to their end-products 2-aminoadipic acid and methionine sulfoxide, respectively.
Acknowledgment
We thank Victor Leverenz for technical assistance in treating rabbit lenses with hyperbaric O2.
This work was supported, in whole or in part, by National Institutes of Health Grants EY07099 (to V. M. M.), EY 02027 (to F. J. G.), and EY 014803 (to F. J. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental materials.
- MCO
- metal-catalyzed oxidation
- ALL
- allysine
- 2-AAA
- 2-aminoadipic acid
- HBO
- hyperbaric oxygen
- CEL
- carboxyethyl lysine
- CML
- carboxymethyl lysine
- MG-H1
- methylglyoxal hydroimidazolone
- WS
- water-soluble fraction
- WI
- water-insoluble fraction
- G-H1
- glyoxal hydroimidazolone
- MetSOX
- methionine sulfoxide
- BSO
- l-buthionine-sulfoximine
- DMF
- dimethylfumarate
- BLPS
- bovine lens water soluble protein
- PBS
- phosphate-buffered saline
- ANOVA
- analysis of variance
- DNP
- 2,4-dinitrophenylhydrazine assay.
REFERENCES
- 1.Truscott R. J. (2005) Exp. Eye Res. 80, 709–725 [DOI] [PubMed] [Google Scholar]
- 2.Lou M. F., Dickerson J. E., Jr., Garadi R. (1990) Exp. Eye Res. 50, 819–826 [DOI] [PubMed] [Google Scholar]
- 3.Garner M. H., Spector A. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sochaski M. A., Jenkins A. J., Lyons T. J., Thorpe S. R., Baynes J. W. (2001) Anal. Chem. 73, 4662–4667 [DOI] [PubMed] [Google Scholar]
- 5.Requena J. R., Levine R. L., Stadtman E. R. (2003) Amino Acids 25, 221–226 [DOI] [PubMed] [Google Scholar]
- 6.Amici A., Levine R. L., Tsai L., Stadtman E. R. (1989) J. Biol. Chem. 264, 3341–3346 [PubMed] [Google Scholar]
- 7.Requena J. R., Chao C. C., Levine R. L., Stadtman E. R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyselová Z., Garcia S. J., Gajdosíková A., Gajdosík A., Stefek M. (2005) Physiol. Res. 54, 49–56 [DOI] [PubMed] [Google Scholar]
- 9.Garland D., Russell P., Zigler J. S., Jr. (1988) Basic Life Sci. 49, 347–352 [DOI] [PubMed] [Google Scholar]
- 10.Saxena P., Saxena A. K., Cui X. L., Obrenovich M., Gudipaty K., Monnier V. M. (2000) Invest Ophthalmol. Vis. Sci. 41, 1473–1481 [PubMed] [Google Scholar]
- 11.Boscia F., Grattagliano I., Vendemiale G., Micelli-Ferrari T., Altomare E. (2000) Invest Ophthalmol. Vis. Sci. 41, 2461–2465 [PubMed] [Google Scholar]
- 12.Fu S., Dean R., Southan M., Truscott R. (1998) J. Biol. Chem. 273, 28603–28609 [DOI] [PubMed] [Google Scholar]
- 13.Biemel K. M., Lederer M. O. (2003) Bioconjug. Chem. 14, 619–628 [DOI] [PubMed] [Google Scholar]
- 14.Akagawa M., Sasaki T., Suyama K. (2002) Eur. J. Biochem. 269, 5451–5458 [DOI] [PubMed] [Google Scholar]
- 15.Peng D. Q., Wu Z., Brubaker G., Zheng L., Settle M., Gross E., Kinter M., Hazen S. L., Smith J. D. (2005) J. Biol. Chem. 280, 33775–33784 [DOI] [PubMed] [Google Scholar]
- 16.Kagan H. M., Williams M. A., Williamson P. R., Anderson J. M. (1984) J. Biol. Chem. 259, 11203–11207 [PubMed] [Google Scholar]
- 17.Sell D. R., Strauch C. M., Shen W., Monnier V. M. (2007) Biochem. J. 404, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornalley P. J., Langborg A., Minhas H. S. (1999) Biochem. J. 344, 109–116 [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed N., Thornalley P. J. (2002) Biochem. J. 364, 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brot N., Weissbach H. (1983) Arch. Biochem. Biophys. 223, 271–281 [DOI] [PubMed] [Google Scholar]
- 21.Nagaraj R. H., Sell D. R., Prabhakaram M., Ortwerth B. J., Monnier V. M. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 10257–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornalley P. J., Battah S., Ahmed N., Karachalias N., Agalou S., Babaei-Jadidi R., Dawnay A. (2003) Biochem. J. 375, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padgaonkar V. A., Leverenz V. R., Fowler K. E., Reddy V. N., Giblin F. J. (2000) Exp. Eye Res. 71, 371–383 [DOI] [PubMed] [Google Scholar]
- 24.Pirie A. (1968) Invest Ophthalmol. 7, 634–650 [PubMed] [Google Scholar]
- 25.Mårtensson J., Steinherz R., Jain A., Meister A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8727–8731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan X., Reneker L. W., Obrenovich M. E., Strauch C., Cheng R., Jarvis S. M., Ortwerth B. J., Monnier V. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16912–16917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis E. A., Guberski D. L., Somogyi-Mann M., Grant M. B. (2000) Free Radic. Biol. Med. 28, 91–101 [DOI] [PubMed] [Google Scholar]
- 28.Lacy F., Kailasam M. T., O'Connor D. T., Schmid-Schönbein G. W., Parmer R. J. (2000) Hypertension 36, 878–884 [DOI] [PubMed] [Google Scholar]
- 29.Devamanoharan P. S., Varma S. D. (1995) Ophthalmic. Res. 27, 39–43 [DOI] [PubMed] [Google Scholar]
- 30.Paolisso G., Di Maro G., Pizza G., D'Amore A., Sgambato S., Tesauro P., Varricchio M., D'Onofrio F. (1992) Am. J. Physiol. 263, E435–E440 [DOI] [PubMed] [Google Scholar]
- 31.Reddy V. N., Giblin F. J., Lin L. R., Dang L., Unakar N. J., Musch D. C., Boyle D. L., Takemoto L. J., Ho Y. S., Knoernschild T., Juenemann A., Lütjen-Drecoll E. (2001) Invest Ophthalmol. Vis. Sci. 42, 3247–3255 [PubMed] [Google Scholar]
- 32.Sellin S., Mannervik B. (1983) J. Biol. Chem. 258, 8872–8875 [PubMed] [Google Scholar]
- 33.Yu N. T., DeNagel D. C., Pruett P. L., Kuck J. F., Jr. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 7965–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan L. A., Lee W., Cowell T., Giblin F., Kantorow M. (2009) Mol. Vis. 15, 985–999 [PMC free article] [PubMed] [Google Scholar]
- 35.Thiagarajan G., Lakshmanan J., Chalasani M., Balasubramanian D. (2004) Invest Ophthalmol. Vis. Sci. 45, 2115–2121 [DOI] [PubMed] [Google Scholar]
- 36.Hazell L. J., Fu H., Dean R. T., Stocker R., Truscott R. J. (2002) Clin. Exp. Optom. 85, 97–100 [DOI] [PubMed] [Google Scholar]