Abstract
A hallmark of a group of neurodegenerative diseases such as Alzheimer disease is the formation of neurofibrillary tangles, which are principally composed of bundles of filaments formed by microtubule-associated protein Tau. Clarifying how natively unstructured Tau protein forms abnormal aggregates is of central importance for elucidating the etiology of these diseases. There is considerable evidence showing that zinc, as an essential element that is highly concentrated in brain, is linked to the development or progression of these diseases. Herein, by using recombinant human Tau fragment Tau244–372 and its mutants, we have investigated the effect of zinc on the aggregation of Tau. Low micromolar concentrations of Zn2+ dramatically accelerate fibril formation of wild-type Tau244–372 under reducing conditions, compared with no Zn2+. Higher concentrations of Zn2+, however, induce wild-type Tau244–372 to form granular aggregates in reducing conditions. Moreover, these non-fibrillar aggregates assemble into mature Tau filaments when Zn2+ has been chelated by EDTA. Unlike wild-type Tau244–372, low micromolar concentrations of Zn2+ have no obvious effects on fibrillization kinetics of single mutants C291A and C322A and double mutant C291A/C322A under reducing conditions. The results from isothermal titration calorimetry show that one Zn2+ binds to one Tau molecule via tetrahedral coordination to Cys-291 and Cys-322 as well as two histidines, with moderate, micromolar affinity. Our data demonstrate that low micromolar zinc accelerates the fibrillization of human Tau protein via bridging Cys-291 and Cys-322 in physiological reducing conditions, providing clues to understanding the relationship between zinc dyshomeostasis and the etiology of neurodegenerative diseases.
Introduction
Tau, a microtubule-associated protein, is the major protein subunit of neurofibrillary tangles (NFTs),2 which are found in a group of neurodegenerative diseases such as Alzheimer disease and frontotemporal lobar degeneration (1, 2). NFTs are mainly composed of bundles of Tau in the form of paired helical filaments (PHFs), straight filaments, or twisted ribbons. It is generally believed that the neuron degeneration in such diseases is very likely to be contributed by the accumulation of these aggregates (3). Thus the characterization of factors involved in abnormal Tau aggregation is of great importance to clarify the etiology of neurodegenerative diseases and assist in the establishment of medical treatment.
Tau is one of the largest proteins without recognizable secondary structure and adopts a natively unfolded structure in solution (4). There are two distinct domains of Tau protein, the projection domain and the microtubule binding domain. The microtubule binding domain mainly contains either three or four imperfect repeats (18 amino acids in length) separated from one another by inter repeats (13–14 amino acids in length) (5). In its normal state, Tau facilitates and stabilizes the assembly of microtubules. But under certain pathological conditions, it will detach from microtubules. Some of its small segments adopt a β-conformation, and further interactions convert them toward formation of aggregates that are rich in β-sheet structures. These aggregates undergo filament nucleation and elongation and form NFTs eventually (1, 2, 6). The microtubule binding repeat region forms the core of filaments while the rest of the protein retains its largely unfolded structure, which forms the fuzzy coat of the filaments (7–9).
Although the mechanism by which Tau protein aggregates is not fully understood, there are increasing evidences showing that a disturbance of brain zinc homeostasis during aging plays a role in the etiology of Alzheimer disease (10–12). Zinc is an integral component of numerous enzymes, transcription factors, and structural proteins. Furthermore, zinc is one of the most abundant transition metals in the brain and is in particularly large concentrations in the mammalian brain with an overall concentration of ∼150 μm (13). The distribution of zinc is not uniform, and up to 15% of brain zinc is located inside presynaptic vesicles (13, 14). Although the cytosolic free concentration of Zn2+ in cultured neurons is generally subnanomolar, in pathological conditions the free concentration of Zn2+ is altered via several pathways such as presynaptic zinc translocation (15).
There is current evidence for a relative increase in intracellular zinc in vulnerable regions of the Alzheimer disease brain (16), and abnormally high levels of zinc at millimolar concentrations have been found in NFTs and senile plaque cores (17, 18). It is estimated that strong activation of Zn2+-containing presynaptic terminals results in transient local synaptic Zn2+ concentrations in the 100–300 μm range (13, 17). Histochemically reactive zinc deposits are also found specifically localized to cerebral amyloid angiopathy deposits and NFT-bearing neurons (19). It has been reported that there are 3- to 5-fold increases in zinc in the cortical and accessory basal nuclei of the amygdala and in the neuropil of Alzheimer disease patients, as compared with age-matched controls (20).
Zinc has been shown to accelerate the aggregation of amyloid β peptides (21, 22), trigger the fibrillization of methionine oxidized α-synuclein (23), and cause an aggregation of Dcp1a protein in an RNA-dependent manner (24). There are also results indicating that Ca2+ and Mg2+ can selectively induce the formation of PHF-Tau aggregation (25), whereas Al3+, Cu2+, and Fe2+/Fe3+ can bind to Tau (26–28). However, whether Zn2+ has an effect on the aggregation of Tau has not been reported so far, which leaves us to answer the physiological question whether the alteration of zinc can affect Tau aggregation and thus play a role in the pathology of neurodegenerative diseases such as Alzheimer disease.
In the present study, by using several biophysical methods, such as thioflavin T binding, atomic force microscopy (AFM), transmission electron microscopy (TEM), and isothermal titration calorimetry (ITC), we investigated the impact of zinc on the aggregation of human Tau fragment Tau244–372. Our results indicated that low micromolar zinc dramatically accelerated fibril formation of wild-type Tau244–372 in the presence of dithiothreitol (DTT), but had no obvious effects on fibrillization kinetics of single mutants C291A and C322A and double mutant C291A/C322A. Further, we demonstrated that Zn2+ bound to Tau molecules via tetrahedral coordination to Cys-291, Cys-322, and two histidines with moderate, micromolar affinity, and thus concluded that low micromolar zinc promoted the fibrillization of human Tau protein via bridging of Cys-291 and Cys-322 in physiological reducing conditions.
EXPERIMENTAL PROCEDURES
Materials
Heparin (average molecular mass = 6 kDa) and thioflavin T (ThT) were purchased from Sigma. DTT was obtained from Ameresco Chemical Co. (Solon, OH). All the metal cations used were chloride forms of analytical grade. All other chemicals used were made in China and were of analytical grade. The buffers used in this study were treated with ion-exchange resins to remove trace amounts of divalent cations present as contaminants in the solutions.
Plasmids and Proteins
The cDNA-encoding human Tau fragment Tau244–372 was amplified using the plasmid for human Tau40 (kindly provided by Dr. Michel Goedert) as a template. The PCR-amplified Tau244–372 was subcloned into pRK172 vector. Single cysteine mutants C291A and C322A and double mutant C291A/C322A of Tau244–372 were generated using primers GCAACGTCCAGTCCAAGGCTGGCTCAAAGG/CCTTTGAGCCAGCCTTGGACTGGACGTTGC for C291A and GTGACCTCCAAGGCTGGCTCATTAGGCAACATC/GATGTTGCCTAATGAGCCAGCCTTGGAGGTCAC for C322A. Single histidine mutants H330A and H362A and histidine-less mutant H268A/H299A/H329A/H330A/H362A were generated in a similar manner. Plasmids containing target sequences were transformed into Escherichia coli BL21 (DE3) strain. The expression of recombinant human Tau fragment Tau244–372 and its mutants was induced with 400 μm isopropyl-β-d-thiogalactopyranoside and cultured for 3 h. Cell pellets of 2-liter culture were collected and re-suspended in 80 ml of buffer A (20 mm phosphate buffer containing 2 mm DTT, pH 7.0) and then sonicated at 250 watts for 30 min. 500 mm NaCl was added into the mixture, and then the mixture was boiled at 100 °C for 15 min. After centrifugation at 17,000 × g for 30 min at 4 °C, supernatant was collected and dialyzed against buffer A extensively. The sample was then loaded to an SP-Sepharose column and washed with 400 ml of buffer A. The target protein was obtained by washing the column using 500 ml of 20 mm phosphate buffer containing 2 mm DTT and 0–400 mm NaCl. The Tau fragment was then concentrated and dialyzed against 50 mm Tris-HCl buffer containing 2 mm DTT (pH 7.5) extensively, and then stored at −80 °C. Purified Tau protein was analyzed by SDS-PAGE with one band and confirmed by mass spectrometry. The concentration of human Tau fragment was determined according to its absorbance at 214 nm with a standard calibration curve drawn by bovine serum albumin as described (29).
ThT Binding Assays
A 2.5 mm ThT stock solution was freshly prepared in 50 mm Tris-HCl buffer (pH 7.5) and passed through a 0.22-μm pore size filter before use to remove insoluble particles. Under standard conditions, 10 μm Tau244–372 was incubated without agitation in 50 mm Tris-HCl buffer (pH 7.5) containing 1 mm DTT and 20 μm ThT with or without Zn2+ at 37 °C for up to 1 h in the presence of fibrillization inducer heparin used in a Tau:heparin molar ratio of 4:1. The fluorescence of ThT was excited at 440 nm with a slit width of 7.5 nm, and the emission was measured at 480 nm with a slit width of 7.5 nm on an LS-55 luminescence spectrometer (PerkinElmer Life Sciences). The preparation of the samples before the first measurement took 1 min.
The polymerization for Tau244–372 and its cysteine mutants in 96-well plates were set up by a mixture of 20 μm Tau protein, 5 μm heparin, 50 μm ThT, and 0–80 μm Zn2+ either in the presence or in the absence of 1 mm DTT in 50 mm Tris-HCl buffer containing 100 mm NaCl (pH 7.5). The reaction components were mixed quickly and immediately read for 8 h (with DTT) or 3 h (without DTT) at 37 °C in SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA) using excitation at 440 nm and emission at 480 nm with a wavelength cut-off at 475 nm. Each sample was run in triplicate or quadruplicate. Kinetic parameters were determined by fitting ThT fluorescence intensity versus time to a sigmoidal equation (30),
where F is the fluorescence intensity, k is the rate constant for the growth of fibrils, and tm is the time to 50% of maximal fluorescence. The initial baseline during the lag time is described by F0. The final baseline after the growth phase has ended is described by A + ct. The lag time is calculated as tm − 2/k.
Sarkosyl-insoluble Tau SDS-PAGE
The Sarkosyl-insoluble Tau experiments were carried out according to the method described by Aoyagi and co-workers (31) with minor changes. Tau polymerization was set up by incubating a mixture of 10 μm Tau244–372, 2.5 μm heparin, 1 mm DTT, and 0–100 μm Zn2+ in 50 mm Tris-HCl buffer (pH 7.5) at 37 °C without agitation. Aliquots (100 μl) of assembly mixtures were taken out and added into 500 μl of 50 mm Tris-HCl buffer (pH 7.5) containing 1% Sarkosyl. The mixture was left at room temperature for 30 min and then centrifuged in an Optima LE-80K ultracentrifuge (Beckman Coulter, Fullerton, CA) at 150,000 × g for 30 min. The supernatant (Sarkosyl-soluble Tau) was removed, and the pellet (Sarkosyl-insoluble Tau) was re-suspended in 50 μl of SDS sample buffer containing 5% 2-mercaptoethanol and subjected to 15% SDS-PAGE. After the electrophoresis the gels were stained with Coomassie Blue.
Transmission Electron Microscopy
The formation of filaments by human Tau fragment was confirmed by electron microscopy of negatively stained samples. Sample aliquots of 10 μl were placed on copper grids and left at room temperature for 1–2 min, rinsed with H2O twice, and then stained with 2% (w/v) uranyl acetate for another 1–2 min. The stained samples were examined using an H-8100 transmission electron microscope (Hitachi, Tokyo, Japan) operating at 100 kV.
AFM
The formation of filaments by human Tau fragment was further confirmed by AFM. Sample aliquots of 10 μl were deposited onto freshly cleaved mica, left on the surface for 10 min, and rinsed with H2O twice. Then the solution was dried in a desiccator for 12 h. AFM images were acquired in tapping mode with an SPM-9500 J3 scanning probe microscope (Shimadzu, Kyoto, Japan). Several regions of the mica surface were examined to confirm that similar structures existed through the sample.
Isothermal Titration Calorimetry
ITC experiments on the interaction of Zn2+ with Tau244–372 and its mutants were carried out at 25.0 °C using an iTC200 titration calorimetry (MicroCal, Northampton, MA). Freshly purified Tau proteins (wild-type Tau244–372, single mutants C291A, C322A, H330A, and H362A, double mutant C291A/C322A, and histidine-less mutant H268A/H299A/H329A/H330A/H362A) were dialyzed against 50 mm Tris-HCl buffer (pH 7.5) containing 1 mm EDTA, overnight at 4 °C and then dialyzed against 50 mm Tris-HCl buffer (pH 7.5) extensively to remove EDTA. A solution of 100–200 μm Tau proteins was loaded into the sample cell (200 μl), and a solution of 2.8–6.0 mm Zn2+ was placed in the injection syringe (40 μl). The first injection (0.3 μl) was followed by 24–27 injections of 1 μl. Dilution heats of Zn2+ were measured by injecting Zn2+ solution into buffer alone and were subtracted from the experimental curves prior to data analysis. The stirring rate was 600 rpm. The resulting data were fitted to a single set of identical sites model using MicroCal ORIGIN software supplied with the instrument, and the standard molar enthalpy change for the binding, ΔbHm0, the dissociation constant, Kd, and the binding stoichiometry, n, were thus obtained. The standard molar free energy change, ΔbGm0, and the standard molar entropy change, ΔbSm0, for the binding reaction were calculated by the fundamental equations of thermodynamics (32).
RESULTS
The Presence of Zn2+ Influenced Tau Aggregation
The enhanced fluorescence emission of the dye ThT has been frequently used for monitoring the kinetics of amyloid fibril formation, which is a specific marker for the β-sheet conformation of fibril structures (33, 34). Because Tau244–372 consists of the four-repeat microtubule binding domain forming the core of PHFs in Alzheimer disease and assembles more readily than full-length Tau protein into filaments in vitro, we employed such a Tau fragment for studying kinetics of Tau fibril formation. As shown in Fig. 1, the kinetic curves of the ThT fluorescence intensity at 480 nm for Tau244–372 fibrillization were consistent with a nucleation-dependent elongation model (35), in which the lag phase corresponded to the nucleation phase, and the exponential part to a fibril growth (elongation) phase.
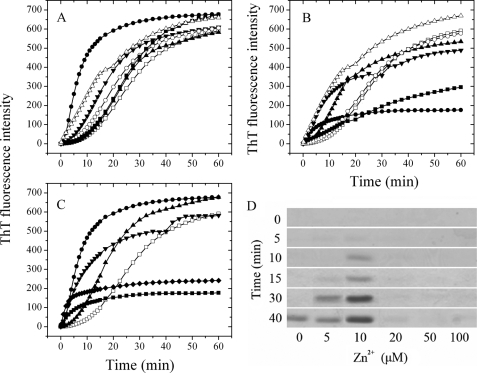
FIGURE 1.
The presence of Zn2+ altered Tau244–372 fibrillization kinetics. A, 10 μm Tau244–372 was incubated with 10 μm cation (open circle, Mg2+; filled triangle, Mn2+; filled inverted triangle, Ca2+; filled circle, Zn2+; filled square, Fe3+; and open triangle, Cu2+) or without cation (open square); B, 10 μm Tau244–372 was incubated with 100 μm cation (open circle, Mg2+; filled triangle, Mn2+; filled inverted triangle, Ca2+; filled circle, Zn2+; filled square, Fe3+; and open triangle, Cu2+) or without cation (open square); C, 10 μm Tau244–372 was incubated with 0–100 μm Zn2+ (open square, 0 μm; filled triangle, 5 μm; filled circle, 10 μm; filled inverted triangle, 20 μm; filled rhombus, 50 μm; and filled square, 100 μm). The buffer used was 50 mm Tris-HCl buffer (pH 7.5) containing 1 mm DTT, 2.5 μm heparin, and 20 μm ThT, and ThT binding assays were carried out at 37 °C. D, 10 μm Tau244–372 was incubated with 0–100 μm Zn2+ containing 2.5 μm heparin and 1 mm DTT in 50 mm Tris-HCl buffer (pH 7.5) at 37 °C. Aliquots were taken at 0, 5, 10, 15, 30, and 40 min, respectively, and then incubated with Tris-HCl buffer containing 1% Sarkosyl followed by centrifuging at 150,000 × g for 30 min. Pellets were re-suspended with sample buffer containing 5% 2-mercaptoethanol and subjected to 15% SDS-PAGE. Gels were stained with Coomassie Blue.
Bivalent cations such as Mg2+, Mn2+, Ca2+, Zn2+, and Cu2+ and trivalent cation Fe3+ were incubated separately with physiological concentrations of Tau244–372 at 37 °C in the presence of DTT. As shown in Fig. 1A, in the absence of cation, the lag phase for wild-type Tau244–372 polymerization was ∼500 s, whereas the addition of Mg2+, Mn2+, Ca2+, Fe3+, and Cu2+ at a low micromolar concentration (10 μm) decreased the lag phase in different ranges. In the presence of 10 μm Zn2+, however, the lag phase was dramatically diminished, indicating that low micromolar concentrations of Zn2+ greatly accelerated fibril formation of wild-type Tau244–372, compared with no Zn2+, and the enhancing effect of Zn2+ was more remarkable than those of other cations such as Mn2+, Fe3+, and Cu2+. Clearly, the nucleation of Tau fragment was much more accelerated by 10 μm Zn2+ than the following step of elongation (Fig. 1A). Meanwhile, when wild-type Tau244–372 was incubated with 100 μm cations, the kinetic curve of Tau244–372 in the presence of Zn2+ was also quite different from those of other cations except Fe3+ (Fig. 1B). The lag phase was too short to be observed, but the maximum intensity became much lower in the presence of 100 μm Zn2+ than that in the absence of cation, indicating the aggregation of Tau244–372 was significantly altered by higher concentrations of Zn2+. Both cases indicated that the presence of Zn2+, compared with other cations, significantly altered the aggregation of Tau in reducing conditions and factors other than electrostatic interactions must play a role in this phenomenon.
To get a better understanding about the effect of zinc on Tau aggregation, we performed ThT binding assays at various concentrations of Zn2+. As shown in Fig. 1C, the addition of 5–20 μm Zn2+ accelerated fibril formation of wild-type Tau244–372 under reducing conditions, but the enhancing effect of Zn2+ was most significant when the concentration of Zn2+ was 10 μm, which is equal to that of Tau fragment. When the concentration of Zn2+ went higher, the kinetic curves still went up instantly, almost without a lag phase, but the final ThT intensities decreased 65 and 76% for 50 and 100 μm Zn2+, respectively (Fig. 1C). The phenomenon that the presence of Zn2+ significantly reduced the lag phase indicated that Zn2+ had a strong enhancing effect on the nucleation of Tau protein. However, smaller exponential growth at higher molar ratios of Zn2+ to Tau suggested fewer filaments but other forms of Tau aggregates were formed, compared with no Zn2+.
To semi-quantify the aggregates of Tau protein formed in the presence of Zn2+ at different concentrations, we carried out Sarkosyl-insoluble SDS-PAGE experiments and assessed Tau244–372 aggregation under reducing conditions by measuring the Sarkosyl-insoluble Tau as described (31). As shown in Fig. 1D, a clear band corresponding to Sarkosyl-insoluble Tau filaments was observed when wild-type Tau244–372 was incubated in the absence of Zn2+ for 40 min, whereas the Sarkosyl-insoluble Tau band was observed when Tau244–372 was incubated with Zn2+ at low micromolar concentrations (5–10 μm) for a much shorter time (15–10 min). Furthermore, when Tau244–372 was incubated for 40 min, the intensity of the Sarkosyl-insoluble Tau band in the presence of 10 μm Zn2+ was remarkably higher than that in the absence of Zn2+. This finding further supports the observation mentioned above that low micromolar concentrations of Zn2+ dramatically accelerate fibril formation of wild-type Tau244–372 in reducing conditions, compared with no Zn2+. At higher concentrations of Zn2+ (50–100 μm), however, no Sarkosyl-insoluble Tau band was observed even at 40 min (Fig. 1D), indicating that fewer filaments but other forms of Tau aggregates, Sarkosyl-soluble Tau aggregates, were formed at higher molar ratios of Zn2+ to Tau under reducing conditions.
Morphology of Tau Aggregates Varied with Molar Ratios of Zn2+ to Tau
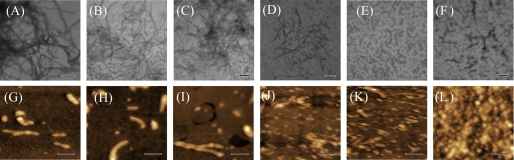
TEM and AFM were employed to study the morphology of wide-type Tau244–372 incubated with Zn2+ at different concentrations for 60 min (Fig. 2). The addition of low micromolar concentrations of Zn2+ (5 or 10 μm) had no significant effect on the morphology of Tau samples monitored by TEM and AFM, and long and branched fibrils as well as a few short filaments (Fig. 2, A–C) and some filaments with a length of 200–500 nm (Fig. 2, G–I) were observed in these three samples. When the concentration of Zn2+ increased (50 or 100 μm) until the molar ratio of Zn2+ to Tau exceeded equality, however, Tau244–372 filaments became much fewer and shorter, and abundant granular aggregates (∼30 nm in diameter) were observed (Fig. 2, E, F, K, and L). Maeda and co-workers (36) have observed similar aggregates and have suggested that such granular Tau aggregates may be an intermediate form of Tau fibrils.
FIGURE 2.
Transmission electron micrographs (A–F) and AFM images (G–L) of Tau244–372 aggregates formed with Zn2+. 10 μm Tau244–372 was incubated with 0–100 μm Zn2+ (A and G, 0 μm; B and H, 5 μm; C and I, 10 μm; D and J, 20 μm; E and K, 50 μm; and F and L, 100 μm) containing 2.5 μm heparin and 1 mm DTT in 50 mm Tris-HCl buffer (pH 7.5) at 37 °C for 60 min. A 2% (w/v) uranyl acetate solution was used to negatively stain the fibrils (A–F). The scale bars represent 200 nm.
Combining the results from TEM and AFM, we concluded that the morphology of wild-type Tau244–372 aggregates varied with molar ratios of Zn2+ to Tau in reducing conditions. At lower molar ratios, Tau aggregates were mainly composed of filaments, which was similar to those in the absence of Zn2+. But at higher molar ratios, filaments became much fewer and shorter, and granular aggregates became prevalent. In other words, higher concentrations of Zn2+ induced wild-type Tau244–372 to form granular aggregates under reducing conditions.
Characterization of the Effect of Zinc on Tau Aggregation at Different Stages
We then checked whether the enhancing effect of Zn2+ on the aggregation of Tau was dependent on the incubation time or different compositions of Tau protein. We added 10 or 100 μm Zn2+ after incubation for 5, 15, and 30 min, when Tau244–372 was no longer pure monomers. As shown in Fig. 3(A–D), Zn2+ added at 15 min in the growth phase or at 30 min in the final equilibrium phase did not facilitate fibril formation of Tau244–372 under reducing conditions; on the contrary, it slowed down or even blocked the growth phase. Furthermore, 10 μm Zn2+ added at 5 min in the lag phase had no obvious effect on fibril formation of Tau244–372, and 100 μm Zn2+ added at 5 min slowed down the growth phase in reducing conditions (data not shown). The above results suggested that the enhancing effect on fibrillization kinetics of Tau by low micromolar concentrations of Zn2+ was mainly due to its direct interaction with Tau monomers, but not protofibrils or fibrils. It is this interaction that accelerated the nucleation of Tau protein.
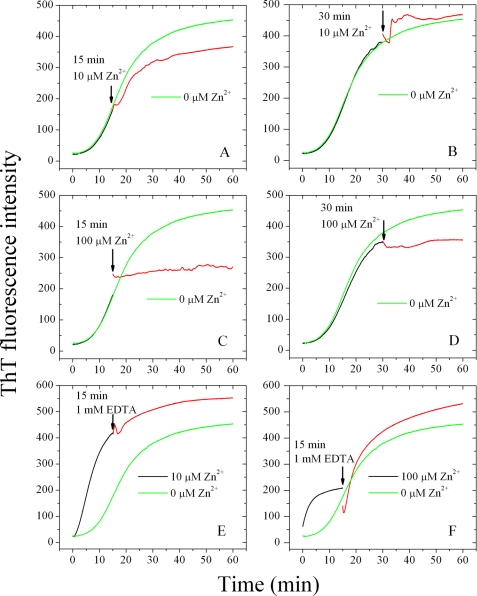
FIGURE 3.
Effect of Zn2+ on Tau244–372 fibrillization at various time points. 10 μm Tau244–372 was incubated without Zn2+ (black curves) and then titrated with 10 μm Zn2+ or 100 μm Zn2+ at 15 min (A and C) or 30 min (B and D) respectively (red curves). E, 10 μm Tau244–372 was incubated with 10 μm Zn2+ (black curves) and then titrated with aliquots of 1 mm EDTA at 15 min (red curves). F, 10 μm Tau244–372 was incubated with 100 μm Zn2+ (black curves) and then titrated with 1 mm EDTA at 15 min (red curves). The beginning of titrations is indicated by black arrows, and the curves are compared with 10 μm Tau244–372 incubated without Zn2+ (green curves). The buffer used was 50 mm Tris-HCl buffer (pH 7.5) containing 1 mm DTT, 2.5 μm heparin, and 20 μm ThT, and ThT binding assays were carried out at 37 °C.
To verify whether the effect of Zn2+ was chelator-reversible, we added 1 mm EDTA into systems containing Tau244–372 incubated with 10 or 100 μm Zn2+ separately. As shown in Fig. 3 (E and F), the addition of EDTA at 15 min in the growth phase did not change fibrillization kinetics of Tau in the presence of 10 μm Zn2+, suggesting that, once the nucleation of Tau had been finished, the binding of Zn2+ to Tau was no longer necessary for the filament elongation and maturation. But in the presence of 100 μm Zn2+, the addition of EDTA at 15 min did alter the fibrillization kinetics by accelerating the elongation of Tau protein remarkably and reaching a final ThT intensity ∼17% larger than that in the absence of Zn2+. Higher concentrations of Zn2+ induced Tau to form granular aggregates under reducing conditions (Fig. 2). Moreover, such non-fibrillar aggregates assembled into mature Tau filaments when Zn2+ had been chelated by EDTA (Fig. 3F).
Effect of Zn2+ on Tau Aggregation under Reducing or Oxidative Conditions
Neuronal cells normally have a reducing environment maintained by an excess of glutathione (1, 5, 37). To mimic the reducing environment present in normal neuronal cells and block the formation of an intramolecular disulfide bond, DTT, a strong reducing agent, was used in this study. We carried out ThT binding assays in the presence and absence of DTT to compare the effect of Zn2+ on Tau aggregation under reducing conditions with that in oxidizing conditions. Additionally, 100 mm NaCl was added into the polymerization mixture to mimic the physiological salinity. It has been reported that Tau aggregation is highly sensitive to elevated ionic strength (38). As shown in Fig. 4A, in the presence of DTT and 100 mm NaCl, the results were quite similar to those obtained in the presence of DTT but without salt (Fig. 1), except that the lag time of Tau fibrillization was much longer than that in the absence of salt as expected. When wild-type Tau244–372 was incubated with low micromolar concentrations of Zn2+, the kinetic curves of Tau fibrillization grew much faster and reached a plateau around 3 h, compared with >8 h in the absence of Zn2+, once again indicating that low micromolar concentrations of Zn2+ dramatically accelerated fibril formation of wild-type Tau244–372 under physiological reducing conditions.
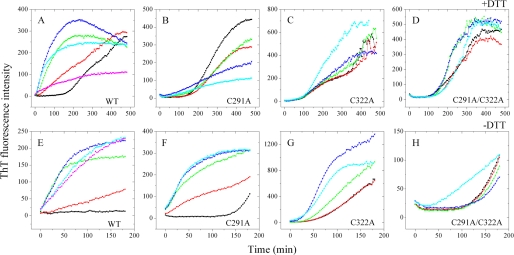
FIGURE 4.
Cys-291 and Cys-322 are key residues in the interaction of Zn2+ with Tau244–372. The top panels show the ThT binding assays of 20 μm wild-type (WT) Tau244–372 (A), 20 μm single mutants C291A (B), and C322A (C) or double mutant C291A/C322A (D) incubated with 0–80 μm Zn2+ (black, 0 μm; red, 4 μm; green, 10 μm; blue, 20 μm; cyan, 40 μm; and magenta, 80 μm) in the presence of 1 mm DTT. The bottom panels show the ThT binding assays of 20 μm WT Tau244–372 (E), 20 μm single mutants C291A (F), and C322A (G) or double mutant C291A/C322A (H) incubated with 0–80 μm Zn2+ (black, 0 μm; red, 4 μm; green, 10 μm; blue, 20 μm; cyan, 40 μm; and magenta, 80 μm) in the absence of DTT. The assays were carried out at 37 °C, and the samples were incubated in 50 mm Tris-HCl buffer (pH 7.5) containing 100 mm NaCl, 5 μm heparin and 50 μm ThT.
While in the absence of DTT (Fig. 4E), the kinetic curve of Tau fibrillization showed no detectable increase within 3 h in the absence of Zn2+. But when Tau244–372 was incubated with different concentrations of Zn2+, the kinetic curves showed instant increases in oxidizing conditions even at higher molar ratios of Zn2+ to Tau. It is quite different from those obtained in reducing conditions, which showed smaller exponential growth and much lower final ThT intensities at higher molar ratios of Zn2+ to Tau (Fig. 4A). This comparison suggests that higher concentrations of Zn2+ induced Tau to form granular aggregates only under reducing conditions.
Cys-291 and Cys-322 Are Key Residues in the Interaction of Zn2+ with Tau
There are two cysteine residues, Cys-291 and Cys-322, in full-length human Tau protein (441 amino acids). They are located in repeats 2 and 3 of the four-repeat microtubule binding domain (37, 39). Little is known about the role of such cysteine residues in Tau assembly, because their substitution with other amino acids has no effect on Tau filament morphology (40). In this study, Tau244–372 mutants containing single and double cysteine mutations were designed, and ThT binding assays using such mutants were performed to provide information about the binding sites of zinc in Tau protein and the role of Cys-291 and Cys-322 in Tau assembly. Table 1 summarizes the kinetic parameters obtained for fibril formation of wild-type Tau244–372 and its cysteine mutants in the presence of low micromolar concentrations of Zn2+ under reducing conditions. Unlike wild-type Tau244–372, 4–10 μm Zn2+ had no obvious effects on fibrillization kinetics of single mutants C291A and C322A and double mutant C291A/C322A in reducing conditions (Fig. 4, B–D, and Table 1), indicating that low micromolar zinc accelerated the fibrillization of human Tau protein via bridging Cys-291 and Cys-322. But higher concentrations of Zn2+ did not have uniform effects on fibrillization kinetics of Tau244–372 mutants under reducing conditions: an inhibitory effect on C291A (Fig. 4B), an enhancing effect on C322A (Fig. 4C), and no obvious enhancing effect on C291A/C322A (Fig. 4D). The above results suggest that, under physiological reducing conditions, Cys-291 and Cys-322 are key residues in the interaction of Zn2+ with Tau protein.
TABLE 1.
Kinetic parameters of Tau244–372 fibrillization in the presence of low micromolar concentrations of Zn2+ as determined by ThT binding assays at 37 °C
Kinetic parameters, k, tm, and the lag time, were determined by fitting ThT fluorescence intensity versus time to Equation 1. The buffer used was 50 mm Tris-HCl buffer (pH 7.5) containing 1 mm DTT, 100 mm NaCl, 5 μm heparin, and 50 μm ThT. Errors shown are ± S.E.
| Tau244–372 | Zn2+ | k | tm | Lag time |
|---|---|---|---|---|
| μm | 10−3min−1 | min | min | |
| WTa | 0 | 37.3 ± 4.0 | 176 ± 4 | 122 ± 10 |
| 4 | 35.1 ± 9.5 | 38.2 ± 11 | 0b | |
| 10 | 17.6 ± 0.6 | 48.0 ± 3.7 | 0b | |
| C291A | 0 | 22.2 ± 0.4 | 253 ± 2 | 163 ± 4 |
| 4 | 21.1 ± 0.4 | 273 ± 3 | 178 ± 5 | |
| 10 | 15.6 ± 0.3 | 257 ± 7 | 129 ± 9 | |
| C322A | 0 | NDc | ND | 100b |
| 4 | ND | ND | 100b | |
| 10 | ND | ND | 70b | |
| DMd | 0 | 16.0 ± 0.5 | 304 ± 8 | 179 ± 12 |
| 4 | 17.8 ± 0.9 | 283 ± 9 | 171 ± 15 | |
| 10 | 26.3 ± 1.3 | 227 ± 3 | 151 ± 7 |
a WT, wild-type Tau244–372.
b Observed from the ThT fluorescence curves directly.
c ND, not determined because the ThT fluorescence data in the present conditions could not be fitted to such a sigmoidal equation.
d DM, double mutant C291A/C322A of Tau244–372.
While under oxidative conditions, the addition of different concentrations of Zn2+ showed remarkable enhancing effects on fibrillization kinetics of wild-type Tau244–372 and single mutants C291A and C322A but had no obvious effect on double mutant C291A/C322A (Fig. 4, E–H), suggesting that, in oxidizing conditions, Cys-291 and Cys-322 are also key residues in the interaction of Zn2+ with Tau protein. The presence of Cys-291 and Cys-322 gives rise to a tendency to form an intramolecular disulfide bond and thus generate compact Tau monomers under oxidative conditions (41, 42). Clearly, the formation of such intramolecular disulfide bonds was unfavorable to fibril formation of Tau244–372, and zinc accelerated filament formation of Tau protein by inhibiting the formation of intramolecular disulfide bonds and enhancing intermolecular ones in oxidative conditions (Fig. 4, E–G).
Thermodynamics of the Binding of Zn2+ to Tau
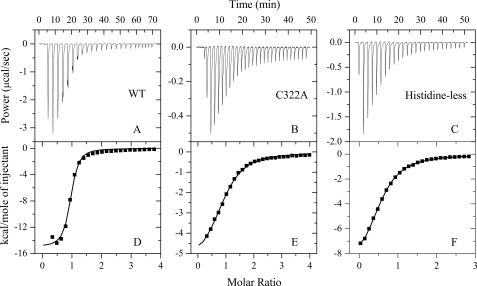
ITC provides a direct route to the complete thermodynamic characterization of non-covalent, equilibrium interactions (32, 43), and DTT concentrations as low as 1 mm can cause severe baseline artifacts due to background oxidation during the titration. Therefore ITC was used to measure the binding affinity of Zn2+ to Tau monomer in the absence of DTT. ITC profiles for the binding of Zn2+ to wild-type Tau244–372, single cysteine mutant C322A, and the histidine-less mutant at 25.0 °C are shown in Fig. 5. The top panels in Fig. 5 representatively show raw ITC curves resulting from the injections of Zn2+ into a solution of wild-type Tau244–372 (Fig. 5A), C322A (Fig. 5B), and histidine-less mutant (Fig. 5C). The titration curves show that zinc binding to wild-type Tau244–372 and its mutants is exothermic, resulting in negative peaks in the plots of power versus time. The bottom panels in Fig. 5 show the plot of the heat evolved per mole of Zn2+ added, corrected for the heat of Zn2+ dilution, against the molar ratio of Zn2+ to wild-type Tau244–372 (Fig. 5D), C322A (Fig. 5E), and histidine-less mutant (Fig. 5F). The calorimetric data were best fit to a model assuming a single set of identical sites. The thermodynamic parameters for the binding of Zn2+ to Tau244–372 are summarized in Table 2. As shown in Table 2, one Zn2+ bound to one wild-type Tau244–372 molecule with a dissociation constant of 3.82 μm. The binding affinities of Zn2+ to single cysteine mutants C291A and C322A were significantly lower than that of wild-type Tau244–372, with a dissociation constant of 9.71 and 20.2 μm, respectively. No binding reaction for Zn2+ with double cysteine mutant C291A/C322A was detected by ITC (Table 2), demonstrating that Cys-291 and Cys-322 are key residues in the interaction of Zn2+ with Tau protein. Because it is known that zinc prefers tetrahedral coordination (44), we wanted to know what the remaining zinc ligands were. As shown in Table 2, the binding affinities of Zn2+ to single histidine mutants H330A and H362A and histidine-less mutant were remarkably lower than that of wild-type Tau244–372, suggesting that His-330 and His-362 are probably the remaining zinc ligands.
FIGURE 5.
ITC profiles for the binding of Zn2+ to wild-type Tau244–372 and its mutants at 25. 0 °C. The top panels represent the raw data for sequential 1-μl injections of Zn2+ (6.0, 2.8, and 4.5 mm for A, B, and C, respectively) into 200 μm wild-type (WT) Tau244–372 (A), 100 μm single mutant C322A (B), and 200 μm histidine-less mutant H268A/H299A/H329A/H330A/H362A in 50 mm Tris-HCl buffer (pH 7.5) (C), respectively. The bottom panels (D, E, and F) show the plots of the heat evolved (kilocalories) per mole of Zn2+ added, corrected for the heat of Zn2+ dilution, against the molar ratio of Zn2+ to Tau244–372. The data (solid squares) were best fitted to a single set of identical sites model, and the solid lines represent the best fit.
TABLE 2.
Thermodynamic parameters for the binding of Zn2+ to Tau244–372 as determined by ITC at 25.0 °C
Thermodynamic parameters, Kd, Δ bHm0, and n, were determined using a single set of identical sites model. The standard molar binding free energy (Δ bGm0) and the standard molar binding entropy (Δ bSm0) for the binding reaction were calculated using Equations 2 and 3, respectively. The buffer used was 50 mm Tris-HCl buffer (pH 7.5). Errors shown are ± S.E.
| Tau244–372 | Kd | n | ΔbHm0 | ΔbGm0 | ΔbSm0 |
|---|---|---|---|---|---|
| μm | kcal mol−1 | kcal mol−1 | cal mol−1K−1 | ||
| WTa | 3.82 ± 0.65 | 0.905 ± 0.012 | −15.0 ± 0.3 | −7.39 ± 0.10 | −25.6 ± 1.3 |
| C291A | 9.71 ± 0.62 | 0.443 ± 0.009 | −10.1 ± 0.27 | −6.83 ± 0.04 | −11.1 ± 1.1 |
| C322A | 20.2 ± 1.2 | 0.922 ± 0.017 | −5.62 ± 0.14 | −6.40 ± 0.04 | 2.62 ± 0.59 |
| DMb | NBc | − | − | − | − |
| H330A | 8.77 ± 1.08 | 0.584 ± 0.012 | −13.7 ± 0.46 | −6.90 ± 0.07 | −22.7 ± 1.8 |
| H362A | 5.92 ± 2.44 | 0.622 ± 0.038 | −3.80 ± 0.27 | −7.13 ± 0.24 | 11.2 ± 1.7 |
| Histidine-lessd | 38.3 ± 0.8 | 0.556 ± 0.004 | −9.75 ± 0.09 | −6.02 ± 0.01 | −12.5 ± 0.3 |
a WT, wild-type Tau244–372.
b DM, double mutant C291A/C322A of Tau244–372.
c NB, no binding observed in the present conditions.
d Histidine-less, histidine-less mutant H268A/H299A/H329A/H330A/H362A.
DISCUSSION
Zn2+ Modulates Tau Aggregation via Bridging Cys-291 and Cys-322
During the past decade, several models describing the fibril formation of human Tau protein have been proposed to characterize the mechanism and key factors in this process (1, 35, 37, 38, 42, 45–47). A typical Tau filament pathway starts with the formation of assembly-competent intermediates, which then form fibrils following classic nucleation-dependent kinetics characterized by a lag period, followed by a period of exponential growth and an asymptotic approach to equilibrium (35, 45). The “driving force” for filament formation by Tau protein involves both the formation of an intermolecular disulfide bond (35) and non-covalent intermolecular interactions such as hydrogen bonds between all pairs of adjacent β-strands (48), electrostatic interactions between the negatively charged heparin and the basic lysine residues of Tau (49), and hydrophobic interactions between Tau molecules and between Tau and heparin (50). Any structural change to enhance the driving force should promote the fibril formation of human Tau molecules. In this study, we demonstrated that low micromolar zinc dramatically accelerated fibril formation of wild-type Tau244–372 but had no obvious effects on fibrillization kinetics of single mutants C291A and C322A and double mutant C291A/C322A and thus suggested that low micromolar zinc accelerated the fibrillization of human Tau protein via bridging Cys-291 and Cys-322 under physiological reducing conditions. In other words, the enhancing effect of low micromolar concentrations of Zn2+ depended on intermolecular disulfide bridges formed by Cys-291 and Cys-322. In contrast, higher concentrations of Zn2+ inhibited Tau fibrillization and induced wild-type Tau244–372 to form granular aggregates but still had no noticeable effect on the double mutant in reductive conditions. The binding of Zn2+ to Tau monomer is an exothermic reaction, which is favorable to the non-covalent intermolecular interactions as described above. Therefore, under reducing conditions, low micromolar zinc enhanced both the formation of intermolecular disulfide bridges between Cys-291 and Cys-322 and these non-covalent intermolecular interactions to promote Tau fibrillization. But higher concentrations of Zn2+ strongly inhibited the formation of intermolecular disulfide bridges between Cys-291 and Cys-322 and thus inhibited Tau fibrillization. All the evidence above strongly supports our finding that Zn2+ modulates Tau aggregation via bridging Cys-291 and Cys-322 in reducing conditions.
Under oxidative conditions (air oxidation), however, a prerequisite for nucleation is the dimerization of Tau, because Tau dimers act as effective building blocks (35), and dimerization and nucleation are the rate-limiting steps for PHF formation (47). Furthermore, a dimeric Tau with an intermolecular disulfide bond is presumably acting as a seed for initiation of Tau polymerization (39), and the presence of PHF6 hexapeptide and one cysteine residue is a minimum requirement for efficient assembly of cysteine-dependent Tau dimer (40). The present study demonstrated that the effect of zinc on Tau assembly can be affected by the oxidative/reductive state of experimental conditions. In oxidizing conditions, the addition of Zn2+ even at high concentrations showed remarkable enhancing effects on fibrillization kinetics of wild-type Tau244–372 and single mutants C291A and C322A but had no obvious effect on the double mutant. This finding suggests that Zn2+ enhances Tau fibrillization by inhibiting the formation of intramolecular disulfide bonds and promoting the formation of the dimeric Tau with an intermolecular disulfide bond through either Cys-291 or Cys-322 under oxidative conditions.
Characterization of the Zinc Binding Sites of Tau
As an essential element, Zn2+ has been found to play a catalytic or structural role in many proteins (13, 51). In catalytic zinc sites, Zn2+ generally forms complexes with water and any three nitrogen, oxygen, and sulfur donors with His being the predominant amino acid chosen. In structural zinc sites, however, Zn2+ is found tetrahedrally coordinated to amino acid residues and bound preferentially to Cys (44). In this case, Zn2+ bound to Tau protein via tetrahedral coordination to Cys-291 and Cys-322 as well as two histidines, which are assumed to be His-330 and His-362, and thus modulated the assembly kinetics and morphology of aggregates of human Tau protein associated with Alzheimer disease. This finding suggests that Zn2+ should play a structural role but not a catalytic role in the aggregation of Tau protein.
Zinc Plays a Significant Role in the Development of Tau Pathology
A hallmark of a group of neurodegenerative diseases such as Alzheimer disease is the formation of NFTs, which are principally composed of bundles of filaments formed by microtubule-associated protein Tau (1–3, 52–54). There is considerable evidence showing that zinc, as an essential element that is highly concentrated in brain, is linked to the development or progression of these diseases (13). Brain zinc is generally divided into two types, protein-bound and loosely bound, the latter also being termed mobile zinc (55). Alterations in zinc metabolism and homeostasis have been reported in such neurodegenerative diseases (10–12). Therefore, further elucidation of the pathological actions of Zn2+ in the brain should result in new therapeutic approaches to these neurodegenerative diseases.
Zn2+ has been reported to bind to several proteins/peptides associated with neurodegenerative diseases, including human amyloid β peptides (21, 56, 57), human prion protein (58, 59) and human α-synuclein (23). Zinc binding has been shown to modulate the aggregation behavior of these amyloidogenic proteins. For instance, zinc binding promotes fibril formation of human amyloid β peptides associated with Alzheimer disease (21, 22). Zn2+ has been used to modulate the assembly kinetics and morphology of congeners of amyloid β peptide (60), and low micromolar zinc significantly reduces amyloid β toxicity (61). Human α-synuclein, a protein associated with Parkinson disease, is intrinsically unfolded (62) and Zn2+ binding triggers the fibrillization of methionine oxidized α-synuclein (23). The abnormal form of prion protein is believed to be responsible for transmissible spongiform encephalopathy (63, 64), and copper and zinc binding modulates the aggregation and neurotoxic properties of the human prion peptide prion protein 106–126 (58). Our work continues the long story of the possible role of metal ions such as zinc in forming fibrils. We demonstrated for the first time that one Zn2+ binds to one Tau monomer via tetrahedral coordination to the two cysteines of human Tau protein, Cys-291 and Cys-322, and two histidines, with a moderate affinity similar to that between Zn2+ and amyloid β (21, 56, 57). We also found that the Tau fibrils formed in the presence of low micromolar Zn2+ showed significant dose-dependent cytotoxicity to SH-SY5Y neuroblastoma cells when SH-SY5Y cells were treated with such fibrils for 24 h (data not shown). Taken together, these results indicate that Zn2+ directly binds to Tau protein via tetrahedral coordination to Cys-291 and Cys-322 as well as two histidines, and thereby modulates Tau aggregation in physiological reducing conditions. Thus, it seems reasonable to speculate that Alzheimer disease, Parkinson disease, and transmissible spongiform encephalopathy share common mechanisms of metal ion (including zinc ion) regulation of amyloidogenic protein aggregation, which might lead to a common (65), or at least one or more overlapping, pathogenic mechanisms.
In addition to this direct interaction between Zn2+ and human Tau protein we observed, it is also known that many of the kinases that phosphorylate Tau (ultimately resulting in hyperphosphorylation and dissociation from the microtubules), such as the extracellular signal-regulated kinase, can be induced by Zn2+ (66, 67). Zinc has a bimodal effect on Tau phosphorylation depending on the zinc concentration and induces glycogen synthase kinase-3β activation and Tau release from microtubules (68). The interaction of Tau protein with its partner S100β is promoted by zinc and inhibited by hyperphosphorylation in Alzheimer disease (69). All the results above suggest that zinc plays a significant role in the onset and development of Tau pathology associated to neurodegenerative diseases by increasing the formation of neurotoxic fibrils of Tau protein.
In conclusion we have shown that: (i) fibril formation of Tau protein from natively unstructured monomers proceeds slowly in the absence of Zn2+; (ii) low micromolar concentrations of Zn2+ dramatically accelerate fibril formation of Tau in both reducing conditions and oxidative conditions; (iii) higher concentrations of Zn2+ induce Tau to form granular aggregates only under reducing conditions, and these non-fibrillar aggregates assemble into mature Tau filaments when Zn2+ has been chelated by EDTA; (iv) one Zn2+ binds to one Tau monomer via tetrahedral coordination to Cys-291 and Cys-322 as well as two histidines, with moderate, micromolar affinity, in oxidizing conditions. Our data demonstrate that low micromolar zinc accelerates the fibrillization of human Tau protein via bridging Cys-291 and Cys-322 in physiological reducing conditions, providing clues to understanding the relationship between zinc dyshomeostasis and the etiology of neurodegenerative diseases. The enhancing effect of zinc on Tau fibrillization occurred at concentrations 10- to 30-fold lower than the 100–300 μm zinc concentration present in synaptic vesicles of the brain, implicating an important role for zinc in Tau aggregation and toxicity in the Alzheimer disease brain.
Acknowledgments
We sincerely thank Dr. Michel Goedert (Laboratory of Molecular Biology, Medical Research Council, Cambridge, UK) for kindly providing the plasmid for human Tau40 and Dr. Frank Shewmaker (NIDDK, National Institutes of Health, Bethesda) for his critical reading of the manuscript. We thank Prof. Guang-Fu Yang (College of Chemistry, Central China Normal University, Wuhan, China), Dr. Sheng Xu (College of Physical Science and Technology, Wuhan University, Wuhan, China), and Dr. Li Li (College of Life Sciences, Wuhan University, Wuhan, China) for their technical assistances on iTC200, AFM, and TEM, respectively.
This work was supported by National Key Basic Research Foundation of China Grant 2006CB910301 and by National Natural Science Foundation of China Grants 30770421 and 30970599.
- NFT
- neurofibrillary tangles
- AFM
- atomic force microscopy
- DM
- double mutant C291A/C322A of Tau244–372
- DTT
- dithiothreitol
- ITC
- isothermal titration calorimetry
- PHF
- paired helical filaments
- TEM
- transmission electron microscopy
- ThT
- thioflavin T
- WT
- wild-type Tau244–372.
REFERENCES
- 1.Kuret J., Chirita C. N., Congdon E. E., Kannanayakal T., Li G., Necula M., Yin H., Zhong Q. (2005) Biochim. Biophys. Acta 1739, 167–178 [DOI] [PubMed] [Google Scholar]
- 2.Skovronsky D. M., Lee V. M., Trojanowski J. Q. (2006) Annu. Rev. Pathol. 1, 151–170 [DOI] [PubMed] [Google Scholar]
- 3.Braak H., Braak E. (1997) Neurobiol. Aging 18, 351–357 [DOI] [PubMed] [Google Scholar]
- 4.Mandelkow E., Song Y. H., Schweers O., Marx A., Mandelkow E. M. (1995) Neurobiol. Aging 16, 347–354 [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg K. J., Ross J. L., Feinstein H. E., Feinstein S. C., Israelachvili J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7445–7450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Nat. Rev. Neurosci. 8, 663–672 [DOI] [PubMed] [Google Scholar]
- 7.Mandelkow E., von Bergen M., Biernat J., Mandelkow E. M. (2007) Brain Pathol. 17, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. (1992) J. Cell Biol. 118, 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4506–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush A. I. (2000) Curr. Opin. Chem. Biol. 4, 184–191 [DOI] [PubMed] [Google Scholar]
- 11.Barnham K. J., Bush A. I. (2008) Curr. Opin. Chem. Biol. 12, 222–228 [DOI] [PubMed] [Google Scholar]
- 12.Cuajungco M. P., Lees G. J. (1997) Brain Res. Rev. 23, 219–236 [DOI] [PubMed] [Google Scholar]
- 13.Weiss J. H., Sensi S. L., Koh J. Y. (2000) Trends Pharmacol. Sci. 21, 395–401 [DOI] [PubMed] [Google Scholar]
- 14.Koh J. Y. (2001) Mol. Neurobiol. 24, 99–106 [DOI] [PubMed] [Google Scholar]
- 15.Frederickson C. J., Koh J. Y., Bush A. I. (2005) Nat. Rev. Neurosci. 6, 449–462 [DOI] [PubMed] [Google Scholar]
- 16.Thompson C. M., Markesbery W. R., Ehmann W. D., Mao Y. X., Vance D. E. (1988) Neurotoxicology 9, 1–7 [PubMed] [Google Scholar]
- 17.Assaf S. Y., Chung S. H. (1984) Nature 308, 734–736 [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson R. W., Cox A. G., McLeod C. W., Marshall P. S., Harper A., Dawson E. L., Howlett D. R. (2005) Anal. Biochem. 346, 225–233 [DOI] [PubMed] [Google Scholar]
- 19.Adlard P. A., Bush A. I. (2006) J. Alzheimers Dis. 10, 145–163 [DOI] [PubMed] [Google Scholar]
- 20.Lovell M. A., Robertson J. D., Teesdale W. J., Campbell J. L., Markesbery W. R. (1998) J. Neurol. Sci. 158, 47–52 [DOI] [PubMed] [Google Scholar]
- 21.Bush A. I., Pettingell W. H., Multhaup G., d Paradis M., Vonsattel J. P., Gusella J. F., Beyreuther K., Masters C. L., Tanzi R. E. (1994) Science 265, 1464–1467 [DOI] [PubMed] [Google Scholar]
- 22.Huang X., Atwood C. S., Moir R. D., Hartshorn M. A., Vonsattel J. P., Tanzi R. E., Bush A. I. (1997) J. Biol. Chem. 272, 26464–26470 [DOI] [PubMed] [Google Scholar]
- 23.Yamin G., Glaser C. B., Uversky V. N., Fink A. L. (2003) J. Biol. Chem. 278, 27630–27635 [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal J., Ginzburg I. (2008) J. Cell Sci. 121, 3253–3260 [DOI] [PubMed] [Google Scholar]
- 25.Yang L. S., Ksiezak-Reding H. (1999) J. Neurosci. Res. 55, 36–43 [DOI] [PubMed] [Google Scholar]
- 26.Shin R. W., Lee V. M., Trojanowski J. Q. (1994) J. Neurosci. 14, 7221–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soragni A., Zambelli B., Mukrasch M. D., Biernat J., Jeganathan S., Griesinger C., Ciurli S., Mandelkow E., Zweckstetter M. (2008) Biochemistry 47, 10841–10851 [DOI] [PubMed] [Google Scholar]
- 28.Sayre L. M., Perry G., Harris P. L., Liu Y., Schubert K. A., Smith M. A. (2000) J. Neurochem. 74, 270–279 [DOI] [PubMed] [Google Scholar]
- 29.Barghorn S., Biernat J., Mandelkow E. (2005) Methods Mol. Biol. 299, 35–51 [DOI] [PubMed] [Google Scholar]
- 30.Chattopadhyay M., Durazo A., Sohn S. H., Strong C. D., Gralla E. B., Whitelegge J. P., Valentine J. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18663–18668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoyagi H., Hasegawa M., Tamaoka A. (2007) J. Biol. Chem. 282, 20309–20318 [DOI] [PubMed] [Google Scholar]
- 32.Xu H., Liang Y., Zhang P., Du F., Zhou B. R., Wu J., Liu J. H., Liu Z. G., Ji L. N. (2005) J. Biol. Inorg. Chem. 10, 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naiki H., Higuchi K., Hosokawa M., Takeda T. (1989) Anal. Biochem. 177, 244–249 [DOI] [PubMed] [Google Scholar]
- 34.Yang F., Jr., Zhang M., Zhou B. R., Chen J., Liang Y. (2006) J. Mol. Biol. 362, 821–834 [DOI] [PubMed] [Google Scholar]
- 35.Friedhoff P., von Bergen M., Mandelkow E. M., Davies P., Mandelkow E. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda S., Sahara N., Saito Y., Murayama M., Yoshiike Y., Kim H., Miyasaka T., Murayama S., Ikai A., Takashima A. (2007) Biochemistry 46, 3856–3861 [DOI] [PubMed] [Google Scholar]
- 37.Friedhoff P., von Bergen M., Mandelkow E. M., Mandelkow E. (2000) Biochim. Biophys. Acta 1502, 122–132 [DOI] [PubMed] [Google Scholar]
- 38.Friedhoff P., Schneider A., Mandelkow E. M., Mandelkow E. (1998) Biochemistry 37, 10223–10230 [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya K., Rank K. B., Evans D. B., Sharma S. K. (2001) Biochem. Biophys. Res. Commun. 285, 20–26 [DOI] [PubMed] [Google Scholar]
- 40.Sahara N., Maeda S., Murayama M., Suzuki T., Dohmae N., Yen S. H., Takashima A. (2007) Eur. J. Neurosci. 25, 3020–3029 [DOI] [PubMed] [Google Scholar]
- 41.Schweers O., Mandelkow E. M., Biernat J., Mandelkow E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8463–8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barghorn S., Mandelkow E. (2002) Biochemistry 41, 14885–14896 [DOI] [PubMed] [Google Scholar]
- 43.Liang Y., Du F., Sanglier S., Zhou B. R., Xia Y., Van Dorsselaer A., Maechling C., Kilhoffer M. C., Haiech J. (2003) J. Biol. Chem. 278, 30098–30105 [DOI] [PubMed] [Google Scholar]
- 44.Lee Y. M., Lim C. (2008) J. Mol. Biol. 379, 545–553 [DOI] [PubMed] [Google Scholar]
- 45.Chirita C. N., Kuret J. (2004) Biochemistry 43, 1704–1714 [DOI] [PubMed] [Google Scholar]
- 46.von Bergen M., Barghorn S., Biernat J., Mandelkow E. M., Mandelkow E. (2005) Biochim. Biophys. Acta 1739, 158–166 [DOI] [PubMed] [Google Scholar]
- 47.Congdon E. E., Kim S., Bonchak J., Songrug T., Matzavinos A., Kuret J. (2008) J. Biol. Chem. 283, 13806–13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D.W., Mohanty S., Irbäck A., Huo S. (2008) PLoS Comput. Biol. 4, e1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sibille N., Sillen A., Leroy A., Wieruszeski J. M., Mulloy B., Landrieu I., Lippens G. (2006) Biochemistry 45, 12560–12572 [DOI] [PubMed] [Google Scholar]
- 50.Jeganathan S., von Bergen M., Mandelkow E. M., Mandelkow E. (2008) Biochemistry 47, 10526–10539 [DOI] [PubMed] [Google Scholar]
- 51.Vallee B. L., Falchuk K. H. (1993) Physiol. Rev. 73, 79–118 [DOI] [PubMed] [Google Scholar]
- 52.Goedert M. (1993) Trends Neurosci. 16, 460–465 [DOI] [PubMed] [Google Scholar]
- 53.Mandelkow E. M., Mandelkow E. (1993) Trends. Biochem. Sci. 18, 480–483 [DOI] [PubMed] [Google Scholar]
- 54.Goedert M., Jakes R., Spillantini M. G., Hasegawa M., Smith M. J., Crowther R. A. (1996) Nature 383, 550–553 [DOI] [PubMed] [Google Scholar]
- 55.Nolan E. M., Lippard S. J. (2009) Acc. Chem. Res. 42, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talmard C., Bouzan A., Faller P. (2007) Biochemistry 46, 13658–13666 [DOI] [PubMed] [Google Scholar]
- 57.Tõugu V., Karafin A., Palumaa P. (2008) J. Neurochem. 104, 1249–1259 [DOI] [PubMed] [Google Scholar]
- 58.Jobling M. F., Huang X., Stewart L. R., Barnham K. J., Curtain C., Volitakis I., Perugini M., White A. R., Cherny R. A., Masters C. L., Barrow C. J., Collins S. J., Bush A. I., Cappai R. (2001) Biochemistry 40, 8073–8084 [DOI] [PubMed] [Google Scholar]
- 59.Gaggelli E., Bernardi F., Molteni E., Pogni R., Valensin D., Valensin G., Remelli M., Luczkowski M., Kozlowski H. (2005) J. Am. Chem. Soc. 127, 996–1006 [DOI] [PubMed] [Google Scholar]
- 60.Dong J., Shokes J. E., Scott R. A., Lynn D. G. (2006) J. Am. Chem. Soc. 128, 3540–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garai K., Sahoo B., Kaushalya S. K., Desai R., Maiti S. (2007) Biochemistry 46, 10655–10663 [DOI] [PubMed] [Google Scholar]
- 62.Fink A. L. (2006) Acc. Chem. Res. 39, 628–634 [DOI] [PubMed] [Google Scholar]
- 63.Prusiner S. B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soto C., Estrada L., Castilla J. (2006) Trends Biochem. Sci. 31, 150–155 [DOI] [PubMed] [Google Scholar]
- 65.Soto C. (2003) Nat. Rev. Neurosci. 4, 49–60 [DOI] [PubMed] [Google Scholar]
- 66.An W. L., Bjorkdahl C., Liu R., Cowburn R. F., Winblad B., Pei J. J. (2005) J. Neurochem. 92, 1104–1115 [DOI] [PubMed] [Google Scholar]
- 67.Harris F. M., Brecht W. J., Xu Q., Mahley R. W., Huang Y. (2004) J. Biol. Chem. 279, 44795–44801 [DOI] [PubMed] [Google Scholar]
- 68.Boom A., Authelet M., Dedecker R., Frédérick C., Van Heurck R., Daubie V., Leroy K., Pochet R., Brion J. P. (2009) Biochim. Biophys. Acta 1793, 1058–1067 [DOI] [PubMed] [Google Scholar]
- 69.Yu W. H., Fraser P. E. (2001) J. Neurosci. 21, 2240–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]