Abstract
Pseudomonas fluorescens CHA0, an antagonist of phytopathogenic fungi in the rhizosphere of crop plants, elaborates and excretes several secondary metabolites with antibiotic properties. Their synthesis depends on three small RNAs (RsmX, RsmY, and RsmZ), whose expression is positively controlled by the GacS-GacA two-component system at high cell population densities. To find regulatory links between primary and secondary metabolism in P. fluorescens and in the related species Pseudomonas aeruginosa, we searched for null mutations that affected central carbon metabolism as well as the expression of rsmY-gfp and rsmZ-gfp reporter constructs but without slowing down the growth rate in rich media. Mutation in the pycAB genes (for pyruvate carboxylase) led to down-regulation of rsmXYZ and secondary metabolism, whereas mutation in fumA (for a fumarase isoenzyme) resulted in up-regulation of the three small RNAs and secondary metabolism in the absence of detectable nutrient limitation. These effects required the GacS sensor kinase but not the accessory sensors RetS and LadS. An analysis of intracellular metabolites in P. fluorescens revealed a strong positive correlation between small RNA expression and the pools of 2-oxoglutarate, succinate, and fumarate. We conclude that Krebs cycle intermediates (already known to control GacA-dependent virulence factors in P. aeruginosa) exert a critical trigger function in secondary metabolism via the expression of GacA-dependent small RNAs.
Introduction
Secondary metabolism occurs in certain bacteria and fungi as part of developmental processes, which are often accompanied by morphological changes (1, 2). In natural environments, secondary metabolites are believed to confer a selective advantage to the producers when these organisms cannot rely on their full growth potential to compete with other organisms (3). Such a role is most plausible for secondary metabolites having antibiotic activities (4). In pure cultures, secondary metabolites are non-essential for the producers and are typically formed when cell population densities are high and growth is restricted. This distinct production phase, sometimes called idiophase, usually follows the phase of optimal growth, also termed trophophase (5). A fundamental question is what triggers the onset of the idiophase. Both extracellular and intracellular signal molecules are known to be involved. For instance, excreted quorum-sensing signal molecules, such as N-acyl-homoserine lactones of Pseudomonas species or γ-butyrolactones of Streptomyces species, positively regulate the expression of antibiotic compounds, and the intracellular alarmone ppGpp is required for antibiotic production in Streptomyces coelicolor under conditions of nitrogen starvation (1, 6). However, these findings do not provide a generally valid picture of how secondary metabolism is initiated in microorganisms. For instance, some Pseudomonas species produce secondary metabolites without N-acyl-homoserine lactones and phosphate-limited S. coelicolor does not rely on ppGpp for antibiotic production (1, 6).
In fluorescent pseudomonads, the Gac/Rsm signal transduction pathway is instrumental for the expression of secondary metabolites. These metabolites contribute effectively to virulence in animal- and plant-pathogenic Pseudomonas species as well as to plant protection in root-colonizing Pseudomonas species having biocontrol activity. Thus, depending on the species, mutants defective in the Gac/Rsm pathway have lost part or all of their virulence or have a reduced ability to suppress plant diseases (7–9). The Gac/Rsm cascade is initiated by the GacS/GacA two-component system (8, 10). At high cell population densities, Pseudomonas species excrete chemically uncharacterized signal molecules that can act as inducers of the Gac/Rsm pathway by favoring phosphorylation of the GacS sensor kinase and hence of the cognate GacA response regulator (11–14). Activated GacA promotes the transcription of two or three non-coding small RNAs (sRNAs),4 depending on the species. In the closely related biocontrol strains Pseudomonas fluorescens CHA0 and Pf-5 (15), GacA controls the expression of three sRNAs, termed RsmX, RsmY, and RsmZ. In the opportunistic human pathogen Pseudomonas aeruginosa, there are only two functionally equivalent, GacA-controlled sRNAs, RsmY and RsmZ. These sRNAs have a high affinity for the RNA-binding protein RsmA and additionally for the RsmA paralogue RsmE in strain CHA0 (11, 12, 16, 17). The RsmA and RsmE proteins repress the translation of genes involved in secondary metabolism during the trophophase. When the RsmX/Y/Z sRNAs are induced in the idiophase, they relieve translational repression of target genes by sequestering the RsmA and RsmE proteins, thereby allowing the synthesis of secondary metabolites (12, 18–20).
We have undertaken the present genetic and metabolic study to find intracellular factors that favor or hinder the expression of the rsmX/Y/Z genes in P. fluorescens CHA0 and to reveal regulatory links that may exist between primary and secondary metabolism. To this end, we have screened mutant libraries of P. fluorescens carrying an rsmZ-gfp fusion (21) and of P. aeruginosa carrying an rsmY-gfp fusion for diminished or enhanced GFP expression. We find that mutational loss of pyruvate carboxylase or fumarase A strongly affects rsmX/Y/Z expression without major changes of growth properties.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
The bacterial strains and plasmids used are listed in Table 1. Strains of Escherichia coli and P. fluorescens were routinely grown in nutrient yeast broth (NYB; 2.5% (w/v) nutrient broth, 0.5% (w/v) yeast extract) with shaking or on nutrient agar plates (4% (w/v) blood agar base, 0.5% (w/v) yeast extract) amended with the following antibiotics when required: ampicillin, 100 μg/ml; gentamicin, 50 μg/ml; kanamycin, 25 μg/ml; spectinomycin, 1 mg/ml; or tetracycline, 25 μg/ml (125 μg/ml for selection of P. fluorescens). Transposon mutants of P. aeruginosa were screened on a medium described by Carmi et al. (22). For antibiotic activity assays, bacteria were grown on glycerol-casamino acids medium (GCM) (23). For metabolic analyses, bacteria were grown in diluted and modified GCM to minimize the effects of the medium on metabolite analysis. The modified medium contained, per liter: casamino acids (Bacto), 1.5 g; glycerol, 1 g; K2HPO4, 0.038 g; MgSO4·7H2O, 0.15 g; FeSO4·7H2O, 1 mg. For testing carbon sources, these were included in the minimal medium of Ornston and Stanier (24). When relevant, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside was added to plates at a final concentration of 0.02%. The inoculation temperatures were 30 °C for P. fluorescens and 37 °C for P. aeruginosa and E. coli. P. fluorescens was grown at 35 °C to improve its capacity to accept DNA originating from E. coli.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| M168 | Wild type | C. Keel |
| E. coli | ||
| DH5α, HB101 | Laboratory strains | Ref. 37 |
| S17-1/λpir | pro thi hsdR recA chromosome::RP4–2 (Tc::Mu Km::Tn7) λpir; Tpr Smr | Ref. 38 |
| P. aeruginosa | ||
| PAO1 | Wild type | ATCC15692 |
| PAO6382 | ΔpvdF | C. Reimmann |
| P. fluorescens | ||
| CHA0 | Wild type | Ref. 39 |
| CHA19 | ΔgacS | Ref. 40 |
| CHA89 | gacA::Kmr | Ref. 41 |
| CHA1201 | pycB::Tn5, insertion 1.6 kb from 5′-end | This study |
| CHA1201C | pycB::Tn5 strain with pycAB+ region in mini-Tn7 | This study |
| CHA1201Cp | pycB::Tn5 strain harboring pME9952 | This study |
| CHA1202 | ΔretS | Ref. 30 |
| CHA1204 | ΔladS | Ref. 30 |
| CHA1313 | ΔgacS pycA::ΩSp/Sm | This study |
| CHA1314 | gacA::KmrpycA::ΩSp/Sm | This study |
| CHA1315 | ΔretS pycA::ΩSp/Sm | This study |
| CHA1316 | ΔladS pycA::ΩSp/Sm | This study |
| CHA1317 | ΔgacS ΔfumA | This study |
| CHA1318 | gacA::Kmr ΔfumA | This study |
| CHA1319 | ΔretS ΔfumA | This study |
| CHA1320 | ΔladS ΔfumA | This study |
| CHA1321 | pycA::ΩSp/Sm | This study |
| CHA1322 | ΔfumA | This study |
| CHA1322C | ΔfumA strain with fumA+ region in mini-Tn7 | This study |
| CHA1322Cp | ΔfumA strain harboring pME9956 | This study |
| Plasmids | ||
| pBluescript II KS | Cloning vector, ColE1 replicon; Apr | Stratagene |
| pHP45Ω | Source of the ΩSp/Smr cassette, ColE1 vector; Apr | Ref. 29 |
| pLM1 | Tn5Gm delivery vector, Gmr derivative of pRL27 | Ref. 27 |
| pME497 | Mobilizing plasmid, IncP-1, Tra, RepA(Ts); Apr | Ref. 42 |
| pME3087 | Suicide vector, ColE1 replicon, Mob; Tcr | Ref. 43 |
| pME3266 | Translational gacS′-′lacZ fusion; Tcr | F. Carruthers |
| pME6031 | pACYC177-pVS1 shuttle vector; Tcr | Ref. 44 |
| pME6060 | Translational aprA'-'lacZ fusion; Tcr | Ref. 45 |
| pME6091 | Transcriptional rsmZ-lacZ fusion; Tcr | Ref. 11 |
| pME6182 | Mini-Tn7 gene delivery vector based on pME3280a, HindIII-SmaI-KpnI-NcoI-SphI cloning site, ColE1 replicon; Gmr Apr | Ref. 30 |
| pME6702 | Translational phlA′-′lacZ fusion under ptac; Tcr | Ref. 17 |
| pME6827 | Translational gacA′-′lacZ fusion; Tcr | C. Reimmann |
| pME6916 | Transcriptional rsmY-lacZ fusion; Tcr | Ref. 16 |
| pME7402 | Transcriptional rsmZ-gfp fusion; Tcr | Ref. 21 |
| pME7415 | Transcriptional rsmY-gfp fusion; Tcr | This study |
| pME7698 | pME6182 containing a transcriptional rsmX-lacZ fusion in mini-Tn7 | B. Humair |
| pME7699 | pME6182 containing a transcriptional rsmY-lacZ fusion in mini-Tn7 | B. Humair |
| pME9951 | pME6182 containing the pycAB genes in mini-Tn7 | This study |
| pME9952 | pME6031 containing the pycAB genes | This study |
| pME9953 | pME3087 containing pycAB region with a 0.5-kb EcoRV deletion in pycA replaced by the 2-kb ΩSp/Sm fragment | This study |
| pME9954 | pME3087 containing a 1.4-kb fumA region with a 0.9-kb deletion in the fumA gene | This study |
| pME9955 | pME6182 containing the fumA gene in mini-Tn7 | This study |
| pME9956 | pME6031 containing the fumA gene | This study |
| pPROBE-TT′ | gfp promoter probe vector; Tcr | Ref. 46 |
| pUX-BF13 | Helper plasmid encoding Tn7 transposition fusions; Apr | Ref. 47 |
DNA Manipulation
Small scale plasmid extraction was carried out with a QIAprep spin miniprep kit (Qiagen); large scale preparations were obtained with a Jetstar kit (Genomed GmbH, Basel, Switzerland). Chromosomal DNA from P. fluorescens was prepared as described previously (25). DNA fragments were purified from agarose gels with a QIAquick gel extraction kit (Qiagen). For localization of Tn5 insertions in CHA0 mutants carrying pME7402, genomic DNA was extracted. Chromosome fragments flanking each Tn5 insertion were amplified by two successive PCR steps (26) and sequenced. Plasmid pME7402 was cured from CHA0 mutants as described previously (21). Oligonucleotides used are listed in supplemental Table S1.
Transposon Tn5Gm Mutagenesis of PAO6382 and Mutant Screening
About 10,000 random Tn5Gm insertions were generated by biparental matings between E. coli S17-1λpir/pLM1 and the pyoverdin-negative P. aeruginosa strain PAO6382. Transposon insertion mutants were selected on nutrient agar containing 50 μg/ml gentamicin and 10 μg/ml chloramphenicol and stored at −80 °C in 96-well microtiter plates in NYB containing 15% (v/v) glycerol and 50 μg/ml gentamicin. The Tn5Gm mutant library was then screened for mutants with altered rsmY expression. As a reporter construct, we chose pME7415 because preliminary tests had shown that detection of GacA-dependent regulation was easier on plates with an rsmYCHA0-gfp fusion than with an rsmYPAO-gfp fusion (data not shown). Plasmid pME7415 was constructed by inserting the rsmYCHA0 promoter region on a 0.31-kb BamHI-PstI fragment (recruited from pME6916) into pPROBE-TT′. Plasmid pME7415 was introduced into the mutant library by conjugation as follows. Mutants were grown overnight in 96-well microtiter plates at 43 °C in NYB and subsequently transferred with a 48-needle replicator onto nutrient agar plates where 100 μl of an NYB-grown overnight culture of E. coli S17-1λpir/pME7415 had been spread. Donor and recipients were incubated together on nutrient agar at 37 °C for 3–4 h and subsequently transferred, with the replicator, onto plates containing Carmi (22) medium and 50 μg/ml gentamicin plus 100 μg/ml tetracycline. To avoid fluorescence due to siderophore production, FeCl3 was added to the selective medium at a final concentration of 100 μm. Plates were incubated at 37 °C, and GFP production was scored regularly by visual inspection in daylight and under UV light. The transposon insertion sites of mutants with altered GFP production were determined by sequence analysis as described previously (27) except that cloning out of the transposon was done with BamHI.
Complementation of the pycB-negative Mutant CHA1201
To restore pycB function in the mutant CHA1201, a 3.8-kb fragment carrying pycA and pycB was amplified with primers PycAB1 and PycAB2, high fidelity DNA polymerase PrimeSTARTM (Takara), and genomic DNA of P. fluorescens CHA0 as a template. The amplified fragment was digested with BamHI and HindIII and cloned into BamHI- and HindIII-digested pBluescript II KS (Stratagene). The insert obtained was confirmed by sequencing and digested with HindIII and BamHI; the BamHI sticky end was filled in with T4 polymerase. This fragment was subcloned into the mini-Tn7 vector pME6182 cleaved at the HindIII and SmaI sites. The resultant plasmid, pME9951, was introduced together with pUX-BF13 into CHA1201 by electroporation. Transposition of the mini-Tn7 carrying the pycAB genes into the chromosome was confirmed by PCR with primers Gm and Glms (28). The resultant strain was named CHA1201C. The pycAB genes were also subcloned into pME6031 (at the BamHI and HindIII sites), and the resulting plasmid, pME9952, was introduced into CHA1201 for complementation. The resultant strain was named CHA1201Cp and used for assaying the chromosomal rsmX-lacZ and rsmY-lacZ fusions carried by mini-Tn7, which occupied the Tn7 attachment site in these strains.
Generation of the pycA-negative Mutant CHA1321
To inactivate the chromosomal pycA gene of P. fluorescens CHA0, we utilized a derivative of the suicide plasmid pME3087. pBluescript II KS carrying the 3.8-kb pycAB fragment mentioned above was digested with EcoRV to replace a 0.5-kb internal region of pycA with the 2-kb ΩSp/Sm cassette from pHP45Ω (29). The resulting plasmid was digested with XbaI and KpnI to excise the mutated pycA gene, which contained 1248 bp upstream and 889 bp downstream of ΩSp/Sm. This fragment was subcloned into pME3087 at the KpnI and XbaI sites to give pME9953. This plasmid was mobilized from E. coli DH5α to P. fluorescens by triparental mating using E. coli HB101/pME497. A pycA::ΩSp/Sm mutant, which was resistant to spectinomycin but sensitive to tetracycline, was obtained after an enrichment for tetracycline-sensitive cells (30), and the ΩSp/Sm insertion was confirmed by PCR in the resulting strain CHA1321.
Generation of the fumA-negative Mutant CHA1322 and Its Complementation
An in-frame deletion in the chromosomal fumA gene of P. fluorescens CHA0 was created as follows. Fragments of ∼700 bp located on each side of the fumA gene were amplified by PCR with primer pairs FumAUF/FumAUR and FumADF/FumADR, annealed, and amplified as a 1.4-kb fragment using primers FumAUF/FumADR. After sequencing, this 1.4-kb fragment was cloned into pME3087 cut with BamHI and HindIII to give pME9954. This plasmid was mobilized from E. coli DH5α by triparental mating with E. coli HB101/pME497. Excision of the vector via a second crossing over was obtained after enrichment for tetracycline-sensitive cells (30), generating the fumA mutant CHA1322. This strain was complemented with a 2.2-kb fragment carrying fumA, which had been amplified by PCR with primers FumAUF and FumADR and subcloned into pME6182 (resulting in plasmid pME9955) to give strain CHA1322C based on the mini-Tn7 method described above. The fumA+ region was also cloned into pME6031 (cut with BamHI and HindIII) and the resulting plasmid, pME9956, was introduced into CHA1322, thereafter named CHA1322Cp.
RNA Extraction and Northern Blot Analysis
RNAs used for Northern blot analysis were isolated using a hot acid phenol extraction protocol (31). RNAs (10 μg/lane) were separated on a denaturing urea-polyacrylamide gel and analyzed by Northern blotting using digoxigenin-labeled probes as described previously (32).
Enzyme Assays
For β-galactosidase assays, P. fluorescens strains were grown at 30 °C in 50-ml flasks containing 20 ml of NYB amended with 0.05% Triton X-100 with shaking at 180 rpm. Specific activities were determined by the Miller method (33). For fumarase assays, exponential phase cultures of P. fluorescens in GCM were harvested at 4 °C by centrifugation at 6,000 × g for 10 min. Cells were washed twice, resuspended in 1 ml of chilled 50 mm sodium phosphate buffer, pH 7.0, containing 1 μg/ml Pefabloc SC (Roche Applied Science), and broken with a VibraCell sonicator (Danbury, CT) in an ice bath for 1 min. Cell debris was removed by centrifugation at 10,000 × g for 10 min. Fumarase activity was measured in the extracts by following the increase in absorbance at 240 nm in the presence of 50 mm malate at room temperature (34). One unit is defined as 1 μmol of fumarate formed per min. Protein concentrations were determined by the Bradford method.
Detection of Antibiotic Activity
Antibiotic activity of P. fluorescens strains grown on GCM plates was determined with Bacillus subtilis M168 as the reporter. Cultures of P. fluorescens were adjusted to A600 nm = 1.5 (corresponding to ∼1.5 × 109 cells/ml), and 5-μl samples were spotted onto the plate. After overnight incubation at 30 °C, cells were killed by UV irradiation on a transilluminator for 5 min. An overlay of B. subtilis revealed antibiotic production by growth inhibition zones.
Plant Disease Suppression and Root Colonization Assays
Ten flasks containing natural sandy loam soil were planted with three cucumber seedlings each and treated with Pythium ultimum and/or P. fluorescens. After 7 days of incubation, the biocontrol activity of each strain was estimated as described previously (16).
LC-HRMS Analysis of Central Metabolites
P. fluorescens strains harboring pME7402 were grown in 20 ml of modified GCM in 50-ml Erlenmeyer flasks with shaking at 180 rpm and 30 °C. For each strain, three cultures were incubated in parallel. Samples were taken from each culture during the exponential trophophase (at A600 nm ≈ 0.5) and the idiophase (at A600 nm ≈ 1.7) when maximal GFP formation was observed. Cells were sampled by fast filtration using RC membrane filters (diameter 47 mm, pore size 0.2 μm; Sartorius Göttingen, Germany) without washing. Subsequently, filters were directly transferred into 8 ml of boiling water for 8 min. An internal standard solution (i.e. a uniformly 13C-labeled metabolite extract (35) from Methylobacterium extorquens AM1 (36)) was added simultaneously. The volumes of the sample and the internal standard were measured by weight. The extracts were cooled on ice, filtered through an RC Sartorius Minisart filter (pore size 0.2 μm), chilled with liquid nitrogen, and freeze-dried. The dried samples were redissolved in 80 μl of aqueous acetonitrile (70% (v/v)) for LC-HRMS analysis as described (36). For each metabolite, the peak intensity of the non-labeled mass peak M0 and that of the uniformly 13C-labeled mass peak MUL were determined, and the intracellular concentrations (μmol/g) were calculated as follows,
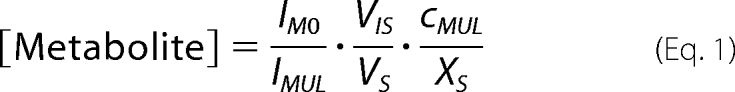 |
where IM0 represents intensity of the metabolite mass peak of the cell extract in sample, IMUL is intensity of the metabolite mass peak of the 13C-labeled internal standard in sample, VS is culture volume of sample, VIS is volume of internal standard added to sample, cMUL is concentration of the 13C-labeled internal standard, and XS is biomass concentration (in g cell dry weight) of sample. Average values and S.D. were determined from three cultures.
Metabolites present in the culture supernatant (citrate, malate, succinate, and amino acids) were determined after removing cells with an RC Sartorius Minisart filter (pore size 0.2 μm). For LC-HRMS analysis, the filtrate was diluted 1:100 using 1 μm sucrose in aqueous acetonitrile (85% (v/v)) as an internal standard. LC-HRMS analysis was performed as described (36). This method was not suited for the analysis of arginine, lysine, cysteine, and tryptophan. The consumption of amino acids was determined by following the decrease of chromatogram peak intensities (relative to the initial peak intensities) for each amino acid.
RESULTS
Transposon Insertion Mutations Affecting the Expression of GacA-dependent sRNAs in Fluorescent Pseudomonads
We used two collections of Tn5 insertion mutants. The first library consists of about 20,000 P. fluorescens CHA0 mutants carrying an rsmZCHA0-gfp fusion on plasmid pME7402 and has previously been used in a search for biocontrol-negative mutants (21). The second library was constructed in this study and consists of about 10,000 P. aeruginosa PAO mutants carrying an rsmYCHA0-gfp fusion on pME7415. These mutants were generated with a gentamicin resistance variant of Tn5 in a pyoverdin-negative background (PAO6382; see “Experimental Procedures”). The rationale for using two different Pseudomonas species and two different sRNA gene reporters is to improve the chances to find regulatory mechanisms that are common to fluorescent pseudomonads. Both collections were screened on rich media for dim and superbright colonies, thus differing with respect to GFP expression from the normally bright fluorescent phenotype seen in the parent strains. In both screens, we used gacA and gacS mutants (dim) as a control. Candidate clones (about 70 from each library) were reexamined for GFP expression in rich liquid media, and, when clear cut phenotypes could be confirmed, the Tn5 insertions were mapped by sequencing. Mutants having insertions in genes with unknown functions were not analyzed further at this stage.
If conditions of suboptimal growth per se were conducive to high expression of secondary metabolism, via the expression of rsmX/Y/Z, we might expect to find a number of small colony variants with a superbright phenotype. However, this did not appear to be the case, although in the P. aeruginosa screen, two independent isolates of this kind were found; both were mutated in the retS gene. The product of this gene has recently been identified as an antagonist of GacS and hence as a negative regulator of rsmZ expression (14), confirming the validity of our approach. Both P. fluorescens and P. aeruginosa screens yielded biotin- and threonine-auxotrophic mutants, which had a slight growth handicap under the conditions used. Both types of mutants were dim rather than superbright. We decided not to examine these further but to concentrate on normally growing mutants.
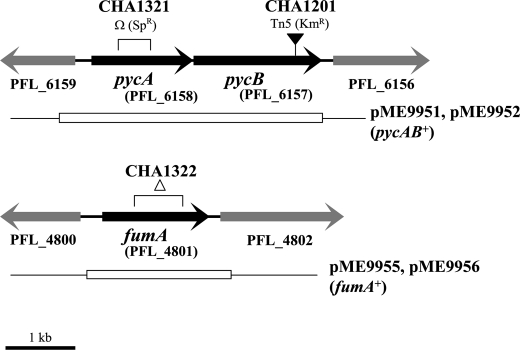
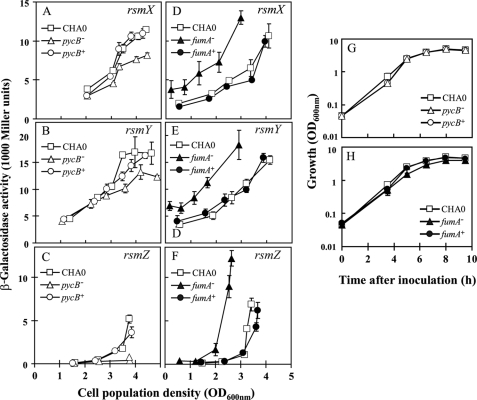
Two independently generated dim mutants of P. fluorescens CHA0 had a Tn5 insertion in the PFL_6157 gene, which appears to form a transcription unit with the upstream PFL_6158 gene (Fig. 1). These genes were originally annotated as oadA (for oxaloacetate decarboxylase subunit) and accC (for a biotin carboxylase), respectively (15). However, it has been shown recently that the PA5435 and PA5436 homologues of P. aeruginosa are in fact the structural genes (pycB and pycA) for pyruvate carboxylase (48), an enzyme that catalyzes the biotin- and ATP-dependent carboxylation of pyruvate, yielding oxaloacetate (49). We cured the pycB::Tn5 mutant CHA1201 of the rsmZ-gfp reporter construct and instead introduced the transcriptional fusions rsmX-lacZ (chromosomal), rsmY-lacZ (chromosomal), or rsmZ-lacZ (on a plasmid). The expression of all three fusions was significantly reduced in the pycB mutant, compared with that in the wild type and in the complemented pycB mutant (Fig. 2, A–C), when these strains were growing in rich medium (NYB). It was important to use chromosomal rsmX-lacZ and rsmY-lacZ reporter fusions; when they were carried by plasmids, their expression of β-galactosidase was too high to be tolerated by the cells. We also constructed a pycA (PFL_6158) ΩSp/Sm insertion mutant, CHA1321. In this strain, the expression of the three GacA-dependent sRNAs was similarly down-regulated (supplemental Fig. S1).
FIGURE 1.
Physical map of the pycA, pycB, and fumA genes in P. fluorescens CHA0 and construction of strains CHA1321 (pycA) and CHA1322 (fumA). ▾, Tn5 insertion in the pycB gene of strain CHA1201; Ω, region replaced by the ΩSp/Sm cassette in strain CHA1321; ▵, region deleted in the fumA of strain CHA1322. The open boxes below indicate the regions used for complementation, either in mini-Tn7 (via pME9951 and pME9955) or in the pME6031 derivatives pME9952 and pME9956, as described under “Experimental Procedures.” PFL gene numbers refer to the genome of P. fluorescens Pf-5 (15).
FIGURE 2.
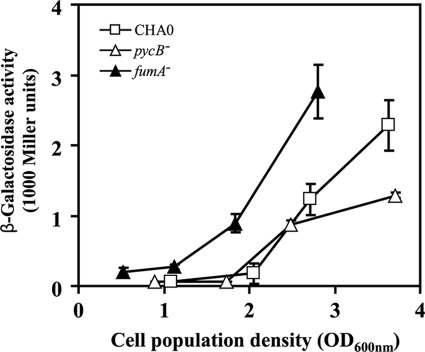
Effect of pycB and fumA mutations on the transcriptional expression of the rsmX, rsmY, and rsmZ genes. The expression of a chromosomal rsmX-lacZ fusion (A and D), that of a chromosomal rsmY-lacZ fusion (B and E), and that of a plasmid-borne rsmZ-lacZ fusion on pME6091 (C and F) were determined in P. fluorescens CHA0 (wild type, open squares), CHA1201 (pycB−, open triangles), CHA1201C/CHA1201Cp (pycB+, open circles), CHA1322 (fumA−, closed triangles), and CHA1322C/CHA1322Cp (fumA+, closed circles). The growth of each strain in the same medium (NYB) was monitored in parallel (G and H). Incubation was carried out in Erlenmeyer flasks as described under “Experimental Procedures.” Measurements were carried out in triplicate. The symbols indicate averages, and the error bars indicate S.D. values. OD600 nm, optical density at 600 nm.
Besides the retS mutants, one more superbright, normally growing P. aeruginosa mutant attracted our attention. It was found to contain Tn5Gm in the PA4333 open reading frame, tentatively annotated as a fumarase (fumA) gene (Pseudomonas Genome Database web site). We constructed a fumA (PFL_4801) deletion mutant in P. fluorescens CHA0, termed CHA1322 (Fig. 1). The rsmX-, rsmY-, and rsmZ-lacZ fusions all showed a strongly elevated expression in this background, compared with the wild type and the complemented fumA mutant (Fig. 2, D–F), indicating that the influence of fumA is similar in both Pseudomonas species. In the rich medium used (NYB), the pycB and fumA mutants grew almost at wild type rates (Fig. 2, G and H).
Phenotypic Characterization of the pycAB and fumA Mutants
In agreement with the growth properties of P. aeruginosa pyruvate carboxylase-negative mutants (48, 49), the P. fluorescens pycA and pycB mutants grew very poorly in minimal media containing glycerol, pyruvate, or glucose as the only carbon source but grew normally in succinate or fumarate media (supplemental Fig. S2) (data not shown). These characteristics confirm that pyruvate carboxylase is an important anaplerotic enzyme also in P. fluorescens. The P. fluorescens fumA mutant, by contrast, was strongly handicapped in minimal media containing fumarate or succinate as the only carbon source, but grew well in glycerol medium (supplemental Fig. S2). Because the PFL_4801 and PA4333 genes had only been tentatively annotated, we measured the fumarase specific activity in P. fluorescens after growth in rich medium. The fumA mutant CHA1322 gave 0.4 ± 0.1 units/mg, whereas the wild type CHA0 and the complemented mutant each had 1.5 ± 0.1 units/mg. The residual fumarase activity in the fumA mutant was probably due to the presence of two additional fumarase isoenzymes, FumC1 (PFL_0907) and FumC2 (PFL_4328). In conclusion, our mutant screen identified two enzymes involved in the function of the Krebs (tricarboxylic acid) cycle (i.e. fumarase and pyruvate carboxylase) as key factors in the regulation of the Gac/Rsm signal transduction pathway, in that both fumA and pycAB mutations had a profound influence on rsmX/Y/Z expression.
Altered sRNA Levels in pycB and fumA Mutants
The results obtained with the rsmX-, rsmY-, and rsmZ-lacZ fusions (Fig. 2) indicate that Krebs cycle intermediates influence the promoter activities of the three GacA-dependent sRNA genes. We validated this finding by determining the levels of RsmZ sRNA by Northern blotting. The RsmZ level was lower in the pycB mutant and higher in the fumA mutant by comparison with the wild type and the complemented mutant (Fig. 3).
FIGURE 3.
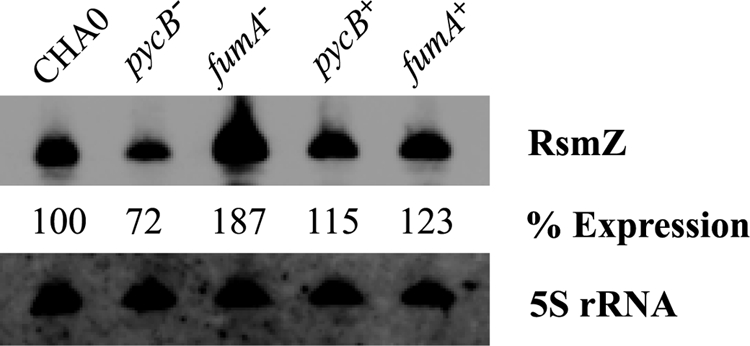
Detection of RsmZ RNA in P. fluorescens CHA0, CHA1201 (pycB−) and CHA1322 (fumA−) by Northern blot. Total RNA was extracted from CHA0 (wild type), CHA1201, CHA1322, CHA1201C (pycB+), and CHA1322C (fumA+) at the end of the exponential phase (A600 nm ≈ 3). As a loading control, 5 S rRNA was revealed in all samples. RsmZ expression levels were quantitated using ImageQuant software, with an estimated error of ±20%.
Antibiotic and Protease Expression in the pycB and fumA Mutants
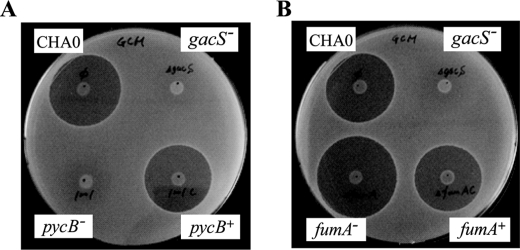
An important GacA-regulated secondary metabolite and biocontrol factor of strain CHA0 is 2,4-diacetylphloroglucinol (20, 41, 43). The translational expression of the 2,4-diacetylphloroglucinol biosynthetic gene phlA was reduced in the pycB mutant CHA1201 and increased in the fumA mutant CHA1322 by comparison with the wild type (Fig. 4), as we expected from the sRNA expression data (Figs. 2 and 3). Moreover, a parallel pattern of antibiotic production was seen in a biotest using B. subtilis as a 2,4-diacetylphloroglucinol-sensitive indicator (Fig. 5). The major GacA-controlled exoprotease of strain CHA0 is the product of the aprA gene (45, 50). We also followed the expression of a translational aprA′-′lacZ fusion in the pycB and fumA mutants; again, the effects of both mutations were consistent with the rsmX/Y/Z expression data (Fig. 6).
FIGURE 4.
Expression of a phlA′-′lacZ fusion. This reporter carried by pME6702 was measured, in triplicate, in P. fluorescens CHA0 (wild type, open squares), CHA1201 (pycB−, open triangles), and CHA1322 (fumA−, closed triangles). The strains were grown in Erlenmeyer flasks containing NYB. The symbols indicate averages, and the error bars indicate S.D. values. OD600 nm, optical density at 600 nm.
FIGURE 5.
Effects of pycB and fumA mutations on antibiotic activity toward B. subtilis. Antibiotic activities of P. fluorescens strains grown on GCM were evaluated by the size of growth inhibition zone of B. subtilis. Antibiotic activities of P. fluorescens CHA0 (wild type), CHA1201 (pycB−), CHA1201C (pycB+), and CHA19 (gacS−) are compared in A. Antibiotic activities of P. fluorescens CHA0 (wild type), CHA1322 (fumA−), CHA1322C (fumA+), and CHA19 (gacS−) are compared in B.
FIGURE 6.
Expression of an aprA′-′lacZ fusion. This reporter carried by pME6060 was measured, in triplicate, in P. fluorescens CHA0 (wild type, open squares), CHA1201 (pycB−, open triangles), and CHA1322 (fumA−, closed triangles). The strains were grown in Erlenmeyer flasks containing NYB. The symbols indicate averages, and the error bars indicate S.D. values. OD600 nm, optical density at 600 nm.
Consequences of pycB and fumA Mutations on Biocontrol Efficacy
Root exudates in the rhizosphere, the natural habitat of P. fluorescens CHA0, are rich in Krebs cycle intermediates and amino acids; sugars are less abundant (51). We wondered how the pycB and fumA mutations would influence the biocontrol efficacy of P. fluorescens in a microcosm under natural soil conditions. In a cucumber/P. ultimum microcosm, the wild type CHA0 afforded excellent protection of the plant from the oomycete, in terms of root weight as well as shoot weight (Table 2). Both the pycB and the fumA mutant were somewhat less effective as biocontrol agents, with ≥95% confidence (Table 2). All bacterial strains reached similar levels of root colonization (Table 2). These results indicate that in vitro levels of secondary metabolites do not translate directly into in vivo biocontrol efficacy. The data also confirm previous work on strain CHA0 showing that any impairment of the Gac/Rsm system generally results in reduced biocontrol efficacy (4, 6) but that constitutive up-regulation of the Gac/Rsm system also negatively affects biocontrol efficacy (40).
TABLE 2.
Contribution of FumA and PycB to the suppression of Pythium damping off and root rot of cucumber by P. fluorescens CHA0 in natural soil
Data represent the means from two individual repetitions of the same experimental setup, with 10 replicates (flasks containing three cucumber plants) per treatment in each experiment. ND, not detected.
| Bacterial strain addeda | Pythium addeda | Surviving plants/flask | Shoot fresh weight/flask | Root fresh weight/flask | Colonization by P. fluorescensb |
|---|---|---|---|---|---|
| % | g | g | log10 colony-forming units/g root | ||
| None | − | 100c | 1.17c | 0.26c | ND |
| CHA0 (wild type) | − | 100c | 1.31c | 0.26c | 7.10 ± 0.20 |
| CHA1322 (ΔfumA) | − | 100c | 1.23c | 0.29c | 7.27 ± 0.17 |
| CHA1201 (pycB::Tn5) | − | 100c | 1.23c | 0.27c | 7.25 ± 0.15 |
| None | + | 19d | 0.21d | 0.05d | ND |
| CHA0 (wild type) | + | 96c | 1.18c | 0.28c | 7.87 ± 0.58 |
| CHA1322 (ΔfumA) | + | 65e | 0.90e | 0.18e | 8.37 ± 0.66 |
| CHA1201 (pycB::Tn5) | + | 78e | 0.94e | 0.18e | 7.91 ± 0.59 |
aP. fluorescens strains were added at 107 colony-forming units/g of natural soil contained within 200-ml flasks (60 g of soil/flask), after planting three 92-h-old, sterile-grown cucumber seedlings per flask. P. ultimum was added as a millet seed inoculum at 2.5 g/kg of soil before planting. Plants were harvested after 7 days.
b The rhizosphere-stable plasmid pME6031 containing a tetracycline resistance determinant (44) was introduced as a selective marker into the bacterial strains to determine their root colonization capacity in natural soil.
c–eMeans within the same column followed by different letters are significantly different (p ≤ 0.05) according to Fisher's protected least squares difference test. Prior to separation of means by the least squares difference test, data of the two individual experiments could be pooled following an analysis of variance of trial-by-treatment interactions.
Metabolite Analysis of the pycB and fumA Mutants
We wished to examine the consequences of the pycB and fumA mutations on intracellular metabolite pools, especially with respect to Krebs cycle intermediates and key metabolites of carbohydrate utilization. This proved to be technically difficult because, on the one hand, simple minimal media could not be used with the mutants and, on the other hand, our commonly used rich media interfered strongly with cell sample preparation. As a compromise, we used a diluted and modified glycerol-casamino acids medium (see “Experimental Procedures”) in which the wild type and the fumA and pycB mutants grew well and showed the same pattern of differential rsmZ-gfp expression as observed in full-strength medium (supplemental Fig. S3). Nevertheless, growth yields were about 2-fold lower, compared with those in full-strength medium (data not shown). Samples were taken during the exponential trophophase (at A600 nm ≈ 0.5) and during the idiophase (at A600 nm ≈ 1.7). From an analysis of amino acids in the culture supernatant, it became evident that upon entry into the idiophase, all three strains had similarly used up their most preferred substrates, such as glutamate, aspartate, proline, histidine, and phenylalanine, whereas they had not exhausted some of the less favorable nutrients, such as leucine, isoleucine, valine, threonine, and methionine (data not shown). Thus, carbon, nitrogen, and sulfur sources were still available to the bacteria, and the oxygen supply never fell below 70% of saturation (data not shown).
The wild type as well as the pycB and fumA mutants had lower intracellular pools of Krebs cycle intermediates, phosphoenolpyruvate, pyruvate, and sugars in the idiophase than in the trophophase (Table 3). However, there were important differential responses in the mutants. In the pycB mutant, the pools of succinate, malate, and 2-oxoglutarate suffered a much stronger decline than in the wild type and in the fumA mutant. In the pycB mutant, the pyruvate pool was only transiently elevated during the trophophase, but not in the idiophase. In the fumA mutant, the fumarate pool was dramatically increased, whereas the pools of succinate, malate, and 2-oxoglutarate were mildly elevated by comparison with the wild type and the pycB mutant (Table 3). Fumarate, succinate, and malate were detectable in the culture supernatant of the fumA mutant but not in the wild type and the pycB mutant (data not shown).
TABLE 3.
Intracellular concentrations of central metabolites determined in cell extracts of P. fluorescens strains CHA0, CHA1322 (fumA−), and CHA1201 (pycB−) during exponential growth (trophophase) and postexponential growth phase (idiophase).
| Metabolite | Phasea | Concentration (μmol/g) |
||
|---|---|---|---|---|
| Wild type | fumA− | pycB− | ||
| μ mol/g | ||||
| Hexose-P | T | 5.4 ± 1.0 | 4.6 ± 0.3 | 4.2 ± 0.3 |
| I | 2.0 ± 0.1 | 2.5 ± 0.5 | 1.7 ± 0.1 | |
| 3-Phosphoglycerate | T | 1.5 ± 0.3 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| I | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.5 | |
| Phosphoenolpyruvate | T | 1.5 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.1 |
| I | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | |
| Pyruvate | T | 9.2 ± 4.9 | 5.7 ± 0.4 | 20b ± 1 |
| I | 2.1 ± 0.2 | 4.5 ± 0.5 | 1.8 ± 0.4 | |
| Citrate + isocitratec | T | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 |
| I | 1.6 ± 0.5 | 1.9 ± 0.3 | 0.8 ± 0.2 | |
| Succinate | T | 7.1 ± 4.4 | 29b ± 5 | 6.4 ± 3.0 |
| I | 4.1 ± 3.3 | 12b ± 3 | 0.9 ± 0.4 | |
| Fumarate | T | 0.4 ± 0.05 | 140b ± 7 | 0.5 ± 0.05 |
| I | 0.2 ± 0.05 | 62b ± 16 | 0.2 ± 0.05 | |
| Malate | T | 0.1 ± 0.05 | 0.3 ± 0.05 | 0.3 ± 0.05 |
| I | <0.1 ± 0.05 | 0.2 ± 0.05 | <0.05 ± 0.05 | |
| 2-Oxoglutarate | T | 2.3 ± 0.1 | 10 ± 0.6 | 1.8 ± 0.05 |
| I | 0.4 ± 0.05 | 0.5 ± 0.1 | 0.1 ± 0.05 | |
a T, trophophase; I, idiophase.
b Values outside linear range.
c Metabolites not separated.
In summary, a direct comparison between the fumA and the pycB mutant in the idiophase reveals the following salient features. A ratio of 15:1 was seen for rsmZ-gfp expression (supplemental Fig. S3), and this was mirrored by ratios of more than 100:1 for fumarate, ∼10:1 for succinate, and ∼5:1 for 2-oxoglutarate and malate (Table 3). It thus appears that the relative pool sizes of fumarate and, to a lesser extent, succinate, 2-oxoglutarate, and malate correlate with RsmZ expression. Whether the pool sizes of these metabolites are a direct cause of differential RsmX/Y/Z expression or simply effects of a metabolic imbalance in the mutants cannot be deduced from this experiment.
Role of the Sensor Kinases RetS, LadS, and GacS
In P. fluorescens and P. aeruginosa, the accessory sensors RetS and LadS have negative and positive effects, respectively, on the GacS sensor (14, 30, 52). Krebs cycle intermediates might conceivably interact with RetS and LadS. However, in both retS and ladS mutant backgrounds, a fumA mutation still positively influenced rsmZ-lacZ expression, and a pycA mutation still negatively affected rsmZ-lacZ expression (supplemental Fig. S4). It is therefore unlikely that RetS and LadS sense Krebs cycle intermediates. In Salmonella enterica, mutational loss of the BarA (GacS) sensor can be compensated by high concentrations of acetate in the growth medium, and it has been hypothesized that acetylphosphate derived from acetate might directly phosphorylate the SirA (GacA) response regulator (53). However, in a P. fluorescens gacS mutant, introduction of a fumA mutation did not elevate the low level of rsmZ-lacZ expression (data not shown), suggesting that there is no compensating bypass activation of GacA by tricarboxylic acid compounds. Moreover, we checked that the fumA and pycB mutations had no effects on the expression of the gacS and gacA genes, as measured with translational ′lacZ fusions (data not shown).
Regulation of sRNA Gene Expression by Krebs Cycle Intermediates
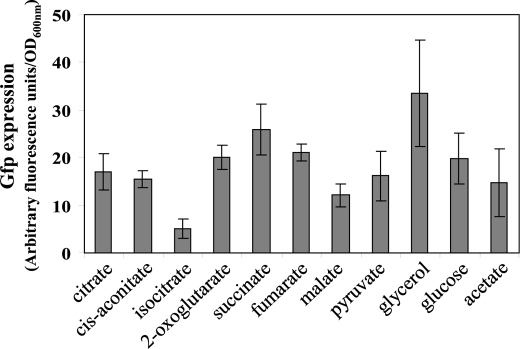
P. fluorescens CHA0, like other fluorescent pseudomonads, grows on most Krebs cycle intermediates as the only carbon source. (An exception is oxaloacetate, which was a poor growth substrate.) We reasoned that Krebs cycle intermediates, when used as the sole organic substrate, should result in elevated intracellular pools. However, it proved difficult to follow growth in liquid minimal media because of strong cell clumping. For this reason, we measured rsmZ-gfp expression (in arbitrary fluorescence units per A600 nm units) in strain CHA0 grown on solid media (Fig. 7). Expression was highest on fumarate, succinate, 2-oxoglutarate, glucose, or glycerol. Interestingly, expression was lowest on isocitrate. Note that in the metabolic analysis described above, we were unable to gauge the impact of isocitrate, because this metabolite was not separated from citrate. Considering the data of Fig. 7, we infer that a high fumarate + succinate + 2-oxoglutarate to isocitrate ratio favors the expression of GacA-dependent sRNAs.
FIGURE 7.
Effect of Krebs cycle intermediates and other carbon sources on rsmZ-gfp expression. Strain CHA0/pME7402 was streaked on solid minimal media containing each compound as the sole carbon source. Concentrations were as follows: citrate, 10 mm; cis-aconitate, 10 mm; isocitrate, 10 mm; 2-oxoglutarate, 12 mm; succinate, 15 mm; fumarate, 15 mm; malate, 15 mm; glycerol, 20 mm; glucose, 10 mm; pyruvate, 20 mm; acetate, 30 mm. The pH was adjusted to pH 6.8 with NaOH, and agarose was added at a concentration of 1.0%. Two days after inoculation, colonies on each plate were scraped and suspended in 0.9% NaCl. The fluorescence (excitation at 480 nm and emission at 520 nm) and the optical density at 600 nm were measured with a Fluostar fluorescence microplate reader (BMG Lab Technologies).
DISCUSSION
Our present study shows that an imbalance in the Krebs cycle can strongly change the expression of GacA-dependent sRNAs in P. fluorescens and hence an ecologically relevant development (i.e. the production of secondary metabolites and biocontrol factors). There is an interesting precedent for this finding. In B. subtilis, a major developmental process, sporulation, depends on the function of the Krebs cycle as well. In this case Krebs cycle intermediates and/or enzymes provide a signal for the phosphorylation of the transcription factor Spo0A, which is the master regulator of sporulation. Strong evidence comes from a triple mutant blocked in citrate synthase, aconitase, and isocitrate dehydrogenase; this mutant sporulates very poorly and lacks Spo0A∼P-mediated functions (54). Furthermore, there is evidence that citrate synthase function may be involved, indirectly, in morphological differentiation and antibiotic biosynthesis of S. coelicolor, although the mechanism by which this effect occurs is not clear (55) and that Krebs cycle function regulates adhesion and virulence factor expression in Staphylococcus aureus (56). Our previous finding that thiamine limitation leads to down-regulation of the Gac/Rsm pathway in P. fluorescens CHA0 may also be due to an underlying metabolic deficiency in the Krebs cycle (21). Thiamine is necessary for pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase activities. Conceivably, the pools of Krebs cycle intermediates, such as citrate, succinate, or fumarate might be negatively affected when thiamine is limiting.
In P. aeruginosa, mutations in Krebs cycle enzymes or in enzymes replenishing the Krebs cycle can strongly affect the expression of the type III secretion system (TTSS). Pyruvate dehydrogenase (aceAB) mutants have lost TTSS function and are non-virulent in a rat model of acute pneumonia (57). A mutation that massively up-regulates histidine utilization enzymes, resulting presumably in elevated glutamate and 2-oxoglutarate pools, also shuts down the TTSS (58). By contrast, P. aeruginosa cells overexpress the TTSS when two citrate synthase isoenzymes (gltA, prpC) are blocked by mutation (59). Acetate, which can be excreted by P. aeruginosa at the end of growth under oxygen limitation (60), does not appear to act as a metabolic signal here, because the enzymes involved in the utilization of acetate via acetyl-CoA are not involved in this regulation of the TTSS (59). It is known that RsmA is a positive regulator and that GacA is a negative regulator of the TTSS (61, 62). Thus, in P. aeruginosa, the Gac/Rsm system has inverse effects on the TTSS, on the one hand, and on the production of secondary metabolites, biofilm polysaccharides, and toxic exoenzymes, on the other hand (63). Given the high degree of conservation of the Gac/Rsm pathway in P. aeruginosa and P. fluorescens (10), it is tempting to speculate that in P. aeruginosa the observed Krebs cycle-dependent control of TTSS expression (57, 59) could be mediated by RsmY and RsmZ. A reciprocal experiment cannot, unfortunately, be carried out in P. fluorescens, because strain Pf-5 of this species lacks a TTSS (15), and most probably strain CHA0 also does.
In Legionella pneumophila, there is evidence that the intracellular alarmone ppGpp influences the activity of the LetS/LetA two-component system, which is homologous to the GacS/GacA system. Stress conditions, carbon source depletion, and amino acid starvation can trigger the synthesis of ppGpp, via the SpoT and RelA enzymes (64). It is unlikely that under our experimental conditions ppGpp would play a major role in the regulation of secondary metabolism of P. fluorescens, because nutrients were not depleted during the idiophase, not even in the dilute medium used for the metabolic analysis (supplemental Fig. S3). Moreover, we did not find any relA or spoT mutants in our screens for dim mutants, although arguably our Tn5 mutagenesis was not saturating. The question of whether Krebs cycle intermediates might modulate the synthesis of the extracellular signal(s) activating GacS cannot be answered at present, because there is no precise biochemical assay for the signal(s), and signal biosynthetic genes have not yet been identified. The energy status of the P. fluorescens cells was not substantially compromised during the idiophase. This can be seen from the fact that the pool sizes of key metabolites, such as hexose-phosphates, 3-phosphoglycerate, and phosphoenolpyruvate, were only about 2-fold lower in the idiophase than in the trophophase (Table 3), whereas the induction of the rsmX/Y/Z genes was much more pronounced during this transition (Fig. 2).
From a biocontrol point of view, it is interesting to note that major root exudate components, such as succinate and fumarate, were among those carbon sources that favored rsmZ expression (Fig. 7). These carbon sources are also preferred growth substrates of fluorescent pseudomonads in general. Including these inexpensive carbon sources in the growth media and formulation mixtures of fluorescent biocontrol pseudomonads may be of practical interest for achieving optimal suppression of root diseases, and such a strategy should be preferable to genetic manipulation of the biocontrol bacteria. Although it was possible to boost the expression of the GacA-dependent sRNAs and antibiotic secondary metabolites under in vitro conditions by introducing a fumA mutation into strain CHA0, this did not result in improved biocontrol efficacy under natural soil conditions (Table 2). This was not surprising, because we had previously found that a constitutively active form of GacS led to overproduction of antifungal secondary metabolites in vitro but not to better biocontrol of Fusarium crown and root rot of tomato (42).
In conclusion, our study on P. fluorescens CHA0 and P. aeruginosa PAO shows that a regulatory link exists between primary and secondary metabolism, via Krebs cycle function and GacA-dependent sRNAs. This regulation does not involve the accessory sensors RetS and LadS but requires functional GacS and GacA. On the one hand, our findings open up new perspectives in the understanding of biocontrol mechanisms of P. fluorescens and in the application of fluorescent pseudomonads as biocontrol agents. On the other hand, our results also shed new light on the observed link between Krebs cycle function and pathogenicity in P. aeruginosa (59).
Acknowledgments
We thank Bérénice Humair for construction of chromosomal reporter strains, Fiona Carruthers for supplying pME3266, Elisabeth Sonnleitner for help with RNA manipulation, Philipp Christen for help with metabolic analysis, Maria Péchy-Tarr for advice on antibiotic assays, Chris Affolter for performing preliminary experiments on mutant isolation in P. aeruginosa, and Benvinda Lopez for sequencing Tn5 insertions.
This work was supported by Swiss National Foundation for Scientific Research Project 3100A0-100180, the Japan Society for the Promotion of Science-Swiss National Foundation for Scientific Research Exchange Program, and an Organisation for Economic Co-operation and Development fellowship (to T. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S4.
- sRNA
- small RNA
- GCM
- glycerol-casamino acids medium
- LC-HRMS
- liquid chromatography high resolution MS
- NYB
- nutrient yeast broth
- TTSS
- type III secretion system
- GFP
- green fluorescent protein.
REFERENCES
- 1.Bibb M. J. (2005) Curr. Opin. Microbiol. 8, 208–215 [DOI] [PubMed] [Google Scholar]
- 2.Yu J. H., Keller N. (2005) Annu. Rev. Phytopathol. 43, 437–458 [DOI] [PubMed] [Google Scholar]
- 3.Vining L. C. (1990) Annu. Rev. Microbiol. 44, 395–427 [DOI] [PubMed] [Google Scholar]
- 4.Haas D., Keel C. (2003) Annu. Rev. Phytopathol. 41, 117–153 [DOI] [PubMed] [Google Scholar]
- 5.Demain A. (1986) Pure Appl. Chem. 58, 219–226 [Google Scholar]
- 6.Dubuis C., Keel C., Haas D. (2007) Eur. J. Plant Pathol. 119, 311–328 [Google Scholar]
- 7.Rahme L. G., Ausubel F. M., Cao H., Drenkard E., Goumnerov B. C., Lau G. W., Mahajan-Miklos S., Plotnikova J., Tan M. W., Tsongalis J., Walendziewicz C. L., Tompkins R. G. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8815–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeb S., Haas D. (2001) Mol. Plant-Microbe Interact. 14, 1351–1363 [DOI] [PubMed] [Google Scholar]
- 9.Haas D., Défago G. (2005) Nat. Rev. Microbiol. 3, 307–319 [DOI] [PubMed] [Google Scholar]
- 10.Lapouge K., Schubert M., Allain F. H., Haas D. (2008) Mol. Microbiol. 67, 241–253 [DOI] [PubMed] [Google Scholar]
- 11.Heeb S., Blumer C., Haas D. (2002) J. Bacteriol. 184, 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay E., Dubuis C., Haas D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17136–17141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubuis C., Haas D. (2007) Appl. Environ. Microbiol. 73, 650–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman A. L., Merighi M., Hyodo M., Ventre I., Filloux A., Lory S. (2009) Genes Dev. 23, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulsen I. T., Press C. M., Ravel J., Kobayashi D. Y., Myers G. S., Mavrodi D. V., DeBoy R. T., Seshadri R., Ren Q., Madupu R., Dodson R. J., Durkin A. S., Brinkac L. M., Daugherty S. C., Sullivan S. A., Rosovitz M. J., Gwinn M. L., Zhou L., Schneider D. J., Cartinhour S. W., Nelson W. C., Weidman J., Watkins K., Tran K., Khouri H., Pierson E. A., Pierson L. S., 3rd, Thomashow L. S., Loper J. E. (2005) Nat. Biotechnol. 23, 873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valverde C., Heeb S., Keel C., Haas D. (2003) Mol. Microbiol. 50, 1361–1379 [DOI] [PubMed] [Google Scholar]
- 17.Reimmann C., Valverde C., Kay E., Haas D. (2005) J. Bacteriol. 187, 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay E., Humair B., Dénervaud V., Riedel K., Spahr S., Eberl L., Valverde C., Haas D. (2006) J. Bacteriol. 188, 6026–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voisard C., Keel C., Haas D., Dèfago G. (1989) EMBO J. 8, 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnider-Keel U., Seematter A., Maurhofer M., Blumer C., Duffy B., Gigot-Bonnefoy C., Reimmann C., Notz R., Défago G., Haas D., Keel C. (2000) J. Bacteriol. 182, 1215–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubuis C., Rolli J., Lutz M., Défago G., Haas D. (2006) Appl. Environ. Microbiol. 72, 2606–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmi R., Carmeli S., Levy E., Gough F. J. (1994) J. Nat. Prod. 57, 1200–1205 [DOI] [PubMed] [Google Scholar]
- 23.Maurhofer M., Reimmann C., Schmidli-Sacherer P., Heeb S., Haas D., Défago G. (1998) Phytopathology 88, 678–684 [DOI] [PubMed] [Google Scholar]
- 24.Ornston L. N., Stanier R. Y. (1966) J. Biol. Chem. 241, 3776–3786 [PubMed] [Google Scholar]
- 25.Gamper M., Ganter B., Polito M. R., Haas D. (1992) J. Mol. Biol. 226, 943–957 [DOI] [PubMed] [Google Scholar]
- 26.O'Toole G. A., Kolter R. (1998) Mol. Microbiol. 28, 449–461 [DOI] [PubMed] [Google Scholar]
- 27.Fox A., Haas D., Reimmann C., Heeb S., Filloux A., Voulhoux R. (2008) Appl. Environ. Microbiol. 74, 1902–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambertsen L., Sternberg C., Molin S. (2004) Environ. Microbiol. 6, 726–732 [DOI] [PubMed] [Google Scholar]
- 29.Fellay R., Frey J., Krisch H. (1987) Gene 52, 147–154 [DOI] [PubMed] [Google Scholar]
- 30.Humair B., González N., Mossialos D., Reimmann C., Haas D. (2009) ISME J. 3, 955–965 [DOI] [PubMed] [Google Scholar]
- 31.Leoni L., Ciervo A., Orsi N., Visca P. (1996) J. Bacteriol. 178, 2299–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González N., Heeb S., Valverde C., Kay E., Reimmann C., Junier T., Haas D. (2008) BMC Genomics 9, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J. H. (1972) Experiments in Molecular Genetics, pp. 352–355, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 34.Hill R. L., Bradshaw R. H. (1969) Methods Enzymol. 13, 91–99 [Google Scholar]
- 35.Wu L., Mashego M. R., van Dam J. C., Proell A. M., Vinke J. L., Ras C., van Winden W. A., van Gulik W. M., Heijnen J. J. (2005) Anal. Biochem. 336, 164–171 [DOI] [PubMed] [Google Scholar]
- 36.Kiefer P., Portais J. C., Vorholt J. A. (2008) Anal. Biochem. 382, 94–100 [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38.Miller V. L., Mekalanos J. J. (1988) J. Bacteriol. 170, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stutz E. W., Défago G., Kern H. (1986) Phytopathology 76, 181–185 [Google Scholar]
- 40.Zuber S., Carruthers F., Keel C., Mattart A., Blumer C., Pessi G., Gigot-Bonnefoy C., Schnider-Keel U., Heeb S., Reimmann C., Haas D. (2003) Mol. Plant-Microbe Interact. 16, 634–644 [DOI] [PubMed] [Google Scholar]
- 41.Laville J., Voisard C., Keel C., Maurhofer M., Défago G., Haas D. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 1562–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voisard C., Rella M., Haas D. (1988) FEMS Microbiol. Lett. 55, 9–14 [Google Scholar]
- 43.Voisard C., Bull C. T., Keel C., Laville J., Maurhofer M., Schnider U., Défago G., Haas D. (1994) in Molecular Ecology of Rhizosphere Microorganisms (O'Gara F., Dowling D. N., Boesten B. eds) pp. 67–89, VCH Weinheim, Germany [Google Scholar]
- 44.Heeb S., Itoh Y., Nishijyo T., Schnider U., Keel C., Wade J., Walsh U., O'Gara F., Haas D. (2000) Mol. Plant-Microbe Interact. 13, 232–237 [DOI] [PubMed] [Google Scholar]
- 45.Blumer C., Heeb S., Pessi G., Haas D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 14073–14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller W. G., Leveau J. H., Lindow S. E. (2000) Mol. Plant-Microbe Interact. 13, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 47.Bao Y., Lies D. P., Fu H., Roberts G. P. (1991) Gene 109, 167–168 [DOI] [PubMed] [Google Scholar]
- 48.Lai H., Kraszewski J. L., Purwantini E., Mukhopadhyay B. (2006) Appl. Environ. Microbiol. 72, 7785–7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phibbs P. V., Jr., Feary T. W., Blevins W. T. (1974) J. Bacteriol. 118, 999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siddiqui I. A., Haas D., Heeb S. (2005) Appl. Environ. Microbiol. 71, 5646–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamilova F., Kravchenko L. V., Shaposhnikov A. I., Makarova N., Lugtenberg B. (2006) Mol. Plant-Microbe Interact. 19, 1121–1126 [DOI] [PubMed] [Google Scholar]
- 52.Workentine M. L., Chang L., Ceri H., Turner R. J. (2009) FEMS Microbiol. Lett. 292, 50–56 [DOI] [PubMed] [Google Scholar]
- 53.Lawhon S. D., Maurer R., Suyemoto M., Altier C. (2002) Mol. Microbiol. 46, 1451–1464 [DOI] [PubMed] [Google Scholar]
- 54.Ireton K., Jin S., Grossman A. D., Sonenshein A. L. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2845–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viollier P. H., Minas W., Dale G. E., Folcher M., Thompson C. J. (2001) J. Bacteriol. 183, 3184–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somerville G. A., Proctor R. A. (2009) Microbiol. Mol. Biol. Rev. 73, 233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dacheux D., Epaulard O., de Groot A., Guery B., Leberre R., Attree I., Polack B., Toussaint B. (2002) Infect. Immun. 70, 3973–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rietsch A., Wolfgang M. C., Mekalanos J. J. (2004) Infect. Immun. 72, 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rietsch A., Mekalanos J. J. (2006) Mol. Microbiol. 59, 807–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eschbach M., Schreiber K., Trunk K., Buer J., Jahn D., Schobert M. (2004) J. Bacteriol. 186, 4596–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulcahy H., O'Callaghan J., O'Grady E. P., Adams C., O'Gara F. (2006) Infect. Immun. 74, 3012–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soscia C., Hachani A., Bernadac A., Filloux A., Bleves S. (2007) J. Bacteriol. 189, 3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toledo-Arana A., Repoila F., Cossart P. (2007) Curr. Opin. Microbiol. 10, 182–188 [DOI] [PubMed] [Google Scholar]
- 64.Molofsky A. B., Swanson M. S. (2004) Mol. Microbiol. 53, 29–40 [DOI] [PubMed] [Google Scholar]