Summary
In Drosophila, the humoral response characterised by the synthesis of antimicrobial peptides (AMPs) in the fat body (the equivalent of the mammalian liver) and the cellular response mediated by haemocytes (blood cells) engaged in phagocytosis represent two major reactions that counter pathogens. Although considerable analysis has permitted the elucidation of mechanisms pertaining to the two responses individually, the mechanism of their coordination has been unclear. To characterise the signals with which infection might be communicated between blood cells and fat body, we ablated circulating haemocytes and defined the parameters of AMP gene activation in larvae. We found that targeted ablation of blood cells influenced the levels of AMP gene expression in the fat body following both septic injury and oral infection. Expression of the AMP gene drosomycin (a Toll target) was blocked when expression of the Toll ligand Spätzle was knocked down in haemocytes. These results show that in larvae, integration of the two responses in a systemic reaction depend on the production of a cytokine (spz), a process that strongly parallels the mammalian immune response.
Keywords: Blood cells, Innate immunity, Drosophila, Toll, Spätzle
Introduction
Insect and mammalian responses to infection share many common features. In humans, innate immunity serves as the first defence line protecting the organism from bacteria, fungi, viruses or parasites. Recognition by the host is directed against non-self determinants that are invariant among various microorganisms (Janeway and Medzhitov, 2002). This phylogenetically ancient safeguard mechanism is mediated by activation of a diverse set of receptors found primarily on monocytes and macrophages, granulocytic and dendritic cells. Following pathogen phagocytosis and destruction, small non-self peptides are displayed on the surfaces of antigen-presenting macrophages and dendritic cells. In addition to triggering adaptive immunity through antigen presentation, macrophage activation initiates an acute phase response characterised by the release of inflammatory cytokines and chemotactic factors that recruit and stimulate immune cells (Janeway and Medzhitov, 2002). Insects have developed efficient host defence mechanisms against microorganisms based primarily on responses of an innate nature. These include phagocytosis and the rapid transient synthesis of potent antimicrobial peptides (AMPs).
In recent years, Drosophila has been used as a model system to address many of the questions pertaining to insect immunity and its parallels to mammalian host defence (reviewed by Wang and Ligoxygakis, 2006; Lemaitre and Hoffmann, 2007). Extensive genetic analysis has shown that production of AMPs – the hallmark of the systemic response – is controlled by the Toll and the IMD (for immune deficiency) signalling pathways. The former is activated primarily but not exclusively following fungal or Gram-positive bacterial infection, whereas the latter is mostly triggered during Gram-negative challenge (Wang and Ligoxygakis, 2006; Lemaitre and Hoffmann, 2007). The crucial feature of both pathways is that their intracellular signalling cascades culminate in the nuclear translocation of fly homologues of the NF-κB transcriptional regulator (reviewed by Wang and Ligoxygakis, 2006; Lemaitre and Hoffmann, 2007). Toll relays a signal resulting in the degradation of the IκB homologue Cactus, whereby the NF-κB homologue Dif is free to move into the nucleus. One of the AMP gene targets of the pathway is drosomycin, widely used as a read-out for Toll activity. The IMD cascade regulates the proteolytic cleavage and activation of Relish, the N-terminal part of which translocates into the nucleus and directs the expression of its transcriptional targets, among which is the AMP gene diptericin. A significant difference in the activation of the two pathways is the fact that Toll signalling is triggered by an endogenous cytokine-like ligand (Spätzle) (Weber et al., 2003) whereas Imd, is activated through the direct recognition of bacterial peptidoglycan by peptidoglycan recognition protein-LC (PGRP-LC) (Kaneko et al., 2004).
Drosophila blood cells (haemocytes) mainly function as phagocytes in both apoptosis and immunity and arise from a lineage strikingly similar to their mammalian counterparts (reviewed by Crozatier and Meister, 2007; Meister, 2004; Evans et al., 2003). By analogy with the mammalian system, circulating haemocytes activated by an invading agent engage in phagocytosis and might stimulate the fat body, which along with oenocytes performs the functions of the liver (Agaisse et al., 2003; Brennan et al., 2007; Gutierrez et al., 2007). According to this hypothesis, priming of the fat body results in the NF-κB-dependent synthesis of antibacterial and antifungal AMPs (see also Mathey-Prevost and Perrimon, 1998). However, data to support such a model and to unravel the mechanism of such priming have been conflicting.
Relevant studies have indicated the existence of a cross-talk between haemocytes and fat body. Upd3, a ligand upstream of the JAK-STAT pathway, was shown to be required in haemocytes for STAT-dependent transcription in the fat body of adult flies after septic injury (Agaisse et al., 2003). In larvae, haemocytes have been found to be essential intermediaries for the epithelia-generated nitric oxide signal to reach the fat body and induce Relish-dependent gene regulation (Foley and O'Farrell, 2003; Dijkers and O'Farrell, 2007). By contrast, an earlier paper had alluded to the fact that in larvae mutant for domino, in which circulating blood cells were largely missing, the AMP response was similar to wild-type larvae after infection by septic injury (Braun et al., 1998). Of note, however, is the observation that these larvae failed to induce diptericin following oral infection by Erwinia carotovora ingestion (Basset et al., 2000).
Here, we have specifically targeted the ablation of blood cells in larvae using the GAL4-UAS system (Brand and Perrimon, 1993). In this ablated background we first assayed for levels of AMP gene expression and bacterial persistence following infection by septic injury or ingestion of bacteria, and then for live AMP gene expression in the fat body tissue (using drs-GFP) under the same conditions. We found that when circulating haemocytes were ablated, bacteria were able to grow unrestrictedly inside the larval body cavity. Moreover, AMP expression in the fat body was not induced properly in the presence of bacteria. We determined one of the signals that are released by haemocytes and required for a wild-type response. We found that production by the haemocytes of the Toll agonist Spätzle, a cytokine-like polypeptide, is required for AMP induction in the fat body. These results indicate that haemocytes contribute to the innate immune response in Drosophila larvae not only with their phagocytic capabilities, but also by orchestrating a cytokine-based regulatory signal in a situation reminiscent of that observed in mammals.
Results
Targeted cell ablation of Drosophila larval haemocytes
We used the GAL4-UAS system (Brand and Perrimon, 1993) to achieve the targeted cell-specific ablation of haemocytes. We used three haemocyte-expressing GAL4 lines, namely hemolectin-GAL4 (hml-GAL4), peroxidasin-GAL4 (pxn-GAL4) and serpentD-GAL4 (srpD-GAL4) (Goto et al., 2003; Galko and Krasnow, 2004; Crozatier et al., 2004). The first is a post-embryonic promoter, whereas the two others are expressed from mid-embryogenesis throughout development (Goto et al., 2003; Galko and Krasnow, 2004; Crozatier et al., 2004) (our unpublished observations). We found that expression of pxn-GAL4 was variable, presenting activity in the fat body as well as in haemocytes (data not shown). We therefore selected pxn-GAL4 lines that expressed the transgene only in blood cells (see Materials and Methods).
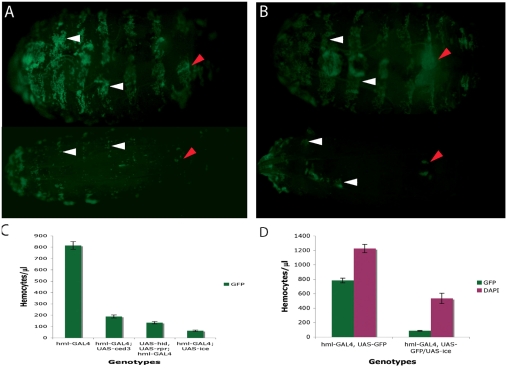
The above-mentioned GAL4 drivers were used to direct expression of pro-apoptotic genes, i.e. UAS-ced3, UAS-ice or both UAS-hid and UAS-reaper (Shigenaga et al., 1997; Zhou et al., 1997) in blood cells. At least two independent insertions were used for each UAS construct to account for position effect variegation (Figs 1 and 2, and data not shown). Haemocytes were visualised by the simultaneous presence of a UAS-GFP transgene. Fig. 1 presents the results comparing UAS-GFP expression between wild-type and ablated larvae (compare Fig. 1A top and bottom panels for hmlGAL4 with Fig. 1B top and bottom panels for pxnGAL4). We performed blood-count measurements (number of blood cells per μl) of circulating haemocytes taken from affected and control animals (see Materials and Methods). Fig. 1C shows the loss of GFP expression (and hence absence of GFP-positive haemocytes) in the different UAS pro-apoptotic lines used. As there was variability in ablation, for subsequent experiments we chose to use the most effective UAS lines, namely ice or the combination of hid, rpr (Fig. 1C).
Fig. 1.
Ablation of haemocytes using the GAL4-UAS system. (A) hmlGAl4 and (B) pxnGAL4 were crossed to a UAS line expressing the human apoptotic gene ICE. The cross was hml-GAL4, UAS-GFP or pxn-GAL4, UAS-GFP × TM6B/UAS-ICE (hml-GAL4, UAS-GFP; UAS-ICE /+ or pxn-GAL4, UAS-GFP; UAS-ICE /+, bottom panels of A and B, respectively). Larvae in top panels are the hmlGAL4, UAS-GFP; TM6B/+ and pxnGAL4, UAS-GFP; TM6B/+ siblings of the ablated larvae, hence their Tubby appearance. Visualisation of ablation through a UAS-GFP transgene showed that circulating blood cells and a large number of sessile haemocytes were eliminated (compare top and bottom panels in both A and B). However, some groups of sessile haemocytes were resistant (white arrowheads). The larval haematopoietic organ (red arrowheads) was not deleted but was severely reduced. (C) Quantification of circulating haemocytes marked with GPF in hmlGAL4, UAS-GFP larvae after expression of C elegans ced-3, Drosophila hid and reaper (hid, rpr) and human ICE measured by the number of cells/μl of blood. The hmlGAL4, UAS-GFP strain (without any of the pro-apoptotic gene UAS constructs) was used as the wild-type control (hml). Six independent experiments were performed. The graph represents the mean values ± s.d. Similar results were obtained with pxnGAL4 and srpD-GAL4 (not shown). (D) Comparison between the total number of circulating haemocytes (DAPI) and those labelled by GFP in hmlGAL4, UAS-GFP larvae (left column) or hmlGAL4, UAS-GFP; UAS-ICE larvae (right column). Approximately 59% of circulating haemocytes were deleted by ablation.
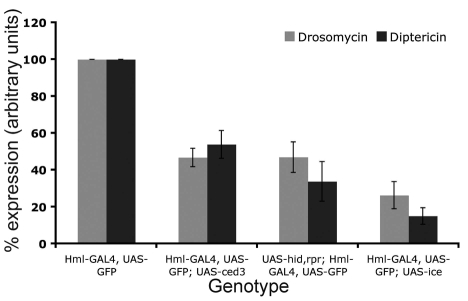
Fig. 2.
Reduced levels of AMP production by the fat body in haemocyte-ablated larvae. Ablated larvae were infected with Gram-positive bacteria (M. luteus; drosomycin expression used as a read-out for the response and assayed 12 hours post-infection) or Gram-negative bacteria (E. coli; diptericin expression used as a read-out for the response and assayed 6 hours post-infection). AMP expression levels were assayed by northern blot and quantified. Four independent experiments were performed. Mean values ± s.d. are presented. The Pearson correlation test was used to establish whether reduction in AMP expression levels correlated well with the extent of haemocyte ablation (compare Fig. 2 with Fig. 1C). The test found a strong correlation between level of ablation and reduction of drs (P=0.03, r=0.87) or dipt (P=0.02, r=0.88). This reduction was not due to the reduced number of haemocytes producing less AMP because, after quantification in dissected tissue, we found that the fat body accounted for 70% of all AMP gene expression (see Table 1).
In the drivers used, GAL4 was expressed in a portion of blood cells (Goto et al., 2003; Galko and Krasnow, 2004) (our unpublished observations). To measure the extent of ablation against the whole population of circulating haemocytes, we independently stained the entire population of circulating haemocytes with DAPI and compared the number of stained blood cells to the population of haemocytes marked by UAS-GFP driven by each GAL4 line. The results (shown in Fig. 1D) indicated a positive correlation between absence of GFP and ablation without influencing the DAPI-only positive population. In control larvae, UAS-GFP driven by hml-GAL4 represented 66% of the DAPI population (on average 800 out of 1200 haemocytes/μl). The ablation reduced this to 7% (on average 50 out of 560 haemocytes/μl), showing that 59% of circulating haemocytes were removed (Fig. 1D). Similar results were obtained with pxn-GAL4, where the total number of blood cells was reduced by 60% when we used UAS-ice (data not shown). Using an antibody against Croquemort (Crq), a haemocyte-specific marker (Franc et al., 1996), we confirmed in wild-type larvae that we were indeed counting blood cells with DAPI (data not shown).
Use of the srpD-GAL4 driver led to a high lethality rate during early larval development. These larvae were devoid of haemocytes and rarely reached the third instar larval stage (data not shown). To test AMP expression following infection, we therefore chose to use the hml-GAL4 and pxn-GAL4 drivers to obtain viable larvae with a severely reduced number of circulating haemocytes.
The paired lobes of the larval haematopoietic organ (lymph gland) in hml-GAL4 or pxn-GAL4 ablated animals were not absent. However, in larvae showing the highest extent of haemocyte ablation (pxn-GAL4, UAS-GFP; UAS-ice and hml-GAL4, UAS-GFP; UAS-ice) these organs were reduced in size (compare lymph glands in upper and lower panels of Fig 1A,B, indicated by red arrowheads). It has been previously postulated that all larval haemocytes are derived from the embryonic anlage (Tepass et al., 1994), whereas lymph gland haemocytes are released at the onset of pupariation following induction by ecdysone (Holtz et al., 2003; reviewed by Crozatier and Meister, 2007; Meister, 2004; Evans et al., 2003). By attempting to ablate both groups of haemocytes (pxn-GAL4) or just post-embryonic haemocytes (hml-GAL4), we observed extensive pupal lethality with pxn-GAL4, UAS-GFP; UAS-ice and hml-GAL4, UAS-GFP; UAS-ice (supplementary material Table S1). Larvae with severely reduced haemocyte numbers were unable to complete metamorphosis. Lethality involving ablation of post-embryonic haemocytes (hml-GAL4) was rescued if larvae were raised in axenic conditions (supplementary material Table S1). However, lethality linked to both embryonic and post-embryonic haemocytes (pxn-GAL4) could not be rescued in such fashion (not shown) revealing a role for blood cells in embryonic development, as recently reported (Defaye et al., 2009). Nevertheless, duration of the three larval stages was not altered in either axenically or conventionally reared animals (data not shown) (Charroux and Royet, 2009). Moreover, no melanotic tumours were observed in response to the induction of apoptosis in blood cells (not shown) (Charroux and Royet, 2009).
Taken together, the above data showed that by using hml-GAL4 to ablate haemocytes there was minimal developmental interference with the immune phenotypes described below. Finally, flies with a reduced number of haemocytes showed susceptibility to bacterial challenge by septic injury (supplementary material Fig. S1C,D), in agreement with recently published results (Defaye et al., 2009; Charroux and Royet, 2009).
Levels of AMP gene expression in ablated larvae
We wanted to determine whether a significant reduction in circulating blood cell number would influence AMP gene expression following infection. We monitored in whole larvae the expression of the AMP gene drosomycin (drs; produced primarily following Gram-positive bacterial challenge) and diptericin (dipt; strongly induced during Gram-negative bacterial infection). Fig. 2 shows the positive correlation between the level of ablation of haemocytes and the observed reduction in drs and dipt (abbreviated hereafter as dipt/drs) gene activation after immune challenge. The lines with the most severe haemocyte reduction showed the most dramatic effect (compare Fig. 1C to Fig. 2).
However, it was conceivable that the correlation between loss of haemocytes and decrease in dipt/drs levels presented in Fig. 2 could be simply because the haemocytes that produced them were extinct. If that were the case, the extent of ablation could still correlate with reduction in production of Dipt and Drs. To confirm the amount of dipt/drs expressed by the constituent parts of larvae, wandering third instar larvae from wild type and the most severely ablated genotype (hml-GAL4; UAS-ice) were dissected 6 hours post-infection for assaying dipt expression and 10 hours post-infection for assaying drs expression. All larval tissues were separated as carefully as possible (see Materials and Methods) to assay for dipt/drs expression. Tissues were divided into three main constituents: the fat body, haemolymph (including circulating haemocytes) and the carcass (remainder of the dissected larva, including gut, trachea, outer cuticle, brain and mouth parts).
Table 1 shows the proportion of dipt/drs expression in haemocytes or fat body in relation to the whole animal. We observed that following infection approximately 70% of expression was linked to the fat body, confirming previous reports that considered this tissue to be the major AMP source (Reichhart et al., 1992; Irving et al., 2005). Our findings indicated that in larvae only 15-25% of dipt/drs total expression was linked to haemocytes. It would therefore be difficult to explain the severe reduction of dipt/drs levels observed in haemocyte-depleted larvae (more than 60% compared to wild type in the most ablated lines; see Fig. 2) on the basis of haemocytes being just a source of AMP production. If this were the case, haemocyte loss would account for only 15-25% of dipt/drs reduction in the whole animal. By contrast, our results indicated that genetic ablation of blood cells influenced the major constituent of dipt/drs induction, which was fat body-derived.
Table 1.
Percentage contribution of different tissues to AMP gene expression following infection in wild-type (hmlGAL4) or ablated larvae (hmlGAL4-UAS-GFP; UASice2-2)
|
Fat body
|
Haemolymph
|
Carcass
|
||||
|---|---|---|---|---|---|---|
| Genotype | % | ± | % | ± | % | ± |
| Drosomycin | ||||||
| Infected hmlGAL4 | 69.0 | 10.0 | 24.0 | 5.6 | 7.0 | 4.3 |
| Infected ablated | 81.5 | 6.7 | 14.6 | 6.7 | 3.9 | 1.0 |
| Diptericin | ||||||
| Infected hmlGAL4 | 76.9 | 6.4 | 15.9 | 4.9 | 7.2 | 2.7 |
| Infected ablated | 79.2 | 11.3 | 10.3 | 5.8 | 10.5 | 5.5 |
Data represent three independent experiments ± s.d. In each case values for whole larvae were set at 100%. See Materials and Methods for experimental details. Diptericin was assayed 6 hours following infection with E. coli while Drosomycin was assayed 12 hours post-infection with Micrococcus luteus. The most significant contribution of AMP for haemocytes (a maximum of 24% for Drosomycin) could not account for the extensive reduction seen in our most haemocyte-depleted lines, where AMP expression in whole larvae was reduced by 85% (see Fig. 2A)
Persistence of bacteria following septic injury in haemocyte-ablated larvae
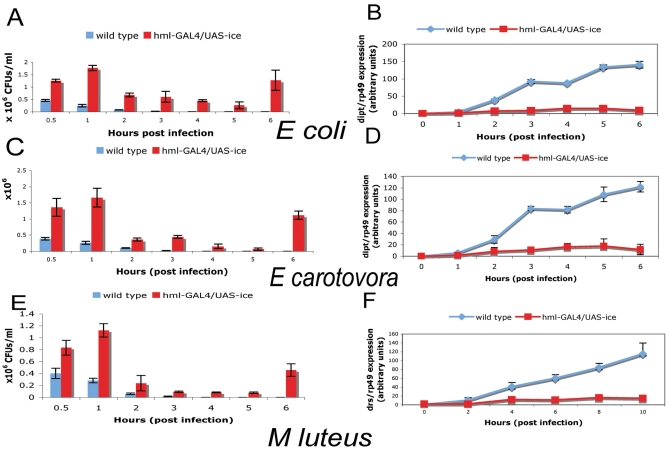
In further experiments, we studied the proliferation of bacteria in larvae with a reduced haemocyte number by measuring colony-forming units per ml (CFU/ml). Fig. 3 summarises results from experiments using the septic injury protocol with Erwinia carotovora carotovora (Ecc) and Escherichia coli (as examples of two Gram-negative bacteria) as well as Micrococcus luteus (as an example of a Gram-positive bacterium). In addition, we used the oral infection protocol developed previously (Basset et al., 2000) to infect haemocyte-ablated larvae with Ecc and monitor bacterial persistence and AMP levels of expression (results shown in Fig. 4). As proxies for AMP gene expression, we again used dipt (Gram-negative bacterial infection) and drs (Gram-positive bacterial challenge). As shown (Fig. 3A,C), in normal larvae infection by both of the Gram-negative bacteria used was contained during the first 20 minutes, keeping the initial load of 2×105 injected bacteria to near extinction in 2 hours. dipt expression reached peak levels at approx 6 hours post-infection (Fig. 3B,D). This delayed activation of AMPs following the elimination of bacteria by blood cells has also been observed in Tenebrio molitor the mealworm beetle (Haine et al., 2008). By contrast, CFUs in haemocyte-ablated larvae went up to 106 and remained stable for at least 1 hour. After 1 hour and until the end of our observation bacteria persisted at 0.4×106-0.6×106. Despite the presence of bacteria however, expression of dipt was poorly induced (Fig. 3B,D). Following infection of wild-type larvae with M. luteus, the bacterial load was cleared quickly with virtually no bacteria after 2 hours (Fig. 3E). The peak of drosomycin expression during our time-course observation was seen at 12 hours following infection (Fig. 3F). In this case as well, haemocyte-ablated larvae did not clear the bacteria (approx 105 bacteria between 2-6 hours were observed, see Fig. 3E). Nevertheless, induction of drs was reduced (Fig. 3F). Results pertaining to AMP induction following septic injury in haemocyte-ablated as compared with normal larvae were extended to defensin (def), as shown in supplementary material Fig. S2.
Fig. 3.
Bacterial persistence versus AMP gene induction in haemocyte-ablated larvae following septic injury. (A,C,E) Proliferation at various times post-infection. In wild-type larvae, bacteria were eliminated within the first hour of infection for E. coli (A) and Ecc (C) or within 2 hours for M. luteus (E). By contrast, haemocyte-depleted larvae failed to efficiently eliminate the bacteria. Although CFUs were reduced within 5 hours post-infection, bacterial proliferation resumed and reached the initial load at the end of the time course (compare blue and red columns in A, C and E at 0.5 and 6 hours post-infection). (B,D,F) Activation of dipt or drs at various times post-infection. Proliferation correlated with a poor activation of dipt (B,D) or drs (F) following infection in haemocyte-ablated larvae (monitored by quantitative real-time PCR). Values in all graphs are mean values ± s.d. of at least three independent experiments. Experiments were done in whole larvae.
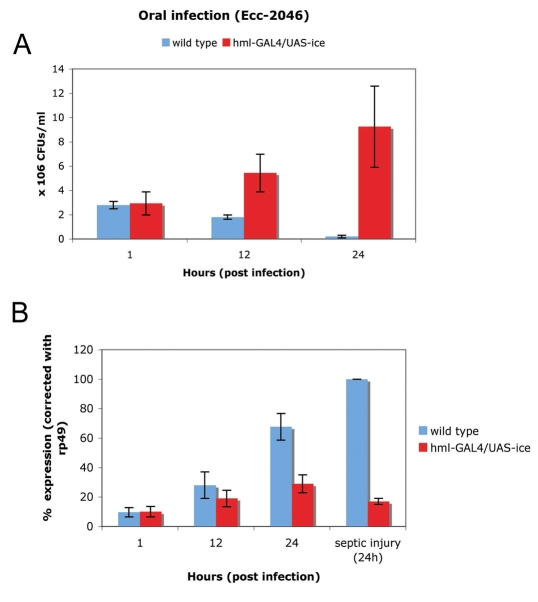
Fig. 4.
Bacterial persistence versus AMP gene induction in haemocyte-ablated larvae following oral infection. (A) During oral infection, wild-type larvae eliminated Ecc-246 within 24 hours. By contrast, haemocyte-depleted larvae could not restrict bacterial proliferation and exhibited elevated CFUs during the same time course. Despite the higher number of CFUs, systemic induction of dipt (measured by northern blot) was severely reduced in comparison to wild-type larvae (B). This showed that the presence of haemocytes was important for the systemic induction of immunity during oral infection. Mean values ± s.d. from at least three independent experiments are presented (northern blots in whole larvae).
Induction of dipt is severely reduced in ablated larvae following oral infection
Oral infection of larvae with Ecc activates Imd-dependent dipt expression in the fat body (Basset et al., 2000). The response starts initially from the gut and is manifested in the fat body 24 hours after challenge, signifying the induction of a systemic host reaction (Basset et al., 2000). Haemocyte-depleted larvae exhibited dramatically reduced levels of dipt following oral infection by Ecc indicating that the presence of blood cells was essential to relay the infection `signal' from the gut to the fat body (Fig. 4B). Moreover, bacteria persisted in greater numbers in these larvae. As shown in Fig. 4A, Ecc CFUs were reduced from 106 to 102 in wild-type larvae during the first 6 hours post-infection as opposed to ablated larvae in which CFUs were stable at approximately 106 throughout the time-course of our experiments. These results correlated with published work (Basset et al., 2000; Charroux and Royet, 2009).
Drs-GFP induction in the fat body is influenced by the absence of blood cells
We then sought to investigate how AMP gene expression was influenced by the absence of circulating haemocytes in live larvae. For this purpose, we used larvae harbouring a drs-GFP transgene (Ferrandon et al., 1998) in a wild-type or haemocyte-ablated genetic background.
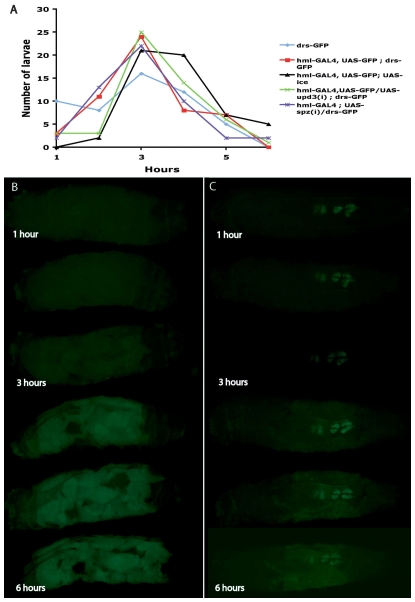
We found that initiation of GFP activation started at the same time in all fat body cells. A graph representing the distribution of the number of larvae according to the time point of first sight of GFP expression following septic injury is shown in Fig. 5A (see Materials and Methods for details). Once activated, intensity of GFP expression increased in the whole tissue over 6 hours (Fig. 5B). The blood cells were not attracted to the site of infection and encountered the bacteria through the rapid flow of haemolymph (not shown). In larvae with ablated haemocytes, although initiation occurred normally (Fig. 5A), GFP expression was low and restricted to only a part of the tissue (Fig. 5C). These results indicated that drs response in the whole fat body required the contribution of haemocytes. It was therefore conceivable that this contribution was achieved by inductive signals, which would orchestrate in fat body cells the propagation of the antimicrobial response in the whole tissue.
Fig. 5.
Fat body expression of drs-GFP in live haemocyte-ablated larvae. (A) Graph showing the distribution of the number of larvae (various genetic backgrounds indicated in colour) initiating expression of drs-GFP in a particular time following immune challenge. As shown, there was essentially no difference between ablated and wild-type larvae in the timing of expression initiation. DD1 is a different chromosomal insertion of drs-GFP used to exclude background specific results. (B,C) Stills from time-lapse imaging of infected wild-type (hmlGAL4/drsGFP) larvae (B) or haemocyte-ablated (hmlGAL4/drsGFP; UAS-ICE larvae) (C) during a 6-hour period. In wild-type larvae, drs-GFP expression initiates and rapidly spreads through the fat body tissue. By contrast, larvae lacking haemocytes do not exhibit the same pattern because initiation of expression occurred but subsequent propagation of expression was not observed. In all frames, posterior is to the left. Similar results were obtained with DD1; hmlGAL4 and DD1; hmlGAL4; UAS-ICE.
Inductive signals sent by haemocytes to the fat body after infection: the case for spätzle
The data presented so far indicated that in larvae, regulation of the fat body antimicrobial response depended on the presence of haemocytes. The system could therefore be used as a tool for studying potential inductive signals that contribute to the control of the immune response during this developmental stage.
DNA microarray studies have shown that the cytokine-like polypeptide Spätzle (Spz), which functions as a ligand for the Toll receptor in Drosophila, is upregulated in larval haemocytes and not in the fat body following infection (Irving et al., 2005). We wanted to determine whether Spz could function as a cue for fat-body-dependent AMP regulation after infection. We used a UAS construct to target spz dsRNA in blood cells or fat body and looked at whether there was a difference in drs induction following infection.
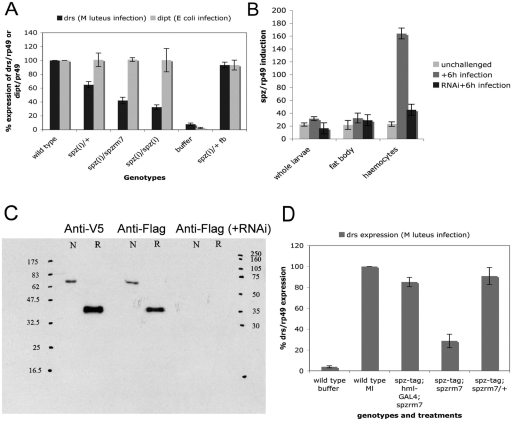
Proteolytically activated Spz initiates an intracellular signalling pathway that culminates in activation of NF-κB-dependent AMP transcription in fat body cells. In spz-deficient larvae, the NF-κB family member DIF is unable to translocate into the nucleus of larval fat body cells (Ligoxygakis et al., 2002). Targeting an UAS-spz(i) transgene to haemocytes resulted in a 40% reduction of drs gene expression levels following infection with M. luteus (Fig. 6A). This decrease became more prominent when the hml-GAL4/UAS-spz(i) configuration was put in trans to a null allele of spz or when two copies of the transgene hairpin and GAL4 driver were used (Fig. 6A). By contrast, silencing spz in the fat body using the lsp2-GAL4 did not significantly influence the levels of expression of the drs gene. Note that dipt was not affected in any of the above configurations following infection with E. coli (Fig. 6A). The efficiency of the knock down at the transcript level was checked by quantitative real time PCR (Fig. 6B) and at the protein level using a UAS-spz-tagged construct (UAS-spztag; see Fig. 6C).
Fig. 6.
Induction of spz is required in larval haemocytes. (A) Targeting a hairpin construct against spz in haemocytes resulted in the reduction of drs expression levels following infection with M. luteus. A further reduction was observed when hairpin expression was combined with a heterozygous recessive mutant background for spz [spz(i)/rm7] at 25°C or two copies of the hairpin and driver construct [spz(i)/spz(i)] reared at 29°C throughout postembryonic development. Expression of UAS-spz(i) in the fat body [fb; spz(i)] through the lsp2-GAL4 line did not influence drs expression levels. Wild-type levels of drs induction were measured by infecting the GAL4 driver line on its own (hml). Because a short-lived AMP activation occurs through wounding, this was measured by injection of phosphate-buffered saline (buffer). Mean values ± s.d. from three independent experiments are presented. Expression of drs was measured 12 hours following infection. Note that induction of dipt (measured 6 hours following E. coli infection) was not affected by the spz knockdown. (B) To support the above findings we measured activation of spz transcription following infection. This was done by using quantitative real-time PCR following infection with M. luteus. We found that spz was predominantly induced in haemocytes and that this infection-dependent activation was severely reduced using a UAS-RNAi construct expressed in haemocytes (hml-GAL4). (C) A tagged version of spz (spztag) was used to explore the potential function of spz as a signal relayed from blood cells to fat body. Western blots of larval haemolymph showed a clear identification of the Spztag band expressed through hml-GAL4. The quantities loaded for each lane were 5 μg for the Anti-V5 lanes and 10 μg for anti-FLAG and anti-FLAG (+RNAi) lanes, respectively. Concomitant expression of a UAS-spzRNAi transgene with the UAS-spztag (anti-FLAG +RNAi lane) sufficiently silenced the latter at the protein level. N, non-reduced samples; R, reduced samples. Concentrations of the antibodies used were 1:10000 for Anti-V5 and 1:500 for Anti-FLAG. (D) Expression of the UAS-spztag using the hml-GAL4 in spz-deficient background (spzrm7) rescued drs induction following infection by septic injury (drs induction assayed by northern blots in whole larvae). Mean values ± s.d. from at least three independent experiments are presented.
The prediction stemming from the results above is that if one expresses spz from the haemocytes in spz– background, one would be able to activate (and rescue) drs expression in the fat body. To confirm this prediction, we constructed a UASp-transgene (Rørth, 1998) containing the full-length spz with V5 and FLAG tags in the N- and C-terminal, respectively (spztag; see Materials and Methods). We first established that the construct was functional by rescuing the embryonic patterning defects (loss of dorsal-ventral polarity) associated with spz deficiency by expressing it through nanos-GAL4 (supplementary material Table S2). In addition, we were able to detect spztag in the haemolymph of both larvae (Fig. 6C) and adults (data not shown; our findings on the regulation of Spz proteolytic cleavage following infection in adults will be presented elsewhere).
We then expressed spztag through hml-GAL4 in a background genetically null for spz (spzrm7) and infected larvae with M. luteus. We assayed drs expression 12 hours post-infection by northern blots in whole larvae. As seen in the graph of Fig. 6D, after infection spzrm7 larvae exhibited a severely reduced level of drs activation (on average 30% of wild-type levels). By contrast, drs was at normal levels in UAS-spztag; hml-GAL4; spzrm7 larvae compared to wild-type controls. This result established that spz-expressing blood cells were able to rescue Toll-dependent drs expression in spz-deficient larvae. As shown in Table 1, the major site of drs production was the fat body. Taken together therefore, our data indicated that Spz from haemocytes was able to restore fat-body induction of drs and brought forward its potential function as a cytokine signal, integrating cellular and humoral responses. This system is in turn reminiscent of the mammalian acute phase response (see Discussion).
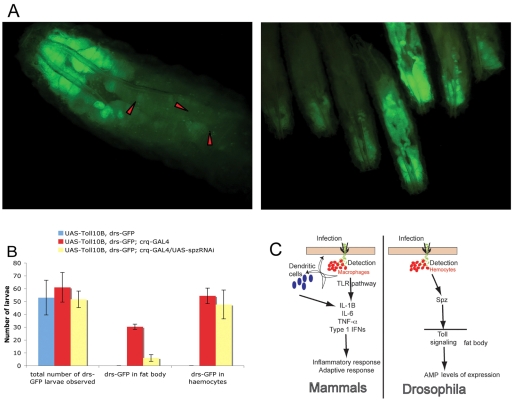
As demonstrated in the laboratory of Michael Levine in 2006, transcriptional regulation of spz is under Toll control. Levine and colleagues showed that a gain-of-function allele of Toll (Toll10B) was able to induce spz expression in the absence of immune challenge (Senger et al., 2006). We expressed the same Toll allele through a UAS construct in the haemocytes using crq-GAL4 (Silva et al., 2007). This is a GAL4 driver expressed in the pattern of croquemort, a gene coding for a macrophage-specific receptor of apoptotic corpses (Franc et al., 1996). We cultured the crosses in axenic conditions (to avoid noise from drs activity caused by the presence of bacteria in the food) in three independent experiments (see Materials and Methods). We found that approximately 50% of drs-GFP, UAS-Toll10B; crq-GAL4 larvae exhibited a characteristic partial activation of drs-GFP in the fat body (Fig. 7). By contrast, drs-GFP, UAS-Toll10B larvae did not exhibit drs-GFP expression in any of our control experiments in axenic conditions (Fig. 7B). These data underlined the ability of Toll10B expressing haemocytes to induce drs in the fat body. Crucially, drs-GFP activity in the fat body was severely reduced when spz expression was knocked-down in haemocytes (drs-GFP, UAS-Toll10B; crq-GAL4/UAS-spzRNAi configuration; Fig. 7B). This result highlighted again the ability of Spz to act as a signal from blood cells to fat body (see Discussion).
Fig. 7.
Expression of Toll10B in larval haemocytes induces drs-GFP in fat body. (A) Expression of UAS-Toll10B using the haemocyte-specific crq-GAL4 (Silva et al., 2007) in axenic conditions of culture induced drs-GFP in both the haemocytes (red arrowheads in magnified larva, left panel) as well as the fat body (right panel; note that the expression was variable but characteristically involved activation at the posterior). (B) Statistical representation of the data, where the number of UAS-Toll10B, drs-GFP; crq-GAL4 larvae expressing GFP in the fat body was significantly reduced when UAS-spzRNAi was included in the configuration. Mean values ± s.d. are from three independent crosses of UAS-Toll10B, drs-GFP with crq-GAL4 in axenic conditions. The total number of third instar larvae observed for each experiment was n1=40, n2=52, n3=67 for UAS-Toll10B, drs-GFP; n1=48, n2=65, n3=70 for UAS-Toll10B, drs-GFP; crq-GAL4 and n1=47, n2=49, n3=59 for UAS-Toll10B, drs-GFP; crq-GAL4/UAS-RNAi. (C) Schematic model for cytokine release in mammals (macrophages) and Drosophila (haemocytes), following an infection. TRL, Toll-like receptor; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon.
Discussion
The results presented here constitute a new paradigm for the study of the host defence in Drosophila (Fig. 7C). This paradigm integrates a pathogen-detection component and a cytokine-mediated response in the control of AMP gene expression in the larval fat body, and point to parallels with the mammalian immune system. In mammals, bacterial infection leads to a systemic reaction called the inflammatory response (see Janeway and Medzhitov, 2002). A major early step towards this response is the release of signalling molecules such as cytokines and chemokines by activated macrophages and dendritic cells (Janeway and Medzhitov, 2002; Akira et al., 2006). After systemic release, these cytokines act at a distance from the site of infection to induce changes in a variety of cells and tissues, namely in blood vessels and the liver.
Specifically, data presented in Fig. 3 is in agreement with the idea that a signal produced by blood cells can modulate the responses of the fat body. Our results directly link Spz to Toll-dependent drs induction in the fat body, indicating that drs transcription levels in this tissue are modulated by a spz RNAi construct expressed in haemocytes. This in turn suggests that spz is secreted by blood cells to activate the Toll receptor in the fat body cells. In agreement with our data, Brennan and colleagues found recently that in psidin mutants defective in digestion of internalised bacteria, expression of the defensin (def) gene was impaired in the fat body (Brennan et al., 2007). Surprisingly, these authors did not notice a similar effect on expression of other AMPs. Further, these authors inferred that Spz was not involved in blood cell communication with the fat body (Brennan et al., 2007) as def was robustly expressed in spz mutants. Our explanation for this discrepancy is as follows. Both Gram-negative and Gram-positive bacteria can induce def expression (DeGregorio et al., 2002). E. coli infection in particular (as these authors used) would stimulate def expression via the IMD pathway, which is not influenced by the absence of Spz (Lemaitre et al., 1996). In the experimental set-up used by Brennan and colleagues, therefore, it was difficult to distinguish whether it was a cytokine or an antigen presentation mechanism (see below) that served as the signal from haemocytes to the fat body.
The question arises as to why Spz is provided by haemocytes if, following a protease-dependent activation in the blood, it binds to Toll receptors in fat body cells. It is conceivable that haemocytes might replenish protein levels that are low after initial cleavage of blood-circulating Spz at the very first stages of pathogen invasion. This is supported by data from genome-wide transcriptional profiling whereby spz was upregulated in blood cells but not in the fat body following infection (Irving et al., 2005).
In the conditions of ablation used, some sessile haemocytes were resistant to ablation, as opposed to their circulating counterparts. It is thought that different blood cell populations have different origins according to the haematopoietic wave they were generated [for embryonic see Tepass et al. (Tepass et al., 1994) and for postembryonic see Holtz et al. (Holtz et al., 2003)] (for a review, see Evans et al., 2003). It is conceivable to hypothesise that time-difference in emergence mirrors a physiological variance that might be sustained (see Márkus et al., 2009). For example, it has been shown recently that overexpression of the Drosophila c-src orthologue Src64B in all haemocytes affects differentiation of sessile haemocytes to lamelocytes but does not influence circulating plasmatocytes in that respect (Williams, 2009).
An issue that still remains to be investigated is the reason that dipt is lost in haemocyte-depleted larvae following Gram-negative bacterial challenge (in both the septic injury and oral infection models). We speculate that nitric oxide (NO) signalling might be a relevant mechanism to explain this phenomenon. NO has been shown to be a haemocyte-dependent signal that regulates AMP activation in the fat body (Foley and O'Farrell, 2003; Djikers and O'Farrell, 2007). Donors of NO were able to activate (in an imd-dependent manner) dipt-GFP in the fat body of wild-type larvae but not in mutants for domino (a haemocyte-deficient condition) contributing to survival against Gram-negative bacteria (Foley and O'Farrell, 2003). It was suggested that although NO signalling emanates from a tissue other than blood cells it needs the latter to exercise its effect in the fat body (Djikers and O'Farrell, 2007). The guts of insects release NO (Foley and O'Farell, 2003; Eleftherianos et al., 2009), and following oral infection of Drosophila larvae with Ecc the gut is the tissue where bacteria activate dipt-GFP prior to fat-body expression and a systemic response (Basset et al., 2000). In our haemocyte-ablated larvae, we observed a severe reduction in systemic dipt following Ecc oral infection. This could be explained by the lack of NO signalling from gut to the fat body due to the absence of haemocytes, which would normally relay the signal. This would in turn be consistent with the recent description of blood cells intimately associated with the larval gut (Charroux and Royet, 2009). Thus, larval tissues that contact a pathogen early (blood cells, gut) might perform a sentinel function and NO might signal pathogen invasion to the fat body.
Our data contradict earlier studies on domino (dom), a mutant with severely reduced number of haemocytes (Braun et al., 1997). The study indicated that AMP gene expression of drs and dipt following infection with Gram-positive or Gram-negative bacteria of dom mutants was normal (Braun et al., 1998). In our hands, dom mutants had a very poor survival rate compared to even our most ablated lines following infection, with 50% of dom larvae dead 4 hours after septic injury (see supplementary material Fig. S3A). Braun and colleagues performed their experiments 6 hours after septic injury (Braun et al., 1998) and at this time point presumably collected only living larvae for northern blot analysis. Here we confirm their results for normal levels of drs or dipt in dom larvae (Braun et al., 1998; data not shown). However, if larvae were collected 3 hours following infection, the level of both AMPs was severely reduced in comparison to wild-type animals or our haemocyte-depleted larvae (supplementary material Fig. S3B); see also the results of Brennan and colleagues for the case of def (Brennan et al., 2007).
Our results from larvae are in disagreement with recently published work using haemocyte-deficient adults (Defaye et al., 2009; Charroux and Royet, 2009). There, blood cell ablation left AMP induction following infection unaffected. It is useful to point out at this time that larvae and adults are two systems with very different physiologies that face a different range of immune challenges. The former contain two organizational centres for blood cells: a haematopoietic organ (the lymph gland) and the reservoir of sessile haemocytes, both of which function as sites of blood cells entering circulation (see Crozatier et al., 2004; Márkus et al., 2009). By contrast, adult flies do not have any such haemocytic organization compartments (see Crozatier and Meister, 2007). In addition to microbes, larvae face parasitism by various wasps (that deposit their eggs in their haemocoele) and entomopathogenic nematodes. To underline the difference in physiology and signalling, larvae that lack both Rel proteins Dif and Dorsal have a severe problem in the survival and function of their haemocytes in addition to reduced fat body responses (Matova and Anderson, 2006), whereas in adults Dorsal is dispensable and Dif is only required for Toll-dependent AMP defences (Rutschmann et al., 2000) and does not affect haemocyte function (Rutschmann et al., 2002).
Our working hypothesis is that in larvae a signal (such as spz) is needed to prime the fat body for Toll-dependent AMP induction. It has been proposed on the basis of the study by Brennan and colleagues (Brennan et al., 2007) that this induction might be dependent on antigen presentation by blood cells (see Hultmark and Borge-Rendberg, 2007), but we found that a cytokine component is involved, at least in part. Our experiments do not exclude that, following initial activation of Toll activity in the fat body by haemocytes, a feedback loop could be established that involves spz being produced in the fat body and used by fat body cells in an autocrine reaction to maintain drs activation. This scenario would explain the transcriptional activity of spz in the fat body. More work would be needed to pinpoint the existence of such a process.
Materials and Methods
Drosophila strains and crosses
All fly stocks were maintained at 25°C on standard flour-treacle-agar medium. Haemocyte-ablated strains were constructed by crossing the previously published GAL4 drivers hemolectin (hml), peroxidasin (pxn), serpentD (srpD) and croquemort-GAL4 (crq-GAL4) (Goto et al., 2003; Galko and Krasnow, 2004; Crozatier et al., 2004; Silva et al., 2007) to UAS lines of apoptotic genes such as ICE (human interleukin-1β-converting enzyme), ced3 (Caenorhabditis elegans death caspase) and hid,rpr (Drosophila melanogaster apoptotic machinery genes) (Shigenaga et al., 1997; Zhou et al., 1997). For targeted transcription knockdown of spz a UAS-RNAi construct (detailed below) was used. Live expression of drs was monitored using a drs-GFP transgene described previously (Ferrandon et al., 1998). The lsp2-GAL4 (lsp2, larval serum protein 2) line was used to drive expression of UAS-spz-RNAi in the larval fat body (Bloomington Stock Centre IN; Lucy Cherbas contributor). The pxn-GAL4, UAS-GFP line (Galko and Krasnow, 2004) was found to express both in haemocytes and fat body so we selected pxn-GAL4, UAS-GFP larvae, which expressed only in the blood cells. We used these larvae to start a culture with the view to establish three independent blood-cells-only pxn-GAL4, UAS-GFP lines. We used all three lines in ablation experiments, and monitored UAS-GFP expression in the course of subsequent generations and for the entire course of the experiments described here. We obtained similar results with all lines (data not shown). Selection was done under a MZFLIII Leica fluorescence stereoscope. Finally, we were aware that the regulatory element directing expression of GAL4 in hmlGAL4 included the open reading frame of tetraspanin (Sinenko and Mathey-Prevot, 2004). However, the similarity of results obtained with all used GAL4 lines indicated that inclusion of tetraspanin was without effect on our observations. For experiments involving spz we used the null allele spzrm7 (Anderson and Nüsslein-Volhard, 1984; Morisato and Anderson, 1994). MetaMorph v6.3r7 (Molecular Devices, USA) software was used to capture images. All images were colour-corrected using Adobe CS2.
Survival studies of Drosophila
For each experiment shown in supplementary material Fig. S1C,D, 20 Drosophila adults were infected with a sharp needle dipped into a concentrated bacterial solution of either E. coli (Gram-negative) or Enterococcus faecalis (Gram-positive). The number of survivors was counted over a 7-day period. Each experiment was repeated five times. Larvae were infected as above and placed in apple-juice plates. They were monitored each hour for a period of 6 hours. Each experiment was repeated four times yielding similar results. Representative data are shown in supplementary material Fig. S3.
Live imaging, microscopy and blood counts
For observing initiation and spreading of drs-GFP expression, wandering third instar larvae from synchronised cultures (Demerec, 1950) were selected and infected using a sharp needle dipped into a concentrated solution of E. coli or M. luteus. The larvae were left to recover on apple-juice agar for 15 minutes and images were captured at hourly intervals. Each larva was cooled for as briefly as possible on a Peltier Stage (Linkam PE 94, UK) before photomicrography and placed on apple-juice agar in a 24-well plate at room temperature until 6 hours post-infection. Initiation of GFP was noted and larvae were assigned as positive for initiation at a particular time point (see Fig. 3A). Fifty larvae were observed for each genotype. Larvae were visualised on a GFP stereo dissecting microscope (Leica MZFIII, UK) and images were captured using KyLink software (v2.0, Japan) and the JVC KY-F75U 3CCD monochrome camera (JVC, Japan). Images were colour-corrected and assembled using Adobe CS2. For haemocyte counts, third instar larvae were washed twice with distilled water and dried. Ten larvae were bled on a glass slide by pulling them apart with two fine forceps. Haemocytes were buffered with 50 μl of Drosophila Schneider's medium (Invitrogen) with 10% fetal bovine serum (Sigma). When necessary, 0.1 μg/μl of DAPI (Sigma) was added to the buffer. The number of haemocytes in a 1 mm grid was counted in an Improved Neubaur haematocytometer under a Nikon Eclipse 80i fluorescent microscope. Five grids were counted for each experiment. Six independent experiments were performed. Fig. 1C,D presents the mean value of these measurements ± standard deviation. Staining of wild-type larval haemocytes with croquemort was done following the protocol described (Franc et al., 1996).
RNA analysis
For whole animal analysis, 20 infected third instar larvae were collected at appropriate times post-infection and frozen at –80°C. To determine the contribution of AMP production in different immuno-competent tissues, 40 larvae from each genotype (wild-type or hml-GAL4; UAS-ice) were recovered 6 hours post-infection and dissected in 50 μl of ice-cold PBS using a fine pair of forceps on ice. The forceps were thoroughly cleaned after each dissection with a clean paper towel and water to avoid tissue cross-contamination. Briefly, each larva was decapitated then carefully rolled inside out on the tip of a forceps. The fat body was carefully dissected and placed in a microfuge tube on ice. To prevent disintegration of the fat body, larvae were dissected in sets of five as quickly as possible and frozen at –80°C. Haemolymph with blood cells (drained and bled into cold PBS during dissection) was pooled together as were the gut and the carcass (containing the cuticle, brain, trachea and salivary glands) before RNA extraction using Trizol (Invitrogen) according to manufacturer's instructions. In each case (whole larvae, fat body, cascass, haemolymph) 20 μg of total RNA were separated on a formaldehyde gel for northern blot analysis as previously described (Ligoxygakis et al., 2002). Northern blot experiments presented in this study were independently repeated at least three times (see respective figure legends). Fig. 2A, Fig. 4A,C and Table 1 show the mean values ± standard deviation. In larvae infected with Gram-negative bacteria, dipt was measured 6 hours post-infection whereas in those infected with Gram-positive bacteria, drs was measured 10 hours post-infection. Quantification of northern blots was performed as previously described (Rutschmann et al., 2000). Briefly, following exposure, signals were measured using a Fuji-film FLA-3000 phosphoimager and normalised against the signal of the loading control, which was a probe for the expression of ribosomal protein 49 (rp49).
Quantitative real-time PCR
Samples were prepared and analysed as previously described (Irving et al., 2005) with the same set of primers for detecting spz expression.
Microbiology
Erwinia carotorova were transformed with an YFP plasmid (Promega) carrying tetracycline antibiotic resistance. Larvae were pricked with a sterile needle dipped in a concentrated solution of bacteria, then left to recover on apple-juice agar. For colony counting, 15 larvae were selected at each time point, washed twice in distilled water, dipped in ethanol to sterilise the outer cuticle and crushed into sterile PBS. Each dilution was plated twice. Four independent experiments were performed. Oral infection with Erwinia carotovora was performed as in Basset et al. (Basset et al., 2000).
UAS-spz RNAi construction
The spätzle RNAi plasmid was constructed by inserting a 520 bp PCR fragment of the spätzle coding sequence (primer sequences available on request) flanked by BamHI and NheI sites between the NheI-BamHI sites (sense) and the XbaI-BglII sites (antisense), respectively. The P{UAS-spz.RNAi} transgene was introduced into white flies by P-element transformation (Rubin and Spradling, 1982).
UAS-spz tagged construction
Double-tagged spz (spztag) was constructed within the pcDNA3.1(+) vector. To clone it into the pUASp vector new restriction sites were required. The spztag ORF was excised as a BamHI-NotI cassette and cloned into the pBluescript vector. The elements contained in the cassette were as follows: BamHI–Leader-peptide–6xHistag–V5tag–Pro-domain–C106–TEV-cleavage-site–FLAGtag–STOP–NotI. spztag was then excised as a KpnI-NotI cassette and cloned directly into the multiple cloning site of pUASp (Rørth, 1998).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/24/4505/DC1
We thank Jules Hoffmann for critical reading of an early version of the manuscript, Michael Galko for the pxn-GAL4, Marie Meister for the srpD-GAL4 and crq-GAL4 lines, Nathalie Franc for the CRQ antibody, Bruno Lemaitre for Erwinia strains, the Bloomington Stock Centre IN for various fly stocks and David Sherratt for the use of the Fuji-film FLA-3000 phosphorimager. This work was supported by Career Establishment Grant G0300170 from the Medical Research Council UK and by New Investigator Grant BBS/B/12326 from the Biological and Biotechnological Sciences Research Council UK to P.L. A.K.H.S. was partly supported by the Yayasan Sarawak Foundation Malaysia. Deposited in PMC for release after 6 months.
References
- Agaisse, H., Petersen, U. M., Boutros, M., Mathey-Prevot, B. and Perrimon, N. (2003). Signalling role of haemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5, 441-450. [DOI] [PubMed] [Google Scholar]
- Akira, S., Uematsu, S. and Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell 124, 783-801. [DOI] [PubMed] [Google Scholar]
- Anderson, K. V. and Nüsslein-Volhard, C. (1984). Genetic analysis of dorsal-ventral embryonic pattern in Drosophila. In Pattern Formation: A Primer in Developmental Biology (ed. G. Malacinski and S. V. Bryant), pp. 269-289. New York: Macmillan.
- Anderson, K. V., Jürgens, G., Nüsslein-Volhard, C. (1985) Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42, 779-789. [DOI] [PubMed] [Google Scholar]
- Basset, A., Khush, R. S., Braun, A., Gardan, L., Boccard, F., Hoffmann, J. A. and Lemaitre, B. (2000). The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. USA 97, 3376-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A. H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Braun, A., Lemaitre, B., Lanot, R., Zachary, D. and Meister, M. (1997). Drosophila immunity: analysis of larval haemocytes by P-element-mediated enhancer trap. Genetics 147, 623-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, A., Hoffmann, J. A. and Meister, M. (1998). Analysis of the Drosophila host defence in domino mutant larvae, which are devoid of haemocytes. Proc. Natl. Acad. Sci. USA 95, 14337-14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, C. A., Delaney, J. R., Schneider, D. S. and Anderson, K. V. (2007). Psidin is required in Drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr. Biol. 17, 67-72. [DOI] [PubMed] [Google Scholar]
- Charroux. B. and Royet, J. (2009). Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc. Natl. Acad. Sci. USA. 106, 9797-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier, M. and Meister, M. (2007). Drosophila haematopoiesis. Cell. Microbiol. 9, 1117-1126. [DOI] [PubMed] [Google Scholar]
- Crozatier, M., Ubeda, J.-M., Vincent, A. and Meister, M. (2004). Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PloS Biol. 2, 1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defaye. A., Evans, I., Crozatier, M., Wood, W., Lemaitre, B. and Leulier, F. (2009). Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate. Immun. 1, 322-334 (DOI: 10.1159/000210264). [DOI] [PubMed] [Google Scholar]
- De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M. and Lemaitre, B. (2002). The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21, 2568-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec, M. (1950). Biology of Drosophila. Wiley, New York.
- Dijkers, P. F. and O'Farrell, P. H. (2007). Drosophila calcineurin promotes induction of innate immune responses Curr. Biol. 17, 2087-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos, I., Felföldi, G., ffrench-Constant, R. H. and Reynolds, S. E. (2009). Induced nitric oxide synthesis in the gut of Manduca sexta protects against oral infection by the bacterial pathogen Photorhabdus luminescens. Insect Mol. Biol. 18, 507-516. [DOI] [PubMed] [Google Scholar]
- Evans. C. J., Hartenstein, V. and Banerjee, U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5, 673-690. [DOI] [PubMed] [Google Scholar]
- Ferrandon, D., Jung, A. C., Criqui, M., Lemaitre, B., Uttenweiler-Joseph, S., Michaut, L., Reichhart, J.-M. and Hoffmann, J. A. (1998). A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 7, 1217-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, E. and O'Farrell, P. H. (2003). Nitric oxide contributes to induction of innate immune responses to Gram-negative bacteria in Drosophila. Genes Dev. 17, 115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc, N. C., Dimarcq, J.-L., Laqueux, M., Hoffmann, J. and Ezekowitz, R. A. (1996). Croquemort, a novel Drosophila haemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 4, 431-443. [DOI] [PubMed] [Google Scholar]
- Galko, M. J. and Krasnow, M. A. (2004). Cellular and genetic analysis of wound healing in Drosophila larvae PLoS Biol. 2, e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, A., Kadowaki, T. and Kitagawa, Y. (2003). Drosophila hemolectin gene is expressed in embryonic and larval haemocytes and its knock down causes bleeding defects. Dev. Biol. 264, 582-591. [DOI] [PubMed] [Google Scholar]
- Gutierrez, E., Wiggins, D., Fielding, B. and Gould, A. (2007). Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445, 277-280. [DOI] [PubMed] [Google Scholar]
- Haine, E. R., Moret, Y., Siva-Jothy, M. T. and Rolff, J. (2008). Antimicrobial defence and persistent infection in insects. Science 322, 1257-1259. [DOI] [PubMed] [Google Scholar]
- Holtz, A., Bossinger, B., Strasser, T., Janning, W. and Klapper, R. (2003). The two origins of hemocytes in Drosophila. Development 130, 4955-4962. [DOI] [PubMed] [Google Scholar]
- Hultmark, D. and Borge-Renberg, K. (2007). Drosophila immunity: is antigen processing the first step? Curr. Biol. 17, R22-R24. [DOI] [PubMed] [Google Scholar]
- Irving, P., Ubeda, J. M., Doucet, D., Troxler, L., Lagueux, M., Zachary, D., Hoffmann, J. A., Hetru, C. and Meister, M. (2005). New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol. 7, 335-350. [DOI] [PubMed] [Google Scholar]
- Janeway, C. A., Jr and Medzhitov, R. (2002). Innate immune recognition Annu. Rev. Immunol. 20, 197-216. [DOI] [PubMed] [Google Scholar]
- Kaneko, T., Goldman, W. E., Mellroth, P., Steiner, H., Fukase, K., Kusumoto, S., Harley, W., Fox, A., Golenbock, D. and Silverman, N. (2004). Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 20, 637-649. [DOI] [PubMed] [Google Scholar]
- Lemaitre, B., Reichhart, J.-M. and Hoffmann, J. A. (1997). Drosophila host defence: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 94, 14614-14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis, P., Bulet, P. and Reichhart, J.-M. (2002). Critical evaluation of the role of the Toll-like receptor 18-Wheeler in the host defence of Drosophila. EMBO Rep. 3, 666-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márkus, R., Laurinyecz, B., Kurucz, E., Honti, V., Bajusz, I., Sipos, B., Somogyi, K., Kronhamn, J., Hultmark, D. and Andó, I. (2009). Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 106, 4805-4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevost, B. and Perrimon, N. (1998). Mammalian and Drosophila blood: JAK of all trades? Cell 92, 697-700. [DOI] [PubMed] [Google Scholar]
- Matova, N. and Anderson, K. V. (2006). Rel/NF-kappaB double mutants reveal that cellular immunity is central to Drosophila host defense. Proc. Natl. Acad. Sci. USA 103, 16424-16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, M. (2004). Blood cells of Drosophila: cell lineages and role in host defence. Cell Microbiol. 5, 573-580. [DOI] [PubMed] [Google Scholar]
- Morisato, D. and Anderson, K. V. (1994). The spätzle gene product encodes a component of the extracellular signalling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell 76, 677-688. [DOI] [PubMed] [Google Scholar]
- Reichhart, J.-M., Meister, M., Dimarq, J.-L., Zachary, D., Hoffman, D. and Hoffmann, J. A. (1992). Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J. 11, 1469-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørth, P. (1998). Gal4 in the Drosophila female germline. Mech. Dev. 78, 113-118. [DOI] [PubMed] [Google Scholar]
- Roth, S., Hiromi, Y., Godt, D. and Nüsslein-Volhard, C. (1991). cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development 112, 371-388. [DOI] [PubMed] [Google Scholar]
- Rubin, G. M. and Spradling, A. C. (1982). Genetic transformation of Drosophila with transposable element vectors. Science 218, 348-353. [DOI] [PubMed] [Google Scholar]
- Rutschmann, S., Jung, A. C., Hetru, C., Reichhart, J.-M., Hoffmann, J. A. and Ferrandon, D. (2000). The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12, 569-580. [DOI] [PubMed] [Google Scholar]
- Rutschmann, S., Kilinc, A. and Ferrandon, D. (2002). The toll pathway is required for resistance to gram-positive bacterial infections in Drosophila. J. Immunol. 168, 1542-1546. [DOI] [PubMed] [Google Scholar]
- Senger, K., Harris, K. and Levine, M. (2006). GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc. Natl. Acad. Sci. USA 103, 15957-15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga, A., Kimura, K., Kobayakawa, Y., Tsujimoto, Y. and Tanimura, T. (1997). Cell ablation by ectopic expression of cell death genes, ced-3 and Ice, in Drosophila. Dev. Growth. Differ. 39, 429-436. [DOI] [PubMed] [Google Scholar]
- Silva, E., Au-Yeung, H. W., Van Goethem, E., Burden, J. and Franc, N. C. (2007). Requirement for a Drosophila E3-ubiquitin ligase in phagocytosis of apoptotic cells. Immunity 27, 585-596. [DOI] [PubMed] [Google Scholar]
- Sinenko, S. A. and Mathey-Prevot, B. (2004). Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated haemocytes. Oncogene 23, 9120-9128. [DOI] [PubMed] [Google Scholar]
- Tepass, U., Fessier, L. I., Aziz, A. and Hartenstein, V. (1994). Embryonic origin of haemocytes and their relationship to cell death in Drosophila. Development 120, 1829-1837. [DOI] [PubMed] [Google Scholar]
- Wang, L. and Ligoxygakis, P. (2006). Pathogen recognition and signalling in the Drosophila innate immune response. Immunobiol. 211, 251-261. [DOI] [PubMed] [Google Scholar]
- Weber, A. N., Tauszig-Delamasure, S., Hoffmann, J. A., Lelièvre, E., Gascan, H., Ray, K. P., Morse, M. A., Imler, J.-L. and Gay, N. J. (2003). Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat. Immunol. 4, 794-800. [DOI] [PubMed] [Google Scholar]
- Williams, M. J. (2009). The c-src homologue Src64B is sufficient to activate the Drosophila cellular immune response J. Innate Immun. 1, 335-339. [DOI] [PubMed] [Google Scholar]
- Zhou, L., Schnitzler, A., Agapite, J., Schwartz, L. M., Steller, H. and Nambu, J. R. (1997). Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc. Natl. Acad. Sci. USA , 94, 5131-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.