Abstract
The maintenance of immune surveillance and the generation of normal immune responses are dependent on leukocyte migration to appropriate lymphoid and nonlymphoid tissues. The process of leukocyte migration occurs through complex and highly regulated interactions between the circulating leukocytes and the vascular endothelium. Multiple families of adhesion molecules as well as specific chemoattractants and their cognate receptors function to stabilize these interactions and induce migration into the tissue. L-selectin is a key adhesion molecule that regulates both the migration of leukocytes at sites of inflammation and the recirculation of lymphocytes between blood and lymphoid tissues. L-selectin-mediated lymphocyte recirculation is required for maintaining the appropriate tissue distribution of lymphocyte subpopulations including naïve and effector subsets such as regulatory T cells. In addition, L-selectin-mediated entry into peripheral lymph nodes is required for optimal induction of lymphocyte homeostatic proliferation during lymphopenia. Importantly, L-selectin has been shown to have both adhesive and signaling functions during leukocyte migration. Specifically, L-selectin is highly efficient at capturing free-flowing leukocytes from the blood and supporting subsequent fast rolling interactions along the vascular endothelium. During rolling, synergistic interactions between L-selectin and integrin functions slow leukocyte rolling velocities allowing for chemoattractant-induced activation and eventual firm adhesion of the leukocyte to the vascular endothelium. Engagement of L-selectin by ligand generates transmembrane signals leading to activation of intracellular signaling pathways, increased integrin binding affinity, and enhanced chemotaxis. L-selectin has also been shown to mediate leukocyte recruitment during chronic inflammatory and autoimmune diseases and thus is a potential therapeutic target for drug development.
Keywords: L-selectin, leukocyte migration, inflammation, adhesion molecules, homeostatic proliferation
1. Introduction
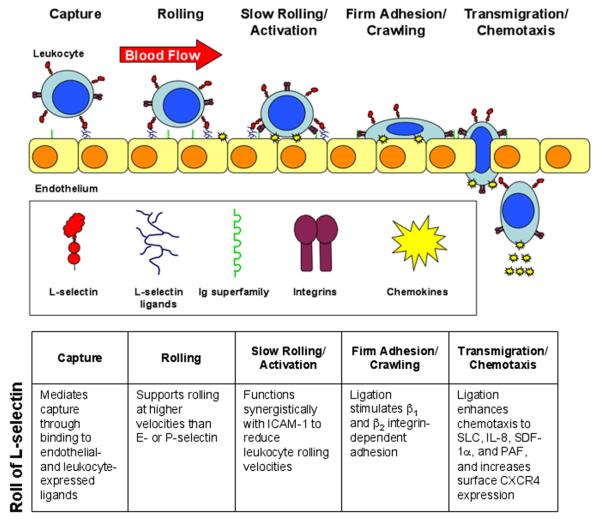
The ability of leukocytes to stably interact with the vascular endothelium is requisite to their ability to enter sites of inflammation. Leukocytes utilize multiple adhesion molecules in a tightly controlled and highly orchestrated process to overcome the high shear stresses encountered within venules and enter the tissues. This process has been termed an “adhesion cascade” and is defined as a series of overlapping and synergistic interactions among different families of adhesion molecules and chemoattractants such as chemokines [1]. Leukocytes must first be captured or tethered from the flowing blood allowing them to roll along the venular wall and become activated by chemoattractants that are displayed on the endothelial surface (Fig. 1). Leukocyte activation results in firm adhesion and arrest and ultimately transendothelial migration into the tissue. Additional steps in this process such as slow rolling, adhesion strengthening and spreading, intravascular crawling and the route of transmigration make this process even more complex [2]. This migratory pathway out of the blood stream is also utilized by lymphocytes as they undergo recirculation through the peripheral lymphoid tissues, such as the lymph nodes and Peyer's patches. Thus, leukocyte migration at sites of inflammation, and lymphocyte recirculation, are integral components of immune surveillance and promote the generation of rapid and effective immune responses.
Figure 1.
L-selectin-mediated leukocyte recruitment. The initial capture of free-flowing leukocytes is mediated by L-selectin on leukocytes binding to endothelial ligands constitutively expressed on HEV, inflamed vessels, or on previously adhered leukocytes (secondary tethering). L-selectin alone mediates fast rolling, but synergistic interactions between L-selectin and integrin functions slow the rolling velocity of the leukocyte, facilitating activation by chemoattractants (e.g., chemokines) presented on the endothelium. Leukocyte activation (through L-selectin ligation and chemokines) induces high affinity integrin-mediated firm adhesion. Ultimately, the leukocyte transmigrates through the vascular endothelium and into the tissue. PAF, platelet-activating factor; CXCR4, CXC chemokine receptor 4.
Leukocyte interactions with the vascular endothelium involve four families of adhesion molecules: selectins, mucins, integrins and immunoglobulin (Ig) superfamily members. The initial capture and rolling of leukocytes is primarily mediated by the selectins (L-, P-, and E-selectin) binding to their respective mucin ligands (for example: glycosylation-dependent cell adhesion molecule-1, GlyCAM-1; P-selectin glycoprotein ligand-1, PSGL-1; E-selectin ligand-1) although the α4 integrins, α4β1 (very late antigen-4, VLA-4) and α4β7, can also mediate capture [3]. The selectins all contain a unique domain structure consisting of an amino terminal calcium-dependent lectin domain, an epidermal growth factor-like domain, and a number of short consensus repeat domains (Fig. 2). While highly homologous in structure, marked differences do occur between the selectins. For example, L-selectin is expressed by all classes of leukocytes, while P- and E-selectin are expressed by inflamed endothelium with P-selectin also being expressed on activated platelets. Unique among the selectins, L-selectin contains a membrane-proximal enzymatic cleavage site that results in the rapid release of L-selectin from the cell surface following leukocyte activation. Cleavage of L-selectin results in the production of a soluble molecule that is functional in vivo [4]. Cleavage is also necessary for the maintenance of appropriate cell-surface L-selectin expression levels and normal leukocyte migration [5]. Furthermore, while L- and P-selectin are very efficient in mediating leukocyte capture and support rolling at relatively fast velocities, E-selectin has very limited capturing ability but does support leukocyte rolling at very slow velocities [6]. Leukocyte rolling can also be influenced by integrin function. Specifically, integrins binding to their Ig superfamily ligands can stabilize rolling interactions and mediate the transition from fast to slow rolling. For example, αLβ2 integrin (leukocyte function-associated antigen-1, LFA-1)/intercellular adhesion molecule-1 (ICAM-1), VLA-4/vascular cell adhesion molecule-1 (VCAM-1), and α4β7 integrin/mucosal addressin cell adhesion molecule-1 (MAdCAM-1) interactions have all been reported to slow leukocyte rolling velocities and ultimately mediate firm adhesion to the endothelium [3, 7, 8].
Figure 2.
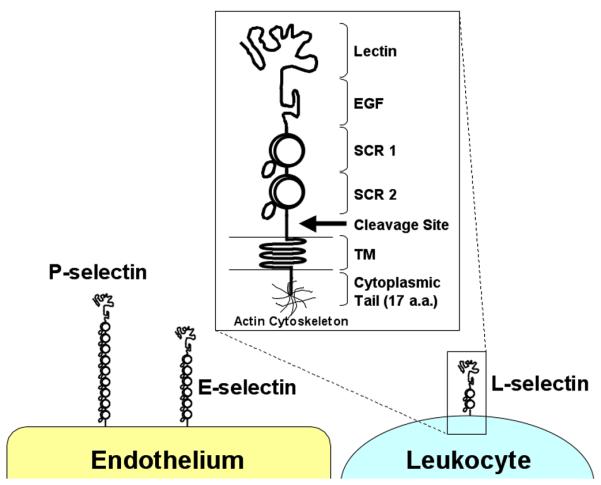
Structure of the human selectins. L-, P-, and E-selectin share a unique structure consisting of the lectin, epidermal growth factor (EGF)-like, short consensus repeat (SCR), transmembrane (TM), and cytoplasmic tail domains. Note the different number of SCR domains among the selectin molecules. a.a., amino acid.
2. L-selectin-mediated leukocyte adhesion and migration
L-selectin mediates leukocyte migration through both adhesive and signaling interactions. Since L-selectin, like all selectins, is constitutively active, its function is largely regulated through the expression of appropriate ligands. Many different ligands for L-selectin have been identified, which are expressed in vascular as well as non-vascular sites [reviewed in 9]. Vascular L-selectin ligands predominantly expressed by specialized venules called high endothelial venules (HEV) located within peripheral lymph nodes (PLN) are identified by the MECA-79 monoclonal antibody (mAb) and are collectively called the peripheral node addressins (PNAd, Table 1). The constitutive expression of L-selectin by naïve lymphocytes and PNAd by HEV within PLNs mediates the continuous recirculation of lymphocytes between blood and lymph. The generation of L-selectin-deficient (L-selectin−/−) mice [10] has clearly demonstrated the dominant role of L-selectin in mediating lymphocyte migration to the PLNs (Fig. 3). This recirculation of lymphocytes is paramount to immune surveillance and the generation of rapid and efficient adaptive immune responses. L-selectin also supports lymphocyte migration to the mesenteric lymph nodes and Peyer's patches through interactions with PNAd and/or MAdCAM-1, which can also serve as L-selectin ligands, expressed on the HEV. In fact, virtually all lymphocyte migration across HEV in lymphoid tissues is controlled by L-selectin and α4β7 integrin function [11].
Table 1.
L-selectin ligands
| Name | Expression | Comments |
|---|---|---|
| GlyCAM-1a | HEV | PNAd subgroup, secreted |
| Sgp200 | HEV | PNAd subgroup, membrane bound and secreted forms |
| CD34 | Vascular endothelium (including HEV) |
PNAd subgroup, non-reactive forms in non- HEV and non-inflamed vessels |
| Podocalyxin | Vascular endothelium (including HEV) |
PNAd subgroup, non-reactive forms in non- HEV and non-inflamed vessels |
| Endomucin | Vascular endothelium (including HEV) |
PNAd subgroup, non-reactive forms in non- HEV and non-inflamed vessels |
| Nepmucin | HEV, some leukocytes | PNAd subgroup, can also mediate binding through Ig domain |
| MAdCAM-1 | HEV, intestinal lamina propria |
Primarily mediates α4β7 integrin binding through Ig domain, L-selectin binding mediated by tissue-specific glycosylation |
| Endoglycan | Leukocytes, vascular endothelium |
Selectin binding similar to PSGL-1 |
| PSGL-1 | Leukocytes | Binds L-, P-, and E-selectin through overlapping regions, mediates secondary tethering |
| CLA | Inflamed endothelium, lymphocyte subsets |
Defined by HECA-452 mAb, expressed on skin-homing lymphocytes |
GlyCAM-1, glycosylation dependent cell adhesion molecule-1; HEV, high endothelial venule; PNAd, peripheral node addressins; Ig, immunoglobulin; MAdCAM-1, mucosal addressin cell adhesion molecule-1; PSGL-1, P-selectin glycoprotein ligand-1; CLA, cutaneous lymphocyte-associated antigen.
Figure 3.
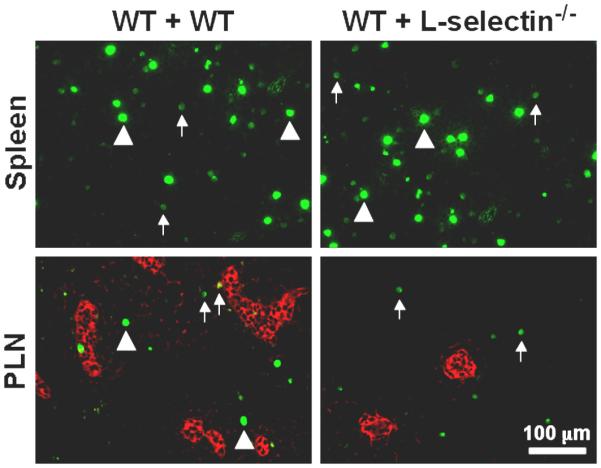
L-selectin mediates lymphocyte migration into PLN. Splenocytes isolated from wild type or L-selectin−/− mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 0.5 μM, CFSEhigh or 0.05 μM, CFSElow) and injected (40 × 106 cells total) intravenously into recipient mice. Thirty minutes after transfer, the spleen and PLNs from recipient animals were harvested, cryosectioned, and immunostained for fluorescence light microscopy. In the left column, all the injected cells were wild type. Both CFSEhigh (bright green, arrowheads) and CFSElow (dull green, arrows) cells were observed in equivalent numbers in the spleen and PLN of the recipient mice. In the right column, L-selectin−/− cells were labeled CFSEhigh and wild type cells were labeled CFSElow. While both CFSEhigh and CFSElow cells were observed in the spleen, only CFSElow cells (wild type) were found within the PLN. Red staining indicates PNAd expression on the HEV.
L-selectin ligands can also be induced on the endothelium of inflamed tissues. Specifically, vascular L-selectin ligands are expressed at cutaneous sites of chronic inflammation [12], acute dermatitis [13], Grave's disease and Hashimoto's thyroiditis [14], rheumatoid arthritis [15], diabetes [16], and asthma [17]. In addition, activation of endothelial cell cultures with pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα) induces L-selectin ligand expression and increased L-selectin-dependent leukocyte adhesion [18]. Interestingly, the cutaneous lymphocyte-associated antigen (CLA), which is a sialyl LewisX-like determinant that is recognized by the mAb HECA-452, is upregulated on inflamed endothelium where it serves as a vascular L-selectin ligand [19]. Leukocytes also express L-selectin ligands, the best studied of these being PSGL-1 [20]. The expression of L-selectin ligands by leukocytes allows for L-selectin-mediated interactions to occur between free flowing leukocytes and leukocytes already attached to the endothelium, a process termed “secondary tethering.” L-selectin-mediated secondary tethering has been shown to be an important mechanism for increasing leukocyte recruitment at sites of inflammation [21]. Therefore, L-selectin ligands expressed on both vascular endothelium and on leukocytes contribute significantly to leukocyte migration.
3. L-selectin-mediated signal transduction
In addition to its adhesive function, L-selectin also serves as a signal transduction molecule. Specifically, engagement of L-selectin results in the activation of intracellular signaling pathways leading to integrin activation and increased leukocyte adhesion to the endothelium [22, 23]. L-selectin signaling also plays an important role in the subsequent chemotaxis of adhered leukocytes. Specifically, during in vivo studies of leukocyte recruitment, L-selectin−/− leukocytes demonstrated significantly reduced emigration away from the blood vessel wall compared to wild type leukocytes [5, 24]. In addition, ligation of L-selectin in the presence of chemoattractants can synergistically increase leukocyte chemotaxis in vitro [25], possibly by increasing surface expression of the corresponding receptor [26]. However, this is unlikely to be the only mechanism since ligation of L-selectin on T cells significantly increased their chemotaxis to secondary lymphoid tissue chemokine (SLC) without affecting surface expression of the receptor for SLC, CC chemokine receptor (CCR) 7 [9]. Thus, L-selectin functions at multiple points during the recruitment process to mediate the efficient migration of leukocytes into the tissue.
4. Role of L-selectin in mediating leukocyte migration to cutaneous sites of inflammation
It is generally considered that most leukocyte migration to cutaneous sites is regulated by the expression of E- and/or P-selectin on dermal venules [reviewed in 27]. Importantly, E-selectin is constitutively expressed at low levels on noninflamed skin vessels and correlates with the presence of large numbers of CLA+ memory T cells in the normal skin [28, 29]. In addition, the importance of the endothelial selectins in mediating cutaneous immune responses and surveillance has been demonstrated with the production of E- and P-selectin double-deficient mice (E/P-selectin−/−) [30]. The striking phenotype of these mice is their significantly increased susceptibility to spontaneous mucocutaneous infections and the development of nonulcerative skin lesions and hair loss. Furthermore, use of the E/P-selectin−/− mice in models of cutaneous inflammation have revealed roles for the endothelial selectins that were not observed in the single-deficient mice (Table 2).
Table 2.
Selectin-deficient mice and cutaneous inflammation
| Molecule | Gross abnormalities of deficient mice |
Role in skin inflammation |
|---|---|---|
| L-selectin | None [10] | L-selectin-deficiency causes delayed skin allograft rejection, reduced DTH reactions, and delayed wound healing [31-34, 36]. |
| P-selectin | None [66] | P-selectin-deficiency has no effect on skin allograft rejection and wound healing, but exhibits variable effects on DTH reactions [31, 67-70]. |
| E-selectin | None [71] | E-selectin-deficiency has no effect on DTH reactions or wound healing [67, 70, 71]. |
| P/E-selectin | Spontaneous mucocutaneous infections, development of nonulcerative skin lesions and hair loss, hypergammaglobulinemia, highly elevated leukocyte counts [30] |
P- and E-selectin double-deficiency significantly reduces DTH reactions and wound healing [67, 68, 70]. |
Despite the prominent role of the endothelial selectins, a number of studies using blocking mAbs or gene-targeted mice have also demonstrated an important role for L-selectin in mediating cutaneous inflammation (Table 2). Specifically, skin allograft rejection was significantly delayed and T cell migration into the graft was reduced in L-selectin−/− mice even with the generation of equivalent cytotoxic T lymphocyte responses [31, 32]. Delayed type hypersensitivity (DTH) responses and contact hypersensitivity responses (CHS) were both found to be significantly reduced in L-selectin−/− mice [32, 33]. Similarly, immediate type hypersensitivity responses were reduced in L-selectin−/− mice including decreased mast cell recruitment to the skin and decreased IgE production [34]. Studies of acute cutaneous immune complex-mediated inflammation demonstrated reduced edema and hemorrhage, decreased neutrophil and mast cell recruitment, and reduced production of TNFα in the absence of L-selectin expression [35]. These results are consistent with the finding of reduced chemokine-induced neutrophil migration into the skin of L-selectin−/− animals [32]. Interestingly, a role for L-selectin in wound healing was revealed in the absence of ICAM-1 expression. Specifically, the additional loss of L-selectin expression in ICAM-1-deficient mice resulted in decreased keratinocyte migration, granulation tissue formation and early neutrophil recruitment beyond that observed for ICAM-1-deficient mice alone [36]. However, optimal wound healing was found to require the cooperative interactions of all three selectins and ICAM-1 [37]. Thus, multiple adhesion pathways can support leukocyte migration into cutaneous sites of inflammation.
5. L-selectin regulates the migration of regulatory T cells
Regulatory T cells (Treg), typically identified as having a CD4+ CD25+ Foxp3+ phenotype, are involved in regulating a broad array of immune functions including inflammatory responses, tolerance, and T cell homeostasis. Treg cells can influence immune responses through both direct cell contact and through cytokine production [38, 39]. Like conventional CD4+ T cells, the majority of Treg cells express L-selectin [40] and utilize its function for migration and maintenance of normal tissue distribution [41]. By contrast, Treg cells have two-fold higher L-selectin mRNA levels and have a higher rate of cell-surface L-selectin turnover than conventional CD4+ T cells [41]. Interestingly, Treg cells maintain an equivalent level of cell-surface L-selectin as conventional CD4+ T cells by having increased enzymatic cleavage. Thus, L-selectin function is required for normal Treg cell migration.
Treg cells also play a significant role in the control of cutaneous inflammation. Treg cell populations found in the skin express a unique phenotype with high levels of L-selectin and CLA, along with the chemokine receptors CCR4 and CCR6 [42]. Numerous reports have indicated that Treg cells control inflammation in CHS reactions [43-45]. Treg cells can regulate inflammation in the skin through multiple mechanisms including cutaneous cytokine production [45, 46], the suppression of mast cell degranulation [47], and/or through a Fas-ligand-dependent mechanism [43]. Importantly, Treg cell migration into inflamed skin is required for the proper resolution of inflammation during CHS responses [44]. In addition, Foxp3-deficient scurfy mice reconstituted with non-skin homing Treg cells developed severe cutaneous inflammation, even though Treg cells were found in normal frequencies and could function in all other tissues examined [48]. Therefore, Treg cells are important in the control of cutaneous inflammation, and Treg cell migration to, and residency in, the skin is necessary for normal cutaneous homeostasis.
6. L-selectin-mediated lymphocyte migration is maintained and required for optimal induction of acute homeostatic proliferation
The maintenance of sufficient lymphocyte numbers is attained through the regulation of cell proliferation and death. Under lymphopenic conditions, both naïve and memory T cells can undergo homeostatic proliferation to boost T cell numbers. Murine models of lymphopenia such as recombination activating gene-1 deficiency (RAG-1−/−), CD3ε deficiency, or irradiation have been widely used to study homeostatic proliferation. Importantly, in humans, lymphopenia can be caused by viral infections such as HIV, exposure to radiation, and chemotherapy. The regulation of T cell homeostatic proliferation is dependent on accessibility to and interaction with a number of factors including cytokines such as interleukin (IL)-7 and IL-15, as well as major histocompatibility complex (MHC)-self peptides [reviewed in 49]. However, the dependence of CD4+ or CD8+ T cells on these factors varies. Interestingly, CD4+ T cells proliferate less efficiently than CD8+ T cells, which may be due to the downregulation of MHC class II expression on dendritic cells induced by increased systemic IL-7 concentrations present during lymphopenia [50]. T cells that have undergone homeostatic proliferation acquire an effector/memory phenotype, including the upregulation of CD44 and IL-2Rβ, and increased memory-like function including the ability to respond to lower antigen doses, the capacity to rapidly secrete interferon γ and become cytotoxic upon cognate antigen stimulation, and the ability to mediate accelerated allograft rejection [51-53]. Furthermore, these cells also show modest decreases in CCR7 expression and chemotaxis to SLC, while upregulating expression of CXCR4 and chemotaxis to stromal cell-derived factor (SDF)-1α [54] However, homeostatically proliferated T cells retain L-selectin expression and preserve the ability to migrate through HEV into PLNs [54]. Therefore, homeostatically proliferated T cells display traits of both naïve and antigen-activated cells. Importantly, recurrent periods of lymphopenia-induced homeostatic proliferation may be linked to the development of autoimmunity presumably due to the selection and proliferation of high-affinity MHC-self peptide reactive T cells [55, 56].
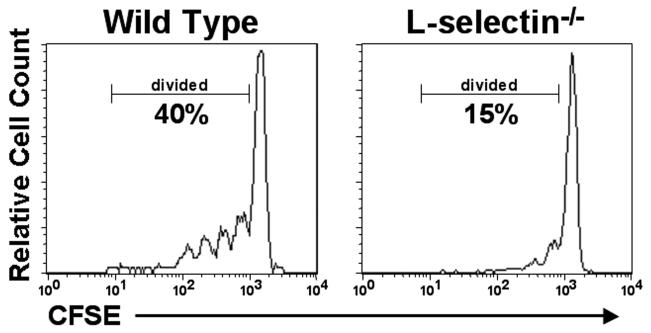
T cell homeostatic proliferation is dependent upon migration into secondary lymphoid tissues to gain access to homeostatic growth factors. Specifically, mice that lack the CCR7 ligands SLC and ELC show a 50-70% reduction in T cell homeostatic proliferation [57]. Current work in our lab indicates that L-selectin-mediated migration into lymph nodes is crucial for optimal induction of homeostatic proliferation. Specifically, intravenous injection of wild type T cells into RAG-1−/− mice resulted in robust homeostatic proliferation while the loss of L-selectin-mediated migration reduced the number of homeostatically proliferated T cells in the spleen and blood by 50-70% (Fig. 4). Interestingly, while few L-selectin−/− transferred T cells were found within the PLN, they demonstrated a similar degree of homeostatic proliferation as the wild type control cells. Furthermore, L-selectin−/− T cells injected subcutaneously (non-HEV-mediated PLN entry) eliminated the defect in homeostatic proliferation observed with intravenous injection. Taken together, these results indicate that homeostatic proliferation occurs primarily in lymph nodes, and that migration to lymph nodes is crucial for access to homeostatic growth factors.
Figure 4.
L-selectin mediates optimal T cell homeostatic proliferation during lymphopenia. Wild type or L-selectin−/− splenocytes were labeled with CFSE (0.25 μM) and injected (5 × 106 cells) intravenously into RAG-1−/− recipient mice. Four days after transfer, the recipient spleen was harvested, immunostained to detect T cells, and analyzed by flow cytometry. CFSE fluorescence is decreased by one half with each cell division thus allowing proliferation to be assessed in vivo. The transfer of wild type cells yielded robust homeostatic proliferation of T cells, while the transfer of L-selectin−/− cells (i.e., excluded from entering the PLN) exhibited much reduced proliferation.
7. L-selectin-directed therapies
The role of L-selectin in mediating the aberrant migration of leukocytes has been demonstrated in numerous autoimmune and inflammatory disorders. Thus, the potential to block L-selectin-mediated adhesion makes it an attractive therapeutic target. However, while both biological and small-molecule L-selectin functional inhibitors have shown great promise in animal models, few of these successes have translated to the effective treatment of human diseases. In fact, only one selectin-directed therapy has so far demonstrated clinical success, the pan-selectin antagonist bimosiamose. Bimosimose is a low molecular weight nonoligosaccharide selectin inhibitor, and was reported to prevent P-, E-, and L-selectin-mediated adhesion in vitro [58, 59]. However, further studies in vivo have indicated that bimosiamose functions primarily by blocking E-selectin-mediated adhesion [60]. Clinical trials using bimosiamose have shown some promise, albeit limited. When bimosiamose was administered via inhalation in asthmatic patients, there was a 50% reduction in late asthmatic reactions following challenge [61]. However, in a separate trial, a single i.v. administration of bimosiamose in asthmatic patients did not attenuate early or late asthmatic responses in response to allergen challenge [62]. Importantly, psoriatic patients treated with bimosiamose showed a reduction in epidermal thickness and lymphocyte infiltration [63]. Therefore, pan-selectin antagonists have shown promise as potential therapeutics for human diseases.
In contrast to pan-selectin antagonists, specific L-selectin-directed therapies have had limited success. Specifically, humanized anti-L-selectin mAbs (aselizumab) were found to significantly increase survival time and decrease mortality in a baboon model of hemorrhagic-traumatic shock [64]. However, in a phase II trial of traumatized patients, treatment with aselizumab resulted in a trend toward higher infection rates, and no significant improvements for any efficacy variables measured [65]. The targeting of Lselectin ligands is another possible therapeutic strategy currently being explored. Importantly, blocking L-selectin ligands using mAb treatment in a sheep asthma model resulted in decreased late phase airway responses and airway hyperresponsiveness following airway challenge [17]. Therefore, it remains to be determined how effective selectin-based therapies will be for the treatment of human disease.
8. Conclusions
L-selectin plays an important role in the complex process of leukocyte recruitment. By functioning as both an adhesion and signaling molecule, L-selectin contributes to both the early adhesive events as well as the later stages of chemotaxis and cell migration. L-selectin ligand expression at sites of inflammation results in L-selectin playing an important role in the development of autoimmune and chronic inflammatory diseases. However much remains to be defined concerning L-selectin function. For example, despite the identification of numerous different ligands recognized by L-selectin, little evidence for a physiologic role for any of these molecules exists. While additional signaling functions for L-selectin are still being described, understanding the role of these functions in vivo is largely lacking. Furthermore, the full contribution of L-selectin to the recruitment of T effector cell populations (e.g., Th1, Th17, Treg) during normal immune responses and disease conditions remains to be determined. As the in vivo function of L-selectin is better understood it is likely that additional roles for this versatile molecule in human health and disease will be described.
Acknowledgements
We thank the National Institutes of Health (HL 05-SC-NIH-1040), the UWM Chancellor's Office, and the UWM Research Growth Initiative for financial support of these studies. We also thank Amy Rymaszewski for critical review of the manuscript, and all members of the lab for their important contributions to this work.
Abbreviations
- CCR
CC chemokine receptor
- CHS
contact hypersensitivity
- CLA
cutaneous lymphocyte-associated antigen
- CXCR4
CXC chemokine receptor 4
- DTH
delayed type hypersensitivity
- E/P-selectin−/−
E- and P-selectin double-deficient
- GlyCAM-1
glycosylation-dependent cell adhesion molecule-1
- HEV
high endothelial venule
- Ig
immunoglobulin
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- LFA-1
leukocyte function-associated antigen-1
- L-selectin−/−
L-selectin-deficient
- MHC
major histocompatibility complex
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- mAb
monoclonal antibody
- PLN
peripheral lymph node
- PNAd
peripheral node addressins
- PAF
platelet-activating factor
- PSGL-1
P-selectin glycoprotein ligand-1
- RAG-1
recombination activating gene-1
- SLC
secondary lymphoid tissue chemokine
- SDF
stromal cell-derived factor
- Treg
regulatory T cells
- TNFα
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late antigen-4
Biography
 Douglas Steeber received his Ph.D. from the University of Wisconsin-Madison in 1995. He trained as a Postdoctoral Fellow under Dr. Thomas Tedder in the Department of Immunology at Duke University from 1995 to 1997. In 1997 he became an Assistant Research Professor in the Immunology department where he continued working until 2003. In 2003 he moved to the Department of Biological Sciences at the University of Wisconsin-Milwaukee as an Assistant Professor. In 2006 he was promoted to Associate Professor within the department. He is currently a UWM Chancellor's Scientist and Director of the Flow Cytometry Facility. Dr. Steeber continues to conduct research in the areas of inflammation and autoimmune disease with a focus on mechanisms of leukocyte recruitment. Other research interests include the active targeting of tumor cells using novel nanoparticles.
Douglas Steeber received his Ph.D. from the University of Wisconsin-Madison in 1995. He trained as a Postdoctoral Fellow under Dr. Thomas Tedder in the Department of Immunology at Duke University from 1995 to 1997. In 1997 he became an Assistant Research Professor in the Immunology department where he continued working until 2003. In 2003 he moved to the Department of Biological Sciences at the University of Wisconsin-Milwaukee as an Assistant Professor. In 2006 he was promoted to Associate Professor within the department. He is currently a UWM Chancellor's Scientist and Director of the Flow Cytometry Facility. Dr. Steeber continues to conduct research in the areas of inflammation and autoimmune disease with a focus on mechanisms of leukocyte recruitment. Other research interests include the active targeting of tumor cells using novel nanoparticles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare there are no conflicts of interest.
Reference
- 1.Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000;22:299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, et al. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–22. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 4.Tu L, Poe JC, Kadono T, Venturi GM, Bullard DC, Tedder TF, et al. A functional role for circulating mouse L-selectin in regulating leukocyte/endothelial cell interactions in vivo. J Immunol. 2002;169:2034–43. doi: 10.4049/jimmunol.169.4.2034. [DOI] [PubMed] [Google Scholar]
- 5.Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, et al. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19:713–24. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 6.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 7.Steeber DA, Campbell MA, Basit A, Ley K, Tedder TF. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Natl Acad Sci, USA. 1998;95:7562–7. doi: 10.1073/pnas.95.13.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadono T, Venturi GM, Steeber DA, Tedder TF. Leukocyte rolling velocities and migration are optimized by cooperative L-selectin and intercellular adhesion molecule-1 functions. J Immunol. 2002;169:4542–50. doi: 10.4049/jimmunol.169.8.4542. [DOI] [PubMed] [Google Scholar]
- 9.Steeber DA, Subramanian H, Grailer JJ, Conway RM, Storey TJ. L-selectin-mediated leukocyte adhesion and migration. In: Ley K, editor. Adhesion molecules: function and inhibition. Birkhauser Verlag AG; Basel: 2007. pp. 27–70. [Google Scholar]
- 10.Arbones ML, Ord DC, Ley K, Radich H, Maynard-Curry C, Capon DJ, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 11.Steeber DA, Tang MLK, Zhang X-Q, Müller W, Wagner N, Tedder TF. Efficient lymphocyte migration across high endothelial venules of mouse Peyer's patches requires overlapping expression of L-selectin and β7 integrin. J Immunol. 1998;161:6638–47. [PubMed] [Google Scholar]
- 12.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol. 1993;143:1688–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Akahori T, Yuzawa Y, Nishikawa K, Tamatani T, Kannagi R, Miyasaka M, et al. Role of a sialyl Lewisx-like epitope selectively expressed on vascular endothelial cells in local skin inflammation of the rat. J Immunol. 1997;158:5384–92. [PubMed] [Google Scholar]
- 14.Kabel PJ, Voorbij HAM, de Haan-Meulman M, Pals ST, Drexhage HA. High endothelial venules present in lymphoid cell accumulations in thyroids affected by autoimmune disease: a study in men and BB rats of functional activity and development. J Clin Endocrin and Metabol. 1989;68:744–51. doi: 10.1210/jcem-68-4-744. [DOI] [PubMed] [Google Scholar]
- 15.Pablos JL, Santiago B, Tsay D, Singer MS, Palao G, Galindo M, et al. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-α/βand TNF-α in cultured endothelial cells. BMC Immunol. 2005;6:6–15. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, et al. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid binding to islet endothelium. J Clin Invest. 1993;92:2509–15. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen SD, Tsay D, Hemmerich S, Abraham WM. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am J Pathol. 2005;166:935–44. doi: 10.1016/S0002-9440(10)62313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spertini O, Luscinskas FW, Kansas GS, Munro JM, Griffin JD, Gimbrone MA, Jr., et al. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991;147:2565–73. [PubMed] [Google Scholar]
- 19.Tu L, Delahunty MD, Ding H, Luscinskas FW, Tedder TF. The cutaneous lymphocyte antigen (CLA) is an essential component of the L-selectin ligand induced in human vascular endothelial cells. J Exp Med. 1999;189:241–52. doi: 10.1084/jem.189.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spertini O, Cordey A-S, Monai N, Giuffrè L, Schapira M. P-selectin glycoprotein ligand-1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J Cell Biol. 1996;135:523–31. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erikksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and artherosclerosis in vivo. J Exp Med. 2001;194:205–18. doi: 10.1084/jem.194.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steeber DA, Engel P, Miller AS, Sheetz MP, Tedder TF. Ligation of L-selectin through conserved regions within the lectin domain activates signal transduction pathways and integrin function in human, mouse, and rat leukocytes. J Immunol. 1997;159:952–63. [PubMed] [Google Scholar]
- 23.Giblin PA, Hwang ST, Katsumoto TR, Rosen SD. Ligation of L-selectin on T lymphocytes activates β1 integrins and promotes adhesion to fibronectin. J Immunol. 1997;159:3498–507. [PubMed] [Google Scholar]
- 24.Hickey MJ, Forster M, Mitchell D, Kaur J, De Caigny C, Kubes P. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J Immunol. 2000;165:7164–70. doi: 10.4049/jimmunol.165.12.7164. [DOI] [PubMed] [Google Scholar]
- 25.Tsang YTM, Neelamegham S, Hu Y, Berg EL, Burns AR, Smith CW, et al. Synergy between L-selectin signaling and chemotactic activation during neutrophil adhesion and transmigration. J Immunol. 1997;159:4566–77. [PubMed] [Google Scholar]
- 26.Ding Z, Issekutz TB, Downey GP, Waddell TK. L-selectin enhances functional expression of surface CXCR4 in lymphocytes: implication for cellular activation during adhesion and migration. Blood. 2003;101:4245–52. doi: 10.1182/blood-2002-06-1782. [DOI] [PubMed] [Google Scholar]
- 27.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong BF, Murphy J-E, Kupper TS, Fuhlbrigge RC. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–81. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- 29.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 30.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, et al. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329–36. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang MLK, Hale LP, Steeber DA, Tedder TF. L-selectin is involved in lymphocyte migration to sites of inflammation in the skin: delayed rejection of allografts in L-selectin-deficient mice. J Immunol. 1997;158:5191–9. [PubMed] [Google Scholar]
- 32.Steeber DA, Tang MLK, Green NE, Zhang X-Q, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and intercellular adhesion molecule-1 pathways. J Immunol. 1999;163:2176–86. [PubMed] [Google Scholar]
- 33.Tedder TF, Steeber DA, Pizcueta P. L-selectin deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–64. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada Y, Hasegawa M, Kaburagi Y, Hamaguchi Y, Komura K, Saito E, et al. L-selectin or ICAM-1 deficiency reduces an immediate-type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J Immunol. 2003;170:4325–34. doi: 10.4049/jimmunol.170.8.4325. [DOI] [PubMed] [Google Scholar]
- 35.Kaburagi Y, Hasegawa M, Nagaoka T, Shimada Y, Hamaguchi Y, Komura K, et al. The cutaneous reverse arthus reaction requires intercellular adhesion molecule 1 and L-selectin expression. J Immunol. 2002;168:2970–8. doi: 10.4049/jimmunol.168.6.2970. [DOI] [PubMed] [Google Scholar]
- 36.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–47. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yukami T, Hasegawa M, Matsushita Y, Fujita T, Matsushita T, Horikawa M, et al. Endothelial selectins regulate skin wound healing in cooperation with L-selectin and ICAM-1. J Leukoc Biol. 2007;82:519–31. doi: 10.1189/jlb.0307152. [DOI] [PubMed] [Google Scholar]
- 38.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1003. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venturi GM, Conway RM, Steeber DA, Tedder TF. CD25+CD4+ regulatory T cell migration requires L-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J Immunol. 2007;178:291–300. doi: 10.4049/jimmunol.178.1.291. [DOI] [PubMed] [Google Scholar]
- 42.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorbachev AV, Fairchild RL. CD4+ T cells regulate CD8+ T cell-mediated cutaneous immune responses by restricting effector T cell development through a Fas ligand-dependent mechanism. J Immunol. 2004;172:2286–95. doi: 10.4049/jimmunol.172.4.2286. [DOI] [PubMed] [Google Scholar]
- 44.Siegmund K, Feuerer M, Siewart C, Ghani S, Haubold U, Dankof A, et al. Migration matters: regulatory T cell compartmentalization determines supressive activity in vivo. Blood. 2005;106:3097–104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ring S, Oliver SJ, Cronstein BN, Enk AH, Mahnke K. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J Allergy Clin Immunol. 2009;123:1287–96. doi: 10.1016/j.jaci.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 46.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–88. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–81. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–65. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–56. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 50.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–57. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–64. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–56. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, et al. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol. 2008;180:3910–8. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 54.Kodera M, Grailer JJ, Karalewitz AP-A, Subramanian H, Steeber DA. T lymphocyte migration to lymph nodes is maintained during homeostatic proliferation. Microsc Microanal. 2008;14:211–24. doi: 10.1017/S1431927608080215. [DOI] [PubMed] [Google Scholar]
- 55.King C, Llic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 56.Calzascia T, Pellegrini M, Lin A, Garza KM, Elford AR, Shahinian A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci USA. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ploix C, Lo D, Carson MJ. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J Immunol. 2001;167:6724–30. doi: 10.4049/jimmunol.167.12.6724. [DOI] [PubMed] [Google Scholar]
- 58.Kogan TP, Dupre B, Bui H, McAbee KL, Kassir JM, Scott IL, et al. Novel synthetic inhibitors of selectin-mediated cell adhesion: synthesis of 1,6-bis[3-(3-carboxymethylphenyl)-4-(2-α–D-mannopyranosyloxy)phenyl]-hexane (TBC-1269) J Med Chem. 1998;41:1099–111. doi: 10.1021/jm9704917. [DOI] [PubMed] [Google Scholar]
- 59.Davenpeck KL, Berens KL, Dixon RAF, Dupre B, Bochner BS. Inhibition of adhesion of human neutrophils and eosinophils to P-selectin by the sialyl Lewis(x) antagonist TBC-1269. Preferential activity against neutrophil adhesion in vitro. J Allergy Clin Immunol. 2000;105:769–75. doi: 10.1067/mai.2000.105121. [DOI] [PubMed] [Google Scholar]
- 60.Hicks AER, Abbitt KB, Dodd P, Ridger VC, Hellewell PG, Norman KE. The anti-inflammatory effects of a selectin ligand mimetic, TBC-1269, are not a result of competitive inhibition of leukocyte rolling in vivo. J Leukoc Biol. 2005;77:59–66. doi: 10.1189/jlb.1103573. [DOI] [PubMed] [Google Scholar]
- 61.Beeh KM, Beier J, Meyer M, Buhl R, Zahlten R, Wolff G. Bimosiamose, an inhaled small-molecule pan-selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double-blind, placebo-controlled clinical cross-over-trial. Pulm Pharmacol Ther. 2006;19:233–41. doi: 10.1016/j.pupt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Avila PC, Boushey HA, Wong H, Grundland H, Liu J, Fahy JV. Effect of a single dose of the selectin inhibitor TBC1269 on early and late asthmatic responses. Clin Exp Allergy. 2004;34:77–84. doi: 10.1111/j.1365-2222.2004.01831.x. [DOI] [PubMed] [Google Scholar]
- 63.Friedrich M, Bock D, Philipp S, Ludwig N, Sabat R, Wolfk K, et al. Pan-selectin antagonism improves psoriasis manifestation in mice and man. Arch Dermatol Res. 2006;297:345–51. doi: 10.1007/s00403-005-0626-0. [DOI] [PubMed] [Google Scholar]
- 64.Schlag G, Redl HR, Till GO, Davies J, Martin U, Dumont L. Anti-L-selectin antibody treatment of hemorrhagic-traumatic shock in baboons. Crit Care Med. 1999;27:1900–7. doi: 10.1097/00003246-199909000-00031. [DOI] [PubMed] [Google Scholar]
- 65.Seekamp A, van Griensven M, Dhondt E, Diefenbeck M, Demeyer I, Vundelinckx G, et al. The effect of anti-L-selectin (aselizumab) in multiple traumatized patients-results of a phase II clinical trial. Crit Care Med. 2004;32:2021–8. doi: 10.1097/01.ccm.0000142396.59236.f3. [DOI] [PubMed] [Google Scholar]
- 66.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P-selectin-deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 67.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–9. [PubMed] [Google Scholar]
- 68.Staite ND, Justen JM, Sly LM, Beaudet AL, Bullard DC. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–9. [PubMed] [Google Scholar]
- 69.Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RO, Wagner DD. Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-selectin-deficient mice. J Exp Med. 1995;181:2277–82. doi: 10.1084/jem.181.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramaniam M, Saffaripour S, Van de Water L, Frenette PS, Mayadas TN, Hynes RO, et al. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–9. [PMC free article] [PubMed] [Google Scholar]
- 71.Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–20. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]