Abstract
The γ subunit of rod-specific cGMP phosphodiesterase 6 (PDE6γ), an effector of the G-protein GNAT1, is a key regulator of phototransduction. The results of several in vitro biochemical reconstitution experiments conducted to examine the effects of phosphorylation of PDE6γ on its ability to regulate the PDE6 catalytic core have been inconsistent, showing that phosphorylation of PDE6γ may increase or decrease the ability of PDE6γ to deactivate phototransduction. To resolve role of phosphorylation of PDE6γ in living photoreceptors, we generated transgenic mice in which either one or both Threonine (T) sites in PDE6γ (T22 and T35), which are candidates for putative regulatory phosphorylation, were substituted with alanine (A). Phosphorylation of these sites was examined as a function of light exposure. We found that phosphorylation of T22 increases with light exposure in intact mouse rods while constitutive phosphorylation of T35 is unaffected by light in intact mouse rods and cones. Phosphorylation of the cone isoform of PDE6γ, PDE6H, is constitutively phosphorylated at the T20 residue. Light-induced T22 phosphorylation was lost in T35A transgenic rods, and T35 phosphorylation was extinguished in T22A transgenic rods. The interdependency of phosphorylation of T22 and T35 suggests that light-induced, post-translational modification of PDE6γ is essential for the regulation of G-protein signaling.
Introduction
Phosphodiesterase 6 (PDE6) enzyme complex consists of catalytic PDE6α and PDE6β as well as regulatory PDE6γ subunits. In dark-adapted retinas, PDE6γ binds to PDE6αβ and inhibits its activity. Upon light exposure, GTP-bound G-protein transducinα (GNAT1) binds to and inhibits PDE6γ, thereby activating PDE6. During photoresponse recovery, GNAT1 is deactivated by hydrolysis of GTP to GDP, permitting PDE6γ to rapidly re-inhibit PDE6αβ [1; 2; 3; 4; 5]. Eventually, the guanylate cyclase-activating proteins GCAP1 and GCAP2 stimulate guanylate cyclases to form more cGMP, thus counteracting the reduction in cGMP caused by PDE6.
In salamander, calcium (Ca2+) has been shown to be necessary and sufficient for light-adaptation at low intensities [6]. The function of Ca2+ sensitive GCAPs on the guanylate cyclase feedback loop is thought to be the most predominant mechanism of adaptation in amphibians. In mice homozygous null for Gcap1 and Gcap2 (Gcap-/-), the response adaptation waveforms are abnormal [7; 8]. However, loss of GCAP activity in mice is not sufficient to abolish adaptation. Although abnormal, Gcap-/- mice response waveforms continue to show accelerated decay in background light [7; 8]. The Weber decline in sensitivity is greater in Gcap-/- than in wild-type mice, but not as much as would be expected if adaptation were completely disrupted. This result indicates that there are additional mechanisms of light adaptation in mice that modulate PDE activity [8; 9].
Acceleration of the response decay from background light accounts for the reduced flash sensitivity of rods during light adaptation. We have recently described a novel mechanism for the reduction in the sensitivity of light adapted mammalian rods that reflects an acceleration of the rate of PDE6 shutoff in the presence of background light [10]. This finding was unexpected because background light does not affect the rate-limiting time constant (tD) for response decay in salamander rods [11; 12] and it has been assumed that mammalian rods behave similarly to salamander rods.
In mammalian rods, GCAP stimulation of cyclase activity accounts for only one half of the extended operating range of light exposed rod cells [7; 13]. The remainder of the response range can be mediated by Ca2+-dependent phosphorylation of PDE6γ [7; 13]. There are two steps in the phototransduction cascade where PDE6γ can influence light adaptation: regulation of GNAT1 and deactivation of PDE6. Although inhibition of PDE6 by PDE6γ primarily involves regulation of the duration of cGMP catalytic activity, several studies have demonstrated that the function of PDE6γ itself may be influenced by phosphorylations. These pathways include phosphorylation of PDE6γ at two sites, threonine 22 (T22) [14; 15; 16; 17; 18] and threonine 35 (T35) [18; 19; 20]. T22 is a consensus site for proline-directed kinases such as cyclin-dependent kinase 5 (CDK5) or MAP kinase, and T35 is a consensus site for serine/threonine kinases such as Protein Kinase A or C. Incubation of frog retinal extracts with the CDK inhibitors olomoucined and roscovitine have been shown to block light-dependent phosphorylation of PDE6 in vitro[15; 17; 19; 21]. Since the portion of the PDE6γ sequence containing these two sites is known to be important for association of PDE6γ with GNAT1 [22; 23; 24] and PDE6αβ [24; 25; 26], phosphorylation of one of these threonines might be expected to alter PDE6 activation or half-life of GNAT1 GTPase.
Several investigators have examined the effects of PDE6γ phosphorylation on the activity of PDE6, but the results of these reports are controversial. Phosphorylation of PDE6γ can increase [19] or decrease the ability of PDE6γ to inhibit purified bovine PDE6 or GNAT1-activated PDE6 [18], respectively. T35 phosphorylation increased inhibition to PDE6αβ [27].
To address these controversies, we generated mice expressing mutant PDE6γ proteins with either T22 or T35 or both replaced with alanine. Alanine substitution eliminates phosphorylation but is likely to be sufficiently conservative to have little effect by itself on the already flexible structure of the PDE6γ molecule. These transgenic mice retain normal levels of other phototransduction proteins [10; 28] such that changes in the physiological and biochemical characteristics of the photoreceptors reflect direct consequences from the lack of phosphorylation. Phosphorylation of these sites was examined as a function of light exposure.
Materials and Methods
Mouse Lines
The mice studied in these experiments were used in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Six-week old MF1 wild-type (WT) and transgenic mice were dark-adapted overnight and sacrificed under infrared conditions.
T22A or T35A point mutation was introduced using a standard site-specific mutagenesis strategy [1; 28; 29; 30].
Materials
Recombinant bovine PDE6γ was a generous gift of Professor Akio Yamazaki. Retinas from overnight dark-adapted mice were placed in a watch glass in Ringer's Buffer (PMSF, phosphatase inhibitor cocktails I+II, Sigma-Aldrich, St. Louis) exposed to flashes, snap frozen in liquid nitrogen, homogenized, and analyzed by immunoblotting.
Phosphorylated PDE6 was detected with phospho-specific antibodies generated in rabbits with PDE6 phospho-peptides {IGGPV(pT)PRKGPPKC} and {KGPPKFKQRQ(pT)RQFKSKPPK} to produce anti-pT22 and anti-pT35 by Abgent Inc., San Diego (dilution 1:1000). PDE6H phospho-peptide {TTGDAPTGPTpTPR} was used to produce polyclonal rabbit anti-pT20 (Abgent Inc., San Diego).
The consistent loading of total PDE6 was verified with rabbit anti-PDE6 antibody (dilution 1:5000, Affinity BioReagents).
The antibodies were tested with phosphorylated and non-phosphorylated peptides and phosphorylated and non-phosphorylated recombinant PDE6. Quantitative measurements of the immunocomplexes were done with an AlphaImager with AlphaImager Software for Windows (AlphaInnotech, San Leandro, CA). Their linearity range was determined (30 - 90 ng total protein), and the equations and regression coefficients (r2) are given as follows (y = IDV, integrated density values):
Rabbit anti-pT22: y = 3.09 * 106 × (ng) − 8.79 * 106 r2 = 0.9103
Rabbit anti-pT35: y = 4.32 * 106 × (ng) − 4.62 * 107 r2 = 0.9558
Rabbit anti-PDE6 : y = 2.14 * 106 × (ng) + 8.92 * 107 r2 = 0.9754
To accurately measure pT22, pT35, and total PDE6γ in retinal lysates, three two-fold dilutions of each lysate were loaded per gel. After densitometry, the densities of the signals from each dilution were compared to determine which sample was in the linear range.
Results
The specificity of antibodies to detect T22 and T35 phosphorylation
The pT22, pT35 and pT20 antibodies only recognized the phosphorylated cognate PDE6γ. The pT22 antibody only recognized MAP kinase-treated recombinant PDE6γ which was phosphorylated at T22 (top row, Fig. 1A and B), while the pT35 antibody only recognized PKA kinase-treated recombinant PDE6γ which was phosphorylated at T35 (second row, Fig. 1A and1B).
Fig. 1.
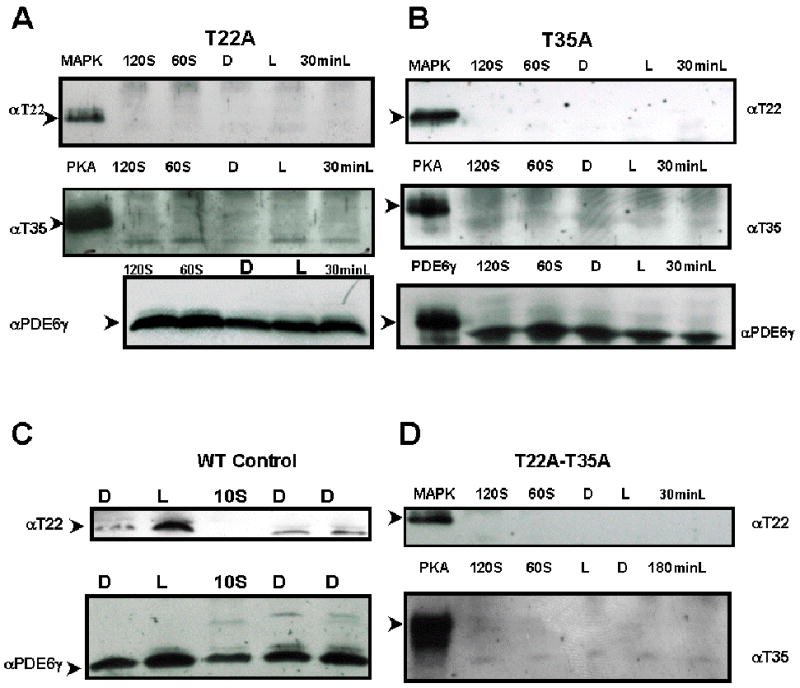
Both anti-pT22 and anti-pT35 antibodies recognized specific residues in phosphorylated PDE6γ.
Immunoblot of mutant transgenic retinal extracts probed with rabbit anti-phosphoT22 antibody recognizing the phosphorylated T22 residue of PDE6γ or rabbit anti-phosphoT35 antibody recognizing the phosphorylated T35 residue of PDE6.
MAPK, mitogen-activated protein kinase phosphorylated PDE6γ marked the expected molecular weight of pT22- PDE6γ at 11 kDa (top row, Fig 1A, B and D).
PKA, protein kinase A phosphorylated PDE6γ marked the expected molecular weight of pT35- PDE6γ (second row, Fig 1A, B and D).
Panel A: T22A transgenic retinal extract. T35 phosphorylation was lost in T22A transgenic rods.
Panel B: T35A transgenic retinal extract. Light induced T22 phosphorylation is lost in T35A transgenic rods.
Panel C: Control wild-type MF1 retinal extract. Light induced T22 phosphorylation was seen.
Panel D: T22A-T35A transgenic retinal extract. Both light-induced T22 and basal T35 phosphorylation was significantly diminished.
Presence of PDE6γ in each sample was confirmed by stripping and re-staining the filter with rabbit anti-native PDE6γ antibody. Reprobing with anti-PDE6γ antibody demonstrated expression of PDE6γ protein in these lysates.
D, overnight dark-adapted retinal extracts; L, light-adapted; 60S, 60 seconds of light exposure to a dark-adapted retina; 120S, 120 seconds of light exposure to a dark-adapted retina; 30minL, 30 minutes of light exposure to a dark-adapted retina; 180 minL, 180 minutes of light exposure to a dark-adapted retina. MAPK, mitogen-activated protein kinase phosphorylated PDE6; PKA, protein kinase A phosphorylated PDE6. Both pT22 and pT35 antibodies only recognized the phosphorylated cognate peptides and anti-pT22 only recognized MAP kinase-treated (phosphorylates T22), while anti-pT35 only recognized PKA kinase-treated (phosphorylates T35) recombinant PDE6γ.
The immunoreactivities of pT22 and pT35 were specific, as they did not produce a signal when used to analyze T22A (Fig. 1A) and T35A (Fig. 1B) mutant retinal extracts, respectively (Fig. 1). Light-induced T22 phosphorylation was neither detected in light (L) nor dark (D) adapted T22A reitnas (Fig. 1A), but T22 phosphorylation was readily seen in light adapted wildtype (WT) controls (Fig. 1C). Similarly, basal constitutive T35 phosphorylation was not detected in light (L) or dark (D) adapted T35A retinas (Fig. 1B) but was readily seen in both light (L) and dark (D) adapted mouse and chicken retinal lysates (Fig. 3).
Fig. 3.
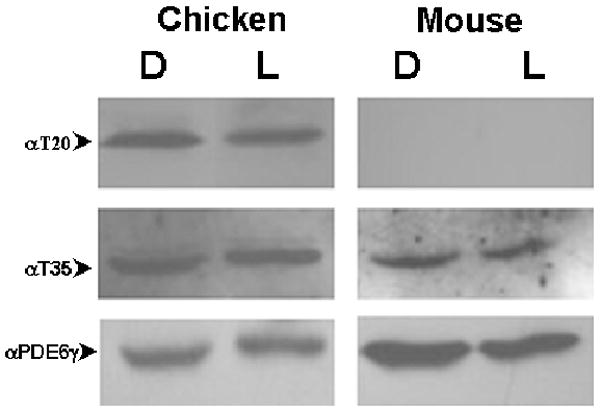
PDE6H phosphorylation in chicken and murine retina.
Immunoblot of normalized dark- and light-adapted chicken and murine retinal extracts probed with rabbit anti-phosphoT20 {TTGDAPTGPT(pT)PR} antibody recognizing the phosphorylated T20 residue of PDE6H or rabbit anti-pT35 antibody recognizing the phosphorylated T35 residue of PDE6γ. Phosphorylated T20 was not detected in mouse retinal extract. Presence of PDE6γ in each sample was confirmed by stripping and re-staining the filter with rabbit anti-native PDE6γ antibody. D, dark-adapted overnight; L, light-adapted to 1.67×10-1 W/cm2 for sixteen hours.
Light induced T22 phosphorylation in intact mouse retinas
Immunoblot analyses of wild-type retinal extracts showed that PDE6γ was phosphorylated at the pT22 site in a light-dependent manner (Fig. 1C and 2), while T35 phosphorylation was not light-dependent (Fig. 3). T22 phosphorylation was 1.5-fold higher in light-adapted as compared to dark-adapted retinas (Fig. 1). Basal T35 phosphorylation levels in dark-adapted retinas were about 7.5% to 38% of total PDE6. T22 phosphorylation levels were 8.89% of the total PDE6 (Table 1).
Fig. 2.
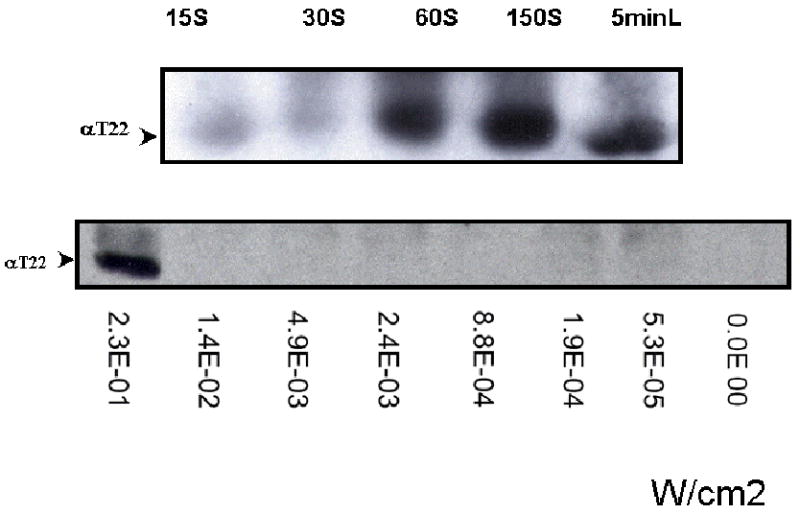
Linearity between T22 PDE6γ phosphorylation and Light Intensities.
Panel A: T22 phosphorylation occurs within one minute of illumination. Retinas were removed and placed in a Petri dish with 5 μl of Ringer buffer to prevent drying-out. The Petri dish was placed under a light source and the dark-adapted retinas were exposed to different irradiances: 2.48× 10-2, 4.93×10-2, 8.63×10-2, 2.33×10-1, 4.73×10-1 (W/cm2) for 15S 30S, 60S, 150S and 5 minutes (5minL), respectively.
Panel B: the dark-adapted retinas were exposed to a single flash of increasing intensities: 5.3× 10-5, 1.9× 10-4, 8.8× 10-4, 2.4× 10-3, 1.4× 10 , and 2.3× 10-1 W/cm2. T22 phosphorylation was not detected until the flash increased to 2.3× 10-1 W/cm2. The irradiance (W/cm2) of the light source was determined with a light meter (Research Radiometer IL1700, International Light Inc., MA) containing a sensor fitted with a photopic filter (Y#23654).
Table 1.
Densitometry assessment of % PDE6 phosphorylation in wild-type (WT) and transgenic retinal extacts. The irradiance (W/cm2), or the light source, was determined with a light meter containing a sensor fitted with a photopic filter. T22A, T35A and T22A-T35A mice received 1.67×10-1 W/cm2. Quantitative measurements of the bands were done with an AlphaImager with Software for Windows (AlphaInnotech, San Leandro, CA). D, dark-adapted; L, light-adaptedl; NS, non-significant.
| T35P (ng) | T22P (ng) | Total PDE6γ (ng) | %T35P/Total PDE6γ | %T22P/Total PDE6γ | |
|---|---|---|---|---|---|
| Illumination at 1.67×10-1 W/cm2 | |||||
| WT L | 19.65 | 11.30 | 82.21 | 33.67% | 13.75% |
| WT D | 30.27 | 7.14 | 80.30 | 37.69% | 8.89% |
| T22A L | 47.27 | NS | 78.13 | 60.50% | |
| T22A D | 36.50 | NS | 96.25 | 37.92% | |
| T35A L | NS | NS | 46.01 | ||
| T35A D | NS | NS | 54.30 | ||
| T22A-T35A L | NS | NS | 47.16 | ||
| T22A-T35A D | NS | NS | 36.43 |
T22 phosphorylation is affected by status of T35 phosphorylation, and vice versa
To explore possible regulatory interactions between the phosphorylation of T22 and T35, the status of T22 and T35 phosphorylation in T35A and T22A mutants, respectively, was examined (Fig. 1). Immunoblots of T35A retinal lysates probed with pT22 antibody failed to detect T22 phosphorylation under dark or light adaptation conditions. Similarly, basal T35 phosphorylation was attenuated in T22A mutants (Fig. 1A). Retinal lysates prepared from mice carrying the double mutations (T22A- T35A) were barely phosphorylated (Fig. 1D).
The mechanisms that control T22 and T35 phosphorylation and the function of these residues exhibit reciprocal characteristics. T22 phosphorylation was enhanced after light adaptation, while T22A and T35A mutations induced opposing effects on PDE6 inactivation kinetics.
Light-dependent phosphorylation of T22 residue in PDE6γ
T22 phosphorylation occurred within 60 seconds of illumination at 8.63×10-2 W/cm2 (Fig. 2A). In Fig. 2B, T22 phosphorylation was not detected until a single flash increased to 2.3× 10-1 W/cm2. Hence, a threshold of light intensity was needed to induce T22 phosphorylation, which remained stable under constant illumination. The level of phosphorylated T22 from dark-adapted retinas stayed relatively stable within 5 min of light adaptation and did not increase significantly with light exposure (Fig. 2).
Immunoblot signals were measured using a densitometer (Personal Densitometer SI 375, Molecular Dynamics). To determine the linear range of the assay, a standard curve analysis was performed of recombinant pT22, and unphosphorylated PDE6γ protein. There was a linear relationship between signal density and amount of protein, generally between 5 ng and 80 ng of recombinant protein (not shown).
The amount of phosphorylated T22 PDE6γ in light and dark-adapted wild-type retinas was quantified (Fig. 1C). Using densitometry, T22P and total PDE6γ signals from a scanned film were measured, and the amounts of protein for each sample were calculated. After normalization for total PDE6γ content, there was a non-significant change in T35P level (0.9-fold) but a more significant increase in T22P level (1.5-fold) in light-adapted lysates.
PDE6H is constitutively phosphorylated in cone-enriched retinal extracts
T20 and T35 are phosphorylation sites in cone PDE6H. Neither basal T20 nor T35 phosphorylation was altered by changes in light intensity on chicken retinas (Fig. 3). The cone-specific pT20 PDE6H antibody did not react to rod-dominant mouse retinal extract (Fig. 3). T35 was constitutively phosphorylated and was light-independent in both cone (chicken) and rod (mouse) enriched retinas (Fig. 3).
The amount of phosphorylated T35 PDE6γ in light and dark-adapted wild-type retinas was quantified (Fig. 3). Using densitometry, T35P and total PDE6γ signals from a scanned film were measured, and the amounts of protein for each sample were calculated. After normalization for total PDE6γ content, there was only a minor increase in T35P level (1.1-fold) in light-adapted lysates.
Discussion
Previous conflicting reports suggest that phosphorylation of T22 can increase [14; 15; 17; 21] or have minimal effect [27] on PDE6γ inhibition of activated PDE6αβ, while T35 phosphorylation increases binding to PDE6αβ [19].
The physiological changes observed in the retinas of T35A mice (sensitivity decrease, amplitude decrease, and sooner initiation of current recovery) are all characteristics of light-adapted rods and are explainable if dephosphorylated PDE6γ has a significant rate of spontaneous dissociation from PDE6αβ in the dark [10; 28]. Such a phosphate transfer could regulate GNAT1-GTP-PDE6 signals without affecting GTP hydrolysis [31]. Specifically, phosphorylation of PDE6γ would induce its release from GNAT1-GTP whereupon it would bind to and inhibit active PDE6 α [15; 17; 21]. If PDE6γ is phosphorylated before it re-inhibits PDE6, a pool of PDE6γ with phosphorylated PDE6 is created, the size of which may depend on the intensity of the illumination.
The apparent inconsistencies in previous studies could, in part, be due to the differences between mammalian and amphibians and/or various in vitro PDE6γ phosphorylation incubation times: 33 °C for 30 min [15] verses room temperature for 5 hours in the presence of GTPγS without an ATP-regenerating system [27]. More importantly, there are limitations of in vitro studies which may be not be sufficiently sensitive to model the rather subtle effects of PDE6γ phosphorylation on the physiology of rod responses. In vitro biochemical experiments assess steady state levels of phosphorylation; whereas suction recording of rods sample dynamic effects of phosphorylation in vivo time scale. In frogs, ∼4% of the total PDE6γ is phosphorylated within 10 seconds when. 03% of the rhodopsin is illuminated [21]. This percentage is significant because the compartmentalization and close proximity of PDE signaling components is likely to enhance protein-protein interactions affected by PDE6γ phosphorylation in the disc membranes.
Light-induced T22 phosphorylation is lost in T35A transgenic rods; T35 phosphorylation is extinguished in T35A transgenic rods. T22 phosphorylation occurred within 60 seconds of illumination at 8.63×10-2 W/cm2 (Fig. 2A). These results indicate an association between T22 and T35 phosphorylation and PDE6 inactivation, which suggests a mechanism for the physiological phenotype of the phosphorylation mutants [10; 28]. Our data are consistent with the idea that T22 is phosphorylated during photoactivation; this phosphorylation would have the effect of improving PDE6 binding affinity to GNAT1. Phosphorylated T22 may represent a low PDE6 affinity state where PDE6 can be activated readily. Continuous noise due to PDE6αβ activity should be significant in this situation, as PDE6γ could more easily attach and detach from PDE6αβ, resulting in the attachment different PDE6γ from the non-PDE6αβ-bound PDE6γ pool. This phenomenon would account for the majority of dark noise [32; 33].
There are two light-dependent phosphoproteins with molecular weights similar to PDE6γ that have been reported [34] in intact frog rod outer segments. “Component II”, a 12-kDa phospho-protein, would represent mono-phosphorylated PDE6γ, whereas “Component I”, a 13-kDa phosphoprotein, would correspond to PDE6γ with phosphates on both T22 and T35. Under saturating illumination, the remaining phosphorylated “component I and II”, which accounts for 35% of the total PDE6γ, could correspond to light-induced phosphorylation at T22. The characteristics of the two frog phosphoproteins match our model for light-dependent phosphorylation of PDE6γ.
Removal of T22 phosphorylation site accelerates the photoresponse whereas removal of the T35 phosphorylation site slows the response [10; 28]. Transgenic rods with nonphosphorylatable T35A saturate prematurely in bright illumination. Astonishingly, light-induced acceleration of the dominant time constant of response recovery is blocked in mice expressing T35A, suggesting that PDE6γ plays an essential role during light adaptation in mammalian rods [10; 28]. Such behavior was unexpected because modulation of the limiting time constant by background light had not been reported to occur in amphibian rods [11; 12]. PDE6γ phosphorylation regulates responses of normal rod, and that the opposite effects of the two phosphorylation sites may play a role in increasing the dynamic response range of these cells.
Light adaptation desensitizes photoreceptors by increasing the amount of light needed to generate a photoresponse. This process is particularly prominent in cones but is also exhibited by rods. Cone-specific PDE6H has two threonine residues, T20 and T35, within known consensus reading frames for kinase recognition. Light induced increase of T20 and T35 phosphorylation cannot be detected (Fig. 3). Neither T20 nor T35 phosphorylation in PDE6H is therefore likely to contribute to light adaptation in cones (Fig. 3).
Control of PDE6γ phosphorylation/dephosphorylation is dependent on PDE6 activity, the canonical phototransduction and adaptation pathways. Results from Fig.s 1 and 2 established the light dependency of phosphorylation of PDE6γ at T22. Light-induced phosphorylation of PDE6γ may provide a resolution to the dilemma of why light adaptation is not completely eliminated in rods lacking both GCAP1 and GCAP2[7; 13].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsang SH, Burns ME, Calvert PD, Gouras P, Baylor DA, Goff SP, Arshavsky VY. Role of the Target Enzyme in Deactivation of Photoreceptor G Protein in Vivo. Science. 1998;282:117–121. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- 2.Tsang SH, Woodruff ML, Chen CK, Yamashita CY, Cilluffo MC, Rao AL, Farber DB, Fain GL. GAP-independent termination of photoreceptor light response by excess gamma subunit of the cGMP-phosphodiesterase. J Neurosci. 2006;26:4472–80. doi: 10.1523/JNEUROSCI.4775-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang SH, Gouras P. Molecular Physiology and Pathology of the Retina. In: Tasman W, Jaeger EA, editors. Duane's Clinical Opthalmology. J.B. Lippincott; Philadelphia: 1996. Chapter 2. [Google Scholar]
- 4.Tsang SH, Gouras P. Photoreceptors and photoreceptor dysfunctions. In: Adelman G, Smith B, editors. Encyclopedia of Neuroscience. Elsevier Science; Amsterdam: 2004. pp. 1633–1644. [Google Scholar]
- 5.Tsang SH, Yamashita CK, Doi K, Salchow DJ, Bouvier N, Mendelsohn M, Gouras P, Farber DB, Goff SP. In vivo studies of the gamma subunit of retinal cGMP-phophodiesterase with a substitution of tyrosine-84. Biochem J. 2001;353:467–74. doi: 10.1042/0264-6021:3530467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutalos Y, Nakatani K, Tamura T, Yau KW. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995;106:863–90. doi: 10.1085/jgp.106.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- 8.Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A. 2001;98:9948–53. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendez A, Chen J. Mouse models to study GCAP functions in intact photoreceptors. Adv Exp Med Biol. 2002;514:361–88. doi: 10.1007/978-1-4615-0121-3_22. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci. 2008;28:2064–74. doi: 10.1523/JNEUROSCI.2973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikonov S, Lamb TD, Pugh EN., Jr The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light-adapted salamander rod photoresponse. J Gen Physiol. 2000;116:795–824. doi: 10.1085/jgp.116.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepperberg DR, Cornwall MC, Kahlert M, Hofmann KP, Jin J, Jones GJ, Ripps H. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992;8:9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- 13.Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A. 2001;98:9948–53. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki A, Moskvin O, Yamazaki RK. Phosphorylation by cyclin-dependent protein kinase 5 of the regulatory subunit (Pgamma) of retinal cgmp phosphodiesterase (PDE6): its implications in phototransduction. Adv Exp Med Biol. 2002;514:131–53. doi: 10.1007/978-1-4615-0121-3_9. [DOI] [PubMed] [Google Scholar]
- 15.Tsuboi S, Matsumoto H, Jackson KW, Tsujimoto K, Williams T, Yamazaki A. Phophorylation of an inhibitory subunit of cGMP phosphodiesterase in Rana catesbiana rod photoreceptors I & II. J Biol Chem. 1994;269:15016–15029. [PubMed] [Google Scholar]
- 16.Matsuura I, Bondarenko VA, Maeda T, Kachi S, Yamazaki M, Usukura J, Hayashi F, Yamazaki A. Phosphorylation by cyclin-dependent protein kinase 5 of the regulatory subunit of retinal cGMP phosphodiesterase. I. Identification of the kinase and its role in the turnoff of phosphodiesterase in vitro. J Biol Chem. 2000;275:32950–7. doi: 10.1074/jbc.M000702200. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi F, Matsuura I, Kachi S, Maeda T, Yamamoto M, Fujii Y, Liu H, Yamazaki M, Usukura J, Yamazaki A. Phosphorylation by cyclin-dependent protein kinase 5 of the regulatory subunit of retinal cGMP phosphodiesterase. II. Its role in the turnoff of phosphodiesterase in vivo. J Biol Chem. 2000;275:32958–65. doi: 10.1074/jbc.M000703200. [DOI] [PubMed] [Google Scholar]
- 18.Paglia MJ, Mou H, Cote RH. Regulation of Photoreceptor Phosphodiesterase (PDE6) by Phosphorylation of Its Inhibitory gamma Subunit Re-evaluated. J Biol Chem. 2002;277:5017–5023. doi: 10.1074/jbc.M106328200. [DOI] [PubMed] [Google Scholar]
- 19.Udovichenko IP, Cunnick J, Gonzalez K, Takemoto DJ. Functional effect of phosphorylation of the photorecptor phosphodiesterase inhibitory subunit by protein kinase C. J Biol Chem. 1994;269:9850–9856. [PubMed] [Google Scholar]
- 20.Xu LX, Tanaka Y, Bonderenko VA, Matsuura I, Matsumoto H, Yamazaki A, Hayashi F. Phosphorylation of the gamma subunit of the retinal photoreceptor cGMP phosphodiesterase by the cAMP-dependent protein kinase and its effect on the gamma subunit interaction with other proteins. Biochemistry. 1998;37:6205–13. doi: 10.1021/bi973087i. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi F, Matsuura I, Kachi S, Maeda T, Yamamoto M, Fujii Y, Liu H, Yamazaki M, Usukura J, Yamazaki A. Phosphorylation by cyclin-dependent protein kinase 5 of the regulatory subunit of retinal cGMP phosphodiesterase. II. Its role in the turnoff of phosphodiesterase in vivo. J Biol Chem. 2000;275:32958–65. doi: 10.1074/jbc.M000703200. [DOI] [PubMed] [Google Scholar]
- 22.Morrison DF, Cunnick JM, Oppert B, Takemoto DJ. Interaction of the gamma-subunit of retinal rod outer segment phosphodiesterase with transducin. Use of synthetic peptides as functional probes. J Biol Chem. 1989;264:11671–11681. [PubMed] [Google Scholar]
- 23.Takemoto DJ, Hurt D, Oppert B, Cunnick J. Domain mapping fo the retinal cyclic GMP phosphdiesterase γ-subunit. Biochem J. 1992;281:637–643. doi: 10.1042/bj2810637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artemyev NO, Hamm HE. Two-site high-affinity interaction between inhibitory and catalytic subunits of rod cyclic GMP phosphodiesterase. Biochem J. 1992;283:273–279. doi: 10.1042/bj2830273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mou H, Cote RH. The Catalytic and GAF Domains of the Rod cGMP Phosphodiesterase (PDE6) Heterodimer Are Regulated by Distinct Regions of Its Inhibitory gamma Subunit. J Biol Chem. 2001;276:27527–34. doi: 10.1074/jbc.M103316200. [DOI] [PubMed] [Google Scholar]
- 26.Guo LW, Grant JE, Hajipour AR, Muradov H, Arbabian M, Artemyev NO, Ruoho AE. Asymmetric interaction between rod cyclic GMP phosphodiesterase gamma subunits and alpha/beta subunits. J Biol Chem. 2005 doi: 10.1074/jbc.M410380200. [DOI] [PubMed] [Google Scholar]
- 27.Paglia MJ, Mou H, Cote RH. Regulation of photoreceptor phosphodiesterase (PDE6) by phosphorylation of its inhibitory gamma subunit re-evaluated. J Biol Chem. 2002;277:5017–23. doi: 10.1074/jbc.M106328200. [DOI] [PubMed] [Google Scholar]
- 28.Tsang SH, Woodruff ML, Janisch KM, Cilluffo MC, Farber DB, Fain GL. Removal of phosphorylation sites of {gamma} subunit of phosphodiesterase 6 alters rod light response. J Physiol. 2007;579:303–12. doi: 10.1113/jphysiol.2006.121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsang SH, Yamashita CK, Lee WH, Lin CS, Goff SP, Gouras P, Farber DB. The positive role of the carboxyl terminus of the gamma subunit of retinal cGMP-phosphodiesterase in maintaining phosphodiesterase activity in vivo. Vision Res. 2002;42:439–45. doi: 10.1016/s0042-6989(01)00213-9. [DOI] [PubMed] [Google Scholar]
- 30.Tsang SH, Woodruff ML, Jun L, Mahajan V, Yamashita CK, Pedersen R, Lin CS, Goff SP, Rosenberg T, Larsen M, Farber DB, Nusinowitz S. Transgenic mice carrying the H258N mutation in the gene encoding the beta-subunit of phosphodiesterase-6 (PDE6B) provide a model for human congenital stationary night blindness. Human Mutation. 2007:243–54. doi: 10.1002/humu.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki A. The GTP-binding protein-dependent activation and deactivation of cyclic GMP phosphodiesterase in rod photoreceptors. Adv Second Messenger Phosphoprotein Res. 1992;25:135–45. [PubMed] [Google Scholar]
- 32.Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–72. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polans AS, Hermolin J, Bownds MD. Light-induced dephosphorylation of two proteins in frog rod outer segments: influence of cyclic nucleotides and calcium. J Gen Physiol. 1979;74:595–613. doi: 10.1085/jgp.74.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]


