Abstract
Current treatments for Parkinson's disease fail to modify disease progression, and the underlying pathogenic mechanisms remain elusive. The identification of specific targets responsible for disease will aid in the development of relevant model systems and the discovery of neuroprotective and neurorestorative therapies. Two promising protein candidates, α-synuclein and LRRK2, offer unique insight into the molecular basis of disease and the potential to intervene in pathogenesis. Although multiple lines of evidence support α-synuclein and LRRK2 as robust targets for therapy, the connection between protein function and neurodegeneration is unclear. Technology capable of mitigating α-synuclein and LRRK2 disease-associated function will ultimately be required before the true value of these proteins as therapeutic targets can be discerned. Antioxid. Redox Signal. 11, 2167–2187.
Parkinson's Disease
A clinical definition of Parkinson's disease (PD) includes cardinal movement-related dysfunction often involving a combination of resting tremor, cogwheel rigidity, and bradykinesia, with a positive response to dopamine-modification therapy. Additional debilitating comorbidities associated with PD include depression, olfactory dysfunction, constipation and other gastrointestinal disturbances, and rapid-eye-movement sleep behavioral disorder that can manifest years before movement-related symptoms (100, 124). The cause of PD remains unknown in the majority of cases, with no potent susceptibilities derived from the environment or common genetic variants responsible for the majority of disease yet identified.
On a pathologic level, PD is defined by a dramatic depletion of neuromelanin-containing, tyrosine-hydroxylase–positive neurons in the substantia nigra pars compacta (SNpc), accompanied by the presence of ubiquitin and α-synuclein (α-syn)-positive proteinaceous inclusion bodies, or Lewy bodies, in the perikarya within remaining neurons in the SNpc and other brain regions and Lewy neurites often present in associated neuronal processes. As careful observations from the clinic continue to expand the spectrum of symptoms associated with disease, the pathologic lesions associated with PD extend to areas of the brain distant and distinct from the substantia nigra. Because in part of the availability of monoclonal antibodies highly specific for pathologic lesions associated with PD (Lewy structures) and the increasing size of brain banks that collect PD-affected tissue, studies demonstrate a progression of disease pathology, with neurodegeneration in the substantia nigra occurring in the mid-stages of the disease process in the majority of cases (17, 44, 101). As with the clinical spectrum associated with PD, variability prevents a strict definition of pathologic staging from applying to all documented cases. However, a six-stage system fits with the majority of pathologically described tissue and ultimately involves much of the central nervous system. Recent pathological studies greatly expand the constituency of selectively vulnerable cell types in PD, obviously extending beyond dopaminergic cells and a dopamine hypothesis of pathogenesis (4).
Drug therapies that serve to restore dopaminergic input in the striatum initially control many movement-related symptoms in the early stages of disease. Drug regimens with efficacy exist for some psychiatric and gastroenterologic comorbidities, all of which depend on access to medical specialists and expensive medications. As the neurodegeneration and pathologic lesions associated with disease progress, drug therapies that initially provide some symptomatic relief ultimately fail to control symptoms, and undesirable side effects become intolerable, invariably resulting in a profound increase in the rate of mortality (45, 161).
Targeted surgical interventions such as deep-brain stimulation provide additional symptomatic relief in some cases (136). Ultimately, therapies that halt or otherwise slow the progression of disease have not been identified. Efforts to elucidate underlying pathogenic mechanisms, with the goal of identifying therapeutic targets to modify the course of disease, are the focus of many laboratories.
Toxins that rapidly and specifically lesion the brain have undeniably provided robust anatomic models of PD in model organisms and allowed deep insight into the nigral-striatal pathway, but manipulating specific and relevant genes may provide the best chance for developing model systems that allow dissection of molecular pathways underlying pathogenesis. However, the utility of proteins associated with PD may extend well beyond the development of model systems in that the proteins themselves could serve as robust targets for therapeutic intervention in pathogenesis. Herein, the tremendous recent progress made toward understanding α-syn and leucine-rich repeat kinase 2 (LRRK2) as therapeutic targets is analyzed and discussed. The cure for PD may ultimately hinge on the existence and identification of specific and rational targets critical for disease pathogenesis.
Therapeutic Targets in Parkinson's Disease
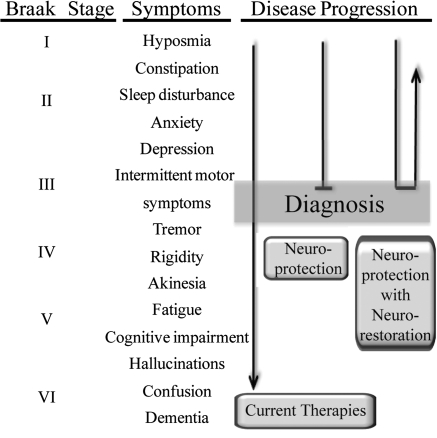
PD progresses over the course of many years, allowing therapeutic intervention at early stages of the disease when symptoms have a more tolerable effect on quality of life. A neuroprotective therapeutic strategy involves protecting neurons that would otherwise continue toward dysfunction and cell death, and a neurorestorative approach involves reviving sick neurons back to normal function and potentially replacing neurons that have already died (Fig. 1). An ideal therapeutic approach would involve both neuroprotective and neurorestorative benefit, helping both newly diagnosed patients and patients with advanced disease. In the current era of successful dopamine-modification therapy, symptoms that may be unrelated to neurodegeneration in the midbrain, such as cognitive decline and dementia in the later stages of disease, have the most dramatic effect on patient mortality and decreased quality of life (86, 235). Thus, therapies that focus exclusively on enhancing or restoring the nigral-striatal pathway or cell death in the substantia nigra will not address the most debilitating aspects of the disease (4).
FIG. 1.
Strategy for PD therapeutics. Braak pathologic stages correspond with progression of symptoms. Due to the nonspecific nature of early symptoms, disease is usually diagnosed at the onset of mild motor symptoms. Current therapies address some of the symptoms but fail to alter disease progression that ultimately involves symptoms difficult or impossible to alleviate with current therapies. Effective neuroprotection therapies would halt the advancement of disease, whereas a neuroprotection strategy coupled with neurorestoration could address the early symptoms associated with disease.
Despite the usual confounding inability to distinguish between cause and effect in postmortem–derived tissue, tremendous efforts have been assigned to addressing abnormalities identified in PD-afflicted brain tissue. Neuroinflammation accompanies sites of neurodegeneration in PD-affected tissue, and nonsteroidal antiinflammatory agents (NSAIDs) may reduce the risk of PD, in addition to other antiinflammatory agents such as minocycline (2, 22, 63, 92, 165, 252). Other therapeutic strategies currently in clinical trials include approaches that target reactive oxygen species (ROS) to limit oxidative stress mechanisms in affected neurons. Coenzyme Q10 enhances mitochondrial electron transport that may address mitochondrial dysfunction and help reduce mitochondrial production of ROS (62, 90, 270). Molecules that more directly quench ROS, such as vitamin E and selenium, have also been evaluated (52, 53, 197). Excessive intracellular calcium and corresponding excitotoxicity pathways are also linked to cell death, and calcium channel blockers are in clinical trials (20, 230). Neuroinflammation, ROS, and excitotoxicity have little specificity to PD because they are linked in some fashion to most neurodegenerative disorders. If it is assumed that we cannot know the fundamental cause of the widespread lesions and degeneration that occur in PD, then perhaps targeting the downstream regulatory events that carry out cell-death programs may slow disease progression. However, the approach may not address the fundamental problem underlying pathogenesis and fail in long-term intervention. Fortunately, molecular genetics has provided unambiguous protein targets causative for disease, at least in the patients that harbor associated mutations.
Genetic Susceptibilities in Parkinson's Disease
The current understanding of PD includes a long initial phase, often dubbed “preclinical,” in which non–movement-disorder symptoms are common but heterogeneous in presentation. Early twin studies suggest that genetic susceptibilities might not play a large role in PD etiology (106). More recent studies show that, in some populations, a single point mutation in the LRRK2 gene causes more than one third of PD cases, with the ancient G2019S mutation reported at a higher frequency in patients without knowledge of PD in their family than in patients aware of a family history of disease, in some populations (91, 130). Genetic studies have therefore illuminated specific causes of PD that promise to aid in the delineation of pathogenesis and the development of therapeutics.
Autosomal-recessive parkinsonism
Families that inherit PD in a manner compatible with autosomal-recessive disease have long been described, with disease usually manifesting earlier than that in the majority of PD cases. Mutations in the parkin gene were described in Japanese juvenile-onset cases of disease, and genetic variation in parkin clearly associates with early-onset PD in multiple ethnicities (115, 208). Apart from the large deletions and rearrangements that inactivate expression, pathogenic point mutations impart deleterious function compatible with a general loss-of-function disease mechanism (226, 255, 259). Likewise, loss-of-function mutations that include nonsense mutations, genomic rearrangements, and missense mutations have also been described in the PINK1 (PTEN-induced kinase-1) and DJ-1 genes in early-onset cases (14, 243). In general, overexpression of these proteins usually imparts cytoprotective properties to cells from a variety of insults (39, 189, 275). Because most effective drug therapies for a variety of human illnesses involve the ablation or reduction of activity of associated targets, dealing with loss-of-function mechanisms usually imparts additional technical demands on therapeutic strategies. Although the autosomal-recessive genes encode clearly relevant proteins in PD, additional work that characterizes underlying cellular pathways in relevant disease models seems necessary before parkin, PINK1, and DJ-1 can be fully realized as potential therapeutic targets.
Autosomal-dominant genes
LRRK2
A genetic locus initially described as associated with late-onset PD in a large Japanese kindred demonstrated association with late-onset autosomal-dominant PD in several additional families, and missense mutations in the LRRK2 gene were identified (61, 182, 277, 278). Sequence analysis of the encoded enzymatic domains revealed an alteration within the activation loop of the kinase domain responsible for a significant percentage of PD disease in many case populations studied (43, 69, 172). In general, common genetic variation in LRRK2 does not appear to contribute heavily to PD susceptibility, with the exception of some Asian populations (13, 217, 232), but in some populations, the missense mutations themselves account for more than one third of PD cases (130, 179). Mutations that clearly segregate with disease in well-described families and are overrepresented in PD cases versus age-matched controls represent a fraction of described LRRK2 variants, and the difficulty in distinguishing benign versus pathogenic variants prevents resolution of the true frequency of LRRK2 mutations in PD.
α-Syn
The first genetic cause for PD was described in a large Italian family that inherited early-onset PD in an autosomal dominant fashion. Subsequently, missense mutations in the α-syn gene were also identified in Greek, German, and Spanish families, with missense mutations localized to the N-terminal half of the protein (121, 191, 272). As opposed to missense mutations in LRRK2, pathogenic missense mutations in the α-syn gene seem confined to only a handful of PD cases worldwide. Again in contrast with LRRK2, genetic variation in the α-syn promoter and other regions of the gene appears to modify susceptibility to PD (54, 148, 166, 265). The identification of genomic multiplications that include α-syn and are causative for PD solidifies the importance of α-syn dosage in PD (215, 216). The main strength that suggests a critical involvement for α-syn in PD does not necessarily lie with human genetic studies; rather, α-syn represents the major protein component of the pathologic structures that define PD-associated lesions in affected regions of the brain (222–224).
LRRK2 Structure and Function
As opposed to α-syn, LRRK2 had not been cloned or thoroughly annotated when missense mutations were identified in PD cases. Bioinformatic prediction strongly suggested that LRRK2 encodes a GTPase-like domain together with a kinase domain, thereby placing LRRK2 within the fraction of the proteome considered modifiable with small molecules or intervention therapies (16, 168). The potential for LRRK2 as a therapeutic target depends not only on activities of the protein associated with PD, but on normal function and the relation between health and disease. For example, generalized inhibition of LRRK2 activity may not be compatible with normal cellular function, or inhibition of one particular aspect of LRRK2 activity may not influence another activity that underlies disease mechanisms. Deep insight into LRRK2-related biology will provide the background necessary for rationally designed therapeutic approaches.
Expression and localization
LRRK2 mRNA displays near ubiquitous localization throughout the mouse and human brain, with particular concentration within neurons of the cortex, striatum, and hippocampus (87, 162, 214, 233). Protein distributes in a likewise manner, detectable in the vulnerable neurons of the substantia nigra pars compacta (81, 214). LRRK2 displays particularly high expression in the kidney and appears to increase in expression on organogenesis and cell maturation (12, 262). LRRK2 adopts a punctate intracellular cytosolic localization that associates with various membranous structures including vesicles, mitochondria, Golgi, and the ER (11). Biochemical fractionation suggests that LRRK2 resides on the cytoplasmic side of membrane-containing organelles without evidence of nuclear or mitochondrial internalization (11, 71, 260). Similar to α-syn, clear orthologues to LRRK2 have been identified in all described mammalian genomes, but in invertebrates, orthologous genes may show closer homology to mammalian LRRK1 than to the LRRK2 gene (150). Close sequence homology and overlapping expression profiles between the LRRK1 and LRRK2 genes suggests redundancy in function (12). LRRK2-deficient mice are viable with no dramatic abnormalities, although detailed reports have not been published. Loss of the LRRK orthologues in Drosophila seems to produce a strain-dependent phenotype, and additional studies will help resolve the controversy (94, 129, 139). Loss of LRRK orthologues in nematodes produces defects in vesicle sorting, whereas loss in slime mold produces defects in chemotaxis (204, 245).
Enzymatic activity of LRRK2
LRRK2 harbors both a GTPase and kinase domain, an extremely rare arrangement found only in LRRK1 and possibly the DAPK1 protein in mammals (16). A solid precedent for GTPase activity dependent modification of protein kinase activity suggests that the enzymatic domains encoded in LRRK2 may function as a self-regulatory apparatus. The most common LRRK2 mutations localize to the kinase domain (amino acid G2019) and the GTPase domain (amino acid R1441), implicating enzymatic output as critical to PD. Beyond a domain-prediction analysis that helps define conserved features and potentially critical residues, in silico approaches do not further predict LRRK2 function in cells. The LRRK2 kinase domain possesses highest sequence homology to the mixed-lineage kinase (MLK) subfamily of MAPKKK proteins but differs in the critical amino acids that define MLK proteins, whereas the GTPase domain displays distinct architecture reminiscent of Rab-like GTPases (153, 261). As one of the largest protein kinases in the mammalian kinome, LRRK2 will necessarily resist characterization by the usual gauntlet of biochemical assays that have historically well served the characterization of other protein kinases. Nevertheless, tremendous advances in a short time have outlined a surprising consensus story for the impact of PD-causing mutations on protein function.
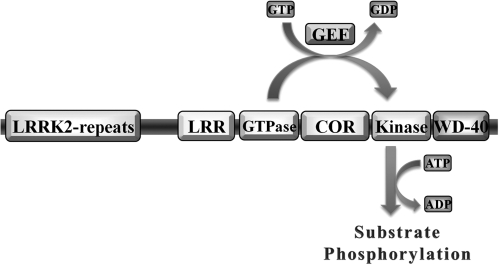
Before a functional description of the LRRK2 protein, a functional description of LRRK1 suggested that intrinsic kinase activity was stimulated on GTPase activation (120). This phenomenon holds true for LRRK2 as well, in which application of GTP or GTPγS spurs modest increases in kinase output (218, 261). However, a clear relation between kinase activity and GTPase activity is defined through the use of specific mutations that ablate either GTPase activity, kinase activity, or both activities. Mutations in the conserved ATP-binding pocket site, in the consensus residues of the activation loop, or mutation within the proton-acceptor site have no apparent effect on GTP binding. In contrast, mutations in the GTPase domain thoroughly ablate kinase activity (95, 218, 261). Thus, LRRK2 functions as a signal-transduction pathway encoded into a single protein. The activity of LRRK2 is perhaps an ancient design, because the orthologue GbpC in slime mold operates in a similar manner, although GbpC also encodes GEF domains upstream of GTPase activity (245). Through functional descriptions of GbpC, LRRK1, and LRRK2 protein, the encoded domains function in a signal cascade ultimately to regulate kinase output (Fig. 2).
FIG. 2.
LRRK2 domain structure and mechanisms of kinase activation. LRRK2 encodes an N-terminal repeat domain, a leucine-rich repeat domain (LRR), and a GTPase, COR, kinase and WD-40–like repeat domain. A guanine nucleotide exchange factor (GEF) would exchange bound-GDP for GTP and activate the GTPase domain, in turn activating the kinase domain, which uses ATP to transfer phosphate groups to protein substrates.
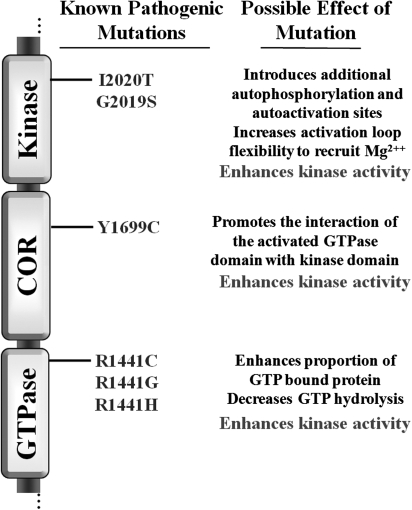
In an initial functional description of the LRRK2 protein, the most common PD-associated mutations, G2019S and R1441C, enhanced kinase activity but failed to alter other basic biochemical properties, such as protein localization and turnover (260). PD-causing mutations in the GTPase domain likewise enhance the proportion of GTP-bound protein in pull-down experiments, whereas mutations in the kinase domain have no effect on the proportion of GTP-bound protein (261). Additional studies demonstrate that PD-causing mutations in the GTPase domain do not result in a higher affinity for GTP; rather, GTP hydrolysis is disrupted because of PD-associated mutations, thereby prolonging GTP-bound states and an activated GTPase domain (79, 131, 134). Missense mutations in the LRRK2 gene that segregate with disease in families and are therefore likely pathogenic variants all result in enhanced kinase activity in vitro, although not all laboratories demonstrate a significant difference between kinase activity associated with wild-type protein and that containing PD-causing mutations (99, 261). Differences in assay protocols and the lack of a relevant substrate and robust kinase-dependent phenotype in cells combine to prevent the assumption that kinase output is the true and only possible functional link between LRRK2 protein and PD. However, the available data, when taken as a whole, suggest that kinase activity represents the final output of activity responsible for pathogenesis (Fig. 3).
FIG. 3.
Functional effect of pathogenic LRRK2 mutations. All currently known PD-causing mutations are listed in relation to the LRRK2 domain structure. Possible functional effects of the mutations are listed.
LRRK2 Target Validation
The implication of LRRK2 as a potential target for neuroprotection strategies in PD clearly originated from human genetic studies. The LRRK2 protein had not been previously identified as a critical mediator of cell-death pathways or mechanisms important in neuron survival before the identification of mutations causative of PD. Further, most individuals with PD do not harbor known LRRK2 mutations, and missense mutations are relatively rare in accounting for a small percentage of PD in most populations. Model systems that demonstrate an LRRK2-dependent phenotype are necessary to validate LRRK2 as an appropriate potential therapeutic target and to help address whether LRRK2 would serve as a general target for therapy in PD, because most individuals living with PD presumably do not harbor coding mutations in LRRK2. Thus, the question of whether LRRK2 represents a valid target in PD cases without LRRK2 mutations may not be fully answered until safe and effective modifiers of LRRK2 action are available in the clinic.
In vitro models
LRRK2 protein is relatively rare in cells compared with other kinases, and overexpression of the human wild-type protein in cell lines or primary neuronal cultures is well tolerated, with relatively minimal increases in markers of cell death or other morphologic indicators (76, 77, 219). In contrast, overexpression of wild-type mixed-lineage kinase 2 protein, the closest kinase to LRRK2 apart from LRRK1, based on sequence similarity of the kinase domain, results in immediate apoptosis (267). However, overexpression of LRRK2 protein with the G2019S mutation results in a significant increase in markers of cell death, including neurite shortening and changes in membrane permeability (76, 145, 219, 261). The effects appear to track with kinase activity, because mutations that ablate kinase activity also nullify the upregulation of markers of toxicity associated with LRRK2-G2019S expression (76, 219, 261). A phenotype derived from WT-LRRK2 protein expression is more subtle, but expression of WT-LRRK2 protein in neurons may leave cells more susceptible to other toxicities, including peroxide exposure (261). Perhaps a more relevant phenotype for target validation would derive from the knockdown of LRRK2 protein or ablation of LRRK2 kinase activity in concert with exposure to agents commonly used in models of PD, such as overexpression of human α-syn or chemical toxins selective for dopaminergic neurons. However, numerous technical challenges include the lack of commercially available antibodies with sufficient specificity to detect human and (more so) mouse LRRK2 protein (12), coupled with incomplete knowledge of the half-life of the endogenous protein in primary cells that constitute in vitro models.
The criteria for relevant in vitro models of PD are still hotly contested because the defining end-point requirements such as cell death, protein aggregation, or other biochemical changes in signaling or metabolism are not agreed on. In vitro data that demonstrate kinase-dependent changes in LRRK2 overexpression paradigms are provocative but not necessarily relevant, unless the endogenous and physiologically important targets for kinase activity are verified in these systems. The fact that a phenotype is observed for PD-associated mutations that link with kinase overactivity does not imply that the protein is correctly functioning in these cells. Ultimately, in vivo model systems that demonstrate LRRK2-dependent phenotype more universally agreed as important for PD (neurodegeneration and inclusion formation in dopaminergic neurons within the SNpc in intact animals) may be required before in vitro model systems can be validated as useful tools for validating therapeutic compounds before use in humans.
In vivo models
Many laboratories interested in developing models of PD have used LRRK2 overexpression in various organisms as a way to understand how mutations in this protein might cause PD. Simultaneously, this work will help validate or exclude LRRK2 as a potential therapeutic target. The LRRK2 gene is highly conserved through evolution, although in many invertebrates, deciding whether the orthologue more closely resembles human LRRK1 or LRRK2 becomes challenging. Overexpression of the Drosophila orthologue of the human LRRK genes (dLRRK) appears well tolerated by Drosophila cells, and loss of the orthologue results in defects in dopaminergic neurons, although this result has not recapitulated in other strains of flies (129, 256). Expression of human WT or G2019S-LRRK2 in Drosophila results in cellular toxicity in both photoreceptor cells and dopaminergic neurons (139). Overexpression of dLRRK containing missense mutations in areas of the protein homologous to PD-associated mutations in the human LRRK2 gene causes significant loss of TH-positive cells, whereas expression of wild-type dLRRK or dLRRK2 with mutations predicted to inactivate kinase activity does not cause similar phenotypes (94). The initial studies in Drosophila suggest that LRRK2, particularly LRRK2 kinase activity, is a valid target important for neurodegeneration.
The identification of PD-causative mutations in the α-syn gene more than10 years ago led to the description of transgenic mice overexpressing human α-syn ∼2.5 years later (152, 191). The last 10 years witnessed numerous dramatic and impressive advances in transgenic technologies. However, since LRRK2 mutations in PD cases were described, transgenic rodents with phenotype have yet to be described in the literature some 4 years later. Problems with cloning and manipulating LRRK2 constructs in combination with downstream expression issues seem to plague the field and may prevent fundamental questions regarding LRRK2 and neurodegeneration from being addressed in a timely fashion. Once the technical issues are resolved, rodent transgenic models may provide a springboard toward identifying and validating not only LRRK2 as a target for disease but also validating potential therapies as they arise to mitigate pathogenic processes.
Targeting LRRK2 Kinase Activity
The unambiguous identification of a protein included in the so-called druggable genome clearly linked with PD susceptibility provides the opportunity to exploit existing technology to move beneficial molecules expediently to the clinic. For example, large panels of active recombinant protein kinases are now commercially available to help define the specificity of kinase inhibitors early in the development process. Protein kinase inhibitors have proven efficacy in the treatment of human disease since the successful application of the first approved kinase inhibitor trastuzumab in cancer therapies. After the approval and successful implementation of the small-molecule imatinib kinase inhibitor spurred the formation of many kinase inhibitor discovery programs that are now a ubiquitous part of the modern pharmaceutical industry. However, protein kinases are far from ideal targets because problems with specificity plague the safety record of inhibitory compounds (64). Additional problems such as loss of sensitivity of the drug due to acquired mutations in cancer-cell targets would presumably not present an issue for the treatment of PD. However, an LRRK2 inhibitor may need to be administered for the remainder of the patient's life, requiring a difficult-to-achieve level of safety from a potential inhibitory compound in the more vulnerable group of older individuals with PD. In addition, compounds would have to cross the blood–brain barrier freely to target the cells of interest.
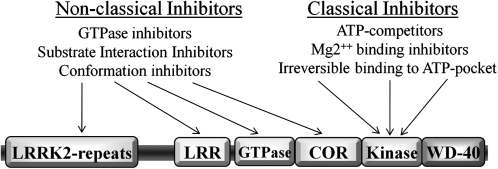
The unique biology of the LRRK2 protein presents both an opportunity for specificity and unique challenges in identifying possible LRRK2 kinase inhibitors (Fig. 4). Classic protein kinase inhibitors might be grouped together as ATP-competitive inhibitors and irreversible inhibitors. The ATP-binding pocket within a protein kinase is an ideal target for compounds that possess drug-like properties, although ATP-binding pockets tend to encode some of the highest sequence homology found between different protein kinases (251). Residues critical to the formation of the ATP-binding pocket are conserved between kinases but not necessarily near in amino acid sequence. The majority of kinases, including LRRK2, have not been described on a structural level. Description of the structure of the LRRK2 ATP-binding pocket and comparison with known ATP-binding pockets of other protein kinases should help shed light on whether ATP-competitive compounds will be feasible for an LRRK2-based therapy. The activation loop of protein kinases, almost always defined as the sequence lying between the DFG … APE canonic sequence motif, have been used as targets for small molecules because the activation loop is critical to kinase activity. The p38 protein kinase inhibitor BIRB796 causes a switch from a “DFG-in” conformation to a “DFG-out” conformation, leading to a steric clash with the phosphate groups of ATP (184). LRRK2 possess a unique activation-loop sequence “DYG,” distinct from the nearly ubiquitous “DFG” found in protein kinase activation loops. The PD-causing G2019S mutation further disrupts this motif to “DYS,” and proves the importance of these particular residues for LRRK2 kinase activity. Theoretically, a small molecule might exist that possesses activity similar to that of BIRB796 by taking advantage of the unique structure of the LRRK2 activation loop in blocking kinase activation in a highly specific manner. Likewise, a small molecule could preferentially interact with the DYS motif in patients that carry the G2019S mutation for customized therapy, in case inhibition of LRRK2 as a whole causes intolerable side effects in humans.
FIG. 4.
Potential routes for LRRK2 kinase inhibition. Potential targets for nonclassic and classic kinase inhibitors are indicated.
LRRK2 protein resides as membrane-associated and freely soluble protein in the cytosol so that LRRK2 ATP-pocket binding compounds must compete with intracellular ATP concentrations to 10 mM to achieve inhibition. In addition to ATP-competitive inhibitors, irreversible inhibitors also represent a viable option for a LRRK2 kinase inhibitor, but the usual concerns of specificity and safety with irreversible inhibitors limit desirability. The safety of inhibiting LRRK2 kinase activity and potentially LRRK1 kinase activity in humans can obviously not be fully described without highly selective and potent inhibitory molecules. In limiting undesirable effects due to the loss of LRRK activity, the available target-validation data and human genetic discoveries suggest that the LRRK2 kinase may not have to be fully inactivated to provide neuroprotection in PD, because the most common pathogenic mutations induce a relatively mild upregulation (around twofold in vitro) of kinase activity. Relevant model systems will prove invaluable in this regard.
If the relatively unique structure of the LRRK2 kinase domain suggests that specific small-molecule inhibitors might exist, the numerous potential mechanisms for disruption of LRRK2 kinase activity through nonclassic inhibition offer another level of opportunity. In vitro data suggest that disruption of GTP-binding or nucleotide exchange within the LRRK2 GTPase domain would necessarily disrupt kinase activity (95, 218, 261). Likewise, allosteric modulators that block a necessary conformational state, such as protein dimerization, could potentially inhibit LRRK2 kinase activity in a highly specific way (78). Substrate-competitive inhibitors would rely on the discovery of robust LRRK2 kinase targets in cells. As yet, even the sites for LRRK2 autophosphorylation have not been mapped, because of the notoriously low activity of LRRK2 in cells and in vitro. However, the numerous technical challenges that will certainly be resolved with time are dwarfed by the potential of LRRK2 as the most exciting and viable target yet identified in PD.
α-Syn Structure and Function
Expression, physiologic function, and interacting molecules
α-Syn is a 140-amino-acid protein identified as a hallmark constituent of Lewy bodies present in a group of neurodegenerative diseases, including PD, multiple system atrophy (MSA), dementia with Lewy bodies (DLB), and diffuse Lewy body disease (DLBD) (9, 72, 224, 253, 266). Despite the implication of nuclear localization in the nomenclature, α-syn is primarily localized to presynaptic terminals in the central nervous system (CNS) (96) and is abundant in brain areas rich in synaptic vesicles and associated with synaptic plasticity, such as the hippocampus, cerebral cortex, and amygdala (133, 196). Early studies in songbirds (Zebra finch) demonstrate a role for α-syn in synaptic plasticity and that song learning coincides with the upregulation of α-syn mRNA (65). The physiologic function of α-syn in normal brain is poorly understood. α-Syn may play a role in neuronal differentiation, regulation of dopamine release, regulation of cell viability, modulation of synaptic transmission, and vesicular recycling (144, 238). Additional roles in cell adhesion, development, regulation of dopamine uptake, and vesicle transport in neurons are described (3, 28, 65).
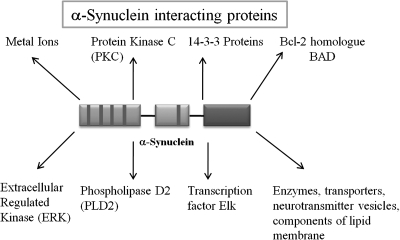
As a highly abundant neuronal protein in the mammalian brain, α-syn interacts with a number of proteins: acting as a high-affinity inhibitor of phospholipase D2 (5, 102), as a regulator for certain enzymes, transporters, and neurotransmitter vesicles (40), promoting oxidative stress (66, 84), and as a regulator for the MAP kinase pathway by forming a complex with transcription factor Elk (97, 98). α-Syn also plays a role in modulating the architecture of membrane lipid components by associating with lipid membranes, fatty acids, detergent micelles, lipid rafts, and lipid droplets (31, 37, 58, 104, 105, 113, 159, 171, 187, 199, 211). Metal ions such as Cu2+ and potentially Fe2+, Al3+, Zn2+, Mg2+, Ca2+, Co2+, Fe3+, Tb3+, and Mn2+ interact with α-syn, although α-syn is not widely regarded as a traditional metalloprotein (18, 238, 266). α-Syn also displays characteristics of chaperone-like proteins and interacts with a family of ubiquitous cytoplasmic chaperones including 14-3-3 proteins, in addition to other abundant proteins like protein kinase C (PKC), the bcl-2 homologue BAD, and extracellular regulated kinase (ERK) (Fig. 5) (176).
FIG. 5.
Interactors of α-syn. Possible co-factors involved in α-syn–mediated toxicity and cell death, and potentially, neuroprotection or neurorestoration or both.
The endogenous function of α-syn has not been clearly delineated through characterization of mice deficient in α-syn expression. The first reports of mice deficient in α-syn demonstrated normal synaptic architecture and brain morphology that led to slight changes in synaptic transmission (3). Additional laboratories have generated α-syn–knockout mice in combination with knockout of the two other synuclein family members in mammals, β-synuclein and γ-synuclein, with little to no apparent phenotype (21, 122, 202, 209). A reproducible phenotype for mice deficient in α-syn includes heightened resistance to the neurotoxin MPTP (36). MPTP, specifically MPP+ generated by MAO-B activity, targets susceptible dopaminergic neurons and can inhibit mitochondrial complex I activity, although the importance of mitochondrial inhibition in initiating cell death remains in question (27, 193, 194, 201, 250, 273). Because the exact mechanism of MPTP action in neurons is not clear, inferring α-syn function via MPTP resistance becomes difficult. The implication that cells containing α-syn may be more susceptible to environmentally derived toxins is provocative, but mice overexpressing α-syn may not be more susceptible to MPTP (200), and, in some cases, are protected against neuronal toxins like paraquat (147). One explanation may involve the lack of functional overlap between mouse α-syn and human α-syn in neurons.
Clear orthologues to α-syn may not exist in lower organisms and invertebrates that would serve as models for study, further hindering efforts to understand the normal function of α-syn in cells. If native α-syn function is important for pathogenesis and that associated function is largely unknown, inserting α-syn into organisms that are normally devoid of the protein without the ability to assess whether α-syn integrates properly into the cell would produce a model system difficult to interpret. α-Syn function may modify crucial physiologic events in mammalian neurons that necessitate high redundancy from other proteins. Conversely, α-syn may play a more generalized role as a dispensable cofactor for a number of diverse cellular pathways present in higher organisms. The lack of a clearly described role for α-syn in cells negatively affects viability as a therapeutic target, because alteration or disruption of α-syn in humans may produce unanticipated and deleterious side effects that outweigh potential benefits.
Primary structure
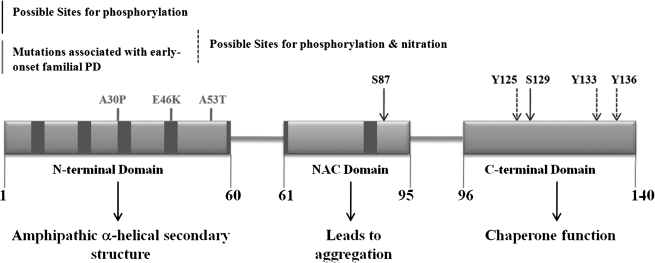
α-Syn is one of the most abundant “natively unfolded” (intrinsically disordered) proteins that likely have different morphologies, including protofibrils, oligomers, and fibrils. Under physiologic conditions, α-syn has little or no ordered structure and possesses noteworthy conformational plasticity (240, 258). The primary structure of α-syn is characterized as follows: (a) Residues 1-60 constitute the N-terminal domain encompassing the conserved imperfect hexameric repeats (KTKEGV) and includes three missense mutations associated with early-onset familial PD (A30P, E46K, and A53T). The conserved 11-residue (XKTKEGVXXXA, X = any amino acid) repeat forms amphipathic secondary α-helical structures on binding to acidic phospholipid membranes, typical of the lipid-binding domains of apolipoproteins (19, 28, 37, 65); (b) the central region (residues 61 to 95) is composed of an extremely hydrophobic, highly aggregation-prone NAC (nonamyloid component) sequence (68, 82, 236); and (c) the hydrophilic C-terminal region consists of highly acidic residues glutamic and aspartic acid, as well as proline, and is responsible for chaperone function (111, 186, 238) (Fig. 6).
FIG. 6.
Structure of α-syn. Primary structure of human α-syn showing the three distinct domains (N-terminal, NAC, and the C-terminal) and their corresponding functions. Dark bars inside protein domains represent the imperfect hexameric KTKEGV repeats. Positions of familial PD mutations are indicated. Arrows (solid and broken), sites of phosphorylation; broken arrow, probable sites for nitration. S129 is an important residue promoting formation of α-syn filaments and oligomers on phosphorylation, due to changes in the hydrophobicity and charge-distribution in the C-terminal region.
Aggregation of α-syn
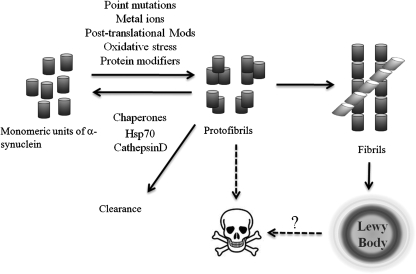
As α-syn is the most abundant protein composing the proteinaceous aggregates that define PD on a pathologic level (224), intense efforts revolve around understanding α-syn accumulation and aggregation. α-Syn may assume oligomeric species through unknown mechanisms, and higher-order α-syn structures usually correlate with α-syn–dependent toxicity in cells (Fig. 7). The specific conformational entities responsible for protection, toxicity, and/or aggregation remain elusive. Factors known to modify α-syn aggregation and/or oligomerization include alterations of the primary amino acid sequence (e.g., PD-associated mutations); c-terminal truncations (34, 110, 158, 170); interactions with metal ions (41, 42, 83, 175, 180, 181, 206, 220, 266); interactions with Aβ peptide (103, 146); interactions with chaperone proteins such as Hsp70 (8, 160); interactions with apolipoprotein E (144), as well as neurotoxins, pesticides, and herbicides (75, 147, 239, 247); organic solvents (169); tyrosine nitration (47, 66, 221); phosphorylation (7, 60); methionine oxidation (70, 89, 242, 269); monoubiquitination (51, 126, 135, 203); interaction with polyanions and polycations (30, 74, 138), oxidative dimmers, and oligomers (221); histones (73); transglutaminase (107); and other protein–protein interactions (238).
FIG. 7.
Aggregatory mechanism of α-syn. The probable mechanism by which α-syn aggregates. In normal cells, α-syn exists in an unfolded and monomeric state, and through disease-associated mechanisms, transitions to a toxic protofibril capable of damaging intracellular membranes. Neuroprotective mechanisms may serve to clear protofibrils by refolding the aggregates back to a monomeric state or by clearing them through the lysosomal or proteasomal degradation pathway. Protofibrils may further oligomerize into fibril structures that ultimately are organized into Lewy bodies. The relation between Lewy bodies and cell death remains unclear.
As in other neurodegenerative disorders and diseases associated with protein aggregation, the role of organized inclusion bodies localized to disease-associated regions in PD-affected tissue remains hotly debated. On the one hand, the inclusion-body organelles may serve to sequester molecules that, for whatever reason, can no longer route through normal metabolism that would otherwise cause cellular dysfunction. Experimental evidence supports this notion, in which the lack of α-syn–inclusion formation associates with toxicity (24, 177). In contrast, the overt formation of inclusions may represent the toxic insult itself composed of molecules that were otherwise nontoxic until association with the inclusion. In the first case, promoting inclusion bodies should facilitate neuroprotection. In the second case, inhibiting inclusion bodies would promote cell toxicity. Both situations may be true, depending on the particular component of the inclusion body in question; for example, sequestration of toxic α-syn species into inclusion bodies may represent cytoprotection, with the cost of sequestering and inactivating other molecules necessary for normal function.
Therapeutically oriented efforts should focus on farther-upstream events as opposed to modifying preexisting inclusion bodies, although this may be a moot point because model systems that demonstrate inclusions with morphologic similarity to Lewy bodies have yet to be developed. One major obstacle to understanding Lewy bodies is the failure to recapitulate the basic morphologic characteristics of Lewy bodies in the context of relevant model systems. Although several model systems describe α-syn– and ubiquitin-positive aggregations in some fraction of cells (51, 126), none can be considered similar to the highly ordered structures found in PD tissue.
As a potential therapeutic target, the accumulation of α-syn into insoluble protein inclusions seems an important event in pathogenesis. A strong case can be made for therapeutically promoting inclusion formation in disease to protect cells from more-soluble toxic species, as well as therapeutic approaches to dissolve inclusions to relieve the cells of a deleterious organelle that disrupts normal function. The dichotomy may not be resolved in the near future and hinders α-syn–antiaggregation strategies as a viable therapeutic approach.
α-Syn Target Validation
The majority of therapies for human disease involve the inhibition of a particular target important for pathogenesis. Many clinical trials fail because the target itself is not a critical component of disease. As a result, pharmaceutical companies now demand more-stringent target-validation studies. Pathologic evidence from human PD tissue nominating α-syn as the primary agent of pathogenesis is circumstantial, whereas human genetic studies firmly place the causative basis with α-syn, but only in a very small proportion of familial and early-onset-disease cases. The idea that α-syn is a robust therapeutic target in PD is provocative but nonetheless requires extensive proof from target-validation studies. More than a decade of research centered on α-syn highlights outstanding questions through the descriptions of numerous model systems.
In vitro model systems
Cell-culture models of α-synuclein provide a valuable route for studying the physiologic and pathologic functions of the protein because they would be potentially amenable to high-throughput translation to identify therapeutic compounds. α-Syn can be highly overexpressed in various cell lines and primary neuronal culture systems. Expression of α-syn containing a PD-associated point mutation in PC12 cells leads to loss of dopaminergic release, alteration of the ubiquitin-dependent degradation system, and autophagic-dependent cell death (228). Overexpression of α-syn in H4-glioma cells results in an upregulation of markers associated with toxicity (160). Delivery of α-syn via HSV-1 transduction in mesencephalic primary cultures likewise results in enhanced toxicity in infected cells (190). However, the goal of achieving a robust model of α-syn–dependent cell death in cultured cells amenable to translation to high-throughput screening remains an elusive goal.
Most described α-syn–overexpression systems in mammalian cells result in very mild to no significant changes in markers of toxicity associated with wild-type α-syn expression. Some culture systems demonstrate neuroprotective mechanisms due to α-syn expression. α-Syn expressed in NT-2/D1 (human teratocarcinoma cell line) and SK-MC (human neuroblastoma cell line) cells delays cell death induced by serum withdrawal, but the effect is reversed via MPP+ (1-methyl-4-phenylpyridinium) exposure (127). In lieu of overt changes in cell-death markers associated with α-syn expression, numerous studies have described potential early phenotypes with possible relevance to pathogenesis. A kinetic basis for intracellular accumulation has been demonstrated by overexpressing Flag- and His-tagged versions of α-syn in PC12 and SH-SY5Y cells (108). Demonstration of α-syn expression and oligomeric intermediates in living cells through bimolecular fluorescence complementation may also provide insight into α-syn mechanisms (178). Because overt cell death caused by α-syn expression has been difficult to achieve in mammalian cells, the future of in vitro model systems will likely involve phenotypes outside of toxicity but focused on particular aspects of α-syn conformation, association, or localization. The relevance of data developed in vitro will require confirmation in successful in vivo model systems.
In vivo models
Animal models involving manipulation of α-syn expression have unquestionably led to a better understanding of the correlation between α-syn, neurotoxicity, aggregation, and neurodegeneration (149). In a transgenic nematode model, overexpression of α-syn results in increased lifespan (248, 249) but impairs survival and function of the eight dopamine-containing cells intrinsic to the animals. MPP+ exposure induces dopamine-neuron death and worm lethality in α-syn transgenic worms. In this model, the major cause of MPP+ toxicity links with ATP depletion (257). The power of Caenorhabditis elegans as a model system lies with rapid and powerful genetic interaction studies to identify protein interactors capable of modifying α-syn action. An RNA interference screen to identify critical proteins involved in α-syn protection identified a number of proteins involved in the endocytic pathway in addition to chaperones and other proteins (123, 246). The usual caveat with α-syn–overexpression model systems, but particularly for models involving organisms that do not natively express an α-syn–like protein, is that overexpressed α-syn might not adopt physiologically relevant cell functionality and that the cell impairment or deficiency has no overlap with the dysfunction occurring in PD. Thus, lower organisms seem an ideal tool for hypothesis generation but ultimately require translation to mammalian systems. Successful examples of translation from yeast to mouse models have highlighted new pathways with potential therapeutic targets (33).
Similar to nematodes, Drosophila does not possess clear homologues to α-syn. In models that involve overexpression of human α-syn, flies demonstrate loss of dopamine neurons associated with progressive loss of motor dysfunctions and the presence of filamentous intraneuronal inclusions (55, 56). These flies also exhibited age-dependent retinal degeneration and premature loss of climbing activity. Induction of chaperone pathways rescues cells from the apparent effects of α-synuclein expression (8). The reproducibility of α-syn–induced dopaminergic cell death in flies has been a matter of contention among different laboratories, with some groups reporting cell shrinkage due to α-syn overexpression in particular dopamine neuron clusters that may be masked as cell loss when counted by using particular methods (46, 188). The utility of Drosophila models of α-syn overexpression remains in question until the technical issues that prevent an understanding of phenotype are clearly defined.
As opposed to reports of dopaminergic cell death in the worm and fly, mouse transgenic models overexpressing human α-syn have not yet demonstrated overt degeneration in substantia nigra neurons. Transgenic mouse models driving α-syn with various promoters such as PDGFβ (152), mouse thymus cell antigen 1:Thy1 (229, 263), TH promoter (154, 200), and prion (PrP) promoter (67, 128) have been described. These transgenic animals demonstrate markedly different phenotypes, making broad-based conclusions difficult to draw. In mice overexpressing human α-syn and α-syn with PD-associated mutations driven by the PrP promoter, phenotype is related to dose, and the PD-mutation A53T demonstrates greater in vivo neurotoxicity as compared with other variants; moreover, these mice develop adult-onset neurodegenerative disease with a progressive motoric dysfunction leading to death (128). Transgenic animals further demonstrate an early phenotype before pathologic lesions form (163, 237). Other important observations from transgenic mouse experiments include loss of straital dopaminergic terminals in case of PDGFβ promoter-WT–α-synuclein expression (152), decreased rotarod performance and the presence of detergent-soluble and -insoluble α-syn species in Thy1-promoter-WT and A53T-α-syn expression (244). Without neurodegeneration in the substantia nigra, the challenge lies with picking a phenotype among the plethora of observations robust enough to screen potential therapies for efficacy and yet possess reasonable homology to mechanisms thought to underlie pathogenesis in human PD. The lack of Lewy body formation in transgenic mice and selective degeneration of substantia nigra neurons might disqualify existing transgenic mice as an appropriate model system for therapeutic testing.
The next generation of transgenic might include conditional and regionally specific expression, or crosses of existing transgenics to mice that modify expression of a critical α-syn modifier. As opposed to that of traditional transgenic mice, neurodegeneration in the substantia nigra due to α-syn expression via viral-vector–based delivery has been described in both rats and mice (114, 119, 141, 149, 227). Viral-based gene transduction in living animals results in acute and targeted gene expression, so-called somatic transgenics. Adeno-associated viral (AAV) vectors and HIV-1–derived lentiviral vectors successfully direct high-levels of α-syn expression and loss of nigral and dopaminergic neurons in rodents (114, 119, 141, 227). Co-delivery of the early-onset PD–associated protein parkin prevents dopaminergic degeneration, but in the same model, delivery of GDNF does not prevent neurodegeneration (140, 142). The authors speculate that GDNF treatment cannot modulate the cellular toxicity related to mutant α-syn accumulation.
Virus-based models suggest α-syn as a viable target for therapeutic intervention. Whether α-syn viral transduction in rodents represents a viable in vivo model in which therapeutic approaches prove efficacy remains speculative. Issues including a high technical proficiency requirement for model implementation, high variation between experiments, interlaboratory variation in reproducing the critical cell death phenotype, and a high degree of labor in counting cells by stereology all prevent widespread use of the model system. Further, the lack of cell death in traditional transgenics might translate to a more cautious approach in interpreting viral-transduction experiments, in which inflammation or viral-transduction pathways may provide a necessary “second-hit” in causing cell death that may or may not have relevance to PD. Alternatively, acute somatic transgenics may not have compensatory pathways that block cell death in the traditional transgenics. Transgenics that conditionally and acutely upregulate α-syn to the levels obtained through viral transduction will help resolve the issues and may provide the most powerful model system.
Therapeutic Modification of α-Syn
In a disease that ultimately involves much of the nervous system, small molecules that provide neuroprotection and neurorestoration to PD-affected brain areas represent the most obvious therapeutic strategy. α-Syn is the major species composing Lewy bodies (222, 223). The aberrant accumulation of α-syn likely plays a major role in the neurodegeneration and progression of PD. From the standpoint that PD manifests as an α-synucleinopathy caused by an overabundance of the protein due to gene multiplication (93), associated noncoding promoter variation that upregulates α-syn expression (25, 26), or protein misfolding that results in enhanced fibrillization and decreased turnover spurred by any number of factors, multiple levels of intervention in the disease process may exist.
Aggregation inhibitors/inhibitors of toxicity
Dramatically different methods and approaches have been described that can suppress α-syn aggregation and provide protection from toxicity in model systems. Blocking the formation of aggregated α-syn structures by inhibition with short synthetic peptides represents one avenue (10). Peptides derived from the N-terminal amino acid sequence (1 to 15) of β-synuclein display neuroprotective activity (264). This peptide sequence may be an antiaggregation factor for α-syn toxicity induced by oxidative stress. When peptide fragments are derived from the α-syn protein itself, some sequences demonstrate the propensity to inhibit fibril formation and toxicity (183). Overall, the peptides may act as β-sheet breakers; the shortest peptide, RGAVVTGR-amide, retains the ability to inhibit α-syn aggregation. In culture systems, a cell-permeable peptide inhibitor of α-syn aggregation inhibited DNA damage induced by Fe2+ in neuronal cells expressing mutant human α-syn (A53T) (183). Therapeutic delivery of β-sheet–breaking peptides to the brain of PD-affected individuals represents a monumental challenge, but small peptides may demonstrate proof of principle in model systems that correlate α-syn aggregation with toxicity.
β-Synuclein (a 134-amino-acid protein) also prevents α-syn aggregation in vitro and in double-transgenic mice (85, 185). Acting as chaperones, β- and γ-synuclein both reduce the rate of α-syn fibrillation and aggregation (48, 238, 241). Hsp70 is a potent protein chaperone and refolding complex also known to inhibit aggregation and fibril formation by preferential binding to prefibrillar species (6, 38, 57, 271). Hsp70 likewise potently reduces α-syn self-interaction in bimolecular fluorescence complementation assays (178). Other chaperones that may play critical roles in anti–α-syn aggregation include torsinA (212, 213, 254), Hsp40 (8), and αB-crystallin (192). Broad therapeutic delivery of protective proteins that target α-syn toxicity and aggregation throughout the brain requires huge advances in current gene-therapy technology, but in the meantime, proteins that protect from α-syn toxicity in relevant model systems will help delineate pathogenesis and provide additional therapeutic targets that in turn may be amenable small-molecule modification.
Human single-chain antibody fragments (scFv) that bind α-syn inhibit toxicity and formation of α-syn–positive fibrils (50). scFv molecules can bind specifically to an oligomeric form of α-syn and prevent aggregation and interaction with the cell membrane, thereby reducing membrane damage and pore formation. Like peptides with an affinity for β-sheets and chaperones with an affinity for unfolded or aggregated proteins, the isolated scFvs ideally bind only to the toxic oligomeric species in the target protein while avoiding the problem of interaction with the potentially benign and abundant natively unfolded α-syn protein. Intrabody therapy, as with most recombinant protein approaches in therapeutics, introduces an additional set of difficult technical challenges in the clinic beyond the question of target relevance.
Inflammation and microglial activation coincide with neurodegeneration in PD and in many models of the disease (125, 156, 157, 274). Microglia inflammation inhibitors and antiinflammatory approaches are under investigation for preventing α-syn toxicity as well as to suppress neuroinflammation in PD (49, 117, 143, 155, 164, 195, 198, 225, 231, 234). Suppressive action by NSAIDs on dopamine quinone formation by interaction of α-syn with microglia and astrocytes either may arrest or effectively slow neurodegeneration (88). α-Syn mutations may induce a proinflammatory phenotype in both microglia and astrocytes, indicating the involvement of cell-surface receptors for both microglia and astrocytes (116, 118). Antagonists for these putative cell-surface receptors (microglial and astrocyte), as well as those for other molecules that regulate microglial activation, including MMP-3 (112), CD40L (23, 173), CCL2 (109), and other chemokines could constitute novel targets for therapeutic intervention.
In another study in which a commercially available compound library was screened, it was found that dopamine and other catecholamines interacted with α-syn protofibrils and inhibited the fibrilization process (32). When antioxidants like sodium metabisulfite were added, the process was reversed, suggesting that fibril inhibition, protofibril accumulation, and monomer modification is a sequel to covalent modification by the dopamine-derived orthoquinone. Other compounds with antioxidative properties such as flavonoid baicalein (276) and some antibiotics like rifampicin (132) are also able to inhibit α-syn fibrillation in vitro and further disaggregate preformed fibrils and soluble oligomers. PD therapeutic agents such as selegiline, dopamine, pergolide, and bromocriptine dose-dependently inhibit the formation of α-syn fibrils and also destabilize the preformed α-syn fibrils (174). The potency of these compounds ranks as follows: selegiline = dopamine > pergolide > bromocriptine. In short, small molecules exist that likely modify α-syn structure in cells. Targeted screens that elucidate molecules with drug-like properties and demonstrate efficacy in relevant model systems will ultimately test the role of aggregation and α-syn protofibril formation in disease pathogenesis.
Reducing α-syn expression
Human genetic studies suggest that α-syn overexpression correlates with PD, at least in the PD cases that have multiplications of the α-syn gene. Targeting α-syn expression itself may be an effective neuroprotective therapy for an α-synucleinopathy. Recent developments highlight the current focus on RNA interference (RNAi)-mediated knockdown of α-syn mRNA, thus offering protection for dopaminergic neuroblastoma cells as well as in in vivo model systems (59, 80, 123, 137, 205). RNAi approaches continue to make strides toward broad use in the clinic, and delivery to the central nervous system will represent one of the most difficult challenges. If α-syn expression is required for pathogenesis, targeting α-syn mRNA should provide neuroprotection or neurorestoration or both. Outside of RNAi approaches, the α-syn promoter may represent a viable target to knockdown α-syn expression. GATA-2 critically induces α-syn expression (207), and inhibitory agents blocking transcription activation may enable therapeutically beneficial lower production of α-syn, at least in those PD patients with α-syn gene multiplications. Transcriptional regulation of α-syn through NGF- and bFGF-mediated signal transduction via the MAP/ERK and PI3 kinase pathways may also serve to regulate α-syn expression and provide neuroprotection (29).
Both the proteasome and lysosomal degradation pathways seem important for clearance of α-syn and α-syn–containing protein aggregates. Enhancing proteosomal function by overexpressing parkin (E3 ubiquitin ligase) reduces α-syn toxicity in model systems, including preservation of TH-positive neurons in the substantia nigra and sparing of TH-positive nerve terminals in the striatum (142, 268). Decreasing α-syn ubiquitination by blocking the E3 ubiquitin-ligase SIAH reduces the amounts of amorphous aggregates formed in cells (51, 203). The action of PD-associated point mutations may impair normal metabolism through chaperone-mediated autophagy (35). Afterward, upregulation of cathepsin D to enhance lysosomal function promotes α-syn degradation and inhibits aggregation and toxicity (210). A small proportion of α-syn also exists outside of the cell and in the cerebrospinal fluid (15, 167). α-Syn vaccination may be another strategy to reduce α-syn protein levels with proof of concept demonstrated in a transgenic model in which immunization reduced aggregation and neurotoxicity (151).
Conclusions
Molecular genetic studies that investigate PD in families that inherit the disease from one generation to the next have identified specific proteins that alone are capable of causing the complex and heterogeneous PD syndrome. Among the linked proteins, α-syn and LRRK2 seem to be the most viable therapeutic targets. Because genetic and functional studies suggest gain-of-function mechanisms at work in α-syn– and LRRK2-related disease, therapies that ablate the aberrant function should provide benefit to at least those patients with mutations but potentially to all PD cases if the proteins are the rate-limiting and critical components to pathogenesis. Most effective therapies in the clinic act by diminishing the activity of the target rather than enhancing the target activity. Many therapeutic approaches for PD currently in clinical trials run the risk of targeting downstream cell-death pathways that, even if efficiently targeted, can be easily compensated through other mechanisms. In comparison, α-syn and LRRK2 likely represent upstream factors, albeit factors that may ultimately serve as very poor targets for intervention because of the issues discussed here.
In the case of LRRK2, small molecules exist that effectively ablate activity in protein kinases that share homology with the LRRK2 kinase domain that are safe in the clinic. The drug Cep-1347 inhibits MLK proteins important for JNK activation and neurodegeneration, although the drug failed to delay disability in early PD (1). Questions of whether MLKs are effective and appropriate targets in PD and whether the protein kinases were actually inhibited in the brain remain open ended. Although LRRK2 is not an MLK protein, small molecules that inhibit LRRK2 activity should exist. As opposed to most protein kinases, LRRK2 offers several opportunities for disruption of kinase activity via the critical internal protein domains that control kinase activity. The overall benefit to small-molecule therapy as opposed to more-targeted therapies like stem cell–replacement therapies and gene therapy in the brain is that the small molecules, if permeable through the blood–brain barrier, should affect the physically widespread regions involved through the course of PD. Issues of safety and specificity are tremendous hurdles but should not be unsurpassable.
The challenge with α-syn as a therapeutic target lies first with deciding what property of the protein to target, and second, what technology might be capable of addressing that property. A reduction of aberrant transcriptional regulation of α-syn that results in overexpression may be a therapeutic approach, although the required technology presents a major challenge. Nevertheless, modifying α-syn gene expression through RNA interference or direct modification of transcription or translation seems like an appropriate approach that avoids the potential complications with targets further downstream. Data from model systems suggest that relatively elusive intermediates in the α-syn–aggregation pathway may be most toxic, although delineation of such species has proved difficult, thereby amplifying the difficulties in identifying strategies specifically to target the toxic species. Likewise, difficulties in the generation of model systems that demonstrate α-syn–dependent phenotypes will also hinder validation of efficacious strategies.
In sum, both α-syn and LRRK2 may be robust targets for therapy in PD. Each protein presents a set of unique challenges that range from target-validation issues to the design of appropriate models that allow identification of therapeutic molecules. α-Syn and LRRK2 challenge some of the central dogma surrounding PD etiology, and the importance of α-syn and LRRK2 in PD will not be fully realized until technology capable of ablating their associated function is translated to PD patients.
Abbreviations Used
- α-Syn
α-synuclein
- AAV
adeno-associated virus
- CNS
central nervous system
- DAPK1
death-associated protein kinase 1
- DLB
dementia with Lewy bodies
- DLBD
diffuse Lewy body disease
- ER
endoplasmic reticulum
- ERK
extracellular-regulated kinase
- GbpC
glucan-binding protein C
- GDNF
glia-derived neurotrophic factor
- GTPγS
guanosine γphosphate
- HSV-1
herpes simplex virus type-1
- LRRK1
leucine-rich repeat kinase 1
- LRRK2
leucine-rich repeat kinase 2
- MAO-B
monoamine oxidase B
- MAP
mitogen-activated protein
- MAPKKK
mitogen-activated protein kinase-kinase-kinase
- MLK
mixed-lineage kinase
- MPP+
1-methly-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSA
multiple system atrophy
- NAC
nonamyloid component
- NSAIDs
nonsteroidal antiinflammatory drugs
- PC12
pheochromocytoma-12
- PD
Parkinson's disease
- PINK1
PTEN-induced kinase-1
- ROS
reactive oxygen species
- scFv
single-chain antibody fragments
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
References
- 1.The Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 2.NINDS NET-PD Investigators. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abeliovich A. Schmitz Y. Farinas I. Choi-Lundberg D. Ho WH. Castillo PE. Shinsky N. Verdugo JM. Armanini M. Ryan A. Hynes M. Phillips H. Sulzer D. Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 4.Ahlskog JE. Beating a dead horse: dopamine and Parkinson disease. Neurology. 2007;69:1701–1711. doi: 10.1212/01.wnl.0000296942.14309.4a. [DOI] [PubMed] [Google Scholar]
- 5.Ahn BH. Rhim H. Kim SY. Sung YM. Lee MY. Choi JY. Wolozin B. Chang JS. Lee YH. Kwon TK. Chung KC. Yoon SH. Hahn SJ. Kim MS. Jo YH. Min DS. alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J Biol Chem. 2002;277:12334–12342. doi: 10.1074/jbc.M110414200. [DOI] [PubMed] [Google Scholar]
- 6.Albani D. Peverelli E. Rametta R. Batelli S. Veschini L. Negro A. Forloni G. Protective effect of TAT-delivered alpha-synuclein: relevance of the C-terminal domain and involvement of HSP70. FASEB J. 2004;18:1713–1715. doi: 10.1096/fj.04-1621fje. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JP. Walker DE. Goldstein JM. de Laat R. Banducci K. Caccavello RJ. Barbour R. Huang J. Kling K. Lee M. Diep L. Keim PS. Shen X. Chataway T. Schlossmacher MG. Seubert P. Schenk D. Sinha S. Gai WP. Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 8.Auluck PK. Chan HY. Trojanowski JQ. Lee VM. Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 9.Baba M. Nakajo S. Tu PH. Tomita T. Nakaya K. Lee VM. Trojanowski JQ. Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 10.Bieler S. Soto C. Beta-sheet breakers for Alzheimer's disease therapy. Curr Drug Targets. 2004;5:553–558. doi: 10.2174/1389450043345290. [DOI] [PubMed] [Google Scholar]
- 11.Biskup S. Moore DJ. Celsi F. Higashi S. West AB. Andrabi SA. Kurkinen K. Yu SW. Savitt JM. Waldvogel HJ. Faull RL. Emson PC. Torp R. Ottersen OP. Dawson TM. Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 12.Biskup S. Moore DJ. Rea A. Lorenz-Deperieux B. Coombes CE. Dawson VL. Dawson TM. West AB. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102. doi: 10.1186/1471-2202-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biskup S. Mueller JC. Sharma M. Lichtner P. Zimprich A. Berg D. Wullner U. Illig T. Meitinger T. Gasser T. Common variants of LRRK2 are not associated with sporadic Parkinson's disease. Ann Neurol. 2005;58:905–908. doi: 10.1002/ana.20664. [DOI] [PubMed] [Google Scholar]
- 14.Bonifati V. Rizzu P. van Baren MJ. Schaap O. Breedveld GJ. Krieger E. Dekker MC. Squitieri F. Ibanez P. Joosse M. van Dongen JW. Vanacore N. van Swieten JC. Brice A. Meco G. van Duijn CM. Oostra BA. Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 15.Borghi R. Marchese R. Negro A. Marinelli L. Forloni G. Zaccheo D. Abbruzzese G. Tabaton M. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- 16.Bosgraaf L. Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Braak H. Ghebremedhin E. Rub U. Bratzke H. Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 18.Brown DR. Interactions between metals and alpha-synuclein: function or artefact? FEBS J. 2007;274:3766–3774. doi: 10.1111/j.1742-4658.2007.05917.x. [DOI] [PubMed] [Google Scholar]
- 19.Bussell R., Jr Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 20.Chan CS. Guzman JN. Ilijic E. Mercer JN. Rick C. Tkatch T. Meredith GE. Surmeier DJ. “Rejuvenation” protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 21.Chandra S. Fornai F. Kwon HB. Yazdani U. Atasoy D. Liu X. Hammer RE. Battaglia G. German DC. Castillo PE. Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H. O'Reilly EJ. Schwarzschild MA. Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 23.Chen K. Huang J. Gong W. Zhang L. Yu P. Wang JM. CD40/CD40L dyad in the inflammatory and immune responses in the central nervous system. Cell Mol Immunol. 2006;3:163–169. [PubMed] [Google Scholar]
- 24.Chen L. Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 25.Chiba-Falek O. Nussbaum RL. Effect of allelic variation at the NACP-Rep1 repeat upstream of the alpha-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum Mol Genet. 2001;10:3101–3109. doi: 10.1093/hmg/10.26.3101. [DOI] [PubMed] [Google Scholar]
- 26.Chiba-Falek O. Touchman JW. Nussbaum RL. Functional analysis of intra-allelic variation at NACP-Rep1 in the alpha-synuclein gene. Hum Genet. 2003;113:426–431. doi: 10.1007/s00439-003-1002-9. [DOI] [PubMed] [Google Scholar]
- 27.Choi WS. Kruse SE. Palmiter RD. Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton DF. George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 29.Clough RL. Stefanis L. A novel pathway for transcriptional regulation of alpha-synuclein. FASEB J. 2007;21:596–607. doi: 10.1096/fj.06-7111com. [DOI] [PubMed] [Google Scholar]
- 30.Cohlberg JA. Li J. Uversky VN. Fink AL. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from alpha-synuclein in vitro. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- 31.Cole NB. Murphy DD. Grider T. Rueter S. Brasaemle D. Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 32.Conway KA. Rochet JC. Bieganski RM. Lansbury PT., Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 33.Cooper AA. Gitler AD. Cashikar A. Haynes CM. Hill KJ. Bhullar B. Liu K. Xu K. Strathearn KE. Liu F. Cao S. Caldwell KA. Caldwell GA. Marsischky G. Kolodner RD. Labaer J. Rochet JC. Bonini NM. Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowther RA. Jakes R. Spillantini MG. Goedert M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 1998;436:309–312. doi: 10.1016/s0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 35.Cuervo AM. Stefanis L. Fredenburg R. Lansbury PT. Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 36.Dauer W. Kholodilov N. Vila M. Trillat AC. Goodchild R. Larsen KE. Staal R. Tieu K. Schmitz Y. Yuan CA. Rocha M. Jackson-Lewis V. Hersch S. Sulzer D. Przedborski S. Burke R. Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidson WS. Jonas A. Clayton DF. George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 38.Dedmon MM. Christodoulou J. Wilson MR. Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280:14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 39.Deng H. Dodson MW. Huang H. Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dev KK. Hofele K. Barbieri S. Buchman VL. van der Putten H. Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 41.Dexter DT. Jenner P. Schapira AH. Marsden CD. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia: the Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol. 1992;32(suppl):S94–S100. doi: 10.1002/ana.410320716. [DOI] [PubMed] [Google Scholar]
- 42.Dexter DT. Wells FR. Lees AJ. Agid F. Agid Y. Jenner P. Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 43.Di Fonzo A. Rohe CF. Ferreira J. Chien HF. Vacca L. Stocchi F. Guedes L. Fabrizio E. Manfredi M. Vanacore N. Goldwurm S. Breedveld G. Sampaio C. Meco G. Barbosa E. Oostra BA. Bonifati V. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet. 2005;365:412–415. doi: 10.1016/S0140-6736(05)17829-5. [DOI] [PubMed] [Google Scholar]
- 44.Dickson DW. Fujishiro H. DelleDonne A. Menke J. Ahmed Z. Klos KJ. Josephs KA. Frigerio R. Burnett M. Parisi JE. Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 45.Driver JA. Kurth T. Buring JE. Gaziano JM. Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70:1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 46.Drobysheva D. Ameel K. Welch B. Ellison E. Chaichana K. Hoang B. Sharma S. Neckameyer W. Srinakevitch I. Murphy KJ. Schmid A. An optimized method for histological detection of dopaminergic neurons in Drosophila melanogaster. J Histochem Cytochem. 2008;56:1049–1063. doi: 10.1369/jhc.2008.951137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duda JE. Giasson BI. Chen Q. Gur TL. Hurtig HI. Stern MB. Gollomp SM. Ischiropoulos H. Lee VM. Trojanowski JQ. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Agnaf OM. Jakes R. Curran MD. Wallace A. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson's disease. FEBS Lett. 1998;440:67–70. doi: 10.1016/s0014-5793(98)01419-7. [DOI] [PubMed] [Google Scholar]
- 49.Eljaschewitsch E. Witting A. Mawrin C. Lee T. Schmidt PM. Wolf S. Hoertnagl H. Raine CS. Schneider-Stock R. Nitsch R. Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 50.Emadi S. Barkhordarian H. Wang MS. Schulz P. Sierks MR. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368:1132–1144. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engelender S. Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease. Autophagy. 2008;4:372–374. doi: 10.4161/auto.5604. [DOI] [PubMed] [Google Scholar]
- 52.Etminan M. Gill SS. Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol. 2005;4:362–365. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- 53.Fariss MW. Zhang JG. Vitamin E therapy in Parkinson's disease. Toxicology. 2003;189:129–146. doi: 10.1016/s0300-483x(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 54.Farrer M. Maraganore DM. Lockhart P. Singleton A. Lesnick TG. de Andrade M. West A. de Silva R. Hardy J. Hernandez D. alpha-Synuclein gene haplotypes are associated with Parkinson's disease. Hum Mol Genet. 2001;10:1847–1851. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- 55.Feany MB. Studying human neurodegenerative diseases in flies and worms. J Neuropathol Exp Neurol. 2000;59:847–856. doi: 10.1093/jnen/59.10.847. [DOI] [PubMed] [Google Scholar]
- 56.Feany MB. Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 57.Flower TR. Chesnokova LS. Froelich CA. Dixon C. Witt SN. Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson's disease. J Mol Biol. 2005;351:1081–1100. doi: 10.1016/j.jmb.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 58.Fortin DL. Troyer MD. Nakamura K. Kubo S. Anthony MD. Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fountaine TM. Wade-Martins R. RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res. 2007;85:351–363. doi: 10.1002/jnr.21125. [DOI] [PubMed] [Google Scholar]
- 60.Fujiwara H. Hasegawa M. Dohmae N. Kawashima A. Masliah E. Goldberg MS. Shen J. Takio K. Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 61.Funayama M. Hasegawa K. Kowa H. Saito M. Tsuji S. Obata F. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 62.Galpern WR. Cudkowicz ME. Coenzyme Q treatment of neurodegenerative diseases of aging. Mitochondrion. 2007;7(suppl):S146–S153. doi: 10.1016/j.mito.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Gao HM. Liu B. Zhang W. Hong JS. Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 64.Garber K. The second wave in kinase cancer drugs. Nat Biotechnol. 2006;24:127–130. doi: 10.1038/nbt0206-127. [DOI] [PubMed] [Google Scholar]
- 65.George JM. Jin H. Woods WS. Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 66.Giasson BI. Duda JE. Murray IV. Chen Q. Souza JM. Hurtig HI. Ischiropoulos H. Trojanowski JQ. Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 67.Giasson BI. Duda JE. Quinn SM. Zhang B. Trojanowski JQ. Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 68.Giasson BI. Murray IV. Trojanowski JQ. Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 69.Gilks WP. Abou-Sleiman PM. Gandhi S. Jain S. Singleton A. Lees AJ. Shaw K. Bhatia KP. Bonifati V. Quinn NP. Lynch J. Healy DG. Holton JL. Revesz T. Wood NW. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 70.Glaser CB. Yamin G. Uversky VN. Fink AL. Methionine oxidation, alpha-synuclein and Parkinson's disease. Biochim Biophys Acta. 2005;1703:157–169. doi: 10.1016/j.bbapap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Gloeckner CJ. Kinkl N. Schumacher A. Braun RJ. O'Neill E. Meitinger T. Kolch W. Prokisch H. Ueffing M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 72.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 73.Goers J. Manning-Bog AB. McCormack AL. Millett IS. Doniach S. Di Monte DA. Uversky VN. Fink AL. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 74.Goers J. Uversky VN. Fink AL. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003;12:702–707. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorell JM. Johnson CC. Rybicki BA. Peterson EL. Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- 76.Greggio E. Jain S. Kingsbury A. Bandopadhyay R. Lewis P. Kaganovich A. van der Brug MP. Beilina A. Blackinton J. Thomas KJ. Ahmad R. Miller DW. Kesavapany S. Singleton A. Lees A. Harvey RJ. Harvey K. Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Greggio E. Lewis PA. van der Brug MP. Ahmad R. Kaganovich A. Ding J. Beilina A. Baker AK. Cookson MR. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J Neurochem. 2007;102:93–102. doi: 10.1111/j.1471-4159.2007.04523.x. [DOI] [PubMed] [Google Scholar]
- 78.Greggio E. Zambrano I. Kaganovich A. Beilina A. Taymans JM. Daniels V. Lewis P. Jain S. Ding J. Syed A. Thomas KJ. Baekelandt V. Cookson MR. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo L. Gandhi PN. Wang W. Petersen RB. Wilson-Delfosse AL. Chen SG. The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res. 2007;313:3658–3670. doi: 10.1016/j.yexcr.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamamichi S. Rivas RN. Knight AL. Cao S. Caldwell KA. Caldwell GA. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson's disease model. Proc Natl Acad Sci U S A. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han BS. Iacovitti L. Katano T. Hattori N. Seol W. Kim KS. Expression of the LRRK2 gene in the midbrain dopaminergic neurons of the substantia nigra. Neurosci Lett. 2008;442:190–194. doi: 10.1016/j.neulet.2008.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han H. Weinreb PH. Lansbury PT., Jr. The core Alzheimer's peptide NAC forms amyloid fibrils which seed and are seeded by beta-amyloid: is NAC a common trigger or target in neurodegenerative disease? Chem Biol. 1995;2:163–169. doi: 10.1016/1074-5521(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 83.Harley A. Cooper JM. Schapira AH. Iron induced oxidative stress and mitochondrial dysfunction: relevance to Parkinson's disease. Brain Res. 1993;627:349–353. doi: 10.1016/0006-8993(93)90341-j. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto M. Hsu LJ. Xia Y. Takeda A. Sisk A. Sundsmo M. Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 85.Hashimoto M. Rockenstein E. Mante M. Crews L. Bar-On P. Gage FH. Marr R. Masliah E. An antiaggregation gene therapy strategy for Lewy body disease utilizing beta-synuclein lentivirus in a transgenic model. Gene Ther. 2004;11:1713–1723. doi: 10.1038/sj.gt.3302349. [DOI] [PubMed] [Google Scholar]
- 86.Hely MA. Morris JG. Reid WG. Trafficante R. Sydney multicenter study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 87.Higashi S. Biskup S. West AB. Trinkaus D. Dawson VL. Faull RL. Waldvogel HJ. Arai H. Dawson TM. Moore DJ. Emson PC. Localization of Parkinson's disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–219. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 88.Hirohata M. Ono K. Morinaga A. Yamada M. Non-steroidal anti-inflammatory drugs have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. Neuropharmacology. 2008;54:620–627. doi: 10.1016/j.neuropharm.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Hokenson MJ. Uversky VN. Goers J. Yamin G. Munishkina LA. Fink AL. Role of individual methionines in the fibrillation of methionine-oxidized alpha-synuclein. Biochemistry. 2004;43:4621–4633. doi: 10.1021/bi049979h. [DOI] [PubMed] [Google Scholar]
- 90.Horstink MW. van Engelen BG. The effect of coenzyme Q10 therapy in Parkinson disease could be symptomatic. Arch Neurol. 2003;60:1170–1172. doi: 10.1001/archneur.60.8.1170-b. author reply 1172–1173. [DOI] [PubMed] [Google Scholar]
- 91.Hulihan MM. Ishihara-Paul L. Kachergus J. Warren L. Amouri R. Elango R. Prinjha RK. Upmanyu R. Kefi M. Zouari M. Sassi SB. Yahmed SB. El Euch-Fayeche G. Matthews PM. Middleton LT. Gibson RA. Hentati F. Farrer MJ. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case-control genetic study. Lancet Neurol. 2008;7:591–594. doi: 10.1016/S1474-4422(08)70116-9. [DOI] [PubMed] [Google Scholar]
- 92.Hunot S. Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(suppl 3):S49–S58. doi: 10.1002/ana.10481. discussion S58–S60. [DOI] [PubMed] [Google Scholar]
- 93.Ibanez P. Bonnet AM. Debarges B. Lohmann E. Tison F. Pollak P. Agid Y. Durr A. Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 94.Imai Y. Gehrke S. Wang HQ. Takahashi R. Hasegawa K. Oota E. Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ito G. Okai T. Fujino G. Takeda K. Ichijo H. Katada T. Iwatsubo T. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 96.Iwai A. Masliah E. Yoshimoto M. Ge N. Flanagan L. de Silva HA. Kittel A. Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 97.Iwata A. Maruyama M. Kanazawa I. Nukina N. alpha-Synuclein affects the MAPK pathway and accelerates cell death. J Biol Chem. 2001;276:45320–45329. doi: 10.1074/jbc.M103736200. [DOI] [PubMed] [Google Scholar]
- 98.Iwata A. Miura S. Kanazawa I. Sawada M. Nukina N. alpha-Synuclein forms a complex with transcription factor Elk-1. J Neurochem. 2001;77:239–252. doi: 10.1046/j.1471-4159.2001.t01-1-00232.x. [DOI] [PubMed] [Google Scholar]
- 99.Jaleel M. Nichols RJ. Deak M. Campbell DG. Gillardon F. Knebel A. Alessi DR. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 101.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2008. Aug 5 [Epub ahead of print]. [DOI] [PubMed]
- 102.Jenco JM. Rawlingson A. Daniels B. Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 103.Jensen PH. Hojrup P. Hager H. Nielsen MS. Jacobsen L. Olesen OF. Gliemann J. Jakes R. Binding of Abeta to alpha- and beta-synucleins: identification of segments in alpha-synuclein/NAC precursor that bind Abeta and NAC. Biochem J. 1997;323:539–546. doi: 10.1042/bj3230539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jensen PH. Nielsen MS. Jakes R. Dotti CG. Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 105.Jo E. McLaurin J. Yip CM. St George-Hyslop P. Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 106.Johnson WG. Hodge SE. Duvoisin R. Twin studies and the genetics of Parkinson's disease: a reappraisal. Mov Disord. 1990;5:187–194. doi: 10.1002/mds.870050302. [DOI] [PubMed] [Google Scholar]
- 107.Junn E. Ronchetti RD. Quezado MM. Kim SY. Mouradian MM. Tissue transglutaminase-induced aggregation of alpha-synuclein: implications for Lewy body formation in Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 2003;100:2047–2052. doi: 10.1073/pnas.0438021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kahle PJ. Neumann M. Ozmen L. Haass C. Physiology and pathophysiology of alpha-synuclein: cell culture and transgenic animal models based on a Parkinson's disease-associated protein. Ann N Y Acad Sci. 2000;920:33–41. doi: 10.1111/j.1749-6632.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- 109.Kalkonde YV. Morgan WW. Sigala J. Maffi SK. Condello C. Kuziel W. Ahuja SS. Ahuja SK. Chemokines in the MPTP model of Parkinson's disease: absence of CCL2 and its receptor CCR2 does not protect against striatal neurodegeneration. Brain Res. 2007;1128:1–11. doi: 10.1016/j.brainres.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 110.Kanda S. Bishop JF. Eglitis MA. Yang Y. Mouradian MM. Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience. 2000;97:279–284. doi: 10.1016/s0306-4522(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 111.Kim TD. Paik SR. Yang CH. Structural and functional implications of C-terminal regions of alpha-synuclein. Biochemistry. 2002;41:13782–13790. doi: 10.1021/bi026284c. [DOI] [PubMed] [Google Scholar]
- 112.Kim YS. Choi DH. Block ML. Lorenzl S. Yang L. Kim YJ. Sugama S. Cho BP. Hwang O. Browne SE. Kim SY. Hong JS. Beal MF. Joh TH. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 113.Kim YS. Laurine E. Woods W. Lee SJ. A novel mechanism of interaction between alpha-synuclein and biological membranes. J Mol Biol. 2006;360:386–397. doi: 10.1016/j.jmb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Kirik D. Rosenblad C. Burger C. Lundberg C. Johansen TE. Muzyczka N. Mandel RJ. Bjorklund A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kitada T. Asakawa S. Hattori N. Matsumine H. Yamamura Y. Minoshima S. Yokochi M. Mizuno Y. Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 116.Klegeris A. Giasson BI. Zhang H. Maguire J. Pelech S. McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20:2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 117.Klegeris A. McGeer EG. McGeer PL. Therapeutic approaches to inflammation in neurodegenerative disease. Curr Opin Neurol. 2007;20:351–357. doi: 10.1097/WCO.0b013e3280adc943. [DOI] [PubMed] [Google Scholar]
- 118.Klegeris A. Pelech S. Giasson BI. Maguire J. Zhang H. McGeer EG. McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Klein RL. King MA. Hamby ME. Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum Gene Ther. 2002;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- 120.Korr D. Toschi L. Donner P. Pohlenz HD. Kreft B. Weiss B. LRRK1 protein kinase activity is stimulated upon binding of GTP to its Roc domain. Cell Signal. 2006;18:910–920. doi: 10.1016/j.cellsig.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 121.Kruger R. Kuhn W. Muller T. Woitalla D. Graeber M. Kosel S. Przuntek H. Epplen JT. Schols L. Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 122.Kuhn M. Haebig K. Bonin M. Ninkina N. Buchman VL. Poths S. Riess O. Whole genome expression analyses of single- and double-knock-out mice implicate partially overlapping functions of alpha- and gamma-synuclein. Neurogenetics. 2007;8:71–81. doi: 10.1007/s10048-007-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuwahara T. Koyama A. Koyama S. Yoshina S. Ren CH. Kato T. Mitani S. Iwatsubo T. A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in {alpha}-synuclein transgenic C. elegans. Hum Mol Genet. 2008;17:2997–3009. doi: 10.1093/hmg/ddn198. [DOI] [PubMed] [Google Scholar]
- 124.Langston JW. The Parkinson's complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 125.Langston JW. Forno LS. Tetrud J. Reeves AG. Kaplan JA. Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 126.Lee JT. Wheeler TC. Li L. Chin LS. Ubiquitination of alpha-synuclein by Siah-1 promotes alpha-synuclein aggregation and apoptotic cell death. Hum Mol Genet. 2008;17:906–917. doi: 10.1093/hmg/ddm363. [DOI] [PubMed] [Google Scholar]
- 127.Lee M. Hyun D. Halliwell B. Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 128.Lee MK. Stirling W. Xu Y. Xu X. Qui D. Mandir AS. Dawson TM. Copeland NG. Jenkins NA. Price DL. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53: T thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee SB. Kim W. Lee S. Chung J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem Biophys Res Commun. 2007;358:534–539. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- 130.Lesage S. Durr A. Tazir M. Lohmann E. Leutenegger AL. Janin S. Pollak P. Brice A. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N Engl J Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 131.Lewis PA. Greggio E. Beilina A. Jain S. Baker A. Cookson MR. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun. 2007;357:668–671. doi: 10.1016/j.bbrc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li J. Zhu M. Rajamani S. Uversky VN. Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 133.Li JY. Henning Jensen P. Dahlstrom A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113:463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 134.Li X. Tan YC. Poulose S. Olanow CW. Huang XY. Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J Neurochem. 2007;103:238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liani E. Eyal A. Avraham E. Shemer R. Szargel R. Berg D. Bornemann A. Riess O. Ross CA. Rott R. Engelender S. Ubiquitylation of synphilin-1 and alpha-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:5500–5505. doi: 10.1073/pnas.0401081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Limousin P. Martinez-Torres I. Deep brain stimulation for Parkinson's disease. Neurotherapeutics. 2008;5:309–319. doi: 10.1016/j.nurt.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu DM. Jin L. Wang H. Zhao HY. Zhao CL. Yang H. RNA interference mediated silencing of alpha-synuclein in MN9D cells and its effects on cell viability. Neurosci Bull. 2008;24:96–104. doi: 10.1007/s12264-008-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu IH. Uversky VN. Munishkina LA. Fink AL. Halfter W. Cole GJ. Agrin binds alpha-synuclein and modulates alpha-synuclein fibrillation. Glycobiology. 2005;15:1320–1331. doi: 10.1093/glycob/cwj014. [DOI] [PubMed] [Google Scholar]
- 139.Liu Z. Wang X. Yu Y. Li X. Wang T. Jiang H. Ren Q. Jiao Y. Sawa A. Moran T. Ross CA. Montell C. Smith WW. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lo Bianco C. Deglon N. Pralong W. Aebischer P. Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson's disease. Neurobiol Dis. 2004;17:283–289. doi: 10.1016/j.nbd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 141.Lo Bianco C. Ridet JL. Schneider BL. Deglon N. Aebischer P. alpha-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci U S A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lo Bianco C. Schneider BL. Bauer M. Sajadi A. Brice A. Iwatsubo T. Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu X. Bing G. Hagg T. Naloxone prevents microglia-induced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience. 2000;97:285–291. doi: 10.1016/s0306-4522(00)00033-6. [DOI] [PubMed] [Google Scholar]
- 144.Lucking CB. Brice A. Alpha-synuclein and Parkinson's disease. Cell Mol Life Sci. 2000;57:1894–1908. doi: 10.1007/PL00000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.MacLeod D. Dowman J. Hammond R. Leete T. Inoue K. Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 146.Mandal PK. Pettegrew JW. Masliah E. Hamilton RL. Mandal R. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer's and Parkinson's in dementia with Lewy body disease. Neurochem Res. 2006;31:1153–1162. doi: 10.1007/s11064-006-9140-9. [DOI] [PubMed] [Google Scholar]
- 147.Manning-Bog AB. McCormack AL. Purisai MG. Bolin LM. Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maraganore DM. de Andrade M. Elbaz A. Farrer MJ. Ioannidis JP. Kruger R. Rocca WA. Schneider NK. Lesnick TG. Lincoln SJ. Hulihan MM. Aasly JO. Ashizawa T. Chartier-Harlin MC. Checkoway H. Ferrarese C. Hadjigeorgiou G. Hattori N. Kawakami H. Lambert JC. Lynch T. Mellick GD. Papapetropoulos S. Parsian A. Quattrone A. Riess O. Tan EK. Van Broeckhoven C. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 149.Maries E. Dass B. Collier TJ. Kordower JH. Steece-Collier K. The role of alpha-synuclein in Parkinson's disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 150.Marin I. Ancient origin of the Parkinson disease gene LRRK2. J Mol Evol. 2008;67:41–50. doi: 10.1007/s00239-008-9122-4. [DOI] [PubMed] [Google Scholar]
- 151.Masliah E. Rockenstein E. Adame A. Alford M. Crews L. Hashimoto M. Seubert P. Lee M. Goldstein J. Chilcote T. Games D. Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 152.Masliah E. Rockenstein E. Veinbergs I. Mallory M. Hashimoto M. Takeda A. Sagara Y. Sisk A. Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 153.Mata IF. Wedemeyer WJ. Farrer MJ. Taylor JP. Gallo KA. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 154.Matsuoka Y. Vila M. Lincoln S. McCormack A. Picciano M. LaFrancois J. Yu X. Dickson D. Langston WJ. McGowan E. Farrer M. Hardy J. Duff K. Przedborski S. Di Monte DA. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- 155.McCoy MK. Martinez TN. Ruhn KA. Szymkowski DE. Smith CG. Botterman BR. Tansey KE. Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McGeer PL. Itagaki S. Boyes BE. McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 157.McGeer PL. Schwab C. Parent A. Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 158.McLean PJ. Kawamata H. Hyman BT. Alpha-synuclein-enhanced green fluorescent protein fusion proteins form proteasome sensitive inclusions in primary neurons. Neuroscience. 2001;104:901–912. doi: 10.1016/s0306-4522(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 159.McLean PJ. Kawamata H. Ribich S. Hyman BT. Membrane association and protein conformation of alpha-synuclein in intact neurons: effect of Parkinson's disease-linked mutations. J Biol Chem. 2000;275:8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 160.McLean PJ. Kawamata H. Shariff S. Hewett J. Sharma N. Ueda K. Breakefield XO. Hyman BT. TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem. 2002;83:846–854. doi: 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- 161.Melamed E. Ziv I. Djaldetti R. Management of motor complications in advanced Parkinson's disease. Mov Disord. 2007;22(suppl 17):S379–S384. doi: 10.1002/mds.21680. [DOI] [PubMed] [Google Scholar]
- 162.Melrose H. Lincoln S. Tyndall G. Dickson D. Farrer M. Anatomical localization of leucine-rich repeat kinase 2 in mouse brain. Neuroscience. 2006;139:791–794. doi: 10.1016/j.neuroscience.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 163.Miller RM. Kiser GL. Kaysser-Kranich T. Casaceli C. Colla E. Lee MK. Palaniappan C. Federoff HJ. Wild-type and mutant alpha-synuclein induce a multi-component gene expression profile consistent with shared pathophysiology in different transgenic mouse models of PD. Exp Neurol. 2007;204:421–432. doi: 10.1016/j.expneurol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 164.Mizuno T. Kuno R. Nitta A. Nabeshima T. Zhang G. Kawanokuchi J. Wang J. Jin S. Takeuchi H. Suzumura A. Protective effects of nicergoline against neuronal cell death induced by activated microglia and astrocytes. Brain Res. 2005;1066:78–85. doi: 10.1016/j.brainres.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 165.Monahan AJ. Warren M. Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson's disease: an autoimmune hypothesis. Cell Transplant. 2008;17:363–372. [PubMed] [Google Scholar]
- 166.Mueller JC. Fuchs J. Hofer A. Zimprich A. Lichtner P. Illig T. Berg D. Wullner U. Meitinger T. Gasser T. Multiple regions of alpha-synuclein are associated with Parkinson's disease. Ann Neurol. 2005;57:535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- 167.Mukaetova-Ladinska EB. Milne J. Andras A. Abdel-All Z. Cerejeira J. Greally E. Robson J. Jaros E. Perry R. McKeith IG. Brayne C. Xuereb J. Cleghorn A. Doherty J. McIntosh G. Milton I. Alpha- and gamma-synuclein proteins are present in cerebrospinal fluid and are increased in aged subjects with neurodegenerative and vascular changes. Dement Geriatr Cogn Disord. 2008;26:32–42. doi: 10.1159/000141039. [DOI] [PubMed] [Google Scholar]
- 168.Muller G. Medicinal chemistry of target family-directed masterkeys. Drug Discov Today. 2003;8:681–691. doi: 10.1016/s1359-6446(03)02781-8. [DOI] [PubMed] [Google Scholar]
- 169.Munishkina LA. Henriques J. Uversky VN. Fink AL. Role of protein-water interactions and electrostatics in alpha-synuclein fibril formation. Biochemistry. 2004;43:3289–3300. doi: 10.1021/bi034938r. [DOI] [PubMed] [Google Scholar]
- 170.Murray IV. Giasson BI. Quinn SM. Koppaka V. Axelsen PH. Ischiropoulos H. Trojanowski JQ. Lee VM. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–8540. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 171.Necula M. Chirita CN. Kuret J. Rapid anionic micelle-mediated alpha-synuclein fibrillization in vitro. J Biol Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- 172.Nichols WC. Pankratz N. Hernandez D. Paisan-Ruiz C. Jain S. Halter CA. Michaels VE. Reed T. Rudolph A. Shults CW. Singleton A. Foroud T. Genetic screening for a single common LRRK2 mutation in familial Parkinson's disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- 173.Okuno T. Nakatsuji Y. Kumanogoh A. Moriya M. Ichinose H. Sumi H. Fujimura H. Kikutani H. Sakoda S. Loss of dopaminergic neurons by the induction of inducible nitric oxide synthase and cyclooxygenase-2 via CD 40: relevance to Parkinson's disease. J Neurosci Res. 2005;81:874–882. doi: 10.1002/jnr.20599. [DOI] [PubMed] [Google Scholar]
- 174.Ono K. Hirohata M. Yamada M. Anti-fibrillogenic and fibril-destabilizing activities of anti-Parkinsonian agents for alpha-synuclein fibrils in vitro. J Neurosci Res. 2007;85:1547–1557. doi: 10.1002/jnr.21271. [DOI] [PubMed] [Google Scholar]
- 175.Ostrerova-Golts N. Petrucelli L. Hardy J. Lee JM. Farer M. Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ostrerova N. Petrucelli L. Farrer M. Mehta N. Choi P. Hardy J. Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Outeiro TF. Kontopoulos E. Altmann SM. Kufareva I. Strathearn KE. Amore AM. Volk CB. Maxwell MM. Rochet JC. McLean PJ. Young AB. Abagyan R. Feany MB. Hyman BT. Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 178.Outeiro TF. Putcha P. Tetzlaff JE. Spoelgen R. Koker M. Carvalho F. Hyman BT. McLean PJ. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS ONE. 2008;3:e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ozelius LJ. Senthil G. Saunders-Pullman R. Ohmann E. Deligtisch A. Tagliati M. Hunt AL. Klein C. Henick B. Hailpern SM. Lipton RB. Soto-Valencia J. Risch N. Bressman SB. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 180.Paik SR. Shin HJ. Lee JH. Metal-catalyzed oxidation of alpha-synuclein in the presence of copper(II) and hydrogen peroxide. Arch Biochem Biophys. 2000;378:269–277. doi: 10.1006/abbi.2000.1822. [DOI] [PubMed] [Google Scholar]
- 181.Paik SR. Shin HJ. Lee JH. Chang CS. Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J. 1999;340(3):821–828. [PMC free article] [PubMed] [Google Scholar]
- 182.Paisan-Ruiz C. Jain S. Evans EW. Gilks WP. Simon J. van der Brug M. Lopez de Munain A. Aparicio S. Gil AM. Khan N. Johnson J. Martinez JR. Nicholl D. Carrera IM. Pena AS. de Silva R. Lees A. Marti-Masso JF. Perez-Tur J. Wood NW. Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 183.Paleologou KE. Irvine GB. El-Agnaf OM. Alpha-synuclein aggregation in neurodegenerative diseases and its inhibition as a potential therapeutic strategy. Biochem Soc Trans. 2005;33:1106–1110. doi: 10.1042/BST20051106. [DOI] [PubMed] [Google Scholar]
- 184.Pargellis C. Tong L. Churchill L. Cirillo PF. Gilmore T. Graham AG. Grob PM. Hickey ER. Moss N. Pav S. Regan J. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002;9:268–272. doi: 10.1038/nsb770. [DOI] [PubMed] [Google Scholar]
- 185.Park JY. Lansbury PT., Jr. Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson's disease. Biochemistry. 2003;42:3696–3700. doi: 10.1021/bi020604a. [DOI] [PubMed] [Google Scholar]
- 186.Park SM. Jung HY. Kim TD. Park JH. Yang CH. Kim J. Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaperone. J Biol Chem. 2002;277:28512–28520. doi: 10.1074/jbc.M111971200. [DOI] [PubMed] [Google Scholar]
- 187.Perrin RJ. Woods WS. Clayton DF. George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 188.Pesah Y. Burgess H. Middlebrooks B. Ronningen K. Prosser J. Tirunagaru V. Zysk J. Mardon G. Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of alpha-synuclein in Drosophila. Genesis. 2005;41:154–159. doi: 10.1002/gene.20106. [DOI] [PubMed] [Google Scholar]
- 189.Petit A. Kawarai T. Paitel E. Sanjo N. Maj M. Scheid M. Chen F. Gu Y. Hasegawa H. Salehi-Rad S. Wang L. Rogaeva E. Fraser P. Robinson B. St George-Hyslop P. Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- 190.Petrucelli L. O'Farrell C. Lockhart PJ. Baptista M. Kehoe K. Vink L. Choi P. Wolozin B. Farrer M. Hardy J. Cookson MR. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 191.Polymeropoulos MH. Lavedan C. Leroy E. Ide SE. Dehejia A. Dutra A. Pike B. Root H. Rubenstein J. Boyer R. Stenroos ES. Chandrasekharappa S. Athanassiadou A. Papapetropoulos T. Johnson WG. Lazzarini AM. Duvoisin RC. Di Iorio G. Golbe LI. Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 192.Pountney DL. Treweek TM. Chataway T. Huang Y. Chegini F. Blumbergs PC. Raftery MJ. Gai WP. Alpha B-crystallin is a major component of glial cytoplasmic inclusions in multiple system atrophy. Neurotox Res. 2005;7:77–85. doi: 10.1007/BF03033778. [DOI] [PubMed] [Google Scholar]
- 193.Przedborski S. Tieu K. Perier C. Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 194.Przedborski S. Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson's disease. Ann N Y Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- 195.Qian L. Block ML. Wei SJ. Lin CF. Reece J. Pang H. Wilson B. Hong JS. Flood PM. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319:44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- 196.Quilty MC. Gai WP. Pountney DL. West AK. Vickers JC. Localization of alpha-, beta-, and gamma-synuclein during neuronal development and alterations associated with the neuronal response to axonal trauma. Exp Neurol. 2003;182:195–207. doi: 10.1016/s0014-4886(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 197.Qureshi GA. Qureshi AA. Memon SA. Parvez SH. Impact of selenium, iron, copper and zinc in on/off Parkinson's patients on L-dopa therapy. J Neural Transm Suppl. 2006;71:229–236. doi: 10.1007/978-3-211-33328-0_24. [DOI] [PubMed] [Google Scholar]
- 198.Ralay Ranaivo H. Craft JM. Hu W. Guo L. Wing LK. Van Eldik LJ. Watterson DM. Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci. 2006;26:662–670. doi: 10.1523/JNEUROSCI.4652-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ramakrishnan M. Jensen PH. Marsh D. Alpha-synuclein association with phosphatidylglycerol probed by lipid spin labels. Biochemistry. 2003;42:12919–12926. doi: 10.1021/bi035048e. [DOI] [PubMed] [Google Scholar]
- 200.Rathke-Hartlieb S. Kahle PJ. Neumann M. Ozmen L. Haid S. Okochi M. Haass C. Schulz JB. Sensitivity to MPTP is not increased in Parkinson's disease-associated mutant alpha-synuclein transgenic mice. J Neurochem. 2001;77:1181–1184. doi: 10.1046/j.1471-4159.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 201.Richardson JR. Caudle WM. Guillot TS. Watson JL. Nakamaru-Ogiso E. Seo BB. Sherer TB. Greenamyre JT. Yagi T. Matsuno-Yagi A. Miller GW. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol Sci. 2007;95:196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- 202.Robertson DC. Schmidt O. Ninkina N. Jones PA. Sharkey J. Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- 203.Rott R. Szargel R. Haskin J. Shani V. Shainskaya A. Manov I. Liani E. Avraham E. Engelender S. Monoubiquitylation of alpha-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J Biol Chem. 2008;283:3316–3328. doi: 10.1074/jbc.M704809200. [DOI] [PubMed] [Google Scholar]
- 204.Sakaguchi-Nakashima A. Meir JY. Jin Y. Matsumoto K. Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007;17:592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 205.Sapru MK. Yates JW. Hogan S. Jiang L. Halter J. Bohn MC. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–390. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 206.Schapira AH. Hartley A. Cleeter MW. Cooper JM. Free radicals and mitochondrial dysfunction in Parkinson's disease. Biochem Soc Trans. 1993;21:367–370. doi: 10.1042/bst0210367. [DOI] [PubMed] [Google Scholar]
- 207.Scherzer CR. Grass JA. Liao Z. Pepivani I. Zheng B. Eklund AC. Ney PA. Ng J. McGoldrick M. Mollenhauer B. Bresnick EH. Schlossmacher MG. GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein. Proc Natl Acad Sci U S A. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Scott WK. Nance MA. Watts RL. Hubble JP. Koller WC. Lyons K. Pahwa R. Stern MB. Colcher A. Hiner BC. Jankovic J. Ondo WG. Allen FH., Jr. Goetz CG. Small GW. Masterman D. Mastaglia F. Laing NG. Stajich JM. Slotterbeck B. Booze MW. Ribble RC. Rampersaud E. West SG. Gibson RA. Middleton LT. Roses AD. Haines JL. Scott BL. Vance JM. Pericak-Vance MA. Complete genomic screen in Parkinson disease: evidence for multiple genes. JAMA. 2001;286:2239–2244. doi: 10.1001/jama.286.18.2239. [DOI] [PubMed] [Google Scholar]
- 209.Senior SL. Ninkina N. Deacon R. Bannerman D. Buchman VL. Cragg SJ. Wade-Martins R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sevlever D. Jiang P. Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sharon R. Goldberg MS. Bar-Josef I. Betensky RA. Shen J. Selkoe DJ. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci U S A. 2001;98:9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shashidharan P. Good PF. Hsu A. Perl DP. Brin MF. Olanow CW. TorsinA accumulation in Lewy bodies in sporadic Parkinson's disease. Brain Res. 2000;877:379–381. doi: 10.1016/s0006-8993(00)02702-5. [DOI] [PubMed] [Google Scholar]
- 213.Shashidharan P. Kramer BC. Walker RH. Olanow CW. Brin MF. Immunohistochemical localization and distribution of torsinA in normal human and rat brain. Brain Res. 2000;853:197–206. doi: 10.1016/s0006-8993(99)02232-5. [DOI] [PubMed] [Google Scholar]
- 214.Simon-Sanchez J. Herranz-Perez V. Olucha-Bordonau F. Perez-Tur J. LRRK2 is expressed in areas affected by Parkinson's disease in the adult mouse brain. Eur J Neurosci. 2006;23:659–666. doi: 10.1111/j.1460-9568.2006.04616.x. [DOI] [PubMed] [Google Scholar]
- 215.Singleton A. Myers A. Hardy J. The law of mass action applied to neurodegenerative disease: a hypothesis concerning the etiology and pathogenesis of complex diseases. Hum Mol Genet. 2004;13:R123–126. doi: 10.1093/hmg/ddh093. [DOI] [PubMed] [Google Scholar]
- 216.Singleton AB. Farrer M. Johnson J. Singleton A. Hague S. Kachergus J. Hulihan M. Peuralinna T. Dutra A. Nussbaum R. Lincoln S. Crawley A. Hanson M. Maraganore D. Adler C. Cookson MR. Muenter M. Baptista M. Miller D. Blancato J. Hardy J. Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 217.Skipper L. Li Y. Bonnard C. Pavanni R. Yih Y. Chua E. Sung WK. Tan L. Wong MC. Tan EK. Liu J. Comprehensive evaluation of common genetic variation within LRRK2 reveals evidence for association with sporadic Parkinson's disease. Hum Mol Genet. 2005;14:3549–3556. doi: 10.1093/hmg/ddi376. [DOI] [PubMed] [Google Scholar]
- 218.Smith WW. Pei Z. Jiang H. Dawson VL. Dawson TM. Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 219.Smith WW. Pei Z. Jiang H. Moore DJ. Liang Y. West AB. Dawson VL. Dawson TM. Ross CA. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sofic E. Riederer P. Heinsen H. Beckmann H. Reynolds GP. Hebenstreit G. Youdim MB. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- 221.Souza JM. Giasson BI. Chen Q. Lee VM. Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers: implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 222.Spillantini MG. Crowther RA. Jakes R. Cairns NJ. Lantos PL. Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 223.Spillantini MG. Crowther RA. Jakes R. Hasegawa M. Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Spillantini MG. Schmidt ML. Lee VM. Trojanowski JQ. Jakes R. Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 225.Sriram K. Miller DB. O'Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- 226.Sriram SR. Li X. Ko HS. Chung KK. Wong E. Lim KL. Dawson VL. Dawson TM. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- 227.St Martin JL. Klucken J. Outeiro TF. Nguyen P. Keller-McGandy C. Cantuti-Castelvetri I. Grammatopoulos TN. Standaert DG. Hyman BT. McLean PJ. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J Neurochem. 2007;100:1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- 228.Stefanis L. Larsen KE. Rideout HJ. Sulzer D. Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21:9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sturchler-Pierrat C. Abramowski D. Duke M. Wiederhold KH. Mistl C. Rothacher S. Ledermann B. Burki K. Frey P. Paganetti PA. Waridel C. Calhoun ME. Jucker M. Probst A. Staufenbiel M. Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson's disease. Lancet Neurol. 2007;6:933–938. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- 231.Takeuchi H. Jin S. Wang J. Zhang G. Kawanokuchi J. Kuno R. Sonobe Y. Mizuno T. Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 232.Tan EK. Zhao Y. Skipper L. Tan MG. Di Fonzo A. Sun L. Fook-Chong S. Tang S. Chua E. Yuen Y. Tan L. Pavanni R. Wong MC. Kolatkar P. Lu CS. Bonifati V. Liu JJ. The LRRK2 Gly2385Arg variant is associated with Parkinson's disease: genetic and functional evidence. Hum Genet. 2007;120:857–863. doi: 10.1007/s00439-006-0268-0. [DOI] [PubMed] [Google Scholar]
- 233.Taymans JM. Van den Haute C. Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–961. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 234.Tripanichkul W. Sripanichkulchai K. Finkelstein DI. Estrogen down-regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication. Brain Res. 2006;1084:28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 235.Truong DD. Bhidayasiri R. Wolters E. Management of non-motor symptoms in advanced Parkinson disease. J Neurol Sci. 2008;266:216–228. doi: 10.1016/j.jns.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 236.Ueda K. Fukushima H. Masliah E. Xia Y. Iwai A. Yoshimoto M. Otero DA. Kondo J. Ihara Y. Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Unger EL. Eve DJ. Perez XA. Reichenbach DK. Xu Y. Lee MK. Andrews AM. Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human alpha-synuclein in mice. Neurobiol Dis. 2006;21:431–443. doi: 10.1016/j.nbd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 238.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 239.Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 240.Uversky VN. Li J. Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 241.Uversky VN. Li J. Souillac P. Millett IS. Doniach S. Jakes R. Goedert M. Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 242.Uversky VN. Yamin G. Souillac PO. Goers J. Glaser CB. Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett. 2002;517:239–244. doi: 10.1016/s0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 243.Valente EM. Abou-Sleiman PM. Caputo V. Muqit MM. Harvey K. Gispert S. Ali Z. Del Turco D. Bentivoglio AR. Healy DG. Albanese A. Nussbaum R. Gonzalez-Maldonado R. Deller T. Salvi S. Cortelli P. Gilks WP. Latchman DS. Harvey RJ. Dallapiccola B. Auburger G. Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 244.van der Putten H. Wiederhold KH. Probst A. Barbieri S. Mistl C. Danner S. Kauffmann S. Hofele K. Spooren WP. Ruegg MA. Lin S. Caroni P. Sommer B. Tolnay M. Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.van Egmond WN. Kortholt A. Plak K. Bosgraaf L. Bosgraaf S. Keizer-Gunnink I. van Haastert PJ. Intramolecular activation mechanism of the dictyostelium LRRK2-homolog Roco protein GbpC. J Biol Chem. 2008;283:30412–30420. doi: 10.1074/jbc.M804265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.van Ham TJ. Thijssen KL. Breitling R. Hofstra RM. Plasterk RH. Nollen EA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vanacore N. Nappo A. Gentile M. Brustolin A. Palange S. Liberati A. Di Rezze S. Caldora G. Gasparini M. Benedetti F. Bonifati V. Forastiere F. Quercia A. Meco G. Evaluation of risk of Parkinson's disease in a cohort of licensed pesticide users. Neurol Sci. 2002;23(suppl 2):S119–S120. doi: 10.1007/s100720200098. [DOI] [PubMed] [Google Scholar]
- 248.Vartiainen S. Aarnio V. Lakso M. Wong G. Increased lifespan in transgenic Caenorhabditis elegans overexpressing human alpha-synuclein. Exp Gerontol. 2006;41:871–876. doi: 10.1016/j.exger.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 249.Vartiainen S. Pehkonen P. Lakso M. Nass R. Wong G. Identification of gene expression changes in transgenic C. elegans overexpressing human alpha-synuclein. Neurobiol Dis. 2006;22:477–486. doi: 10.1016/j.nbd.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 250.Virmani A. Gaetani F. Binienda Z. Xu A. Duhart H. Ali SF. Role of mitochondrial dysfunction in neurotoxicity of MPP+: partial protection of PC12 cells by acetyl-L-carnitine. Ann N Y Acad Sci. 2004;1025:267–273. doi: 10.1196/annals.1316.033. [DOI] [PubMed] [Google Scholar]
- 251.Vulpetti A. Bosotti R. Sequence and structural analysis of kinase ATP pocket residues. Farmaco. 2004;59:759–765. doi: 10.1016/j.farmac.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 252.Wahner AD. Bronstein JM. Bordelon YM. Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- 253.Wakabayashi K. Hayashi S. Kakita A. Yamada M. Toyoshima Y. Yoshimoto M. Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- 254.Walker RH. Brin MF. Sandu D. Gujjari P. Hof PR. Warren Olanow C. Shashidharan P. Distribution and immunohistochemical characterization of torsinA immunoreactivity in rat brain. Brain Res. 2001;900:348–354. doi: 10.1016/s0006-8993(01)02302-2. [DOI] [PubMed] [Google Scholar]
- 255.Wang C. Tan JM. Ho MW. Zaiden N. Wong SH. Chew CL. Eng PW. Lim TM. Dawson TM. Lim KL. Alterations in the solubility and intracellular localization of parkin by several familial Parkinson's disease-linked point mutations. J Neurochem. 2005;93:422–431. doi: 10.1111/j.1471-4159.2005.03023.x. [DOI] [PubMed] [Google Scholar]
- 256.Wang D. Tang B. Zhao G. Pan Q. Xia K. Bodmer R. Zhang Z. Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol Neurodegener. 2008;3:3. doi: 10.1186/1750-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang YM. Pu P. Le WD. ATP depletion is the major cause of MPP+ induced dopamine neuronal death and worm lethality in alpha-synuclein transgenic C. elegans. Neurosci Bull. 2007;23:329–335. doi: 10.1007/s12264-007-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Weinreb PH. Zhen W. Poon AW. Conway KA. Lansbury PT., Jr. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 259.West A. Periquet M. Lincoln S. Lucking CB. Nicholl D. Bonifati V. Rawal N. Gasser T. Lohmann E. Deleuze JF. Maraganore D. Levey A. Wood N. Durr A. Hardy J. Brice A. Farrer M. Complex relationship between Parkin mutations and Parkinson disease. Am J Med Genet. 2002;114:584–591. doi: 10.1002/ajmg.10525. [DOI] [PubMed] [Google Scholar]
- 260.West AB. Moore DJ. Biskup S. Bugayenko A. Smith WW. Ross CA. Dawson VL. Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.West AB. Moore DJ. Choi C. Andrabi SA. Li X. Dikeman D. Biskup S. Zhang Z. Lim KL. Dawson VL. Dawson TM. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 262.Westerlund M. Belin AC. Anvret A. Bickford P. Olson L. Galter D. Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: implications for Parkinson's disease. Neuroscience. 2008;152:429–436. doi: 10.1016/j.neuroscience.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 263.Wiessner C. Allegrini PR. Rupalla K. Sauer D. Oltersdorf T. McGregor AL. Bischoff S. Bottiger BW. van der Putten H. Neuron-specific transgene expression of Bcl-XL but not Bcl-2 genes reduced lesion size after permanent middle cerebral artery occlusion in mice. Neurosci Lett. 1999;268:119–122. doi: 10.1016/s0304-3940(99)00392-4. [DOI] [PubMed] [Google Scholar]
- 264.Windisch M. Wolf H. Hutter-Paier B. Wronski R. The role of alpha-synuclein in neurodegenerative diseases: a potential target for new treatment strategies? Neurodegener Dis. 2008;5:218–221. doi: 10.1159/000113707. [DOI] [PubMed] [Google Scholar]
- 265.Winkler S. Hagenah J. Lincoln S. Heckman M. Haugarvoll K. Lohmann-Hedrich K. Kostic V. Farrer M. Klein C. alpha-Synuclein and Parkinson disease susceptibility. Neurology. 2007;69:1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4. [DOI] [PubMed] [Google Scholar]
- 266.Wright JA. Brown DR. Alpha-synuclein and its role in metal binding: relevance to Parkinson's disease. J Neurosci Res. 2008;86:496–503. doi: 10.1002/jnr.21461. [DOI] [PubMed] [Google Scholar]
- 267.Xu Z. Maroney AC. Dobrzanski P. Kukekov NV. Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21:4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yamada M. Mizuno Y. Mochizuki H. Parkin gene therapy for alpha-synucleinopathy: a rat model of Parkinson's disease. Hum Gene Ther. 2005;16:262–270. doi: 10.1089/hum.2005.16.262. [DOI] [PubMed] [Google Scholar]
- 269.Yamin G. Glaser CB. Uversky VN. Fink AL. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J Biol Chem. 2003;278:27630–27635. doi: 10.1074/jbc.M303302200. [DOI] [PubMed] [Google Scholar]
- 270.Young AJ. Johnson S. Steffens DC. Doraiswamy PM. Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectrum. 2007;12:62–68. doi: 10.1017/s1092852900020538. [DOI] [PubMed] [Google Scholar]
- 271.Yu F. Xu H. Zhuo M. Sun L. Dong A. Liu X. Impairment of redox state and dopamine level induced by alpha-synuclein aggregation and the prevention effect of hsp70. Biochem Biophys Res Commun. 2005;331:278–284. doi: 10.1016/j.bbrc.2005.03.148. [DOI] [PubMed] [Google Scholar]
- 272.Zarranz JJ. Alegre J. Gomez-Esteban JC. Lezcano E. Ros R. Ampuero I. Vidal L. Hoenicka J. Rodriguez O. Atares B. Llorens V. Gomez Tortosa E. del Ser T. Munoz DG. de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 273.Zeng BY. Iravani MM. Lin ST. Irifune M. Kuoppamaki M. Al-Barghouthy G. Smith L. Jackson MJ. Rose S. Medhurst AD. Jenner P. MPTP treatment of common marmosets impairs proteasomal enzyme activity and decreases expression of structural and regulatory elements of the 26S proteasome. Eur J Neurosci. 2006;23:1766–1774. doi: 10.1111/j.1460-9568.2006.04718.x. [DOI] [PubMed] [Google Scholar]
- 274.Zhang W. Wang T. Pei Z. Miller DS. Wu X. Block ML. Wilson B. Zhou Y. Hong JS. Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 275.Zhou W. Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 276.Zhu M. Rajamani S. Kaylor J. Han S. Zhou F. Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 277.Zimprich A. Biskup S. Leitner P. Lichtner P. Farrer M. Lincoln S. Kachergus J. Hulihan M. Uitti RJ. Calne DB. Stoessl AJ. Pfeiffer RF. Patenge N. Carbajal IC. Vieregge P. Asmus F. Muller-Myhsok B. Dickson DW. Meitinger T. Strom TM. Wszolek ZK. Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 278.Zimprich A. Muller-Myhsok B. Farrer M. Leitner P. Sharma M. Hulihan M. Lockhart P. Strongosky A. Kachergus J. Calne DB. Stoessl J. Uitti RJ. Pfeiffer RF. Trenkwalder C. Homann N. Ott E. Wenzel K. Asmus F. Hardy J. Wszolek Z. Gasser T. The PARK8 locus in autosomal dominant parkinsonism: confirmation of linkage and further delineation of the disease-containing interval. Am J Hum Genet. 2004;74:11–19. doi: 10.1086/380647. [DOI] [PMC free article] [PubMed] [Google Scholar]