Abstract
Objective
To identify biomarkers of growth hormone (GH) and insulin-like growth factor 1 (IGF-1) action in human serum.
Background
The search for new markers of GH activity has received extensive attention given that the current biomarkers (IGF-1, IGFBP-3 and collagen peptides) show substantial variability in the population, and are not reliably predictive of either the physiologic effects of GH therapy or the detection of GH abuse by athletes. GH releasing hormone (GHRH) is a polypeptide synthesized in the hypothalamus that binds to receptors on pituitary somatotropes to promote the synthesis and release of GH. Serum GH and IGF-1 levels have been shown to increase with administration of GHRH or CJC-1295, a long acting GHRH analog.
Design
Sera from 11 healthy young adult men before and one week after CJC-1295 injection were analyzed by two-dimensional gel electrophoresis for proteomic changes. Serum proteins displaying significant changes before and after treatment were subsequently identified using mass spectrometry. In addition, correlations between these proteins and GH or IGF-1 levels were evaluated.
Results
Two protein spots that displayed decreased intensities after treatment were identified as an apolipoprotein A1 isoform and a transthyretin isoform. Three protein spots upregulated by CJC-1295 treatment included beta-hemoglobin, a C-terminal fragment of albumin, and a mix of an immunoglobulin fragment and another C-terminal albumin fragment. A linear relationship was found between the spot containing immunoglobulin and albumin fragments and IGF-1 levels.
Conclusions
Although the molecular mechanisms linking the identified proteins to GH and IGF-1 biological activity remain to be clarified, the results suggest that they represent potential biomarkers of GH and/or IGF-1 action.
Keywords: serum proteomics, biomarkers, growth hormone, IGF-1, GHRH analog, apolipoprotein A1, transthyretin, albumin, hemoglobin
1. Introduction
Growth hormone releasing hormone (GHRH) is a 44-amino acid peptide synthesized in the hypothalamus. At the pituitary gland, GHRH binds to receptors on somatotropes to promote synthesis and release of GH. Together with somatostatin and ghrelin, GHRH is a key participant in the regulation of pulsatile GH secretion.
The administration of recombinant human GH (rhGH) has been approved by the FDA and various international regulatory agencies for several indications both in children (GH deficiency (GHD), Turner syndrome, children born short for gestational age, Prader-Willi syndrome, idiopathic short stature, etc.) and in adults (GHD, HIV-associated lipodystrophy, etc.) [1]. Treatment with rhGH is routinely performed as one subcutaneous daily injection, thus it does not resemble the physiological pulsatile pattern of GH release. On the other hand, the administration of GHRH or its analogs results in increased levels of GH in serum and more importantly, maintenance of the episodic pattern of GH secretion. In addition, contrary to rhGH administration which contains only the 22kDa isoform of GH, therapy with GHRH-type molecules should result in the secretion of all forms of GH that are produced in the pituitary somatotropes (including post-translationally modified isoforms whose functions remain unknown [2]).
A major obstacle in assessing the efficacy of GH therapy is the lack of a consistent biological serum biomarker. Levels of IGF-1 and IGFBP-3 are widely used to monitor the efficacy of GH therapy, but unfortunately, there is considerable variability in responses (growth velocity, final stature, glucose tolerance, insulin levels, etc.) with a lack of consistent correlation between the measured response and the serum levels of GH, IGF-1, IGFBP-3, etc. Collagen peptides are also used as biomarkers of GH activity, but again their reliability as predictors of treatment efficacy is variable [3]. The abuse of rhGH by athletes to improve performance is also a major concern, and underscores the need for new and more specific biomarkers of GH action [3-7]. Moreover, the finding of new biomarkers will expand our understanding of the molecular mechanism and physiologic effects of GH action.
In a previous study, the long-acting GHRH analog CJC-1295 was shown to promote an increase in GH and IGF-1 levels in sera of healthy young adult men one week after administration, without affecting the pulsatility of GH secretion [8]. The extended half-life of the compound (8 – 10 days in humans) is due to its ability to bind to endogenous serum albumin through a free thiol group forming a covalent disulfide bond [9]. The present study was designed to identify biomarkers of GH and/or IGF-1 action in serum samples obtained before and one week after administration of CJC-1295 to healthy subjects. The samples were analyzed using a proteomic approach to search for serum proteins and protein isoforms that respond to increases in GH or IGF-1. Five protein spots displaying significant changes after treatment were identified and their serum levels evaluated for correlations to GH and IGF-1 serum concentrations.
2. Materials and Methods
The subjects and procedures followed for drug administration and GH and IGF-1 measurements have been previously reported [8].
2.1. Subjects and serum samples
A subset of 11 healthy men that were part of a larger cohort previously described [8] were selected for this study. Individuals in this subset had a BMI lower than 25 kg/m2 (22.1 ± 1.6, mean ± SD), and ages 20-34 (25.2 ± 5.1, mean ± SD). Serum samples were obtained as described previously [8]. Briefly, control (pre-treatment) samples were obtained during an overnight 12-hr period (1900 h – 0700 h) using an indwelling forearm venous catheter connected to tubing that allowed for collection of samples every 20 min (a total of 37 samples) in an adjacent room. After the collection period was completed, 60-90 μg/kg of CJC-1295 (ConjuChem Inc., Montreal, Canada) were administered to the subjects in a single subcutaneous injection. The serum sampling procedure was repeated in an identical manner one week after injection of the drug. The study was performed using a protocol approved by the University of Illinois at Chicago Institutional Review Board and all subjects gave informed, written consent. The protocol also was approved by the Ohio University Institutional Review Board. This part of the study was conducted in the General Clinical Research Center of the University of Illinois Medical Center.
2.2. GH and IGF-1 measurements (Performed at the University of Illinois at Chicago)
Serum levels of GH and IGF-1 for the subjects have been reported previously [8]. Mean GH levels recorded were based on all the serum samples obtained during the 12 h sampling period. The lowest GH level measured throughout that period was also recorded. Pulsatility was assessed as well. The IGF-1 level was calculated as the mean of the first three serum samples obtained in each period.
2.3. Proteomic analysis
These procedures have been described previously [10-12].
2.3.1. Sample preparation (Performed at Ohio University)
All samples were stored frozen at -80°C for 1.5 years and shipped frozen (dry ice) from Chicago, IL to Athens, OH for proteomic analysis. The initial serum sample obtained from each subject from the pre-treatment period and the initial sample from the post-treatment period were selected for analysis. Protein concentration was measured by the Bradford method and showed comparable values for all samples with no significant differences before and after treatment (P= 0.943 in a two-tailed paired t-test). Therefore, 25μl of each selected serum sample was albumin-depleted using a Montage Albumin Deplete kit (Millipore, Billerica, MA) and diluted in sample buffer containing 7M urea, 2M thiourea, 1% w/v SB 3-10, 3% w/v CHAPS, 0.25% v/v Bio-Lyte 3/10 ampholytes (Bio-Rad Laboratories Inc., Hercules, CA), and 1.5% v/v protease inhibitor cocktail (Sigma, St. Lewis, MO). Disulfide bonds were reduced by addition of tributylphosphine and sulfhydryl groups were alkylated with iodoacetamide.
2.3.2. Two-dimensional gel electrophoresis (2DE) (Performed at Ohio University)
Samples treated as described above were subjected to 2DE as described previously [10-12]. For the first dimension, 300μl of diluted and treated samples were loaded onto IPG strips (isoelectric point (pI) 3-10 linear, Bio-Rad) and placed into a PROTEAN IEF cell (Bio-Rad) for isoelectric focusing consisting of 12 hours of active rehydration followed by separation at 4 000 volts for 60 000 volt hours. Next, strips were equilibrated for 30 min in buffer containing 0.375M Tris-HCl pH 8.8, 6M urea, 2% w/v SDS, and 20% v/v glycerol and loaded on 12.5% polyacrylamide gels. SDS-PAGE was run in a PROTEAN II XL cell (Bio-Rad) at 75 mA/gel. After fixing and washing, gels were stained using SYPRO Orange (Molecular Probes, Inc., Eugene, OR) and scanned in a PharosFX Plus Molecular Imager (Bio-Rad) with an excitation wavelength of 488 nm and emission detected at 605 nm.
2.3.3. Image analysis (Performed at Ohio University)
Spot matching was performed using the image analysis software PDQuest Advanced v. 8.0 (Bio-Rad) and all matches were examined manually. Spot intensities were normalized to total image density in each gel which depended on the total protein content of the sample.
2.3.4. Mass Spectrometry (MS)
Protein spots displaying significant (P<0.05) intensity changes before and after treatment were manually excised from the gels and sent to the Michigan Proteome Consortium at the University of Michigan for analysis by mass spectrometry (MS) and tandem-MS (MS/MS) using matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) and MALDI-TOF-TOF.
2.3.4.1. Protein digestion (Performed at the Michigan Proteome Consortium)
All protein digestions were performed in the wells of a 96-well ‘U’ bottom dish using a Packard Mass Prep robot. Briefly, the gel plugs containing individual protein spots were destained using acetonitrile and 100mM ammonium bicarbonate. The proteins were reduced with 10mM DTT and then alkylated with 55mM iodoacetamide. Digestion was carried out for 4 hours at 40°C using 200ng of Trypsin Gold (Promega, Madison, WI). Thirty microliters of 2% acetonitrile, 1% formic acid was added to each well to stop the digestion. After 30 minutes the liquid in the digest wells was transferred to the wells of a PCR (‘extraction’) plate.
2.3.4.2. Concentration and spotting of gel digest extracts (Performed at the Michigan Proteome Consortium)
For MS and MS/MS analyses, 5μl of alphacyano-4-hydroxycinnaminic acid (5 mg/ml in 50% acetonitrile, 0.1% TFA, 2mM ammonium citrate) matrix was added to the 30μl of digest extract for each well of the extraction plate. The samples were dried and 5μl of 50% acetonitrile/0.1% TFA was added back into the extraction well. This solution (0.5μl) was hand-spotted on a 192-well MALDI target and allowed to dry in atmosphere.
2.3.4.3. MS analysis (Performed at the Michigan Proteome Consortium)
Mass spectra were acquired on an Applied Biosystems 4 800 Proteomics Analyzer (TOF/TOF). MS spectra were acquired in Reflector Positive Ion mode. Peptide masses were acquired for the range from 800-3 500 Da. MS spectra were summed from 2 000 laser shots using an Nd-YAG laser operating at 355 nm and 200 Hz. Internal calibration was performed using a minimum of 3 trypsin autolysis peaks.
2.3.4.4. MS/MS analysis (Performed at the Michigan Proteome Consortium)
Tandem MS spectra were acquired in MS/MS 2kV Positive mode. Spectra were acquired for 6 000 laser shots or until 5 peptide fragment ions reached a S/N of 100, whichever was less. Fragmentation of the peptides was induced by the use of atmosphere as a collision gas with a pressure of ~ 6 ×10-7 torr and collision energy of 2kV. Spectra were subjected to a 7-point Gaussian smooth prior to peak picking, and peaks with a minimum mass of 20 to 60 Da below the precursor mass were used. A maximum of 65 peaks were selected for database searching with a minimum S/N of 10, and a maximum peak density of 50 peaks per 200 Da.
2.3.5. Protein identification (Performed at Ohio University)
Protein identities were established using the MS and MS/MS data obtained and the online softwares Mascot [13] and MS-Seeker [14]. This procedures have been described previously [10-12]. Search parameters included the following: MS: database: NCBInr; taxonomy: Homo sapiens; enzyme: trypsin; missed cleavages allowed: 1; fixed modifications: none; protein mass: not specified; peptide tolerance: ±1.2 Da; mass values: MH+; monoisotopic/average: monoisotopic. Tandem MS: database: NCBInr; taxonomy: Homo sapiens; enzyme: trypsin; missed cleavages allowed: 1; fixed modifications: none; Quantitation: none; peptide tolerance: ±1.2 Da; MS/MS tolerance: ±0.6 Da; Peptide charge: 1+; monoisotopic/average: monoisotopic; Precursor m/z: not specified; Instrument: MALDI-TOF-TOF. Variable modifications that were included in separate and combined submissions for both MS and MS/MS were Acetyl (K), Carbamidomethyl (C), Deamidated (NQ), Oxidation (M), Phospho (ST), Phospho (Y), Sulfo (S), Sulfo (T), Sulfo (Y). The criteria used for assessment of protein identity were a minimum match of two MS/MS fragments with significant scores. Also, when MS hits were available, it was required that all fragments matched in MS/MS hits were included among the fragments matched by the MS data.
2.4. Statistical analysis
All intensity data were log-transformed. Protein spots that followed a normal distribution (P<0.05 in a Shapiro-Wilk test) with equal variances (P<0.05 in a t-test for homogeneity of variance for two dependent samples [15]) were compared between the two groups using a two-tailed paired t-test (corresponding values of t10 and P are reported for significant spots, see results for spots A, B and D). The remaining spots were analyzed using the non-parametric Wilcoxon signed-ranks test (z and P values are reported for significant spots, see results for spots C and E). Relationships to mean and lowest GH and to IGF-1 values were tested using correlation tests: Pearson (for normal distributions) and Spearman (for non-normal data). All tests were performed using SPSS v. 14.0. P-values lower than 0.05 were considered significant.
3. Results
3.1. Individual serum proteome patterns
The subjects’ serum proteomes studied displayed marked heterogeneity in the low molecular weight range (< 25 kDa) when resolved by 2DE. The patterns observed were conserved in each subject at the two timepoints studied and consisted mainly of the presence or absence of two of the spot “trains” usually observed in that region (Fig. 1). The term spot “train” refers to a group of protein spots that have approximately the same molecular weight and varying isoelectric points. These characteristics result in the spots appearing in a horizontal line on the 2D-gel (see examples highlighted in Fig. 1). Although the spots in a train may contain entirely different proteins that have similar molecular weight and isoelectric point, trains are usually suspected to contain isoforms of the same protein carrying different post-translational modifications such as increasing number of phosphorylations.
Fig. 1.
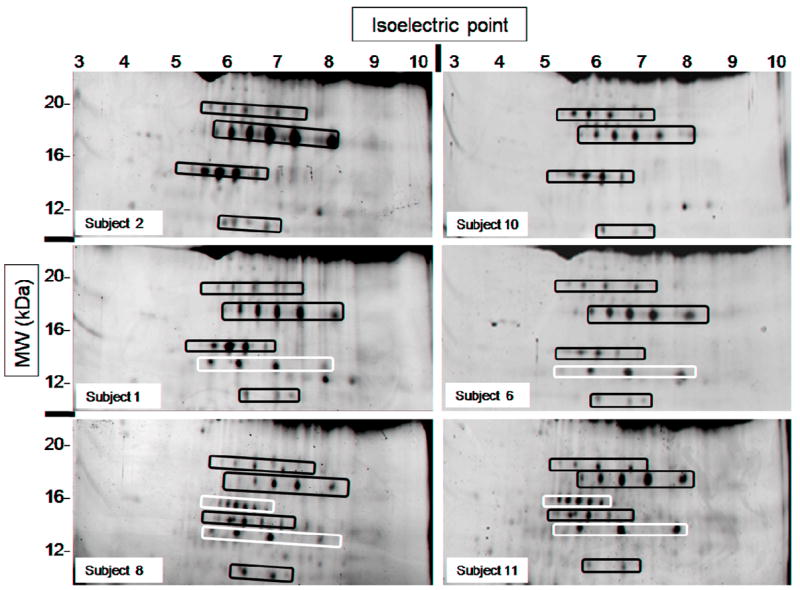
Low molecular weight (~10-25 kDa) portion of six gels showing marked heterogeneity in their spot patterns. All images belong to different subjects (see labels) and correspond to the pre-treatment state; these patterns were consistent for each subject before and after treatment. Spot “trains” marked in black were present in samples from all subjects. Spot “trains” marked in white were present in only some of the subjects studied.
As generally observed in 2D-gels, the high molecular weight region displayed low resolution, with very high amounts of protein. Thus, no spots were analyzed in this region (> 50 kDa). For the same reason, the dark train observed at ~25 kDa and a pI ~6.0 to 9.5 (where light chains of immunoglobulins are found) was not analyzed. A total of 192 protein spots were analyzed in all remaining regions of the gel images (Fig. 2).
Fig. 2.
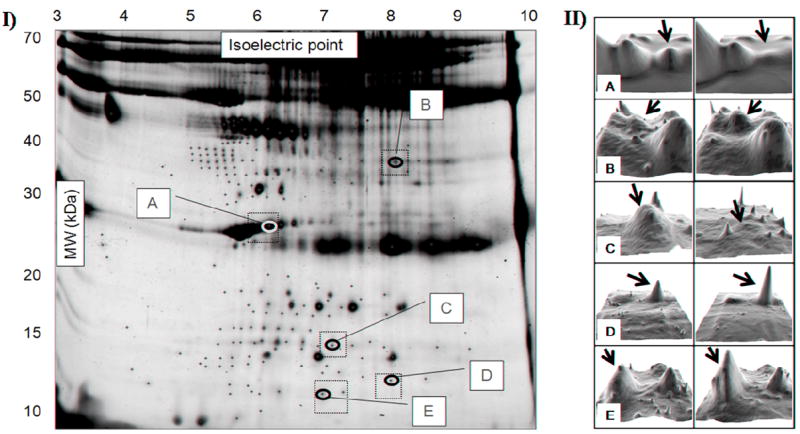
I. Representative two-dimensional serum gel. A total of 192 spots were analyzed (plus signs), with 97 being present in all 11 subjects. Five of those displayed significant changes in intensity one week after CJC-1295 administration (labeled A to E). (Dotted squares outline the gel areas that are shown in II). II. Representative 3D images of spots A to E showing changes in intensity before (left) and after (right) treatment. Images were obtained using the 3D Viewer tool from PDQuest, which converts spot intensity data to topographical peaks and valleys. For each spot, left and right images belong to the same subject.
3.2. Serum proteome changes after treatment with CJC-1295
The serum levels of GH (both mean and lowest) and IGF-1 measured in the 11 subjects showed significant increases one week after administration of CJC-1295 [8]. Therefore, the intensities of the 192 spots detected were analyzed for significant changes in intensity between the two time points. Because the goal of this study was to uncover biomarkers of clinical relevance, we focused on the protein spots that were present in the sera of all subjects. Only 97 spots fulfilled that criteria and, among them, five (A to E) were found to display significant intensity differences between the two time points (Fig. 2). The intensities of spots A to E before and after administration of the GHRH analog are shown in Fig. 3.
Figure 3.
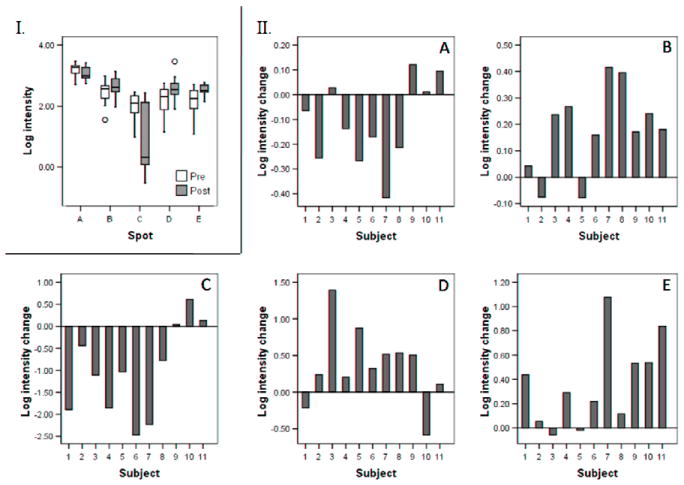
Log intensity changes for spots A to E. I. Box plots showing the distribution of log intensity values before and after treatment. II. Spot intensity changes (log intensity after treatment minus log intensity before treatment) in individual subjects.
The protein identities of spots A to E were determined using MS and tandem MS. Table 1 shows the identity matches, scores and sequence coverage obtained using Mascot [13] and MS-Seeker [14] for each of the five spots. Spot A (MW ~28, pI ~6.3), which was decreased after treatment (t10=2.27, P=0.047, Fig. 3), was identified as an isoform of apolipoprotein A1 (ApoA1). Spot B (MW ~35, pI ~8.1), which increased after treatment (t10=3.60, P=0.005, Fig. 3), matched two different protein identities, suggesting that it contains a mixture of two proteins: a fragment of an immunoglobulin and an albumin fragment. Spot C (MW ~15, pI ~7.2) contained an isoform of transthyretin (TTR) which was decreased with CJC-1295 administration (z=2.31, P=0.021, Fig. 3). Spot D (MW ~12, pI ~8.0) increased after administration (t10=2.24, P=0.049, Fig. 3) and was identified as beta-hemoglobin. Finally, spot E (MW ~11, pI ~7.0) was identified as an additional albumin fragment and was increased after treatment (z=2.58, P=0.010, Fig. 3).
Table 1.
Mass spectrometry identity matches for spots A-E.
| Spot | Gel MW | Gel pI | MS identity match | Replicates identified | Max sequence coverage (%) | MS/MS identity match | Replicates identified | Max # of MS/MS hits (out of 8 total) | Max sequence coverage (%) |
|---|---|---|---|---|---|---|---|---|---|
| A | 28 | 6.3 | Apolipoprotein A-I | 6 | 80 | Apolipoprotein A-I | 6 | 6 | 25 |
| B | 35 | 8.1 | Immunoglobulin heavy chain constant region | 1 | 33 | Immunoglobulin heavy chain constant region | 6 | 4 | 12 |
| Albumin | 6 | 4 | 8 | ||||||
| C | 15 | 7.2 | Transthyretin | 4 | 81 | Transthyretin | 6 | 4 | 34 |
| D | 12 | 8.0 | Hemoglobin Beta | 6 | 75 | Hemoglobin Beta | 7 | 6 | 40 |
| E | 11 | 7.0 | Albumin | 6 | 3 | 7 |
3.3. Correlation to GH and IGF-1
The observed changes in spot intensities for spots A to E were compared to the changes in mean GH, lowest GH and IGF-1 levels in each subject. Changes in the intensity of spot B (mixture of immunoglobulin fragment and albumin fragment) showed a significant linear correlation to changes in IGF-1 levels in the 11 subjects (Pearson’s r2=0.668; F1,9=18.09; P= 0.002; Fig. 4). No other significant correlations were found between changes in intensity and changes in IGF-1 or lowest or mean GH levels before and after treatment.
Figure 4.
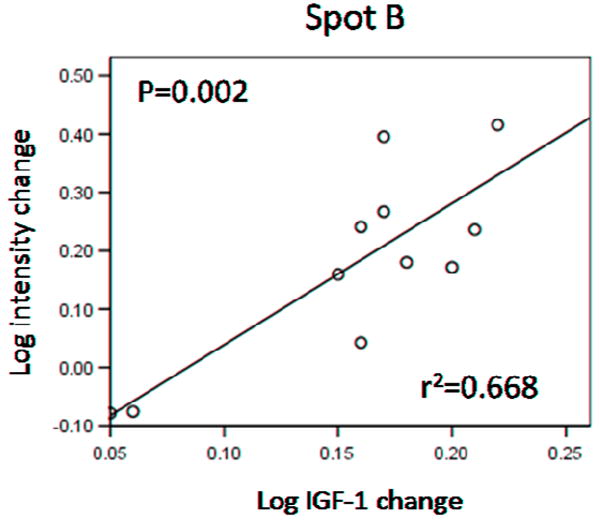
Log intensity changes in spot B displayed a linear correlation with log changes in IGF-1 levels. R2 and P values for the curve are shown on the graph.
4. Discussion
The analysis of the serum proteome of 11 healthy male subjects revealed marked heterogeneity among gel images from different subjects, which might be partly explained by the alleles of haptoglobin present in each subject [16, 17]. Given that this study was aimed at finding clinically relevant protein biomarkers of GH and/or IGF-1, the criteria used for selection of spots were not only statistical significance between before and after drug administration, but also that the spots were present in the gels of all subjects. Five spots (A to E, Fig. 1) fulfilled these criteria, displaying significant intensity changes after one week of increased GH and IGF-1 secretion induced by treatment with CJC-1295. These spots were further analyzed by MS to reveal their identity.
Spot A (MW ~28, pI ~6.3), was identified as ApoA1, which is a major component of high density lipoproteins (HDL) in serum, and facilitates transport of cholesterol from the tissues to the liver. According to the SWISS-2DPAGE database, there are several isoforms of ApoA1 in that region of the 2D-gel [17]. The post-translational modification that results in the shift in position of the different isoforms has not been described. Thus, the 2DE approach has and will continue to uncover isoforms of many known serum proteins. The fact that only one of those isoforms is decreased in our study could be related to the activity of that particular isoform resulting from the specific modification it carries. In a study on GHD children, both ApoA1 and HDL-cholesterol levels were found to be similar to healthy controls independently of GH replacement therapy [18, 19]. In contrast, Blackett et al. [20] reported a decrease in ApoA1 levels in GHD children after five weeks of GH replacement therapy. In addition, a decrease in GH after treatment of adult acromegalic patients has been reported to increase ApoA1 levels [21, 22]. These results are consistent with the decrease we detected in an isoform of ApoA1 after administration of the GHRH analog, although total ApoA1 levels were not evaluated in this study.
The MS data obtained for spot B (MW ~35, pI ~8.1), matched two different protein identities: an immunoglobulin fragment and a C-terminal albumin fragment, suggesting that the spot contains a mixture of both protein fragments. The intensity of this spot was increased after treatment, although it is not clear if one of the proteins or both are responsible for this change in intensity. Because the samples were depleted of albumin before running the 2DE, it seemed unexpected to find a fragment of albumin in one of the protein spots. A possible explanation for the presence of a fragment of albumin in the gels could be based on the type of affinity column used for albumin depletion. If the albumin antibodies used recognized the N-terminal region of albumin, then C-terminal fragments would not be bound by the antibodies and would be eluted with the remainder of the serum proteins. Therefore, the fragment of albumin contained in spot B, which does not include the N-terminal region of albumin (data not shown) managed to escape the depletion step. The increased levels in serum of this albumin fragment can be explained by GH’s effect on liver to increase albumin mRNA transcription [23]. On the other hand, an increase in an immunoglobulin fragment also may reflect the actions of GH and IGF-1 to enhance immune function [24], although no other immunoglobulin components were found to be affected in this study.
Interestingly, a significant linear correlation was found between the intensity changes observed for spot B and the changes in serum IGF-1 levels (Fig. 4). Unfortunately it is not clear from our results whether the albumin fragment or the immunoglobulin fragment or both are responsible for this correlation. Further analysis is necessary to resolve this question, perhaps through the use of IPG strips with a smaller pI range that would yield higher resolution in the first dimension (isoelectric focusing) to hopefully separate the two proteins into two different spots.
Another protein reported to be subject to transcriptional regulation by GH signaling is TTR, which was identified as spot C (MW ~15, pI ~7.2). Expression of TTR in the liver is under the control of hepatocyte nuclear factor 6 (HNF-6), a transcription factor of the ONECUT class, which is activated upon GH stimulation and in turn activates expression of TTR [25, 26]. Although TTR levels were expected to increase after treatment with the GHRH analog, the detected spot intensity actually decreased. Given that there are numerous isoforms of TTR in serum when analyzed on 2D-gels [17], the fact that this particular isoform decreased does not necessarily contradict the premise of TTR activation by GH. The detected decrease in spot C could be due to a shift in the ratio of post-translational modifications found on TTR, which in turn could result in variations of function. Changes in TTR isoforms have also been found in the serum of GH transgenic mice (Ding and Kopchick, unpublished results). The observed decrease in abundance could also be related to increased turnover of this specific TTR isoform. TTR is a thyroid hormone binding protein, thought to transport thyroxin to the brain. It also binds retinol binding protein (RBP) decreasing its renal clearance [16]. There are many isoforms of TTR on a serum 2D-gel, but apart from glycosylation, no other post-translational modifications have been described that could explain the shifts in positions of these isoforms on the gel.
Spot D (MW ~12, pI ~8.0), which contains beta-hemoglobin, was increased after CJC-1295 administration. Hemoglobin alpha-chain was recently reported as a novel biomarker of GH action by Chung et al. [27]. To our knowledge there are yet no reports on beta-hemoglobin level changes due to GH or IGF-1.
Another albumin fragment was identified in spot E (MW ~11, pI ~7.0). This albumin fragment was different in size from that found in spot B but also contained the C-terminus of the protein (data not shown). This is consistent with the proposed explanation for escape from depletion during affinity chromatography. As stated above, albumin gene transcription in the liver is activated by GH [23], which would explain the increased levels seen for this spot after treatment.
Except for the linear relationship with IGF-1 levels found for spot B, no other correlations with IGF-1 or GH levels were found for the remaining spots. In the case of GH levels, they were determined and averaged over a prolonged period of time (12 hours at night), and the possibility still exists that measurements over a longer period (24 hours) might show better correlations with the proteins detected in this study. Also, it is worth mentioning that despite the well established role of IGF-1 as a downstream biomarker of GH action, a previous report [8] found no correlation between the changes in IGF-1 and those in GH levels (lowest or mean). It follows then that a lack of correlation between GH and the proteins we have found in this study does not rule out the significance of their relationship with GH.
In summary, proteins found to change after one week of increased GH and IGF-1 secretion induced by treatment with CJC-1295 were ApoA1, TTR, beta-hemoglobin, two albumin fragments and an immunoglobulin fragment. Although the relationship of the identified proteins to GH and IGF-1 biological activity remains to be clarified further, the results suggest that they represent potential biomarkers of GH and/or IGF-1 action. Future studies will help elucidate the molecular mechanisms that link them to these hormones and may lead to their use in detection assays for assessment of GH’s therapeutic efficacy and illicit use via doping.
Acknowledgments
This work was supported in part by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by grants from WADA, the Diabetes Research Initiative at Ohio University, and ConjuChem Inc. J.J.K. is also supported in part by NIH (AG019899-06, DK075436-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Association of Clinical Endocrinologists Growth Hormone Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in adults and children--2003 update. Endocr Pract. 2003;9:64–76. doi: 10.4158/EP.9.1.64. [DOI] [PubMed] [Google Scholar]
- 2.Zhan X, Giorgianni F, Desiderio DM. Proteomics analysis of growth hormone isoforms in the human pituitary. Proteomics. 2005;5:1228–41. doi: 10.1002/pmic.200400987. [DOI] [PubMed] [Google Scholar]
- 3.Nelson AE, Ho KK. A robust test for growth hormone doping--present status and future prospects. Asian J Androl. 2008;10:416–25. doi: 10.1111/j.1745-7262.2008.00395.x. [DOI] [PubMed] [Google Scholar]
- 4.Erotokritou-Mulligan I, Bassett EE, Bartlett C, et al. The effect of sports injury on insulin-like growth factor-I and type 3 procollagen: implications for detection of growth hormone abuse in athletes. J Clin Endocrinol Metab. 2008;93:2760–3. doi: 10.1210/jc.2007-2801. [DOI] [PubMed] [Google Scholar]
- 5.Holt RI, Sonksen PH. Growth hormone, IGF-I and insulin and their abuse in sport. Br J Pharmacol. 2008;154:542–56. doi: 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powrie JK, Bassett EE, Rosen T, et al. Detection of growth hormone abuse in sport. Growth Horm IGF Res. 2007;17:220–6. doi: 10.1016/j.ghir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Bidlingmaier M, Wu Z, Strasburger CJ. Problems with GH doping in sports. J Endocrinol Invest. 2003;26:924–31. doi: 10.1007/BF03345245. [DOI] [PubMed] [Google Scholar]
- 8.Ionescu M, Frohman LA. Pulsatile secretion of growth hormone (GH) persists during continuous stimulation by CJC-1295, a long-acting GH-releasing hormone analog. J Clin Endocrinol Metab. 2006;91:4792–7. doi: 10.1210/jc.2006-1702. [DOI] [PubMed] [Google Scholar]
- 9.Teichman SL, Neale A, Lawrence B, Gagnon C, Castaigne JP, Frohman LA. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91:799–805. doi: 10.1210/jc.2005-1536. [DOI] [PubMed] [Google Scholar]
- 10.List EO, Berryman DE, Palmer AJ, et al. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics. 2007;7:1140–9. doi: 10.1002/pmic.200600641. [DOI] [PubMed] [Google Scholar]
- 11.Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4:1311–8. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Kopchick JJ, List EO, Kohn DT, Keidan GM, Qiu L, Okada S. Perspective: proteomics--see “spots” run. Endocrinology. 2002;143:1990–4. doi: 10.1210/endo.143.6.8882. [DOI] [PubMed] [Google Scholar]
- 13.Mascot - www.matrixscience.com.
- 14.MS-Seeker - www.msseeker.org.
- 15.Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. CRC Press; Boca Raton, FL: 1997. p. 719. [Google Scholar]
- 16.The UniProt Consortium. The Universal Protein Resource (UniProt) 2009. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkm895. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogland C, Mostaguir K, Sanchez JC, Hochstrasser DF, Appel RD. SWISS-2DPAGE, ten years later. Proteomics. 2004;4:2352–6. doi: 10.1002/pmic.200300830. [DOI] [PubMed] [Google Scholar]
- 18.Lanes R, Soros A, Gunczler P, et al. Growth hormone deficiency, low levels of adiponectin, and unfavorable plasma lipid and lipoproteins. J Pediatr. 2006;149:324–9. doi: 10.1016/j.jpeds.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer GB, Greger NG, Fesmire JD, Blackett PR, Wilson DP, Frindik JP. Lipids and apolipoproteins in growth hormone-deficient children during treatment. Metabolism. 1994;43:1457–61. doi: 10.1016/0026-0495(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 20.Blackett PR, Weech PK, McConathy WJ, Fesmire JD. Growth hormone in the regulation of hyperlipidemia. Metabolism. 1982;31:117–20. doi: 10.1016/0026-0495(82)90121-4. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson C, Drake WM, Wieringa G, Yates AP, Besser GM, Trainer PJ. Serum lipoprotein changes following IGF-I normalization using a growth hormone receptor antagonist in acromegaly. Clin Endocrinol (Oxf) 2002;56:303–11. doi: 10.1046/j.1365-2265.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- 22.Oscarsson J, Wiklund O, Jakobsson KE, Petruson B, Bengtsson BA. Serum lipoproteins in acromegaly before and 6-15 months after transsphenoidal adenomectomy. Clin Endocrinol (Oxf) 1994;41:603–8. doi: 10.1111/j.1365-2265.1994.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Chen M, Zheng G, et al. Transcriptional activation by growth hormone of HNF-6-regulated hepatic genes, a potential mechanism for improved liver repair during biliary injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G357–66. doi: 10.1152/ajpgi.00581.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun. 2007;21:384–92. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahuna O, Fernandez L, Karlsson H, et al. Expression of hepatocyte nuclear factor 6 in rat liver is sex-dependent and regulated by growth hormone. Proc Natl Acad Sci U S A. 1997;94:12309–13. doi: 10.1073/pnas.94.23.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samadani U, Costa RH. The transcriptional activator hepatocyte nuclear factor 6 regulates liver gene expression. Mol Cell Biol. 1996;16:6273–84. doi: 10.1128/mcb.16.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung L, Clifford D, Buckley M, Baxter RC. Novel biomarkers of human growth hormone action from serum proteomic profiling using protein chip mass spectrometry. J Clin Endocrinol Metab. 2006;91:671–7. doi: 10.1210/jc.2005-1137. [DOI] [PubMed] [Google Scholar]


