Abstract
Understanding endothelial cell (EC) differentiation is a step forward in tissue engineering, controlling angiogenesis, and endothelial dysfunction. We hypothesized that epigenetic activation of EC lineage specification genes is an important mediator of embryonic stem cell (ESC) differentiation into EC. Mouse ESC was differentiated by removing leukemia inhibitory factor (LIF) from the maintenance media in the presence or absence of the specific DNA methyltransferase (DNMT) inhibitor 5′-aza-2′-deoxycytidine (aza-dC). Expression of EC specification and marker genes was monitored by quantitative PCR, western, immunocytochemistry, and flow cytometry. Functionality of differentiated EC was assessed by angiogenesis assay. The methylation status in the proximal promoter CpGs of the mediators of EC differentiation VEGF-A, BMP4, and EPAS-1 as well as of the mature EC marker VE-cadherin was determined by bisulfite sequencing. ESC differentiation resulted in repression of OCT4 expression in both the absence and presence of aza-dC treatment. However, significant increase in angiogenesis and expression of the mediators of EC differentiation and EC-specific genes was only observed in aza-dC-treated cells. The DNMT inhibition-mediated increase in EC specification and marker gene expression was not associated with demethylation of these genes. These studies suggest that DNMT inhibition is an efficient inducer of EC differentiation from ESC.
Keywords: embryonic stem cell, DNA methylation, endothelial cells, Differentiation
INTRODUCTION
EC have been shown to play a central role in vasculogenesis during development and in angiogenesis [1, 2]. EC-mediated angiogenesis also plays a key role in tumor development and metastasis [3-5], and endothelial dysfunction has been found to be an independent predictor of cardiovascular diseases [6, 7]. Besides their potential role in regenerative medicine, EC are an important tool for studying vasculogenic and angiogenic mechanisms involved in the pathogenesis of vascular disease, cancer, and diabetic retinopathy. In addition, endothelial signaling in developmental organogenesis appears to be universal in vertebrates [8], making them important for tissue engineering. ESC are a better source of endothelial cells compared with other endothelial cell origins, due to their low immunogenicity [9], high proliferation potential, and pluripotency. Furthermore, although somatic stem cells can home to sites of injured endothelium [10], the number and functional activity of endothelial progenitors is reduced in patients with disease risk factors such as age, smoking, hypertension, hyperlipidemia, diabetes, coronary heart disease, ischemic heart failure, and long-term statin treatment [11-13].
Several studies have shown that ESC can be induced to differentiate into EC in vitro through embryoid body formation on LIF-free growth media in combination with some type of mixture of matrix proteins and growth factors [14-16]. However, even in the presence of growth factors, differentiation of EC from ESC is not robust. The development of a productive method of isolating endothelial cells from ESC is critical for therapeutic and tissue engineering applications. Moreover, the upstream regulators of the growth factor genes and the EC-specific genes in the context of differentiation are not well understood. Recent studies have shown that BMP4 and VEGF have synergistic effects in the differentiation of embryonic stem cells into the endothelial lineage [17-19] and are sufficient to induce the differentiation [20].
In mammalian cells, epigenetic genomic DNA methylation modifications at CpG dinucleotides, carried out by DNMTs, has been shown to induce gene expression repression. Epigenetic mechanisms have been suggested for the dynamic regulation of lineage specification genes in embryonic stem cell differentiation. Indeed the specific DNMT inhibitor aza-dC has been shown to induce the differentiation of hESC into mesodermal cells such as cardiomyocytes [21, 22]. This effect could not be achieved by the other differentiation agents such as DMSO or retinoic acid [21]. However, little is known about the role of DNMT inhibition in the regulation of differentiation of ESC into other mesodermal lineages such as the EC. We present data showing that indeed, DNMT inhibition induces mESC to differentiate into the endothelial lineage, through activation of the EC differentiation inducer genes such as BMP4, VEGF, and EPAS-1.
MATERIALS AND METHODS
Cell culture and differentiation
The Pluristem™ 129S6 Murine ESC line derived from the 129/S6/SvEv mice strain (Millipore, Billerica, MA) was used. The ESC were adapted to serum- and feeder-free culture conditions and were routinely maintained in ESGRO COMPLETE™ serum-free clonal grade media (Millipore, Billerica, MA) on gelatin-coated plates. For adaptation, undifferentiated ES cells were initially grown on a monolayer of mitomycin-inactivated primary mouse embryonic fibroblasts (MEF) from the SF-1 mice strain (Charles River, Wilmington, MA) in Pluristem™ 129S6 cell medium (Millipore) containing 1000 units/ mL of mice leukemia inhibitory factor (mLIF=ESGRO, Millipore, Billerica, MA). The ESC were then transferred and routinely maintained on gelatin-coated plates in the ESGRO COMPLETE™ serum-free clonal grade media (Millipore, Billerica, MA). To induce differentiation, ESC were plated in 6-well plates at a concentration of 3.5×105 cells/well, and treated with 1 μM or 3 μM of aza-dC (Sigma, St. louis, MO) or the vehicle (0.00076% DMSO) in ESGRO COMPLETE™ basal media (Millipore, Billerica, MA) lacking LIF. Cultures were exposed to aza-dC at the beginning of treatment and returned to basal medium without the reagent after 24 h of treatment. The media was replaced every other day, and differentiation was monitored for 15 days. Mouse brain endothelial cells (mBEC, ATCC#: CRL-2299, Manassas, VA) were grown on DMEM (Invitrogen, San Diego, California) containing 10% FBS.
Quantitative RT-PCR analysis
Total cellular RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, California). cDNA was synthesized using the advantage RT for PCR kit (Clontech, Mountain View, California). The expression of endothelial-specific genes such as vascular endothelial cadherin (VE-cadherin), endothelial-specific receptor tyrosine kinase-2 (Tie-2), Von Willebrand factor (vWF ), angiogenic gene such as Angiopoietin-1 (Ang-1), and the inducers of EC differentiation such as bone morphogenetic protein 4 (BMP-4), vascular endothelial growth factor A (VEGF-A), and endothelial PAS domain 1 (EPAS-1) were quantified by real-time PCR analysis using Roche light cycler and Fast Start DNA master Syber Green I assay kit (Roche, Indianapolis, Indiana). Primer sequences and PCR conditions are available upon request. Results are normalized over GAPDH expression.
Immunofluorescence staining
Cells were grown in 6-well plates and the immunostaining was performed in the culture plates. Cells were fixed in 4% paraformaldehyde for 20 minutes at room temperature, permeabilized with methanol for 5 minutes and blocked in 5% BSA. Cells were incubated with the primary antibody for VE-cadherin (1:20, BD Biosciences, San Jose, California), Oct4 (1:100, Santa Cruz Biotechnology, Santa Cruz, California), Tie2 (1:100, Santa Cruz Biotechnology, Santa Cruz, California), and vWF (1:50, Santa Cruz Biotechnology, Santa Cruz, California ) overnight at 4°C. After washing, cells were incubated at room temperature for 1h in the species-specific secondary antibodies and were observed using an Olympus BX-60 or IX-71 fluorescence microscope (Olympus, Center Valley, PA). mBEC served as a positive control.
Western Blotting
Total protein extracts were obtained using the RIPA lysis buffer and 150 μg proteins per sample (30μg for mBEC) were electrophoresed on 4-20% SDS/PAGE (Biorad, Hercules, California). Proteins were transferred into nitrocellulose and blocked with 5% non-fat dry milk and incubated with VE-cadherin (1:250, BD Biosciences, San Jose, California) or Oct3/4 (1:100, Santa Cruz Biotechnology, Santa Cruz, California) or ß-actin (1:1000, Santa Cruz). After washing, the blots were incubated for 1h with horseradish peroxidase-conjugated anti-mouse or anti-rat IgG antibody (1:500, Invitrogen, San Diego, California) and visualized by enhanced chemiluminescence.
Flow cytometry
About 1×106 cells were blocked in 100 μL of 5% BSA and incubated 30 minutes with VE-cadherin antibody or the respective isotype control (BD Biosciences, San Jose, California). After washing cells were incubated with anti-rat IgG-Alexa Fluor 488 antibody for an hour, washed and analyzed in a Guava Easy Cyte flow cytometer using Cytosoft 3.4 software (Guava Technologies, Hayward, California).
Angiogenesis Assay
The angiogenesis assay was performed on matrigel coated 96-well plates using the In Vitro Angiogenesis Assay kit (Millipore, Billerica, MA). 0.05mL of diluted ECMatrix solution was added to each well and allowed to solidify at 37°C for at least an hour. About 0.2 mL of 5×104 to 1×105 cells were plated on the matrix and incubated at 37°C and were observed at different time intervals. mBEC were used as positive controls.
Bisulfite genomic sequencing
Genomic DNA was extracted using the DNeasy kit (Qiagen, Valencia, California). Bisulfite conversion was performed on 1 μg of genomic DNA using the EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA). CpG islands in the promoters and first exon of VE-cadherin, BMP4, VEGF-A, and EPAS-1 were predicted using MethPrimer [23]. Primers to amplify the predicted regions were designed using the same software. Primer sequences for bisulfite sequencing and PCR conditions are available upon request. Amplified regions from the bisulfite converted genomic DNA were ligated into pGEM-T Easy vector (Promega, Madison, WI) and used to transform E. coli. 10 plasmids from 10 colonies per treatment were sequenced with the T7 promoter primer. BiQ Analyzer software [24] was used for analysis, visualization and quality control of the DNA methylation data from bisulfite sequencing.
Statistical Analysis
Data on the effect of treatment on mRNA expression and % cells expressing VE-cadherin was analyzed by analysis of variance (ANOVA). Comparisons of CpG methylation status between treatments at each individual CpG site and at all the CpG sites interrogated were performed by the chi-square test. Calculations were performed with the statistical analysis system (SAS, version 8.01, SAS Inc., Cary, NC). ANOVA and chi-square test were performed by the general linear model procedure and post-hoc multiple mean comparisons were performed by the Bonferoni test. P values ≤ 0.05 were considered to be statistically significant. Unless otherwise stated, results are presented as mean ± SEM.
RESULTS
Inhibition of DNMT induces the ESC to adopt an EC-like morphology
The colony morphology characteristic of ESC (Fig. 1A) changed into a heterogeneous population of differentiated cells that still contained diffuse colonies in vehicle-treated cells (Fig. 1B-D). ESC treated with the DNMT inhibitor (aza-dC) lost their colony morphology and many of the cells adopted a flat cobblestone-like morphology characteristic of EC [25] (Fig. 1E-J).
Figure 1. Morphological changes of mESC after treatment with the DNMT inhibitor aza-dC.
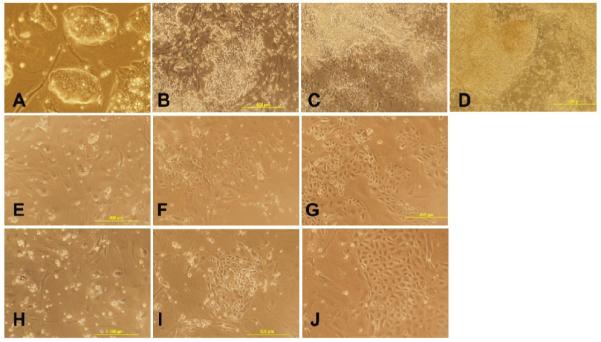
The ESC were exposed to vehicle, 1μM or 3 μM aza-dC for 24 hours in ESGRO COMPLETE™ basal media lacking LIF. Cultures were returned to basal medium without aza-dC after 24 h of treatment and photographed after 3, 7, and 15d of treatment at 100x magnification. (A) mESC. (B, C, D) ESC treated with vehicle at 3, 7, and 15d of treatment, respectively. (E, F, G) ESC treated with 1 μM aza-dC at 3, 7, and 15d of treatment, respectively. (H, I, J) ESC treated with 3 μM aza-dC at 3, 7, and 15d of treatment, respectively.
Inhibition of DNMT down-regulates mRNA expression of the pluripotency marker gene Oct4 and up-regulates mRNA expression of EC marker and angiogenic genes in the differentiated ESC
To confirm that the cells that adopted the EC-like morphology following aza-dC treatment were EC, we quantified mRNA levels of the pluripotency marker gene Oct4 [26] and of EC marker and angiogenic genes in ESC and after 3d, 7d, and 15d of treatment. Relative to ESC, Oct4 expression was significantly down-regulated in both vehicle- and aza-dC-treated cells by day 3 (Fig. 2A). However, expression of EC marker and angiogenic genes were extremely low in ESC and vehicle-treated cells relative to aza-dC-treated cells. Relative to vehicle-treated cells, expression of vWF was up-regulated by more than 3-fold (Fig. 2B), while VE-cadherin increased by 20-fold (Fig. 2C), and Tie-2 by 25-fold (Fig. 2D) reaching a maximum at 15 days of differentiation. Similarly, expression of the angiogenic gene Angiopoeitin-1 was also very low in vehicle-treated ESC but increased significantly (25-folds) in aza-dC-treated cells (Fig. 2E). Because aza-dC was withdrawn from culture media 24h after treatment, its effect tended to wear off at 1μM relative to 3μM aza-dC.
Figure 2. Effect of DNMT inhibition by aza-dC on expression of pluripotency marker, EC marker, and angiogenic genes.
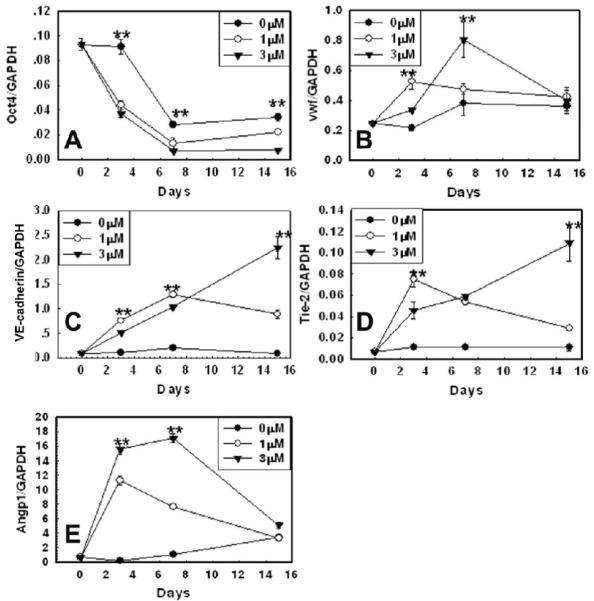
The ESC was exposed to vehicle or 1 μM or 3 μM aza-dC for 24 hours in ESGRO COMPLETE™ basal media lacking LIF. Cultures were returned to basal medium without aza-dC after 24 h of treatment and cells were collected after 3, 7, and 15d of treatment. mRNA expression of the pluripotency marker gene Oct4 (A), the EC marker genes Vwf (B), VE-cadherin (C), Tie-2 (D), and the angiogenic gene Angiopoietin-1 (E), was determined by quantitative real time PCR. Values are normalized over those of GAPDH (n=6). ** indicates a p value ≤ 0.01, for vehicle- vs. aza-dC-treated cells (ESC vs. vehicle- and aza-dC-treated cells for Oct4).
Inhibition of DNMT down-regulates protein expression of the pluripotency gene Oct4 and up-regulates protein expression of EC marker genes in the differentiated ESC
To further confirm that the DNMT inhibition induced ESC differentiation into EC, protein levels of Oct4, Tie-2, vWF, and VE-cadherin were determined by immunofluorescence staining. Protein levels of the pluripotency marker Oct4 were high in ESC, but decreased upon induction of differentiation especially by aza-dC treatment (Fig. 3A-C). Protein levels of the EC-specific markers VE-cadherin (Fig. 3D-F), vWF (Fig. 3H-J), and Tie-2 (Fig. 3L-N) were undetectable in ESC and vehicle-treated ESC but were observed in aza-dC-treated samples. mBEC (Fig. 3G, K, O) were used as positive controls for the markers of EC. Protein levels of the mature pluripotency marker Oct4 and of the mature EC marker VE-cadherin were also determined by western analysis (data not shown).
Figure 3. Immunocytochemical analysis of expression of the pluripotency gene Oct4 and EC marker genes VE-cadherin, vWF and Tie2 in response to DNMT inhibition in ESC.
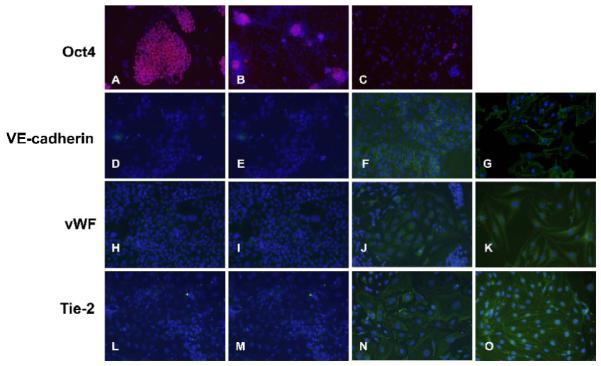
The ESC were exposed to vehicle or 1 μM or 3 μM aza-dC for 24 hours in ESGRO COMPLETE™ basal media lacking LIF. Cultures were returned to basal medium without aza-dC after 24 h of treatment and cells were stained as described in Methods after 15d of treatment. Nuclei are stained in blue by DAPI. Images were captured at 200x magnification. Oct4 :(A) ESC, (B) vehicle-treated ESC, (C) ESC treated with 3 μM aza-dC. VE-cadherin : (D) ESC, (E) vehicle-treated ESC, (F) ESC treated with 3 μM aza-dC, (G) mBEC. vWF : (H) ESC, (I) vehicle-treated ESC, (J) ESC treated with 3 μM aza-dC (K) mBEC. Tie2 : (L) ESC, (M) vehicle-treated ESC (N) ESC treated with 3 μM aza-dC (O) mBEC. mBEC were used as positive controls. The experiment was repeated three times and representative data is presented.
A significant number of cells express the mature EC marker gene VE-cadherin following the DNMT inhibition-mediated ESC differentiation
Flow cytometric quantification of the proportion of cells expressing the VE-cadherin protein, a specific marker of mature EC, was performed to determine the extent of DNMT inhibition-mediated ESC differentiation into mature EC. Whereas 16% of cells expressed VE-cadherin (Fig. 4D) following aza-dC treatment, cells expressing VE-cadherin were undetectable in ESC (Fig. 4B) and very low (5%) in vehicle-treated ESC (Fig. 4C). About ninety percent of mBEC used as positive control expressed the marker (Fig. 4A).
Figure 4. Flow-cytometric analysis of the proportion of cells expressing the EC marker gene VE-cadherin in response to DNMT inhibition in ESC.
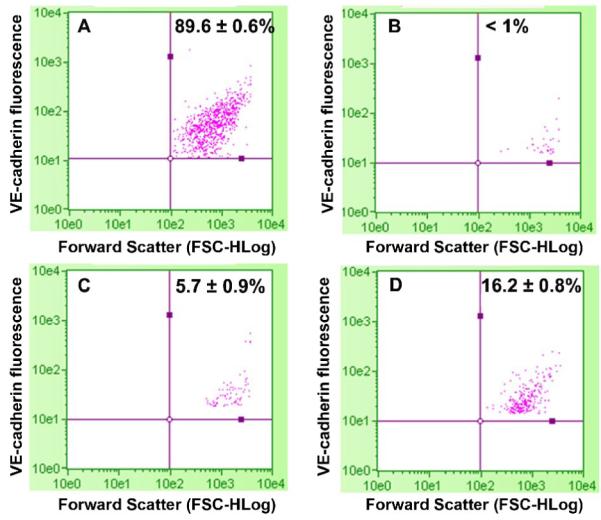
The ESC were exposed to vehicle or 3 μM aza-dC for 24 hours in ESGRO COMPLETE™ basal media lacking LIF. Cultures were returned to basal medium without aza-dC after 24 h of treatment and cells were collected after 11d of treatment for flow cytometry analysis as described in Methods. (A) mBEC, (B) ESC, (C) ESC treated with Vehicle, and (D) ESC treated with 3 μM aza-dC. mBEC were used as positive controls. Values are means (n=3) ± SE). The experiment was repeated three times and representative data is presented.
Inhibition of DNMT induces the differentiated ESC to form vascular-like structures
Angiogenesis is the process where new blood vessels sprout out of preexisting vessels and this process involves the recruitment and proliferation of EC [27, 28]. In vitro angiogenesis assay has shown that mature ECs form a capillary-like network when plated on matrigel [29]. We used a matrigel-based angiogenesis assay (Millipore) to define the functionality of the ESC-derived ECs. Although the expression of the pluripotency gene Oct4 was almost equally decreased in both vehicle- and aza-dC-treated ESC (Figs. 2B vs. 2C and 3B vs. 3C), vascular-like network formation was only observed in aza-dC-treated cells (Fig. 5D) and in mBEC (Fig. 5A), used as positive control. No such network was observed in ESC and vehicle-treated ESC (Fig. 5B, 5C).
Figure 5. Angiogenesis assay for functional analysis of the EC derived from ESC by DNMT inhibition.
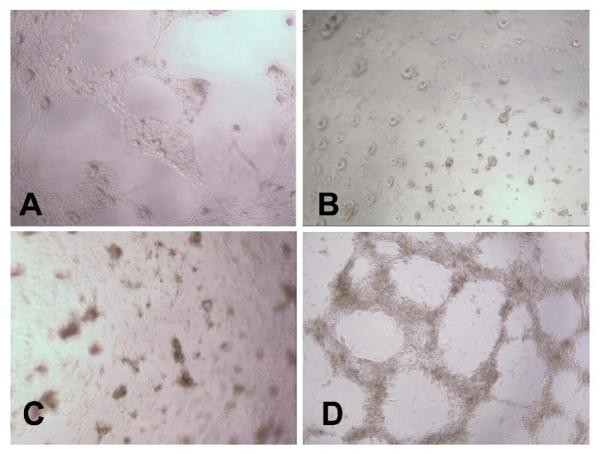
The ESC were exposed to vehicle or 3 μM aza-dC for 24 hours in ESGRO COMPLETE™ basal media lacking LIF. Cultures were returned to basal medium without aza-dC after 24 h of treatment. After 11 days of differentiation, 100,000 cells per well of a 96-well plate were plated on matrigel and incubated at 37°C for 16 h. Vascular-like network formation was captured by phase contrast microscopy at 100x magnification. (A) mBEC used as positive control, (B) ESC, (C) vehicle-treated ESC, and (D) ESC treated with 3 μM aza-dC. mBEC were used as positive controls. The experiment was repeated three times and representative data is presented.
Inhibition of DNMT up-regulates expression of the inducers of EC differentiation in the differentiated ESC
Expression of the inducers of EC differentiation BMP4, VEGF-A, and EPAS-1 was extremely low in ESC and vehicle-treated cells relative to aza-dC-treated cells. Relative to vehicle-treated cells, expression of BMP4 increased by 13 fold (Fig. 6A), EPAS-1 by 6-fold (Fig. 6B), and VEGF-A by 3-fold (Fig. 6C).
Figure 6. Effect of DNMT inhibition by aza-dC on expression of inducers of endothelial cell differentiation.
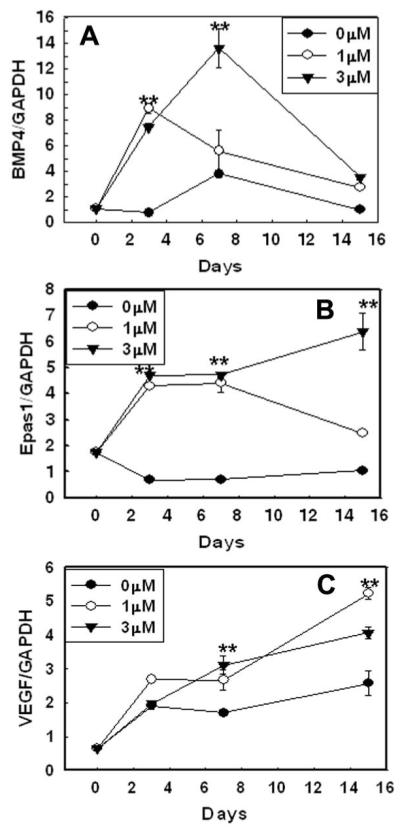
The ESC were exposed to vehicle or 1 μM or 3 μM aza-dC for 24 hours in ESGRO COMPLETE™ basal media lacking LIF. Cultures were returned to basal medium without aza-dC after 24 h of treatment and cells were collected after 3, 7, and 15d of treatment. mRNA expression of the EC inducer genes (A) BMP4, (B) EPAS-1 and (C) VEGF was determined by quantitative real time PCR. Values are normalized over those of GAPDH (n=6). ** indicates a pvalue ≤0.01, for vehicle- vs. aza-dC-treated cells (ESC vs. vehicle- and aza-dC-treated cells).
Expression of the mature EC marker gene VE-cadherin and of the inducers of EC differentiation BMP4, EPAS-1 and VEGF-A was not correlated to their methylation status
Aza-dC activated expression of EC-marker genes and the inducers of EC differentiation and induced ESC to differentiate into EC. This suggested a DNA demethylation-dependent mechanism of up-regulation of expression of these genes. To test the hypothesis we determined the methylation status of these genes during the differentiation process, comparing ESC to vehicle- and aza-dC-treated ESC, and to mature EC (mBEC). Analysis of the bisulfite-converted sequences revealed that the conversion efficiency was more than 98%, greater than the 90% accepted by most softwares designed to analyze bisulfite-converted DNA. Analysis of the sequences showed that the CpG Island in the VE-cadherin promoter was fully methylated in the ESC, vehicle-treated ESC, and aza-dC-treated ESC and even in the mBEC where it is highly expressed (Table 1). Conversely, all the three inducers of EC differentiation had low methylation that did not differ between treatments ESC (Table 1). The lack of change in methylation status was observed at individual CpG sites and at all CpG sites analyzed together.
Table 1. Percentage CpG methylation in the proximal promoter of the mature EC marker VE-cadherin and the EC differentiation inducers BMP4, VEGF-A and EPAS-1.
| Number of CG sites* | ESC | ESC+Vehicle | ESC +aza-dC | mBEC | |
|---|---|---|---|---|---|
| VE-cadherin | 14 | 100 | 98 | 90 | 94 |
| BMP4 | 10 | 9 | 15 | 17 | 11 |
| EPAS-1 | 7 | 0 | nd | nd | nd |
| VEGF-A | 42 | 4 | nd | 2 | 2 |
The number of CG sites analyzed for each gene are indicated. Ten clones were analyzed for each CG site. The data is the average percentage methylation over all CpG sites analyzed.
DISCUSSION
Differentiation of endothelial cells from embryonic stem cells in vitro is very inefficient and producing a sufficient number of these cells in vitro for cell therapy and tissue and organ engineering remains a challenge. Indeed the best results have been obtained with VEGF treatment, which induces only up to 2% of embryonic stem cells to express mature EC markers [30]. Previous studies have documented the importance of epigenetic changes such as DNA methylation in gene regulatory networks during ESC differentiation. We postulated that EC differentiation from ESC could be enhanced by inhibiting DNA methylation and reversing the repression of genes that specify endothelial cell fate determination.
In the present report, we provide for the first time evidence that inhibition of DNMT by aza-dC can induce differentiation of mouse ESC into the endothelial lineage in vitro in adherent culture. This conclusion is based on the morphological features (Fig. 1) and on the observation that whereas expression of endothelial markers such as Tie-2, vWF, and VE-cadherin and angiogenic genes such as Angiopoeitin-1 was low-to undetectable in ESC and vehicle-treated ESC, expression of these marker genes was increased by several fold following aza-dC treatment both at mRNA and protein levels (Figs. 2 and 3). In addition, FACS analysis of the proportion of cells expressing VE-cadherin, a specific marker of mature EC [14, 25], not expressed in ESC [31], revealed that whereas 16% of cells expressed VE-cadherin following aza-dC treatment, 0% and less than 5% of cells expressed VE-cadherin in ESC and vehicle-treated ESC, respectively (Fig. 4).
Previous studies using the EB method of differentiating ESC have reported that in differentiation media, ES cells can spontaneously differentiate in vitro into endothelial progenitor cells. However, the differentiation into cells expressing the mature EC markers such as VE-cadherin has been found to be up to 2%, and a subsequent subculture on growth factors that have been found to stimulate growth of EC have been used to obtain a significant number of cells expressing VE-cadherin. Up to 36% of cells positive for VE-cadherin were obtained under these conditions [25]. To our knowledge, the present investigation is the first to report a significant induction of ESC differentiation into EC that does not involve the use of endothelial growth factors. It is also the first to establish the role of DNMT inhibition and the potential role of DNA methylation in the differentiation of mESC into the endothelial cell lineage in adherent culture. These differentiated EC cells were functional. Indeed, angiogenesis assay revealed that ESC formed vascular network-like structures only following aza-dC treatment (Fig. 5). We compared the angiogenic potential of the ESC-derived ECs relative to adult EC using mouse brain vascular EC (mBEC) and found that the ESC-derived EC had a better angiogenic potential than the mBEC. However, in our hands, the angiogenic potential of mBEC was not as good as that of HUVECs either (data not shown). MCcloskey et al [32] reported that VE-cadherin in the ESC-derived EC did not localize as well at the cell-cell junctions as in the mature endothelial cells. However, in the DNMT inhibition-induced differentiation, VE-cadherin correctly localized in cell-cell junctions (Fig 3F).
To begin to define the mechanisms by which DNMT inhibition induces ESC to differentiate into EC, we determined the expression of the endothelial differentiation inducers and their response to DNMT inhibition. Factors involved in mesoderm formation such as FGF-2 [33], TGF-beta family of factors such as BMP4 [34], have been found to play a critical role in the induction of the endothelial cell precursors (angioblasts). Angioblasts arise from the mesoderm through the action of factors such as Indian hedgehog (IHH) [35]. IHH signaling in this context is mediated by factors such as BMP4 and it cooperates with FGF2 signaling to activate flk-1 expression [1, 35]. The flk-1+ endothelial precursors are very responsive to VEGF, which plays a central role in growth and differentiation of angioblasts into mature endothelial cells and subsequent vasculogenesis and angiogenesis [36]. The sequential role of FGF2, BMP4, and VEGF in the differentiation of embryonic cells into endothelial cells is in agreement with recent reports of synergistic effects of BMP4 and VEGF in the induction of hemangioblastic commitment and development from hESCs [19] and the differentiation of ESC into EC [17-19]. Pearson et al. [20] found that though addition of FGF2 and Activin A to BMP4 could increase expression of hemangioblast precursors relative to BMP4 alone, there was no statistical difference in the production of committed endothelial/hematopoietic precursors when ES cells are differentiated with BMP4 alone or with BMP4, Activin A and bFGF. They also found that although VEGF was not required for the hemangioblast specification, it induces the proliferation of precursor cells and increases the number of hemangioblasts. They concluded that VEGF-A and BMP4 are sufficient to induce the differentiation of ESC into EC. Zhang et al. [37] showed that both FGF and TGF-β/Nodal/activin signaling pathways are involved in BMP-4 initiated mesoderm induction. BMP4 is able to complete the EC differentiation program because at optimal concentration, BMP-4 may induce the expression of VEGF or other VEGF family members [38]. Interestingly, expression of VEGF-A, BMP4, as well as EPAS-1, a gene that up-regulates VEGF expression [39] were strongly stimulated by DNMT inhibition (Fig. 6), suggesting a mechanism of robust induction of ESC differentiation into EC by DNMT inhibition.
The cytosine analog aza-dC is an effective DNA hypomethylating agent that has been shown to decrease DNMT activity in cells and cause extensive demethylation of 5-methylcytosine residues [40, 41]. The fact that aza-dC induced mESC to differentiate into EC suggested a DNA demethylation-dependent mechanism of activation of the EC differentiation inducer genes or of the EC marker genes. We tested the hypothesis that aza-dC-induced activation of VEGF-A, BMP4, EPAS-1, and the mature EC-specific gene VE-cadherin by demethylation of CGs in the proximal promoters of these genes. Surprisingly, all the EC differentiation inducers had proximal promoter CpG islands with very low methylation levels even in ESC where they are not expressed. The methylation was not affected by DNMT inhibition and was not different to that of adult EC (mBEC, used as positive expression control) where these genes are highly expressed (Table1). Conversely, the CpG island in the VE-cadherin promoter was fully methylated even in the mBEC and the aza-dC-treated ESC that highly expressed VE-cadherin, and there was no difference in methylation status between these cells and ESC that did not express VE-cadherin (Table 1). DNA methylation has been shown to repress target gene expression by DNA methylation-mediated binding of proteins with the methyl cytosine-binding domain (MBDs, MeCP-1, and MeCP-2) which recruit repressors such as histone deacetylases and the H3-lysine 9-specific methyltransferases [42, 43], leading to chromatin compaction and gene inactivation. However, DNMT is able to bind directly and recruit the co-repressors such as histone deacetylases [44], H3K9/H3K27/H4K20 methyltransferases [45], or even H3K4/H3K79 demethylases and repress transcription of target genes in a CpG methylation-independent manner. This process may be operational here or an undefined upstream mediator may be the target for demethylation.
Overall, this study demonstrates for the first time that DNMT inhibition induces the differentiation of ESC into EC more efficiently than any other known mechanism through up-regulation of expression of the major inducers of EC differentiation. Our EC differentiation strategy, coupled with selection and subculture techniques such as those described by McCloskey et al. [46] could result in the production of homogeneous populations of mature EC for tissue engineering and other therapeutic applications. Future studies will elucidate the mechanisms of up-regulation of expression of the inducers of EC differentiation by DNMT inhibition. In addition, since BMP signaling must be modulated for efficient mesoderm differentiation [37], future studies will elucidate the mediator role of BMP receptor subtypes and of BMP antagonists.
ACKNOWLEDGEMENTS
This work was funded by the NIH/NCRR/RCMI Grant G12-RR03034, the NIH/NHLBI grant 5 K01 HL084725-02, and the MSM Cardiovascular Center of Excellence grant 5R25HL003676. We thank Dr. Steve Stice’s lab for help with MEF and Jerry Manlove-Simmons for technical help with Flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Poole TJ, Finkelstein EB, Cox CM. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev Dyn. 2001;220:1–17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [2].Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93:1253–1263. [PubMed] [Google Scholar]
- [3].Ackerstaff E, Artemov D, Gillies RJ, Bhujwalla ZM. Hypoxia and the presence of human vascular endothelial cells affect prostate cancer cell invasion and metabolism. Neoplasia. 2007;9:1138–1151. doi: 10.1593/neo.07568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guo Y, Wang S, Hoot DR, Clinton SK. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18:408–417. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- [5].Elzarrad K, Haroon A, Reed D, Al-Mehdi AB. Early incorporated endothelial cells as origin of metastatic tumor vasculogenesis. Clin Exp Metastasis. 2009 doi: 10.1007/s10585-009-9257-8. [DOI] [PubMed] [Google Scholar]
- [6].Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- [7].Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- [8].Levenberg S, Zoldan J, Basevitch Y, Langer R. Endothelial potential of human embryonic stem cells. Blood. 2007;110:806–814. doi: 10.1182/blood-2006-08-019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- [10].Dong C, Goldschmidt-Clermont PJ. Endothelial progenitor cells: a promising therapeutic alternative for cardiovascular disease. J Interv Cardiol. 2007;20:93–99. doi: 10.1111/j.1540-8183.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- [11].Liguori A, Fiorito C, Balestrieri ML, Crimi E, Bruzzese G, Williams-Ignarro S, D’Amora M, Sommese L, Grimaldi V, Minucci PB, Giovane A, Farzati B, Ignarro LJ, Napoli C. Functional impairment of hematopoietic progenitor cells in patients with coronary heart disease. Eur J Haematol. 2008;80:258–264. doi: 10.1111/j.1600-0609.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- [12].Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- [13].Hristov M, Fach C, Becker C, Heussen N, Liehn EA, Blindt R, Hanrath P, Weber C. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis. 2007;192:413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- [14].Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- [15].Kaufman DS, Lewis RL, Hanson ET, Auerbach R, Plendl J, Thomson JA. Functional endothelial cells derived from rhesus monkey embryonic stem cells. Blood. 2004;103:1325–1332. doi: 10.1182/blood-2003-03-0799. [DOI] [PubMed] [Google Scholar]
- [16].Boyd NL, Dhara SK, Rekaya R, Godbey EA, Hasneen K, Rao RR, West FD, 3rd, Gerwe BA, Stice SL. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 2007;232:833–843. [PubMed] [Google Scholar]
- [17].Nakayama N, Lee J, Chiu L. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 2000;95:2275–2283. [PubMed] [Google Scholar]
- [18].Bai H, Chen K, Arzigian M, Wang ZZ. Sequential roles of BMP-4, VEGF and FGF-2 in generation of CD34+ vascular progenitor cells from human embryonic stem cells under animal product-free condition. Cell Res. 2008;18:S108–S108. [Google Scholar]
- [19].Lu SJ, Luo C, Holton K, Feng Q, Ivanova Y, Lanza R. Robust generation of hemangioblastic progenitors from human embryonic stem cells. Regen Med. 2008;3:693–704. doi: 10.2217/17460751.3.5.693. [DOI] [PubMed] [Google Scholar]
- [20].Pearson S, Sroczynska P, Lacaud G, Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- [21].Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- [22].Yoon BS, Yoo SJ, Lee JE, You S, Lee HT, Yoon HS. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation. 2006;74:149–159. doi: 10.1111/j.1432-0436.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- [23].Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- [24].Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21:4067–4068. doi: 10.1093/bioinformatics/bti652. [DOI] [PubMed] [Google Scholar]
- [25].Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- [27].Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- [28].Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- [29].Murray JD. On the mechanochemical theory of biological pattern formation with application to vasculogenesis. C R Biol. 2003;326:239–252. doi: 10.1016/s1631-0691(03)00065-9. [DOI] [PubMed] [Google Scholar]
- [30].Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- [31].Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McCloskey KE, Smith DA, Jo H, Nerem RM. Embryonic stem cell-derived endothelial cells may lack complete functional maturation in vitro. J Vasc Res. 2006;43:411–421. doi: 10.1159/000094791. [DOI] [PubMed] [Google Scholar]
- [33].Saxton TM, Pawson T. Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc Natl Acad Sci U S A. 1999;96:3790–3795. doi: 10.1073/pnas.96.7.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- [35].Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- [36].Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002;129:1881–1892. doi: 10.1242/dev.129.8.1881. [DOI] [PubMed] [Google Scholar]
- [37].Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, Ding M, Deng H. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- [38].Tokuda H, Hatakeyama D, Shibata T, Akamatsu S, Oiso Y, Kozawa O. p38 MAP kinase regulates BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts. Am J Physiol Endocrinol Metab. 2003;284:E1202–1209. doi: 10.1152/ajpendo.00300.2002. [DOI] [PubMed] [Google Scholar]
- [39].Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haaf T, Schmid M. Experimental condensation inhibition in constitutive and facultative heterochromatin of mammalian chromosomes. Cytogenet Cell Genet. 2000;91:113–123. doi: 10.1159/000056830. [DOI] [PubMed] [Google Scholar]
- [41].Cheng X. DNA modification by methyltransferases. Curr Opin Struct Biol. 1995;5:4–10. doi: 10.1016/0959-440x(95)80003-j. [DOI] [PubMed] [Google Scholar]
- [42].Fujita N, Watanabe S, Ichimura T, Tsuruzoe S, Shinkai Y, Tachibana M, Chiba T, Nakao M. Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression. J Biol Chem. 2003;278:24132–24138. doi: 10.1074/jbc.M302283200. [DOI] [PubMed] [Google Scholar]
- [43].Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- [44].Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat Genet. 2000;25:338–342. doi: 10.1038/77124. [DOI] [PubMed] [Google Scholar]
- [45].Esteve PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, Carey MF, Pradhan S. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 2006;20:3089–3103. doi: 10.1101/gad.1463706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McCloskey KE, Stice SL, Nerem RM. In vitro derivation and expansion of endothelial cells from embryonic stem cells. Methods Mol Biol. 2006;330:287–301. doi: 10.1385/1-59745-036-7:287. [DOI] [PubMed] [Google Scholar]


