Abstract
Diffuse large B-cell lymphoma (DLBCL) is a very infrequent neoplasm in the pediatric age group; therefore there are very few studies on the immunophenotype or genetics of these cases. We studied a series of 16 patients with nodal DLBCL occurring in patients between 10 and 18 years of age. The cases were classified according to the 2008 World Health Organization classification criteria, with application of immunohistochemistry for the detection of CD10, BCL-6 and MUM1 proteins to divide the lymphomas into germinal center and non-germinal center types. In addition, TCL1, BCL-2 expression, and the Ki-67 proliferation index were evaluated by immunohistochemistry, and c-MYC and BCL-2 translocations were evaluated by FISH. All these parameters were correlated with clinical features and outcome. Our study revealed that centroblastic morphology and the germinal center type of DLBCL are more prevalent in these young patients (63%), with 37% containing a c-MYC translocation. Only one case showed a BCL-2 translocation, reflecting a double-hit case with features intermediate between DLBCL and Burkitt lymphoma. We found a higher frequency of BCL-2 expression than previously reported, with no direct influence on the outcome of the disease in univariate or multivariate analysis. The expression of TCL1 has not been specifically studied in nodal pediatric DLBCL before; we found a 31% incidence of TCL1 expression. MUM1 expression was observed in 44% of the cases and these positive cases showed a significant negative impact on clinical outcome. TCL1 is directly and significantly associated with the presence of c-MYC and a high proliferative index. The germinal center and non-germinal center subtypes showed significant differences for both overall survival and disease-free interval. C-MYC translocation was found in 37% of patients, and had a favorable impact on clinical outcome. We conclude that nodal pediatric and adolescent DLBCL are mainly of the germinal center type, with a generally good outcome in spite of the frequent expression of BCL-2 and the presence of c-MYC translocation. TCL1 expression seems to be associated with a good clinical outcome, while MUM1 expression predicts a poor clinical outcome.
Keywords: pediatric non-Hodgkin lymphoma, diffuse large B-cell lymphoma, TCL-1, germinal center-like DLBCL (GCB), non-germinal center-like DLBCL, c-MYC and BCL-2
INTRODUCTION
Malignant non-Hodgkin lymphomas (NHL) account for about 6 to 8% of pediatric malignancies (31, 37) and diffuse large B-cell lymphoma (DLBCL) comprises about 10% of pediatric and adolescent NHL (5, 32). It is now recognized that DLBCL represents a heterogeneous group of lymphoid neoplasms without common genetic features. Gene-expression profiling (GEP) analyses have identified two robust and highly reproducible DLBCL subtypes, germinal center cell-like (GC) and activated B-cell-like (ABC) (3). Hans and coworkers (13) established an immunohistochemistry algorithm with a strong correlation with the groups identified by GEP, combining CD10, BCL-6 and MUM-1 expression to recognize two groups of DLBCL, germinal center-like B cell lymphoma (GCB) and non-GCB lymphoma.
There have been only a few studies on the immunophenotype or genetics of pediatric DLBCL (8, 10, 31, 33). In the larger series of pediatric DLBCL reported, the cases were mainly extranodal disease. It has been shown that 60 to 85% of pediatric DLBCL have a GCB subtype in comparison to 45% in adult patients (31, 33). BCL-2 expression is detectable in 28 to 40% of the cases according to different studies (10, 25, 31). However, breaks in the BCL-2 gene or fusion with IGH by translocation t(14;18) seem to be virtually absent in pediatric DLBCL (8, 31), and breaks in BCL-6 gene are also very rare. In contrast, translocations involving MYC has been described in 25% to 35% of pediatric DLBCL (8, 25) in contrast to 5-10% observed in adults (2, 24).
The transcription factor MUM-1 (MUM1/IRF4, multiple myeloma-1/interferon regulatory factor 4 protein) is considered to be a histogenetic marker, expressed in the final step of intra-GC B-cell differentiation and in post-GC B-cells (11, 29). Inside the GC, expression of MUM1 begins at centrocyte stage and is maintained during post-GC maturation, in contrast to BCL-6 expression, which is observed immediately after the B-cell enters the GC and is maintained until GC exit (9). There are different phenotypic groups of cells in normal lymphoid tissues; the post-germinal phenotype (MUM1+/BCL-6 −/CD10−) differs from that of most GC cells (MUM1−/BCL-6+/CD10+) and from mantle cells (MUM1−/BCL-6−/CD10−). Unlike normal GC B-cells, in which the expression of MUM1 and BCL-6 is mostly mutually exclusive, approximately 50% of MUM1-positive DLBCL co-express BCL-6 (9, 38).
C-MYC is a proto-oncogene which functions as a transcription factor, involved in regulating cell proliferation and maturation activated by transcriptional deregulation. C-MYC repression is required for normal plasma cell differentiation. Deregulation of c-MYC prevents B-cell differentiation and has oncogenic activity (12, 14). Pediatric DLBCL has been reported to have a higher incidence of c-MYC translocation in comparison to adult cases (10, 14, 25).
The proto-oncogene TCL1 (T-cell leukemia 1) is a powerful B-cell oncogene and has been implicated in the pathogenesis of various types of mature B-cell lymphomas (16). Burkitt lymphoma (BL) is the type with the most consistently high expression of TCL1 protein, a tumor whose normal counterpart is the mostly TCL1-negative germinal cell (1). Other types of lymphomas, including precursor cell B and T-cell lymphoma, show great variability in TCL1 expression. DLBCL in adult patients has been reported to be TCL-1 positive in 18 to 60% of cases, especially in cases of the activated B-cell type (27, 28). TCL-1 has been described in close association with c-MYC translocation in aggressive B-cell lymphomas in adults, being expressed in 86% of c-MYC-translocation-positive cases versus 40% in c-MYC negative cases in the Rodig et al. study (34). In B-cell neoplasms, the TCL1 protein expression occurs in the consistent absence of TCL1 chromosomal abnormalities (15). There is no information about TCL1 expression in pediatric cases. The largest series of pediatric DLBCL include mainly extranodal cases, without a separate analysis on nodal cases (20, 31, 33). In adults, nodal and extranodal DLBCL have been considered to represent different neoplasms (31, 37). In this study, we have evaluated only nodal cases of pediatric and adolescent DLBCL, with the goal of analyzing the immunophenotype, specifically determining germinal versus non-germinal center type, in relationship to the presence of c-MYC translocation, TCL1 expression and correlation with the clinical course.
MATERIALS AND METHODS
Case selection and clinical data
Sixteen cases of nodal DLBCL from children and adolescent patients between 10 to 18 years old were obtained retrospectively from the files of Consultoria em Patologia, a large reference consultation service in anatomic pathology located in Botucatu, Sao Paulo State, Brazil between June 2004 and December 2008. All 16 cases had available slides and paraffin blocks for review. Extranodal cases of DLBCL were excluded as they may represent a different disease compared to nodal DLBCL. Clinical data, including gender, age at diagnosis, anatomic location, stage, laboratory tests, treatment and follow-up were obtained from the referring hematologists/oncologists. Available hematoxylin and eosin stained slides of each case were reviewed and representative areas were selected for TMA. A morphologic subclassification of the cases was performed, considering variants included in 2008 WHO classification (37).
Treatment
All patients received chemotherapy. The patients were stratified by risk factors (stage and LDH level). Ten cases received the NHL-BFM 90 (Berlin-Frankfurt-Munster, BFM) based protocol with reduction of the methotrexate dose from 5mg/m2 to 2 mg/m2 in the group with more intensive courses in patients in stage IV or with LDH more than double the normal value. Four patients received CHOP protocol, one case received HyperCVAD (HCVAD) and another received ICE protocol. As a second line one of the patients who received BFM 90 underwent ICE-R, two of the patients receiving CHOP received as second line CHOP-R (one) or Rituximab (one). The patient treated with ICE subsequently received Rituximab and later underwent autologous stem cell transplantation. Three patients were treated with radiotherapy due to bulky tumor mass (Table 2).
Table 2.
Clinical features, laboratory tests, treatment, follow up and immunohistochemical and genetic features of 16 cases of nodal pediatric DLBCL including GC and non-GC types.
| Case n° | Sex | Age | AL | S | LDH | BM | CNS | MC | IP | QT | RTX | RT | BMT | FUP | CD20 | CD10 | BCL6 | BCL2 | MUM1 | TCL1 | MIB | CMYC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 10 | C,I | IV | NA | P | N | BRT | GC | CHOP×8 | YX8 | N | N | 19 | P | N | P | P | N | N | 50% | N |
| 2 | M | 15 | A | I | NO | N | N | CB | GC | BFM×4 | N | N | N | 6 | P | N | P | P | N | N | 90% | N |
| 3 | M | 16 | M | II | H<2 | N | N | CB | GC | CHOP×6 | N | Y | N | 38 | P | P | N | P | N | N | 80% | I |
| 4* | M | 15 | I | II | NA | N | N | UC | GC | HCVAD | N | N | N | 18 | P | P | P | P | N | P | 80% | P |
| 5 | F | 13 | A | III | NO | N | N | CB | GC | BFM×6 | N | N | N | 36 | P | P | P | N | N | P | 90% | P |
| 6 | M | 10 | C | II | NO | N | N | BRT | GC | BFM×4 | N | N | N | 31 | P | N | P | N | N | N | 50% | P |
| 7 | M | 16 | RP | II | NO | N | N | CB | GC | BFM×4 | N | N | N | 24 | P | P | P | N | P | P | 80% | N |
| 8 | F | 12 | C, A | IV | H<2 | N | P | CB | GC | BFM×6 | N | Y | N | 19 | P | N | P | N | N | N | 40% | N |
| 9 | M | 16 | M, C | IV | H<2 | P | N | CB | GC | BFM×6 | N | Y | N | 40 | P | N | P | N | N | N | 80% | N |
| 10 | M | 15 | M, A,I |
IV | NO | P | N | CB | GC | ICE×4 | YX6 | N | AT | 18 | P | N | P | P | N | N | 70% | P |
| 11 | M | 15 | A, RP |
III | H>2 | N | N | CB | NO-GC | BFM×5 | ICEYX5 | N | N | 12 | P | N | N | P | P | N | 70% | N |
| 12 | F | 15 | M | III | NA | N | N | CB | NO-GC | CHOP×2 | N | N | N | 1 | P | N | P | P | P | N | 40% | N |
| 13 | F | 17 | M, C | II | H<2 | N | N | CB | NO-GC | BFM×6 | N | N | N | 12 | P | N | P | P | P | N | 30% | N |
| 14 | F | 18 | C | IV | H>2 | N | P | IB | NO-GC | CHOP×8 | CHOP YX8 |
N | N | 36 | P | N | P | P | P | N | 90% | N |
| 15 | F | 12 | RP, I, A |
IV | H>2 | N | N | CB | NO-GC | BFM×6 | N | N | N | 44 | P | N | P | N | P | P | 80% | P |
| 16 | M | 18 | RP | III | N | N | N | CB | NO-GC | BFM×8 | N | N | N | 48 | P | N | P | P | P | P | 80% | N |
Age: in years; AL: anatomic location; S: clinical stage; LDH: lactate deshidrogenase; BM: bone marrow; CNS: central nervous system; MC: morphological classification; IP: immunophenotype; QT: chemotherapy; RTX: rituximab; RT: radiotherapy; BMT: bone marrow transplantion; FUP: follow up; F: female; M: male; C: cervical; I: inguinal; A: axillar; M: mediastinum; RP: retroperitonium; NA: not available; NO: normal; H: high: <2 less than double normal, >2 higher than double normal; P: positive; N: negative; I: inconclusive; Y: yes; CB: centroblastic; IB: immunoblastic; BRT: T-cell rich B-cell lymphoma; UN: unclassified; GC: germinal center type; NO-GC: non-Germinal center type; AT: autologous;
case 4 revealed BCL2 translocation by FISH.
Tissue microarray construction
One tissue microarray (TMA) block was constructed, using a tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA). Three tumor cores of 0.6 mm that had been taken from the original paraffin blocks represented each individual case. Serial sections of 3μm were cut from the tissue array blocks and used for the immunohistochemical and fluorescence and non-fluorescence in situ hybridization analysis. Proper positive and negative controls cores for each marker were also included in the array block to provide an assessment of the adequacy of the antibodies used in the immunohistochemical study.
Immunohistochemistry and in situ hybridization
Immunohistochemical studies were performed on the TMA using the Novolink polymer® (Novocastra, Newcastle Upon Tyne, UK) as the detection system, and an epitope-retrieval method was applied as needed for each specific antibody; diaminobenzidine (DAB) was the chromogen. The primary antibodies are shown in Table 1. MUM-1 was considered positive when greater than 20% tumor nuclei staining was seen, according to Falini et al (9). For CD10, BCL-6, BCL-2 and TCL-1 a 30% cut off was used. To evaluate staining at least 10 high-power fields including tumor were evaluated. The Ki-67 proliferative index (PI), using the monoclonal antibody MIB1, was assigned a percentage value that was calculated by scoring 500 tumor cell nuclei for the presence of nuclear expression. Sections from the TMA were examined for the expression of the Epstein-Barr viral RNA by in situ hybridization using the EBER-1 probe, as previously described (4).
Table 1.
Primary antibodies used.
| Antigen | Clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| CD45RB | PD7 | 1:500 | MW CB | Dako |
| CD20 | L26 | 1:1,200 | MW CB | Dako |
| CD3 | SP7 | 1:200 | S CB | Neomarkers |
| CD10 | 56C6 | 1:100 | S CB | Novocastra |
| CD5 | 4C7 | 1:1,600 | S CB | Novocastra |
| CD138 | B-B4 | 1:800 | PC CB | Zymed |
| Bcl-2 | 124 | 1:400 | MW CB | Dako |
| Bcl-6 | PG-B6P | 1:100 | T+ S TRIS | Dako |
| IRF4/MUM-1 | MUM1P | 1:1,200 | S CB | Dako |
| TCL-1 | 1-21 | 1:9,600 | PC EDTA | Zymed |
| Ki-67 | MIB-1 | 1:4,800 | PC CB | Dako |
DAKO, Carpinteria, CA, USA; Lab Vision Corporation, Fremont, CA, USA, Novocastra, United Kindom; Becton-Dickinson Biosciences, Mountain View, CA, USA; Zymed. Heat-induced epitope retrieval was employed; MW: microwave oven; PC: pressure cooker; S: steamer; T: trypsin and CB: citrate buffer.
Fluorescence in situ hybridization (FISH)
FISH analysis was performed in each case using a 3μm-thick tissue section of the array block, using probes specific for BCL2 and c-MYC rearrangements, as previously described (7, 31). The t(14;18)(q32;q21) translocation was analyzed using a commercial Dual ISI IGH/BCL2 probe (Vysis, Downers Grove, IL, USA). For the detection of breakpoints (splits) in the c-MYC locus, an LSI MYC Dual Color Break-apart Rearrangement probe (Vysis, Abbott AG, USA) was applied. The slides were evaluated using spectrum orange and spectrum green filters (Chroma Technology GmbH, Fuerstenfeldbruck, Germany) on a Zeiss Axio Imager M1 fluorescence microscope (Carl Zeiss AG, Germany) using the assistance of Isis FISH Imaging Software (Metasystems, Altlussheim, Germany). A positive case was defined when the mean number of positive tiles detected was 3 standard deviations above the mean of a negative control (reactive lymphoid tissue). This threshold established was 15.9% for BCL2 translocation and 2.19% for c-MYC (the mean of the negative control group was 7.8% and 0.73% respectively).
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis until death or date of last follow-up. Survival curves were estimated by the Kaplan-Meier method, using the Breslow test to analyze the statistical differences between the groups. Analyses were carried out using EpiInfo 2000. Life tables were produced in order to adjust life curves and disease-free survival. The Cox proportional hazards model was used to assess which of those markers retained significant prognostic value. Contingency tables were analyzed using Pearson chi-square statistic. Multinomial Logistic Regression (MLR) was applied to evaluate all markers at the same time (holistic approach). Group means were compared using ANOVA technique with Fischer-F statistics. The significance level used in the study was 10% due to the small number of patients.
RESULTS
The 16 cases studied included 7 girls and 9 boys. The mean age was 14.5 years (range 10 to 18 years). All cases had a nodal presentation; in two cases the lymph nodes were located in the mediastinum, there were four cases with both peripheral lymphadenopathy and mediastinal involvement, and ten cases with superficial and/or retroperitoneal lymphadenopathy. The clinical and laboratory findings are summarized in Table 2.
Morphologically, twelve cases showed centroblastic features, one was immunoblastic, two were T-cell histiocyte-rich B-cell type and one case was considered intermediate between DLBCL and BL, a provisional entity according to the 2008 WHO classification. This case was classified as intermediate between DLBCL and BL as it expressed BCL-2 protein, the proliferation index equal to 80%, lower than expected to be BL and by the presence of BCL2 translocation together with c-MYC by FISH (36).
In all cases the tumor cells were CD20-positive and CD3-negative. CD10 was positive in 4 cases (24%), BCL-6 was positive in 14 cases (87%), BCL-2 was positive in 10 cases (63%), MUM-1 was positive in 7 cases (44%) and TCL-1 was positive in 5 cases (31%). Using the immunophenotypic classification of Hans et al (13), GCB was defined as all cases expressing CD10 and those cases that were CD10 negative, BCL-6 positive and MUM-1 negative (Figures 1 and 2). All cases that were CD10 negative and MUM1 positive irrespective of BCL-6 were considered to be of non-germinal center-like subtype (non-GCB). According to these criteria, we found 10 DLBCL of GCB (63%) and 6 cases of non-GCB (37%) (Table 3). There was no significant difference in sex distribution and in the mean age between the GCB (13.8 years) and the non-GCB (14.1 years) groups of patients, χ2 =0.830, p=0.362.
Figure 1.
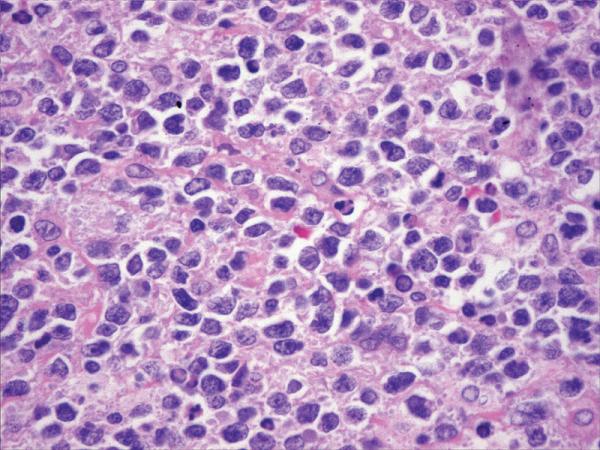
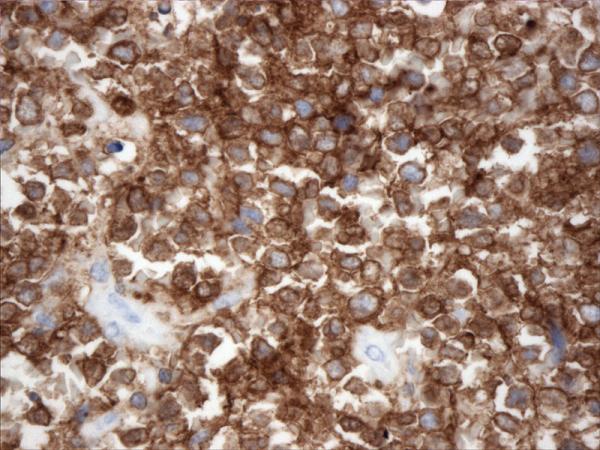
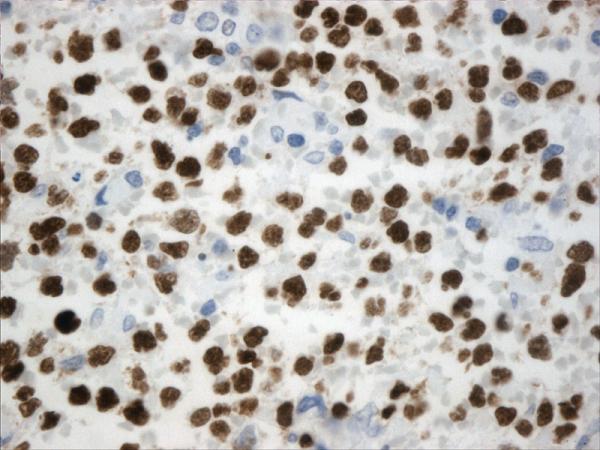
Histopathology and immunohistochemistry findings of GCB of DLBCL. A. Hematoxilin-eosin 400X, B. CD10 positive (400X), C. BCL-6 positive (400X), D. TCL1 positive (400X), BCL-2 positive (400X),
Figure 2.
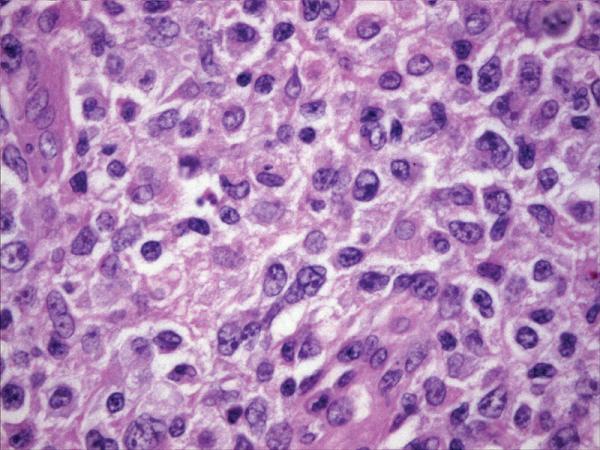
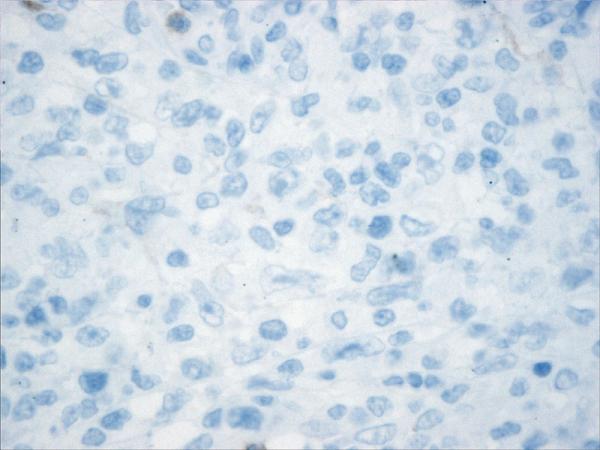
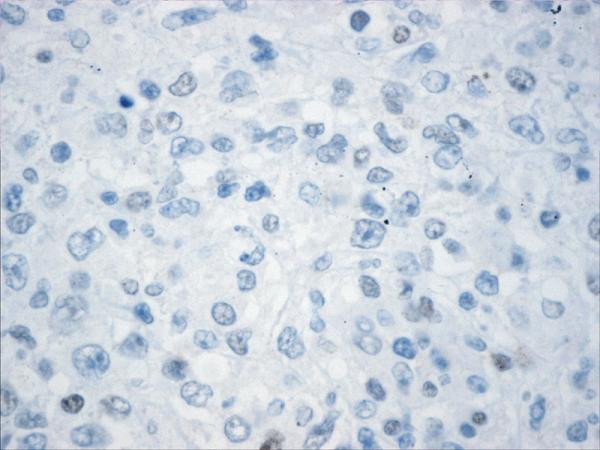
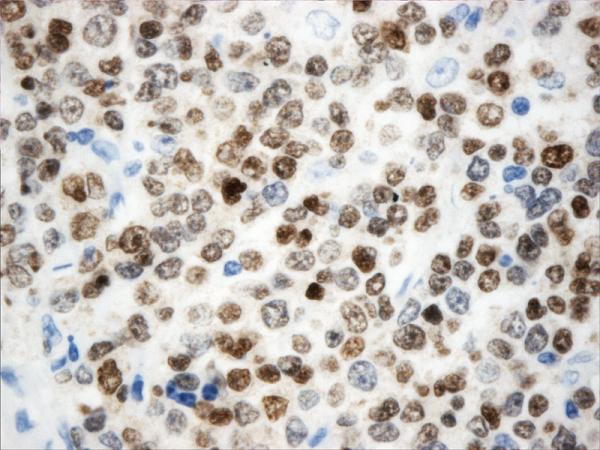
Histopathology and immunohistochemistry findings of non-GCB of DLBCL. A. Hematoxylin-eosin 400X, B.CD10 negative (400X). C. BCL-6 negative (400X), D. MUM-1 positive (400X),
Table 3.
Immunophenotype and FISH C-MYC analysis in relation to the subtypes (GCB or non-GCB) in 16 cases of pediatric DLBCL.
| GCB | Non-GCB | All cases + | p | |
|---|---|---|---|---|
| CD10 | 4 | 0 | 4 | 0.002 |
| BCL-6 | 9 | 5 | 14 | NS |
| MUM-1 | 1 | 6 | 7 | 0.003 |
| BCL-2 | 5 | 5 | 10 | NS |
| FISH C-MYC | 5* | 1 | 6 | NS |
| 10 | 6 | 16 | ||
one had an inconclusive result; GC- Germinal center; NS: not significant
The centroblastic type was the most common morphologic variant found in both groups. MUM-1 was co-expressed with CD10 and BCL-6 in only one case in the GCB group (10%). All cases of the non-GC-type group expressed MUM-1.
The mean Ki-67-proliferation index was 71%, with a range of 30-90%. The mean proliferation index was 72% in the GCB and was 65% in the non-GCB, without statistical significant difference. BCL-2 was expressed in 10 cases, five of 10 cases of GCB (50%) and 5 of 6 cases of the non-GCB (83%), without significant association. TCL-1 expression was observed in five cases, (31%), including four from the GCB and one from the non-GCB. FISH analysis showed six cases (37%) with a detectable c-MYC translocation, including five in the GCB group, and one in the non-GCB (Table 3). There was a statistical tendency to associate c-MYC with GC but the number of cases was too small to confirm this correlation. One case had an inconclusive result for c-MYC translocation by FISH. It is interesting to note that one of the two cases of TCRBCL showed c-MYC translocation.
BCL2 translocation was only present in the case of unclassified type of lymphoma with features intermediate between DLBCL and BL. As this case also showed c-MYC translocation it would be considered a double-hit lymphoma.
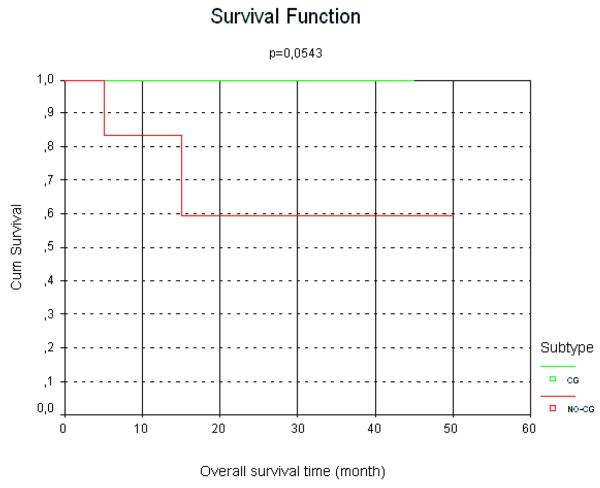
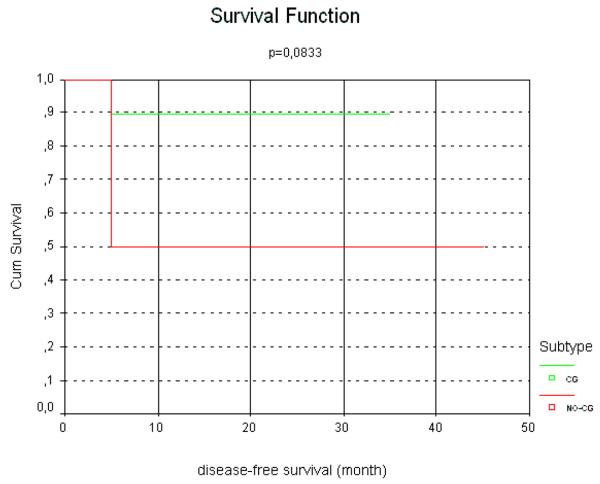
TCL-1 positive cases presented with c-MYC translocation in 80%, resulting in a direct and significant association between TCL1 expression and the presence of c-MYC translocation (2. 2,813 p=0.009). The mean proliferation index of these cases was higher in comparison to the TCL-1-negative cases (mean values of 85 vs 61% respectively) a finding that was also statistically significant (2. 3.366, p=0.067). Sixty-six percent of the c-MYC positive cases presented with TCL-1 co-expression. All cases were negative for EBV using in situ hybridization for EBV-EBER (including the TCL-1 positive cases). In respect to the clinical follow-up, all GCB cases were alive, including nine without evidence of disease and only one with persistent disease, with a median follow-up of 26 months. Patients with GCB lymphoma had a significantly better survival rate and longer interval free of disease, as shown in figures 3 and 4. Two patients of the non-GCB cases died of disease, one patient had persistent disease at last follow-up (he is currently on second-line therapy) and three were alive without disease at last follow-up. One of the patients who died achieved a complete remission with first-line chemotherapy but presented with an early relapse 10 months after finishing the chemotherapy regime. In this patient the second line protocol including rituximab was introduced without any response. The other non-GCB patient died in the second cycle of a CHOP protocol due to disease progression (Table 2). Although CHOP is not widely used in the treatment of pediatric lymphomas overall, in some parts of Brazil CHOP is used in some cases of DLBCL in older children and adolescents and sometimes in younger patient as well.
Figure 3.
Kaplan-Meier plot of overall survival of pediatric DLBCL according to germinal-center or non-germinal center immunophenotypes
Figure 4.
Kaplan-Meier plot of survival free of disease of pediatric DLBCL according to germinal-center or non-germinal center immunophenotypes.
When cases were compared by clinical stage, the differences in survival and interval free of disease between stages I/II versus III/IV did not reach statistically significant levels. For patients with stage III and IV there was no significant difference in outcome between GCB and non-GCB immunophenotype.
In cases with bone marrow (BM) involvement there was diffuse infiltration of large cells in solid sheets in the two centroblastic-morphologic-type cases and few large lymphoid neoplastic cells in the case of T-cell rich B-cell lymphoma variant. All three patients with BM involvement belonged to the GCB group, and are alive without any evidence of disease. Two patients had central nervous system involvement, one from each phenotypic group; the one of GCB-type is alive with disease, while the patient with a non-GCB-phenotype is dead.
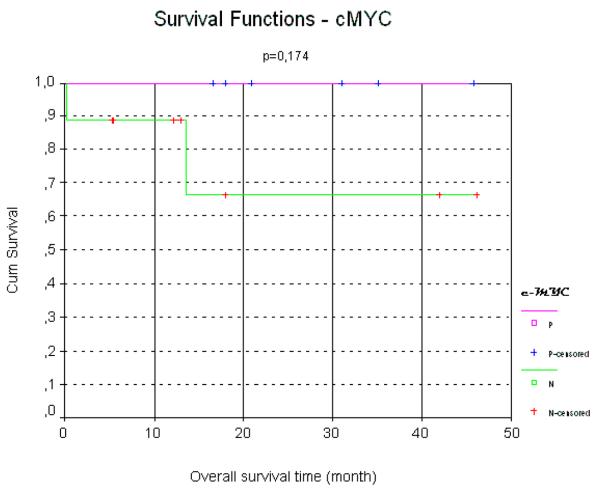
Among the MUM-1 positive cases, 57% were alive without disease, as compared to 89% in the MUM-1 negative group; the difference in overall survival was significantly better for MUM1 negative cases. In contrast, 100% of TCL1-positive cases were alive without disease as compared to 64% in the TCL1-negative group. The presence of a c-MYC positive result by FISH showed a favorable impact on survival (Figure 5).
Figure 5.
Presence of c-MYC and correlation with a favorable impact in survival in pediatric DLBCL.
DISCUSSION
DLBCL in the pediatric group is a rare neoplasm and usually has an extranodal presentation (31). DLBCL constitutes about 10 to 20% of juvenile and pediatric NHL (7, 14, 23, 33). There are few reports in the literature dealing exclusively with nodal DLBCL in this age group (31). This is especially true of studies in which the cases of pediatric DLBCL are divided in GCB and non-GCB types (31).
Hans et al. (13) demonstrated that the expression of CD10, BCL-6, MUM1, BCL-2, and cyclin D2 are each predictive of survival in DLBCL, and that the results for CD10, BCL-6, and MUM1 can be combined to divide DLBCL into GCB and non-GCB subgroups, with an outcome similar to that predicted by cDNA microarray analysis.
CD10 expression in DLBCL varies from 39 to 100% in different series (7, 10, 14, 31). We found a lower frequency of CD10 expression with 24% of our cases being positive for this marker. However it must be kept in mind that in these series, that used the same anti-CD10 antibody, most of the cases were extranodal without enough data to determine the frequency of expression in nodal cases alone (25, 31). BCL-6 expression was observed in 75 to 90% of cases in the literature (7, 10, 25, 31); in agreement, we detected BCL-6 expression in 87% of our cases.
Our cases showed a higher frequency of BCL-2 (63%) expression than previously reported series, 63% vs 29-40%, (7, 10, 14, 25, 31). It has been reported that BCL-2 expression in pediatric patients does not indicate a poor prognosis (31), in agreement with what we found in our series. It has also been established in adult patients that the adverse outcome associated with BCL-2 expression is observed only in non-GCB lymphomas (17). In our series all the patients who had BCL-2 expression and died of disease belonged to the non-GCB type.
The GCB type is more prevalent in our nodal pediatric cases than the non-GCB, but the difference is less evident than in the series of mixed nodal and extranodal pediatric DLBCL (25, 31).
Most of the reported cases of DLBCL have a Ki-67 proliferation index greater than 40% (14, 25, 31). In our cases the mean values of Ki-67-proliferation index was 71%, with a range of 30 to 90%.
According to the literature, c-MYC translocation has been found in 35% of nodal pediatric DLBCL, a higher frequency than that reported in nodal DLBCL occurring in adult patients (5%) (14, 25). We found 37.5% of our pediatric cases to have a translocation involving c-MYC: without a significant negative impact on outcome. Although our results show a tendency for the association between presence of c-MYC with GCB, it would be necessary to study a larger number of cases to confirm it.
Cytogenetic studies reporting abnormalities in T-cell histiocyte-rich B-cell lymphoma are rare (21). To the best of our knowledge, no other similar case of a T-cell/histocyte-rich B-cell lymphoma with a c-MYC translocation has been previously reported in pediatric patients.
In adults c-MYC protein expression in DLBCL has been associated with more aggressive disease, but the protein can be induced by mechanisms other than cytogenetically identifiable translocations (25). Also a high proliferation index in DLBL in adults is related to a worse prognosis (35). It is interesting to note that in a series of pediatric DLBCL in which most of the cases presented with a moderate to high proliferation index the cases showed excellent prognosis (25). Miles et al also found significant differences in survival between the GCB and non-GCB phenotypes of DLBCL, but these differences disappeared after adjustment for risk group (25).
TCL-1-positive lymphomas were initially considered to be related to EBV infection (18), believing that the virus stimulates TCL-1 expression, such as in EBV-positive cases of BL. In our series, there were 5 of 16 cases (31%) of pediatric DLBCL with TCL1 protein expression and all cases were EBV negative. The significance of TCL1 expression should be clarified in a larger series but it has been proposed as a potential therapeutic target based in experimental studies (1). It has been reported that TCL-1 positive cases show a higher proliferation index (15, 34). In our series, the TCL-1 positive cases had a mean proliferation index of 85%, significantly higher than TCL-1 negative cases (mean value 61%), although the significance of this difference should be evaluated and confirmed in a larger number of pediatric TCL1-positive DLBCL cases. Similarly, the high frequency of c-MYC translocation in TCL-1 positive cases may suggest a more aggressive type of lymphoma, but this was not confirmed, since all of our TCL1-positive cases had a good clinical outcome, in contrast to other studies (34). DLBCL occurring in pediatric patients usually has a good prognosis, with 90% of patients surviving more than two years and 72% surviving more than 5 years (6, 22, 26, 36). We demonstrated a significantly difference in survival between pediatric DLBCL of GCB and non-GCB types (Kaplan-Meier survival analysis, P < 0.05, log rank test).
In conclusion, in our series of pediatric DLBCL the GCB was the most common phenotype and had a better outcome, which was independent of the presence of c-MYC translocation or TCL1 expression. When the expression pattern of BCL-2, TCL-1 and MUM-1 is combined with the results of c-MYC translocation, the cases appeared to cluster into groups. One group had detectable c-MYC translocation and was mostly TCL1 positive; almost all of these cases belonged to the GCB phenotype and had a good outcome. There was a second group with the worst prognosis, showing expression of MUM1 and BCL-2, corresponding to the non-GCB phenotype. These findings could reflect a spectrum, with one group of cases more related to Burkitt lymphomas and another more related to adult DLBCL, showing that pediatric DLBCL are not as homogeneous as previously suggested (31). BCL-2 expression alone does not correlate with survival, even when only non-GCB cases are considered, in contrast to results reported in adult patients (30). TCL-1 expression in pediatric and adolescent DLBCL may be a good prognostic indicator, however this finding needs further confirmation.
Acknowledgments
The authors would like to thank Drs. Jozina Maria A. Agareno, Romana A. Alves Barbosa, João Paulo Biguetti, Mauricio B. Nunes, Teresa Cristina Cardoso Fonseca, Guilherme Coelho, Maria Cecilia Ferro, Marina Kishima, Isis Magalhães, Flávia Nogueira S. de Araujo, Ana Flávia C. de Oliveira, Silvia Saueressig and Daniel O. Sezerino for providing the clinical information. We also acknowledge the outstanding service of Consultoria em Patologia staff for skillful technical assistance. This study was partially supported by NIH R01CA1219-09 (WHJr).
Footnotes
Disclosure/Conflict of Interest
No conflict of interest to declare.
REFERENCES
- 1.Aggarwal M, Villuendas R, Gomez G, et al. TCL1A expression delineates biological and clinical variability in B-cell lymphoma. Mod Pathol. 2009;22:206–215. doi: 10.1038/modpathol.2008.148. [DOI] [PubMed] [Google Scholar]
- 2.Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt's, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510–518. doi: 10.1200/JCO.2000.18.3.510. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 4.Bacchi MM, Bacchi CE, Alvarenga M, Miranda R, Chen YY, Weiss LM. Burkitt's lymphoma in Brazil: strong association with Epstein-Barr virus. Mod Pathol. 1996;9:63–67. [PubMed] [Google Scholar]
- 5.Burkhardt B, Zimmermann M, Oschlies I, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39–49. doi: 10.1111/j.1365-2141.2005.05735.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, McClintock S, Cleveland RP, et al. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol. 2004;28:464–470. doi: 10.1097/00000478-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Chuang SS, Ye H, Du MQ, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558–564. doi: 10.1309/EQJR3D3V0CCQGP04. [DOI] [PubMed] [Google Scholar]
- 8.Dave SS, Fu K, Wright GW, et al. Lymphoma/Leukemia Molecular Profiling Project. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 9.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084–2092. [PubMed] [Google Scholar]
- 10.Frost M, Newell J, Lones MA, Tripp SR, Cairo MS, Perkins SL. Comparative immunohistochemical analysis of pediatric Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Clin Pathol. 2004;121:384–392. doi: 10.1309/8WYN-VUTG-V9RP-HUQH. [DOI] [PubMed] [Google Scholar]
- 11.Gaidano G, Carbone A. MUM1: a step ahead toward the understanding of lymphoma histogenesis. Leukemia. 2000;14:563–566. doi: 10.1038/sj.leu.2401748. [DOI] [PubMed] [Google Scholar]
- 12.Gualco G, Queiroga EM, Weiss LM, Klumb CE, Harrington WJ, Jr, Bacchi CE. Frequent expression of multiple myeloma 1/interferon regulatory factor 4 in Burkitt lymphoma. Hum Pathol. 2009;40:565–571. doi: 10.1016/j.humpath.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 14.Heerema NA, Bernheim A, Lim MS, et al. State of the art and future needs in cytogenetic/molecular genetics/arrays in childhood lymphoma: summary report of workshop at the First International Symposium on childhood and adolescent non-Hodgkin lymphoma, April 9, 2003, New York City, NY. Pediatr Blood Cancer. 2005;45:616–622. doi: 10.1002/pbc.20552. [DOI] [PubMed] [Google Scholar]
- 15.Herling M, Patel KA, Hsi ED, et al. TCL1 in B-cell tumors retains its normal B-cell pattern of regulation and is a marker of differentiation stage. Am J Surg Pathol. 2007;31:1123–1129. doi: 10.1097/PAS.0b013e31802e2201. [DOI] [PubMed] [Google Scholar]
- 16.Hoyer KK, French SW, Turner DE, et al. Dysregulated TCL1 promotes multiple classes of mature B cell lymphoma. Proc Natl Acad Sci U S A. 2002;99:14392–14397. doi: 10.1073/pnas.212410199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal J, Neppalli V, Wright G, et al. BCL-2 expression is a prognostic marker for the activated B-cell-like type of diffuse large-B-cell lymphomas. J Clin Oncol. 2006;24:961–968. doi: 10.1200/JCO.2005.03.4264. [DOI] [PubMed] [Google Scholar]
- 18.Kiss C, Nishikawa J, Takada K. Tcell leukemia I oncogene expression depends on the presence of Epstein Barr virus in the virus carrying-Burkitt lymphoma lines. PNAS. 2003;100:4813–4818. doi: 10.1073/pnas.0730710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lida S, Rao P, Butler M, et al. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat Genet. 1997;17:226–230. doi: 10.1038/ng1097-226. [DOI] [PubMed] [Google Scholar]
- 20.Liu YH, Xu FP, Zhuang HG, et al. Clinicopathologic significance of immunophenotypic profiles related to germinal center and activation B-cell differentiation in diffuse large B-cell lymphoma from Chinese patients. Hum Pathol. 2008;39:875–884. doi: 10.1016/j.humpath.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Lones MA, Cairo MS, Perkins SL. T-cell-rich large B-cell lymphoma in children and adolescents: a clinicopathologic report of six cases from the Children's Cancer Group Study CCG-5961. Cancer. 2000;88:2378–2386. doi: 10.1002/(sici)1097-0142(20000515)88:10<2378::aid-cncr24>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Lones MA, Perkins SL, Sposto R, et al. Large-cell lymphoma arising in the mediastinum in children and adolescents is associated with an excellent outcome: a Children's Cancer Group report. J Clin Oncol. 2000;18:3845–3853. doi: 10.1200/JCO.2000.18.22.3845. [DOI] [PubMed] [Google Scholar]
- 23.Lones MA, Raphael M, Perkins SL, et al. Mature B-cell lymphoma in children and adolescents: International group pathologist consensus correlates with histology technical quality. J Pediatr Hematol Oncol. 2006;28:568–574. doi: 10.1097/01.mph.0000212980.67114.a5. [DOI] [PubMed] [Google Scholar]
- 24.McClure RF, Remstein ED, Macon WR, et al. Adult B-cell lymphomas with Burkitt-like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol. 2005;29:1652–1660. doi: 10.1097/01.pas.0000180442.87022.08. [DOI] [PubMed] [Google Scholar]
- 25.Miles RR, Raphael M, McCarthy K, et al. Pediatric diffuse large B-cell lymphoma demonstrates a high proliferation index, frequent c-Myc protein expression, and a high incidence of germinal center subtype: Report of the French-American-British (FAB) international study group. Pediatr Blood Cancer. 2008;51:369–374. doi: 10.1002/pbc.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora J, Filippa DA, Thaler HT, Polyak T, Cranor ML, Wollner N. Large cell non-Hodgkin lymphoma of childhood: Analysis of 78 consecutive patients enrolled in 2 consecutive protocols at the Memorial Sloan-Kettering Cancer Center. Cancer. 2000;88:186–197. doi: 10.1002/(sici)1097-0142(20000101)88:1<186::aid-cncr26>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama I, Murao S, Kitazawa S, Azumi A, Yamamoto M, Maeda S. Activation of the TCL1 protein in B cell lymphomas. Pathol Int. 2000;50:191–199. doi: 10.1046/j.1440-1827.2000.01023.x. [DOI] [PubMed] [Google Scholar]
- 28.Narducci MG, Pescarmona E, Lazzeri C, et al. Regulation of TCL1 expression in B- and T-cell lymphomas and reactive lymphoid tissues. Cancer Res. 2000;60:2095–2100. [PubMed] [Google Scholar]
- 29.Nguyen H, Hiscott J, Pitha P. The growing family of interferon regulatory factors. Cytok Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 30.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppä S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009 May 15; doi: 10.1038/modpathol.2009.73. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Oschlies I, Klapper W, Zimmermann M, Krams M, Wacker HH, Burkhardt B, Harder L, Siebert R, Reiter A, Parwaresch R. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) Multicenter Trial. Blood. 2006;107:4047–4052. doi: 10.1182/blood-2005-10-4213. [DOI] [PubMed] [Google Scholar]
- 32.Raetz E, Perkins S, Davenport V, Cairo MS. B large-cell lymphoma in children and adolescents. Cancer Treat Rev. 2003;29:91–98. doi: 10.1016/s0305-7372(02)00108-1. [DOI] [PubMed] [Google Scholar]
- 33.Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br J Haematol. 2008;142:329–347. doi: 10.1111/j.1365-2141.2008.06988.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodig SJ, Vergilio JA, Shahsafaei A, Dorfman DM. Characteristic expression patterns of TCL1, CD38, and CD44 identify aggressive lymphomas harboring a MYC translocation. Am J Surg Pathol. 2008;32:113–122. doi: 10.1097/PAS.0b013e3180959e09. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez E, Chacon I, Plaza MM, et al. Clinical outcome in diffuse large B-cell lymphoma is dependent on the relationship between different cell-cycle regulator proteins. J Clin Oncol. 1998;16(5):1931–9. doi: 10.1200/JCO.1998.16.5.1931. [DOI] [PubMed] [Google Scholar]
- 36.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2008. World Health Organization Classification of Tumours. [Google Scholar]
- 38.Tsuboi K, Iida S, Inagaki H, et al. MUM1/IRF4 expression as a frequent event in mature lymphoid malignancies. Leukemia. 2000;14:449–456. doi: 10.1038/sj.leu.2401696. [DOI] [PubMed] [Google Scholar]