Abstract
Innate and adaptive immunity affect the pathogenesis of Parkinson's disease (PD). In particular, activation of microglia influences degeneration of dopaminergic neurons. Cell-to-cell interactions and immune regulation critical for neuronal homeostasis also influence immune responses. The links between T cell immunity and nigrostriatal degeneration are supported by laboratory, animal model, and human pathologic investigations. Immune-associated biomarkers in spinal fluids and brain tissue of patients with idiopathic or familial forms of PD provide means to improve diagnosis and therapeutic monitoring. Relationships between oxidative stress, inflammation, and immune-mediated cell death pathways are examined in this review as they are linked to PD pathogenesis. Harnessing the immune system by drugs or by vaccination remain promising future therapeutic options. Antioxid. Redox Signal. 11, 2151–2166.
Introduction
Parkinson's disease (PD) is second only to Alzheimer's disease as the most prevalent neurodegenerative disease. Its onset and progression is affected by host genetic factors, environmental cues, age, and the engagement of host innate and adaptive immune responses (62, 69, 161). How the immune system affects the onset and progression of PD and what processes serve to control onset and progression of movement dysfunctions has only recently been investigated.
Inflammation is a self-defensive reaction against pathogenic stimuli or injury, that is, under normal conditions, reparative to the host process. A well-controlled immune response to infection, environmental toxins, or injury is helpful as it protects the host by clearance of debris or pathogens, and promotes healing. However, when chronically sustained and dysregulated, inflammation can lead to significant tissue and cellular damage (116). Such events are regulated, in large measure, through the innate immune system comprising cellular elements that include mononuclear phagocytes (MP) [monocytes, microglia, macrophages and dendritic cells]; natural killer (NK) cells; and neutrophils; as well as regulatory humoral elements such as complement, cytokines, and a host of other secretory factors that control host surveillance and homeostasis in a nonspecific manner (97). Apropos the innate immune system, microglia are the resident phagocytic cells of the central nervous system (CNS), constituting 20% of the total glial population, and as such represent the first line of immune defense in the brain. Microglia are distributed throughout the brain and are highly mobile, capable of clearing damaged neurons, plaques, and infectious agents. When microbial pathogens cross the blood-brain barrier (BBB), microglia react to destroy the infectious agents before inflicting host tissue damage. As antibodies are normally too large to cross the BBB, microglia serve to recognize foreign factors, phagocytocize and destroy them through phagolysosomal fusion mechanisms, as well as acting as immune effectors and antigen presenting cells. Although necessary for foreign cell surveillance, the microglia, once activated, may be directly involved in neurotoxicity that is linked to uncontrolled inflammatory responses. In an inflammatory state many aspects of the “immune privileged” state of the brain break down and disease can quickly ensue. Indeed, an activated microglial response is strongly associated with dopaminergic cell loss in PD and neuronal dysfunction in degenerative diseases of the CNS (27, 66, 93, 132, 154, 162).
On the other hand, the adaptive immune system is highly specialized; is comprised of cells with specific immunologic effector, regulatory, and memory capabilities (T lymphocytes and B lymphocytes) that specifically eliminate or prevent pathogenic insults; but is activated by the “nonspecific” innate immune system. The CNS has traditionally been considered “immune privileged” and protected through the BBB, which prevents toxins and infections from reaching the CNS. We now know that both the innate and cell-mediated immune processes are highly active in PD (11, 107).
Innate Immunity
Overview
Innate immunity consists of the immune mechanisms that are encoded in the germ line possessed at birth, and work in a “nonspecific” manner, for immediate defense, against microbial infection. These mechanisms include removal of foreign substances by phagocytes, recruitment of additional immune cells to the site of infection through cytokine and chemokine production, activation of the complement cascade, and processing and presentation of antigens for activation of the adaptive immune response. The innate immune system functions through the nonspecific, generic recognition of common cell signaling pathways shared through a host of endogenous and exogenous threats called pathogen-associated molecular patterns (PAMPs). These are recognized by toll-like receptors (TLRs), which are expressed by microglia, astrocytes, oligodendrocytes, and neurons (16, 17, 36, 51, 85, 107). Engagement of TLRs contributes to neuroinflammation by activating signaling cascades that result in pro-inflammatory cytokine and chemokine production, as well as affecting BBB permeability.
Microglia
Microglia actively monitor the CNS environment by continual movement of their fine processes in the healthy brain (106), key in immune surveillance functions. In the brain cortex, microglial processes and protrusions directly contact astrocytes, neuronal cells bodies, and blood vessels, suggesting close communication (106) that allows microglia to react to brain insults quickly. With a multitude of TLRs, cytokine and chemokine receptors, and ion channels, microglia are sensitive to changes in their extracellular environment from a wide variety of stimuli that range from pathogens to aggregated proteins to alterations in ion homeostasis. Detection of disturbances within the neuron microenvironment induces microglia to become activated and affect a graded response (84). Upon activation, microglia proliferate, undergo morphogenesis, and increase cell volume with extension of their processes. These morphological changes are in stark contrast to resting microglia, which have a small cell body and ramified processes (162).
Among the many innate immune functions, activated microglia are well adapted for the induction of inflammation, cytokine-mediated and antibody-dependent cell cytotoxicity (ADCC), and regulation of T cell responses through antigen presentation (3). In the alerted state, microglia demonstrate increased IgG reactivity, and upregulation of complement receptors and cell adhesion molecules such as lymphocyte function associated antigen (LFA)-1, intercellular adhesion molecule (ICAM)-1 cluster of differentiation (CD)54, vascular cell adhesion molecule (VCAM)-1 (CD106), and CD1 (84). Indeed, increased expression of ICAM-1 and its counter receptor LFA-1 were identified and revealed aggregates of reactive microglia along with infiltrating LFA-1+ leukocytes in postmortem PD brains, as well as monkeys that received MPTP-injections indicative of a sustained inflammatory process in PD (98).
Activated microglia are known to produce a variety of toxic substances that, in addition to killing infectious agents, can accelerate neuronal injury and death. These toxic substances include reactive oxygen species (ROS), reactive nitrogen species (RNS), proinflammatory cytokines, and prostaglandins. Several molecules upregulate microglial secretory responses including lipopolysaccharide (LPS), interferon gamma (IFN-γ), amyloid beta (β-amyloid), CD40 ligand (CD40L), chemokines, neurotransmitters, gangliosides, and proteases such as thrombin, tissue plasminogen activator (tPA), and matrix metalloproteinase-3 (MMP-3) (1, 4, 79, 102, 135, 139, 149). Large numbers of human leukocyte antigen (HLA)-DR-positive reactive microglia are found in the substantia nigra (SN) of patients with PD and parkinsonism with dementia. Additionally, activated microglia are present in the SN and/or striatum of animals used in models of PD such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-, 6-hydroxydopamine (6-OHDA)-, and medial forebrain bundle axotomy-induced parkinsonism (15, 117, 131, 132). Uncontrolled microglial activation is toxic to neurons, due in part to release of pro-inflammatory factors including, but not limited to interleukin one beta (IL-1β), tumor necrosis factor alpha (TNF-α), IL-6, nitric oxide (NO), prostaglandin E2 (PGE2), and superoxide radical. The SN contains the highest concentration of microglia in the brain, especially in the ventral tier of the pars compacta (78, 88), making this region especially susceptible to altered microglial activation responses. Abnormally high levels of IL-1β and TNF-α found in the plasma and cerebral spinal fluid in patients with PD supports this contention (13, 56). Reactive microglia are highly localized, found close to cell bodies of dead or injured dopaminergic neurons in the SN and not to their degenerating termini (99), suggesting a retrograde mechanism for neuronal death. Cell death can also result from loss of trophic support stemming from microglia-induced neuritic beading or synaptic stripping along dendrites (125, 138). These findings suggest a direct link between dopaminergic neuronal death in PD and microglial activation. Indeed, postmortem examinations demonstrate that neuronal degeneration in PD is associated with a substantive gliosis linked to activated microglia, and has also been shown in MPTP-induced parkinsonism in primates, rodents, and humans (27, 86, 94, 96, 144). Moreover, activated microglia have been shown phagocytosing dying dopaminergic cells correlated with α-synuclein (α-syn) deposition in neuronal inclusions (49). Importantly, in vivo and in vitro studies demonstrated that microglia become activated in response to overexpression of α–syn or nitrated and aggregated forms of α-syn, a major component of Lewy bodies found in the brains of PD patients (119, 130).
Complement
Complement activation, a biochemical cascade that serves to facilitate pathogen clearance can also affect neural function. Although typically initiated through antibody deposition (classical pathway), the complement system is also considered an arm of the innate immune response. Increased mRNA levels of complement components are found in PD-affected brain regions (95) and complement components, including all constituents of the membrane attack complex (MAC) (164, 165), have been found intracellularly on Lewy bodies and on oligodendroglia in the SN in sporadic PD. IgG present in PD patients and recombinant human C5a synergistically induced dopaminergic neurodegeneration in rat mesencephalic neuron-glia cultures, while either alone was minimally toxic. IgG from unaffected individuals did not affect dopaminergic neurotoxicity (160). Such toxicity is mediated by microglia (160). Activated complement cascades promote inflammation and opsonization, facilitating phagocytosis. Complement may also be lytic to cells by inserting itself into viable cell membranes causing cytosolic leakage resulting in cell death. Cell membrane leakage could possibly increase the release of aggregated α-syn and other inflammatory mediators into the extracellular milieu to engage adjacent glial cells and propagate the inflammatory response.
Oxidative Stress, Inflammation, and Nigrostriatal Degeneration
Although the etiological event for idiopathic PD remains enigmatic, positive risk factors suggest the involvement of a multitude of factors including genetics, environmental exposure to toxins, and aging. Collectively, these influence the tempo and progression of disease (105). The single greatest risk factor currently identified is age, which suggests cumulative CNS damage for disease pathogenesis. However, considerable variation among degenerative lesions in the SN of PD patients compared to aged non-PD patients, suggests that aging and the disease processes underlying PD may occur independently (141). A significant body of evidence implicates ROS and RNS as possible initiating factors. The brain tissue itself is especially sensitive to oxidative damage, as this tissue accounts for 20% of the total oxygen demand of the body and is rich in peroxidizable fatty acids (20:4 and 22:6), while within the SN and basal ganglia, antioxidant defenses (i.e., catalase, superoxide dismutase, glutathione, and glutathione peroxidase) are the most sparse (43); and the microglia, a primary source for reactive oxygen species which contribute to neuronal degeneration, are the greatest in number. Figure 1 illustrates the consequences of microglial activation and oxidative stress on neuronal physiology in PD. In part due to dopamine metabolism by endogenous enzymes such as monoamine oxidases (MAO) or by autooxidation that can yield H2O2 and dopamine-quinones, the neurons in the SN are especially vulnerable to oxidative stress (57, 58, 129, 133). Furthermore, the presence of transition metals, such as iron, has been shown to accelerate the auto-oxidation of dopamine (133). Thus, the breakdown products of dopamine exacerbate inflammation and tissue damage by feeding H2O2 into the ROS cycle and/or by dopamine-quinone modification of protein sulfhydryl groups via nucleophilic additions. Subsequently, overproduction of free radicals such as superoxide and peroxynitrite feed into the ROS cycle to create an imbalance in the oxidation/reduction capacity of cells. Increased free radicals, without adequate antioxidant buffers in the SN, then react with proteins and nucleic acids to alter their functions, induce lipid peroxidation, or inhibit enzymes of the electron transport chain, eventually contributing to neuronal injury and death.
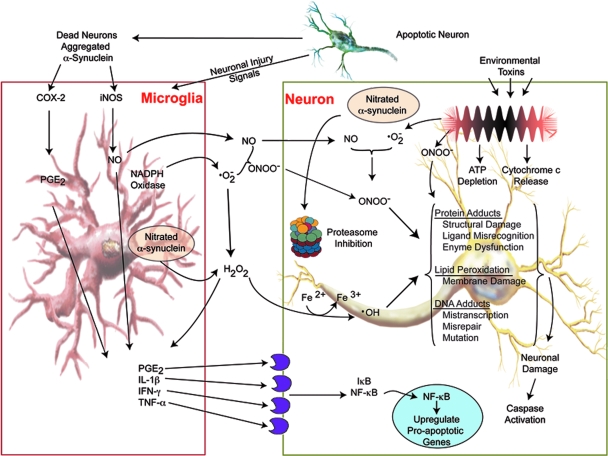
FIG. 1.
Oxidative Stress and PD pathobiology. Free radicals can arise as a result of glial cell activation, mitochondrial dysfunction, or protein aggregation. Increased microglial activation is attributable to increased neuronal cell death and cell debris including aggregated proteins. Microglial-derived NO and superoxide (•O−2) species react in extracellular spaces to form peroxynitrite (ONOO−). Peroxynitrite readily crosses cell membranes where it contributes to lipid peroxidation, DNA damage, and nitrotyrosine formation in α-syn and other cellular proteins. Damaged proteins are targeted to cellular proteosomes for degradation via the ubiquitin pathway. Excessive protein damage caused by oxidants and disruptions in the ubiquitin pathways may overload or inhibit protein degradation quality control measures leading to the accumulation of damaged proteins in cells. When reactive species exceed antioxidant defenses, oxidative stress is generated, destroying molecular structures, such as proteins, lipids, and DNA, causing irreversible and detrimental damage, neuronal cell injury and death. Adapted from Gao et al. (47). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Activation of mitochondrial-dependent programmed cell death pathways are found in postmortem PD brains and in rodent models of PD (113, 114, 156). The mitochondrial apoptotic pathway involves mitochondrial outer membrane permeabilization leading to the release of cytochrome c, apoptosis-induced factor (AIF), endonuclease G, second mitochondria-derived activator of caspases (Smac), and high temperature requirement protein A2 (HTRA2)(151). Inhibition of complex I of the electron transport chain (ETC), for instance with MPTP, results in a time-dependent and region specific increase in the soluble pool of cytochrome c in the mitochondrial intermembrane space that can be released into the cytosol by programmed cell death agonists such as Bax (113). This occurs together with the release of caspase-9 and -3. Bax regulates SN pars compacta (SNpc) dopaminergic cell death associated with these caspases since their release coincides with Bax upregulation and translocation to the mitochondria, and caspase activation is prevented by genetic ablation of Bax (114, 155). Blocking caspase-9 or Apaf-1 also provides some degree of dopaminergic neuroprotection (101, 157). The importance of these events have been confirmed in PD as activation of Bax, caspase-9, and caspase-3 are detected in SNpc dopaminergic neurons of postmortem PD brains (64, 142, 157). Additionally, reduction of complex I activity by 30% was described in idiopathic PD patients (110, 124). Complex I inhibition results in depletion of ATP and the inevitable impairment of all ATP-dependent cellular processes, as well as blocking the flow of electrons along the ETC which increases generation of free radicals that increase oxidative stress. Specifically, complex I inhibition causes oxidative damage by peroxidation of the inner mitochondrial lipid cardiolopin which affects the binding of cytochrome c to the inner mitochondrial membrane, leading to increases in the soluble cytochrome c pool of the mitochondrial intermembrane space. These factors leading to complex I inhibition most probably sensitize neurons to cell death agonists such as Bax.
Oxidative stress also damages mitochondria and proteosomes, while functional loss of the proteosome can also contribute to increased oxidative stress and induce neural apoptosis. Both oxidative stress and proteosome inhibition act in concert to promote protein fibril formation and accumulation of protein aggregates. Indeed, misfolded proteins are found in inclusion bodies associated with several neurodegenerative diseases (40, 63, 74, 121). In PD, the normally soluble, unfolded protein α-syn is found in intraneuronal cytoplasmic inclusions or “Lewy bodies” in aggregated form along with ubiquitin, and lipids. Alpha-synuclein structure contains a central hydrophobic region that contributes to its propensity to aggregate, but oxidative stress induced nitration also contributes to α-syn aggregation and protofibril formation. Dopamine stabilizes α-syn protofibrils by forming a dopamine-α-syn adduct (24). Normally, misfolded proteins are ubiquitinated and degraded by the proteosome, but inhibition of this mechanism by oxidative stress allows greater accumulation of aggregated proteins. Furthermore, oxidative modification of α-syn can lead to self-aggregation and aggregation of other proteins, as well as damage to the ubiquitin–proteosome system. Importantly, aggregated α-syn has been shown to activate microglia leading to enhanced secretion of ROS (118, 145, 167). Activation of microglia by nitrated α-syn (N-α-syn) may also diminish protective mechanisms against oxidative stress by lowering the cellular glutathione buffering capacity as demonstrated by diminished GSH levels, GSH/GSSG ratios, and total glutathione levels from microglia stimulated with N-α-syn (119). The role of proteolytic stress in PD pathology is further supported by the observation that excess levels of parkin substrate proteins are found in a nonubiquitinated state in PD patients with mutations in the parkin gene (encodes an ubiquitin E3 ligase), and these mutations are associated with autosomal recessive juvenile parkinsonism (104). Overexpression of α-syn in models and duplication or triplication in the wild-type gene in PD patients are associated with neurodegeneration and microglial activation, possibly because of the inability of the proteosome to handle the increased number of misfolded proteins. Taken together, these findings illustrate the close relationship between oxidative and proteolytic stress, microglial activation, and inflammation that may contribute to neuronal injury and cell death in PD.
Adaptive Immunity
The adaptive immune system provides the ability to recognize specific pathogens and mount stronger responses with each encounter due to immunological memory. An adaptive immune response ensues when the innate immune system encounters a pathogen, recognized by PAMPs, and links the adaptive immune response through antigen presentation of the foreign protein. Antigen presenting cells (APC) phagocytose or endocytose foreign pathogens, then process and present foreign antigen complexed with surface major histocompatibility complex (MHC) molecules that are recognized by CD4+ (helper) or CD8+ (cytotoxic) T cells. T cells become fully activated when the APC provides appropriate co-stimulatory molecules. Activated CD4+ T cells can in turn recruit other T cells and B cells to sites of inflammation propagating the immune response.
Naïve T cells and B cells are normally precluded from entry into the CNS; however, in a neuroinflammatory state, activated glial cells secrete factors which disrupt the BBB and allow entry of adaptive immune components (11, 42, 69). Increased expression of cellular adhesion molecules, and the induction of chemokine gradients by glia, direct leukocytes to sites of inflammation (7). Indeed, T cell infiltration has been found in CNS tissues of PD patients (98), and adoptively transferred immune splenocytes into MPTP-treated mice results in significant infiltration into the brain and localization within the inflamed SN (11).
T cells and neuropathobiology
Several studies support a role for an adaptive immune response in the etiology of PD. Recent evidence from our laboratory suggests that nitrated α-syn activate peripheral leukocytes in draining lymphoid tissue (11). The aforementioned study demonstrated in the MPTP model the necessity for T cells in dopaminergic neurodegeneration and that dopaminergic neuronal loss was exacerbated by T cells for induced adaptive immune responses towards nitrated but not native α-syn, thus suggesting a causal link between T cell infiltration and sustained microglial activation associated with PD. Figure 2 illustrates a possible mechanism for N-α-syn-mediated adaptive immune responses in potentiating microglial activation and exacerbating neuronal death. Increased numbers of CD8+ T cells are found in close proximity to activated microglia and degenerating neurons within the SN in PD patients (94). The presence of T cell subsets at levels exceeding those typically found in the CNS and in lower ratios found in the periphery suggests a role in PD more profound than that associated with surveillance. In addition, aberrations in peripheral lymphocyte subsets are detectable in PD patients. Total numbers of lymphocytes in PD cohorts have been shown to be diminished by 17%, while CD19+ B cells were diminished by 35% and CD3+ T cells were diminished by 22% (10). Among CD3+ T cells, numbers of CD4+ T cells were diminished by 31% whereas numbers of CD8+ T cells were not significantly changed. A greater loss of naïve helper CD4+ T cells (CD45RA+) and either unchanged or increased effector/memory helper T cell subset (CD29+ or CD45RO+) was observed (10). A selective loss of CD4+CD45RA+ cells was also observed in diseases such as multiple sclerosis and Down's syndrome, suggesting a common immunological abnormality in neurological disorders (26, 41). Increased mutual co-expression of CD4 and CD8 by CD45RO+ T cells, as well as upregulation of CD25 (α-chain of the IL-2 receptor), TNF-α receptors, and significant downregulation of IFN-γ receptors suggested that these T cell subsets from PD patients are activated. In addition to CD4+ and CD8+ T cells that express αβ T cell receptors, another T cell subset exists with pathological relevance to PD. Elevated T cell populations expressing γδ T cell receptors have been described in the CSF of patients with PD (42) and are thought to play a regulatory role in CNS inflammation (115). Moreover, a greater proportion of the γδ T cells were CD25+ in the CSF suggesting a preferential activation of this T cell subset within the CSF compartment in PD patients (42). Increased proportions of memory and activated T cell subsets observed in PD patients relative to controls suggest a causal role in PD etiology.
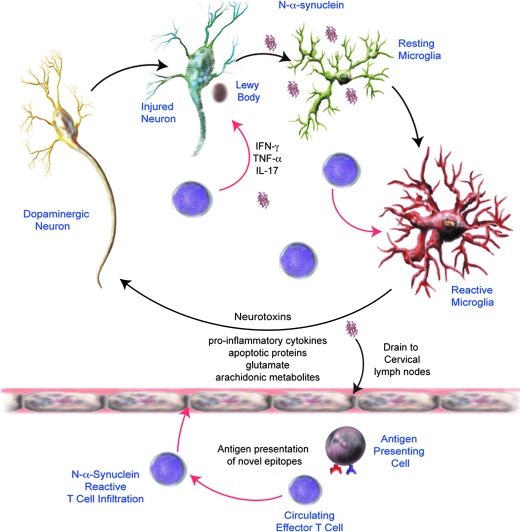
FIG. 2.
Nitrated-α-syn-mediated PD immunopathology. A hallmark feature of PD is the presence of Lewy body neuronal inclusions consisting of nitrated-α-syn (N-α-syn). As the neurons die, the inclusions are released into the extracellular environment where the protein aggregates (most notably N-α-syn) interact with adjacent microglia and initiate an activation cascade. Recent evidence from our own laboratories suggests that N-α-syn in the brain can also drain to cervical lymph nodes where the protein can initiate an adaptive immune response. Antigen presenting cells would present synuclein as a neo-epitope to T cells present in lymphoid tissues. The ongoing inflammatory processes facilitate infiltration of autoreactive T cells into the brain, exacerbating microglial activation and accelerating neuronal death.
Humoral immunity
Humoral immunity may play a role in the initiation or regulation of inflammation in PD. Opsonization of a cell or damaged neuron can more easily target it for phagocytosis and degradation by phagocytic macrophages and can activate the complement system, a major mediator of immune/inflammatory reactions. In both idiopathic and genetic cases of PD, IgG immunolabeled dopaminergic neurons were found associated with an increased number of activated microglia expressing the high affinity IgG receptor, FcγRI and were strongly associated with a more progressive state of neurodegeneration (108). IgG is closely associated with Lewy bodies. Microglia in the SN expressing the FcγRI receptor phagocytose IgG-immunopositive neurons (108). In addition, deletion of the FcγR by genetic ablation protects mice from microglial activation and dopaminergic cell death (67).
Natural regulatory T cells
Following an immune response, once the foreign pathogen/antigen is removed, suppression of the active effector immune response is necessary so that the cytotoxic affects of inflammation do not have a profound effect on self-tissues. Several mechanisms exist by which the immune response can be regulated. Among the various cell types involved, there exists a naturally occurring T cell subtype that functions to prevent immune responses towards self-peptides: the regulatory T cell (Treg). The naturally occurring Tregs are generated in the thymus and constitutively express CD4 and CD25 in addition to the transcription factor FOXP3 (forkhead box p3) that is required for their development, maintenance, and function (44, 72). CD4+CD25+ Tregs play an important role in preventing autoimmune diseases such as type I diabetes, and limiting chronic inflammatory diseases such as asthma and inflammatory bowel disease (25, 123, 128, 163). Tregs are widely recognized to be capable of controlling innate immune reactivity and suppressing both CD4+ and CD8+ effector T cell responses as well as B cell responses to both self and foreign antigens (122). Thus, Tregs have a critical role in immune homeostasis. Tregs exhibit regulatory activity by suppression of immune responses by secretion of anti-inflammatory cytokines such as IL-10 and TGF-β (65, 76), by cytolysis (61), by metabolic disruption (109), and by modulation of dendritic cell maturation or function through CTLA4 ligation (136, 140) (Fig. 3). Effector cells such as B cells or T cells, myeloid and APCs, including microglia, are inactivated or neutralized through the actions of Tregs (20, 134, 146).
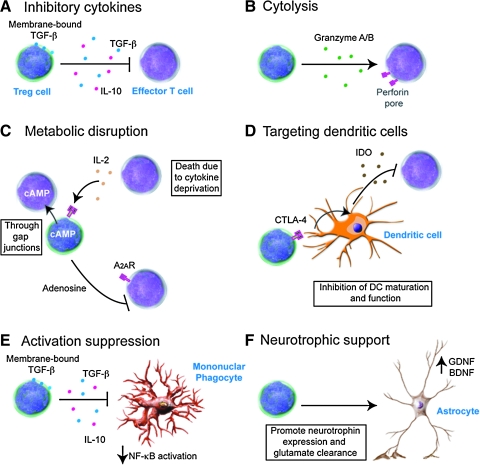
FIG. 3.
Tregs and neuroimmunity. Tregs are proposed to have several mechanisms of action to suppress immune reactivity depending on the target effector cells. Albeit in vitro, Treg-mediated suppression of effector T cell responses is primarily through cell-to-cell contact; several mechanisms of action are postulated for Treg-mediated suppression of effector T cells in vivo. These mechanisms include cytokine-mediated inhibition of activation (A), induction of apoptosis either through a granzyme/perforin-dependant mechanism (B), or through disruption of metabolic function or IL-2 competition (C), or indirectly by inducing tolerance through modulation of dendritic cell activation (D). Recent evidence supports a role for Tregs in modulating mononuclear phagocyte activation through both cytokine dependent and independent mechanisms (E). Moreover, Tregs are proposed to influence astrocytes to promote neurotrophin expression and glutamate clearance (F). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
CTLA4 is a critical negative regulator of T cell responses and is instrumental in maintaining immunological tolerance. This co-receptor has been shown to inhibit T cell activation, IL-2 production, and cell cycle progression (147). One mechanism by which CTLA4 can mediate T cell responses is through the competition with CD28 for CD80/86 binding which leads to decreased activation. Binding of CD28 by CD80/86 in the presence of TCR engagement stimulates proliferation of T cells and production of IL-2 (91). However, in the absence of CD28 co-stimulation, activated T cells become anergic to antigens or become apoptotic (50, 166), but when CTLA4 binds these same ligands, T cell activation is restricted (112, 158). In addition, signaling through the cytoplasmic tail of CTLA4 and modulation of TCR signaling leads to cell cycle disruption and suppression of IL-2 production. Furthermore, dendritic cell function can be conditioned to express indoleamine 2,3-dioxygenase (IDO) through interactions between CTLA4 and CD80 and CD86, resulting in suppression of effector T cells (147, 158). As one might expect, CTLA4 and Foxp3 expression are strongly correlated, yet Foxp3 alone is not sufficient for Treg activity. Treatment of human CD25− T cells with IL-2 in the absence of CTLA4 upregulated Foxp3 expression in cells without suppressive capacity; however CTLA4 transfection into CD25−FoxP3− T cells produced suppressive T cells without Foxp3 (168). These data suggest that CTLA4 is required for suppressive function, but not Foxp3 even though they are both expressed after T cell activation (168). CTLA4 may also be involved in suppression through disruption of CD28 signaling at the immunological synapse and by interfering with lipid raft formation (28, 111). Furthermore, CTLA4 can reduce the contact period between T cells and APCs and lead to decreased proinflammatory cytokine production and T cell proliferation (127).
The mechanism by which Tregs suppress metabolic function in effector cells includes the induction of apoptosis by competition for and deprivation of IL-2, transfer of cAMP into effector T cells through membrane gap junctions, or by activation of the adenosine receptor 2A (A2AR), binding which has also been shown to enhance Treg generation via promotion of TGF-β expression and inhibition of IL-6 expression (82). However, much work is still needed to understand the mechanism(s) responsible for Treg induced suppression of Teffs, as it is possible that there are multiple mechanisms that are context dependent or differentially functional for each Treg subset. In addition, Tregs have also been shown to promote neurotrophic support by inducing astrocytes to increase expression of BDNF and GDNF (12, 118) and may promote glutamate clearance (48). Understanding the Treg mechanism of action is of great importance considering their capacity to control immunity and inflammation, events strongly associated with the pathogenesis of PD.
In addition to natural CD4+CD25+ Tregs, effector T cells can be converted into inducible regulatory T cells capable of controlling peripheral T cell responses, largely based on their potential to produce regulatory cytokines following antigen priming (65). These inducible Tregs include type 1 Treg (Tr1), in which the suppressive function is cell-contact independent and involves IL-10, and Th3 cells that produce primarily TGF-β (23, 120). Induced Tregs may also work by a granzyme B and perforin-dependent manner, as evidenced by human induced Tregs expressing these cytolytic factors (61). These populations do not typically express Foxp3. Other T cell populations with immune-regulatory properties have been described and include IFN-γ producing Th1 cells and IL-4 producing Th2 cells (80). In mice, adaptive Tregs can be induced via the upregulation of Foxp3 in the presence of cytokines, especially TGF-β, however, whether this also true in humans is controversial, but recent data suggests that CTLA4 expression is required (168).
Neuroimmune Pharmacology
Several lines of evidence in both animal studies and clinical trials suggest that manipulation of various aspects of the immune response may provide substantial neuronal protection (12, 23, 32, 118, 154). The possible mechanisms include decreased microglial activation, increased neurotrophic support, inhibition of pro-inflammatory T cell responses, and enhanced clearance of aberrant proteins (Fig. 4).
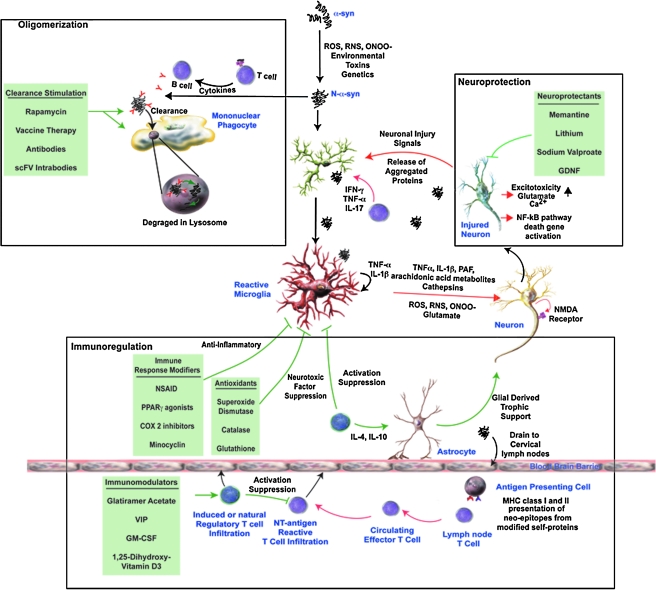
FIG. 4.
Therapeutic Strategies for PD. Several targeted approaches are currently available or proposed for the treatment of PD. The first of these targets the aggregated or misfolded proteins themselves in an effort to prevent oligomerization induce neuronal damage and microglial activation. Vaccine-induced antibodies or intracellular-produced single chain antibodies directed against misfolded proteins, or drugs (e.g., rapamycin) that stimulate phagocytosis by mononuclear phagocytes, and lysosomal degradation has been proposed for the clearance of extracellular and intracellular aggregated proteins. Alternatively, drugs that affect inflammatory responses, directly inhibit neurotoxicity (by inhibiting apoptotic pathways or excitotoxcity), or promote neuroprotection (e.g., glial derived neurotrophic factor, GDNF) have shown to be effective in animal models for human disease. Finally, efforts are being made to modulate the immune response to aggregated proteins through either immune modifiers or antioxidants that attenuate microglial activation, with the observation that disease can either be exacerbated by effector T cells specific for neo-epitopes or ameliorated by regulatory T cells. Therefore, therapeutic intervention by immunomodulators or adjuvants to induce or upregulate regulatory T cell responses can inhibit ongoing adaptive and innate immune responses and prevent further neurodegeneration.
Immune modulation as therapy
One potential target in PD is cyclooxygenase type 2 (COX-2). COX-2 has been shown to be upregulated in SN pars compacta dopaminergic neurons in both PD patients and in animal models. Pre-treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) is protective against MPTP and 6-OHDA-induced nigrostriatal dopaminergic degeneration in mice (66, 143). Furthermore, recent studies have shown that the risk of developing PD is reduced in humans who use daily NSAIDs, particularly ibuprofen. However, the mechanism by which this protection occurs and what specific drug and dosage regimen that is best for prevention of PD is still not completely understood. Like other tissues in the body, the COX isoforms have a heterogeneous distribution in the brain. COX-1 and COX-1b are detected in microglial cells, while COX-2 is found in neuronal and glial cells, and significant levels of COX are expressed by astrocytes (71, 81). The normally low levels of COX-2 expressed in the nigral dopaminergic neurons is upregulated in both PD patients and experimental models of PD (29, 37, 38, 144, 150, 154, 159). Furthermore, knockout mice that do not express COX-2 exhibit less neuronal death following ischemia, challenge with NMDA (73), and MPTP (37, 38, 154, 159). Moreover, COX-2 inhibition through pharmacologic and genetic manipulation has been shown to protect the entire nigrostriatal pathway (154).
Prostaglandin E2 (PGE2) production and free radical generation is a prominent feature in COX-2 neurotoxicity of SN dopaminergic neurons as evidenced by their detection in experimental models and in PD postmortem tissues (33, 34, 144, 150, 154). PGE2 is thought to mediate COX-2 neurotoxicity through activation of EP1/EP3 receptors leading to disruption of Ca2+ homeostasis and excitotoxicity (19, 77). Indeed, the concentration of PGE2 has been shown to almost double following MPP+ (active metabolite of MPTP) induced COX2 upregulation, a process dependent on microglial interaction with dopaminergic neurons (144, 159). The microgliosis induced by MPP+ involves the release of inflammatory factors, including PGE2 that enhances COX-2 activity in dopaminergic neurons leading to further neurodegeneration; and increased gliosis (131, 144, 154, 159). Overall, these data suggest that COX-2 may play a significant role in microglial activation and amplification of the inflammatory response that leads to a fierce cycle of neurodegeneration.
Inhibition of prostaglandin synthesis is an action of many NSAIDs, but not all of NSAID's therapeutic affects are well understood. NSAIDS have also been shown to inactivate nuclear factor-kappa B (NF-κB) and factor activator protein-1 (AP-1) (5, 35, 60, 83, 100, 152) and may be able to scavenge ROS and RNS, thus blocking their detrimental effects (6, 60). Moreover, ibuprofen and indomethacin are activators of the peroxisome proliferator-activated receptor-γ (PPARγ), a transcription factor that antagonizes the activity of NF-κB, AP-1, signal transducer, and activator of transcription-1 (STAT-1) and nuclear factor of activated T cells (NFAT) (75, 90), and thus is associated with regulation of inflammatory cytokines (75). Furthermore, the NSAIDs acetylsalicyclic acid (ASA) and paracetamol were shown to be able to block MPP+-induced inhibition of complex I and mitochondrial ETC activity with subsequent reduction of superoxide generation (92). However, some NSAIDs (indomethacin, ibuprofen, ketoprofen, or diclofenac) may enhance MPP+ induced neurodegeneration under some conditions, possibly by inhibition of multidrug resistance proteins and blocking the efflux of MPP+ (103). The stark differences in the effects of these NSAIDs may be due to the differences in dose and duration of treatment, observational window, toxins used, or the differences in experimental conditions. Still, there is strong evidence that ASA, salicylic acid (SA), ibuprofen, and COX-2 selective inhibitors exert an overall neuroprotective effect, although the mechanism(s) through which they act remains obscure. Despite evidence of inflammation in the brains of PD patients and strong evidence in animal models, NSAIDs have not yet been formally tested in humans and the data from epidemiological studies analyzing the association between NSAID use and PD have shown conflicting results (14, 21, 22, 68, 126, 148).
Immunomodulation of adaptive immunity and therapeutic responses
While research continues into the possible use of NSAIDs for treatment of PD, much interest lies in the mechanism through which Tregs modulate inflammation and whether they can be harnessed as a cell-based treatment modality. Others and we demonstrated that in models of neurodegeneration, induction of a regulatory T cell response attenuates microglial activation and promotes neuronal survival (8, 9, 12, 18, 45, 118). Based on these data, we suggest that induction of Tregs may be used to modulate immune responses, possibly through interactions with the peripheral and CNS immune systems to provide neuroprotection. We previously demonstrated that immune cells from mice immunized with Copolymer-1 (Cop-1; Copaxone, glatiramer acetate) attenuate microglial responses and protect against MPTP induced dopaminergic neurodegeneration (12, 87). T cell depletion abrogated this protection, and later works showed that indeed CD4+ cells were responsible for the observed neuroprotection (87). Cop-1 is thought to preferentially induce Th2 and Th3 Tregs that secrete anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β (2), and to promote the conversion of CD4+CD25− T cells to CD4+CD25+ Tregs through induction of Foxp3 (70).
Furthermore, adoptive transfer of CD3-activated CD4+CD25+ Tregs to MPTP-intoxicated mice provided >90% protection of the nigrostriatal system (118). This protective response was cell number dependent, and paralleled the modulation of microglial responses and upregulation of glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) and TGF-β, as well as downregulation of proinflammatory cytokines such as TNF-α. The neuroprotective effect by Tregs was shown in laboratory tests to function through modulation of microglial oxidative stress and cytotoxicity and was also operational following removal of Tregs from culture prior to stimulation, suggesting the afferent modulation of the microglial phenotype is plausible (118). Treg infiltration into the midbrain was implicated with the observation of enhanced expression of anti-inflammatory cytokines IL-10 and TGF-β that paralleled increased Foxp3 expression within the SN. Preliminary studies using single photon emission computerized tomography (SPECT) confirmed infiltration of Tregs into the SN following MPTP intoxication (unpublished observation). Adoptive transfer of activated CD4+ T cells induced neuroprotective activities in a mouse model of amyotrophic lateral sclerosis (ALS) (9).
Methods of generating Tregs for therapeutic modalities are of great interest for use in autoimmune diseases, for induction of transplantation tolerance, or in diseases that have an inflammatory component such as PD, ALS, Huntington's disease (HD), and HIV-associated dementia (HAD). In addition to Cop-1 which has already been proven to be effective in multiple sclerosis, other tolerogenic molecules exist that have been demonstrated to promote production of Tregs and may be useful as a therapeutic tool to control immunity. One such molecule is 1a, 25-dihydroxyvitamin D3, shown to activate lymphocytes and induce T cell tolerance. Furthermore, co-administration of specific antigen results in antigen-specific tolerance when administered with 1α, 25-dihydroxyvitamin D3 (59). Granulocyte macrophage colony-stimulating factor (GM-CSF) also has similar effects (46, 153). Both molecules are thought to generate Tregs and stimulate the production of TGF-β and IL-10 (137). Recent data supporting the importance of CTLA4 in Treg function demonstrated that enhanced selective engagement of CTLA4 on T cells by antigen-presenting DCs resulted in the induction of antigen specific CD4+CD25+FoxP3+ and CD4+CD25−TGF-β1+ adaptive Tregs. Moreover, generation of adaptive Tregs has been demonstrated through the in vivo delivery of a CTLA4 specific ligand along with self-antigen, resulting in protection against autoimmune disease (52).
Other molecules that may be useful in Treg generation are neuropeptides. These are also produced by lymphocytes, especially Th2 cells, in response to mitogenic and inflammatory stimuli, and promotes Th2 responses in vivo (31, 53, 54, 89). They can expand Tregs ex vivo and convert CD4+CD25− effector T cells to Tr1 regulatory T cells. Indeed, neuropeptides have been utilized to generate antigen-specific Tregs for promotion of self-tolerance and suppression of autoimmune disorders in different animal models (30, 39, 55).
In our prior works, transfer of spleen cells from mice immunized with nitrated α-syn (N4YSyn) to MPTP-treated mice demonstrated an increased loss of dopaminergic cell bodies in the SNpc and nerve termini in the striata (11). In contrast, induction of a regulatory T cell response following MPTP-intoxication was shown to promote neuroprotection and was associated with reduced microglial activation either by adoptive transfer of CD3-activated Tregs (118) or induction of a regulatory T cell response by glatiramer acetate (12, 87) or by vasoactive intestinal peptide (VIP) (32) in the MPTP mouse model of PD. Neuroprotection provided by neuropeptides may be due to suppression of the adaptive immune response following MPTP intoxication. Indeed, T cells of the adaptive immune system significantly contributed to neurodegeneration in MPTP-intoxicated mice (11), whereas the severe combined immunodeficient mouse is not susceptible to MPTP-induced neuronal degeneration. The interaction of Tregs with effector T cells by direct cell contact or via soluble factors may boost attenuation of microglial function, thus providing increased neuroprotection. This protection may be due to increased regulatory cytokines or induction of new mediators and cellular functions related to suppression, but only secreted when stimulated by Teffs.
Conclusions
Evidence for an active immunological role in PD abounds. The causal role of microglia and inflammation in the loss of dopaminergic neurons was shown to be a key player in this process. Furthermore, the role of the immune system in regulation of homeostasis in the brain has come to the forefront in the quest to understand the mechanism behind the localized microglial activation and its detrimental effect on dopaminergic neurons in the SN. Oxidative stress is involved in the perpetuation of inflammation and may be a potential trigger of the initial inflammatory response that eventually leads to a vicious cycle of gliosis associated with dopaminergic cell death. There also appears to be many different sources of oxidative stress associated with PD. The effect of oxidation on α-syn has also been shown to contribute to this process through aggregation and introduction of neo-epitopes on self-proteins. Links between the innate and adaptive arms of the immune system are active in PD, and various immune processes may contribute to dopaminergic cell death and microglial activation. Moreover, infiltration of the brain by activated immune cells and the secretion of inflammatory cytokines have been shown to be associated with PD.
There are many mechanisms that may be involved in the death of dopaminergic neurons within the inflammatory environment of the parkinsonian SN in PD that appear to involve mitochondrial dysfunction, inflammation, oxidative stress, and proteasome dysfunction, any of which may be the initial trigger, but many or all may eventually become engaged at some point in the disease process. NSAIDs such as ASA, SA, ibuprofen, and COX-2 inhibitors may still hold promise for prevention of PD, although more research is warranted to demonstrate their efficacy. The use of antioxidants, neuroprotectants, therapies that improve clearance of modified and misfolded proteins, or immunomodulatory compounds may still provide possible treatment options for PD. Of considerable interest are the immunomodulating abilities of Tregs. We propose that modulation of Treg cells can be utilized as a therapeutic modality in PD and warrants further investigation. Thus, further advances in understanding the entire picture of PD immunopathology are necessary for the development of new treatments.
Abbreviations Used
- ADCC
antibody-dependent cell cytotoxicity
- AIF
apoptosis induced factor
- ALS
amyotrophic lateral sclerosis
- AP-1
activator protein 1
- Apaf-1
apoptotic protease activating factor 1
- APC
antigen presenting cell
- ASA
acetylsalicylic acid (aspirin)
- A2AR
adenosine receptor 2A
- α-syn
alpha-synuclein
- BBB
blood–brain barrier
- BDNF
brain-derived neurotrophic factor
- CDX
cluster of differentiation (X = 1 = 28 = 80 = etc.)
- CD40L
cluster of differentiation 40 ligand;
- CNS
central nervous system
- COX-1
cyclooxygenase type 1
- COX-2
cyclooxygenase type 2
- CTLA4
cytotoxic T-lymphocyte antigen 4
- ETC
electron transport chain
- FOXP3
forkhead box p3
- GDNF
glial cell-derived neurotrophic factor
- GSH
glutathione
- GSSG
glutathione oxidized form
- HA
human immunodeficiency virus-associated dementia
- HD
Huntington's disease
- HLA
human leukocyte antigen
- HTRA
high temperature requirement protein A2
- ICAM-1
intercellular adhesion molecule 1
- IDO
indoleamine 2,3-dioxygenase
- IFN-γ
interferon gamma
- IL-X
interleukin X (X = 4 = 5 = 10 = etc.)
- IPEX
immune dysregulation polyendocrinopathy enteropathy X-linked syndrome
- LFA-1
lymphocyte function-associated antigen 1
- LPS
lipopolysaccharide
- MAC
membrane attack complex
- MAO
monoamine oxidase
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase-3
- MPP+
1-methyl-4-phenyl pyridinium (active metabolite of MPTP)
- MPTP−
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- N
nitric oxide
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor-kappa B
- NSAI
nonsterodial anti-inflammatory drugs
- 6-OHDA
6-hydroxydopamine
- PAMPS
pathogen-associated molecular patterns
- PD
Parkinson's disease
- PGE2
prostaglandin E2
- PPARγ
peroxisome proliferator-activated receptor-γ
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Smac
second mitochondria-derived activator of caspases
- SNp
substantia nigra pars compacta
- SPECT
single photon emission computerized tomography
- STAT-1
signal transducer and activator of transcription-1
- TCR
T cell receptor
- TGF-β
transforming growth factor beta
- TLRs
Toll-like receptors
- TNFα
tumor necrosis factor alpha
- tPA
tissue plasminogen activator
- VCAM-1
vascular cell adhesion molecule 1
References
- 1.abd-el-Basset E. Fedoroff S. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. J Neurosci Res. 1995;41:222–237. doi: 10.1002/jnr.490410210. [DOI] [PubMed] [Google Scholar]
- 2.Aharoni R. Teitelbaum D. Leitner O. Meshorer A. Sela M. Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci USA. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 4.Aloisi F. Penna G. Polazzi E. Minghetti L. Adorini L. CD40–CD154 interaction and IFN-gamma are required for IL-12 but not prostaglandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol. 1999;162:1384–1391. [PubMed] [Google Scholar]
- 5.Amann R. Egger T. Schuligoi R. Heinemann A. Peskar BA. Sodium salicylate enhances the expression of cyclooxygenase-2 in endotoxin-stimulated human mononuclear cells. Eur J Pharmacol. 2001;433:129–134. doi: 10.1016/s0014-2999(01)01488-1. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma M. Nishibayashi–Asanuma S. Miyazaki I. Kohno M. Ogawa N. Neuroprotective effects of nonsteroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem. 2001;76:1895–1904. doi: 10.1046/j.1471-4159.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 7.Babcock AA. Kuziel WA. Rivest S. Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakalash S. Shlomo GB. Aloni E. Shaked I. Wheeler L. Ofri R. Schwartz M. T-cell-based vaccination for morphological and functional neuroprotection in a rat model of chronically elevated intraocular pressure. J Mol Med. 2005;83:904–916. doi: 10.1007/s00109-005-0689-6. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R. Mosley RL. Reynolds AD. Dhar A. Jackson–Lewis V. Gordon PH. Przedborski S. Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS ONE. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bas J. Calopa M. Mestre M. Mollevi DG. Cutillas B. Ambrosio S. Buendia E. Lymphocyte populations in Parkinson's disease and in rat models of parkinsonism. J Neuroimmunol. 2001;113:146–152. doi: 10.1016/s0165-5728(00)00422-7. [DOI] [PubMed] [Google Scholar]
- 11.Benner EJ. Banerjee R. Reynolds AD. Sherman S. Pisarev VM. Tsiperson V. Nemachek C. Ciborowski P. Przedborski S. Mosley RL. Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benner EJ. Mosley RL. Destache CJ. Lewis TB. Jackson–Lewis V. Gorantla S. Nemachek C. Green SR. Przedborski S. Gendelman HE. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum–Degen D. Muller T. Kuhn W. Gerlach M. Przuntek H. Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 14.Bower JH. Maraganore DM. Peterson BJ. Ahlskog JE. Rocca WA. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: A case-control study. Neurology. 2006;67:494–496. doi: 10.1212/01.wnl.0000227906.99570.cc. [DOI] [PubMed] [Google Scholar]
- 15.Brecknell JE. Dunnett SB. Fawcett JW. A quantitative study of cell death in the substantia nigra following a mechanical lesion of the medial forebrain bundle. Neuroscience. 1995;64:219–227. doi: 10.1016/0306-4522(94)00370-k. [DOI] [PubMed] [Google Scholar]
- 16.Bsibsi M. Persoon–Deen C. Verwer RW. Meeuwsen S. Ravid R. Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 17.Bsibsi M. Ravid R. Gveric D. van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 18.Butovsky O. Koronyo–Hamaoui M. Kunis G. Ophir E. Landa G. Cohen H. Schwartz M. Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci USA. 2006;103:11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson NG. Neuroprotection of cultured cortical neurons mediated by the cyclooxygenase-2 inhibitor APHS can be reversed by a prostanoid. J Neurosci Res. 2003;71:79–88. doi: 10.1002/jnr.10465. [DOI] [PubMed] [Google Scholar]
- 20.Cederbom L. Hall H. Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Chen H. Jacobs E. Schwarzschild MA. McCullough ML. Calle EE. Thun MJ. Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 22.Chen H. Zhang SM. Hernan MA. Schwarzschild MA. Willett WC. Colditz GA. Speizer FE. Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 23.Chen W. Jin W. Hardegen N. Lei KJ. Li L. Marinos N. McGrady G. Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway KA. Rochet JC. Bieganski RM. Lansbury PT., Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 25.Coombes JL. Robinson NJ. Maloy KJ. Uhlig HH. Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 26.Crucian B. Dunne P. Friedman H. Ragsdale R. Pross S. Widen R. Alterations in levels of CD28−/CD8+ suppressor cell precursor and CD45RO+/CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin Diagn Lab Immunol. 1995;2:249–252. doi: 10.1128/cdli.2.2.249-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czlonkowska A. Kurkowska–Jastrzebska I. Czlonkowski A. Peter D. Stefano GB. Immune processes in the pathogenesis of Parkinson's disease: A potential role for microglia and nitric oxide. Med Sci Mon. 2002;8:RA165–177. [PubMed] [Google Scholar]
- 28.Darlington PJ. Baroja ML. Chau TA. Siu E. Ling V. Carreno BM. Madrenas J. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Meira Santos Lima M. Braga Reksidler A. Marques Zanata S. Bueno Machado H. Tufik S. Vital MA. Different parkinsonism models produce a time-dependent induction of COX-2 in the substantia nigra of rats. Brain Res. 2006;1101:117–125. doi: 10.1016/j.brainres.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Delgado M. Chorny A. Gonzalez–Rey E. Ganea D. Vasoactive intestinal peptide generates CD4+CD25+ regulatory T cells in vivo. J Leuk Biol. 2005;78:1327–1338. doi: 10.1189/jlb.0605299. [DOI] [PubMed] [Google Scholar]
- 31.Delgado M. Ganea D. Cutting edge: Is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001;166:2907–2912. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- 32.Delgado M. Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- 33.Di Matteo V. Benigno A. Pierucci M. Giuliano DA. Crescimanno G. Esposito E. Di Giovanni G. 7-nitroindazole protects striatal dopaminergic neurons against MPP+-induced degeneration: An in vivo microdialysis study. Ann NY Acad Sci. 2006;1089:462–471. doi: 10.1196/annals.1386.015. [DOI] [PubMed] [Google Scholar]
- 34.Di Matteo V. Pierucci M. Di Giovanni G. Di Santo A. Poggi A. Benigno A. Esposito E. Aspirin protects striatal dopaminergic neurons from neurotoxin-induced degeneration: An in vivo microdialysis study. Brain Res. 2006;1095:167–177. doi: 10.1016/j.brainres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Dong Z. Huang C. Brown RE. Ma WY. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J Biol Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farina C. Krumbholz M. Giese T. Hartmann G. Aloisi F. Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z. Li D. Fung PC. Pei Z. Ramsden DB. Ho SL. COX-2-deficient mice are less prone to MPTP-neurotoxicity than wild-type mice. Neuroreport. 2003;14:1927–1929. doi: 10.1097/00001756-200310270-00009. [DOI] [PubMed] [Google Scholar]
- 38.Feng ZH. Wang TG. Li DD. Fung P. Wilson BC. Liu B. Ali SF. Langenbach R. Hong JS. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329:354–358. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez–Martin A. Gonzalez–Rey E. Chorny A. Martin J. Pozo D. Ganea D. Delgado M. VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Ann NY Acad Sci. 2006;1070:276–281. doi: 10.1196/annals.1317.026. [DOI] [PubMed] [Google Scholar]
- 40.Finkbeiner S. Mitra S. The ubiquitin-proteasome pathway in Huntington's disease. Sci World J. 2008;8:421–433. doi: 10.1100/tsw.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiszer U. Mix E. Fredrikson S. Kostulas V. Link H. Parkinson's disease and immunological abnormalities: Increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol Scand. 1994;90:160–166. doi: 10.1111/j.1600-0404.1994.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 42.Fiszer U. Mix E. Fredrikson S. Kostulas V. Olsson T. Link H. gamma delta+ T cells are increased in patients with Parkinson's disease. J Neurol Sci. 1994;121:39–45. doi: 10.1016/0022-510x(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 43.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JD. Gavin MA. Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 45.Frenkel D. Huang Z. Maron R. Koldzic DN. Moskowitz MA. Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Gangi E. Vasu C. Cheatem D. Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 47.Gao HM. Liu B. Zhang W. Hong JS. Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 48.Garg SK. Banerjee R. Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol. 2008;180:3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 49.Giasson BI. Duda JE. Murray IV. Chen Q. Souza JM. Hurtig HI. Ischiropoulos H. Trojanowski JQ. Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 50.Gimmi CD. Freeman GJ. Gribben JG. Gray G. Nadler LM. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glezer I. Simard AR. Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 52.Glinka Y. Chang Y. Prud'homme GJ. Protective regulatory T cell generation in autoimmune diabetes by DNA covaccination with islet antigens and a selective CTLA-4 ligand. Mol Ther. 2006;14:578–587. doi: 10.1016/j.ymthe.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Gomariz RP. De La Fuente M. Hernanz A. Leceta J. Occurrence of vasoactive intestinal peptide (VIP) in lymphoid organs from rat and mouse. Ann NY Acad Sci. 1992;650:13–18. doi: 10.1111/j.1749-6632.1992.tb49088.x. [DOI] [PubMed] [Google Scholar]
- 54.Gomariz RP. Lorenzo MJ. Cacicedo L. Vicente A. Zapata AG. Demonstration of immunoreactive vasoactive intestinal peptide (IR-VIP) and somatostatin (IR-SOM) in rat thymus. Brain Behav Immun. 1990;4:151–161. doi: 10.1016/0889-1591(90)90017-k. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez–Rey E. Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131:1799–1811. doi: 10.1053/j.gastro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez–Scarano F. Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Ann Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 57.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- 58.Graham DG. Tiffany SM. Bell WR., Jr. Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14:644–653. [PubMed] [Google Scholar]
- 59.Gregori S. Giarratana N. Smiroldo S. Uskokovic M. Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 60.Grilli M. Pizzi M. Memo M. Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 61.Grossman WJ. Verbsky JW. Barchet W. Colonna M. Atkinson JP. Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Hancock DB. Martin ER. Vance JM. Scott WK. Nitric oxide synthase genes and their interactions with environmental factors in Parkinson's disease. Neurogenetics. 2008;9:249–262. doi: 10.1007/s10048-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardy J. Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 64.Hartmann A. Hunot S. Michel PP. Muriel MP. Vyas S. Faucheux BA. Mouatt-Prigent A. Turmel H. Srinivasan A. Ruberg M. Evan GI. Agid Y. Hirsch EC. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawrylowicz CM. O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nature Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 66.He Y. Appel S. Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909:187–193. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- 67.He Y. Le WD. Appel SH. Role of Fcgamma receptors in nigral cell injury induced by Parkinson disease immunoglobulin injection into mouse substantia nigra. Exp Neurol. 2002;176:322–327. doi: 10.1006/exnr.2002.7946. [DOI] [PubMed] [Google Scholar]
- 68.Hernan MA. Logroscino G. Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology. 2006;66:1097–1099. doi: 10.1212/01.wnl.0000204446.82823.28. [DOI] [PubMed] [Google Scholar]
- 69.Hisanaga K. Asagi M. Itoyama Y. Iwasaki Y. Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Arch Neurol. 2001;58:1580–1583. doi: 10.1001/archneur.58.10.1580. [DOI] [PubMed] [Google Scholar]
- 70.Hong J. Li N. Zhang X. Zheng B. Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoozemans JJ. Rozemuller AJ. Janssen I. De Groot CJ. Veerhuis R. Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain. Acta Neuropathol. 2001;101:2–8. doi: 10.1007/s004010000251. [DOI] [PubMed] [Google Scholar]
- 72.Hori S. Nomura T. Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 73.Iadecola C. Niwa K. Nogawa S. Zhao X. Nagayama M. Araki E. Morham S. Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwatsubo T. Pathological biochemistry of alpha-synucleinopathy. Neuropathology. 2007;27:474–478. doi: 10.1111/j.1440-1789.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 75.Jiang C. Ting AT. Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 76.Joetham A. Takeda K. Taube C. Miyahara N. Matsubara S. Koya T. Rha YH. Dakhama A. Gelf EW. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. Journal of immunology. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 77.Kawano T. Anrather J. Zhou P. Park L. Wang G. Frys KA. Kunz A. Cho S. Orio M. Iadecola C. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nature Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 78.Kim WG. Mohney RP. Wilson B. Jeohn GH. Liu B. Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YS. Kim SS. Cho JJ. Choi DH. Hwang O. Shin DH. Chun HS. Beal MF. Joh TH. Matrix metalloproteinase-3: A novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.King C. Sarvetnick N. Organ-specific autoimmunity. Curr Opin Immunol. 1997;9:863–871. doi: 10.1016/s0952-7915(97)80191-4. [DOI] [PubMed] [Google Scholar]
- 81.Kis B. Snipes JA. Isse T. Nagy K. Busija DW. Putative cyclooxygenase-3 expression in rat brain cells. J Cerebr Blood Flow Metab. 2003;23:1287–1292. doi: 10.1097/01.WCB.0000090681.07515.81. [DOI] [PubMed] [Google Scholar]
- 82.Kobie JJ. Shah PR. Yang L. Rebhahn JA. Fowell DJ. Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 83.Kopp E. Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 84.Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 85.Lafon M. Megret F. Lafage M. Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29:185–194. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 86.Langston JW. Ballard P. Tetrud JW. Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 87.Laurie C. Reynolds A. Coskun O. Bowman E. Gendelman HE. Mosley RL. CD4+ T cells from Copolymer-1 immunized mice protect dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neuroimmunol. 2007;183:60–68. doi: 10.1016/j.jneuroim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 88.Lawson LJ. Perry VH. Dri P. Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 89.Leceta J. Martinez C. Delgado M. Garrido E. Gomariz RP. Expression of vasoactive intestinal peptide in lymphocytes: A possible endogenous role in the regulation of the immune system. Adv Neuroimmunol. 1996;6:29–36. doi: 10.1016/s0960-5428(96)00001-0. [DOI] [PubMed] [Google Scholar]
- 90.Lemberger T. Desvergne B. Wahli W. Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Ann Revf Cell Develop Biol. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 91.Lenschow DJ. Walunas TL. Bluestone JA. CD28/B7 system of T cell costimulation. Ann Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 92.Maharaj DS. Saravanan KS. Maharaj H. Mohanakumar KP. Daya S. Acetaminophen and aspirin inhibit superoxide anion generation and lipid peroxidation, and protect against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats. Neurochem Internatl. 2004;44:355–360. doi: 10.1016/s0197-0186(03)00170-0. [DOI] [PubMed] [Google Scholar]
- 93.McGeer PL. Itagaki S. Akiyama H. McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- 94.McGeer PL. Itagaki S. Boyes BE. McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 95.McGeer PL. McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord 10 Suppl. 2004;1:S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 96.McGeer PL. Schwab C. Parent A. Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 97.Medzhitov R. Janeway C., Jr. Innate immunity. New Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 98.Miklossy J. Doudet DD. Schwab C. Yu S. McGeer EG. McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 99.Mirza B. Hadberg H. Thomsen P. Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience. 2000;95:425–432. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- 100.Mitchell JA. Saunders M. Barnes PJ. Newton R. Belvisi MG. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Molecular Pharmacology. 1997;51:907–912. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 101.Mochizuki H. Hayakawa H. Migita M. Shibata M. Tanaka R. Suzuki A. Shimo-Nakanishi Y. Urabe T. Yamada M. Tamayose K. Shimada T. Miura M. Mizuno Y. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc Natl Acad Sci USA. 2001;98:10918–10923. doi: 10.1073/pnas.191107398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moller T. Hanisch UK. Ransom BR. Thrombin-induced activation of cultured rodent microglia. J Neurochem. 2000;75:1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- 103.Morioka N. Kumagai K. Morita K. Kitayama S. Dohi T. Nonsteroidal anti-inflammatory drugs potentiate 1-methyl-4-phenylpyridinium (MPP+)-induced cell death by promoting the intracellular accumulation of MPP+ in PC12 cells. J Pharmacol Exp Ther. 2004;310:800–807. doi: 10.1124/jpet.104.065300. [DOI] [PubMed] [Google Scholar]
- 104.Murakami T. Shoji M. Imai Y. Inoue H. Kawarabayashi T. Matsubara E. Harigaya Y. Sasaki A. Takahashi R. Abe K. Pael-R is accumulated in Lewy bodies of Parkinson's disease. Ann Neurol. 2004;55:439–442. doi: 10.1002/ana.20064. [DOI] [PubMed] [Google Scholar]
- 105.Nagatsu T. Sawada M. Cellular and molecular mechanisms of Parkinson's disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol. 2006;26:781–802. doi: 10.1007/s10571-006-9061-9. [DOI] [PubMed] [Google Scholar]
- 106.Nimmerjahn A. Kirchhoff F. Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 107.Olson JK. Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunology. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 108.Orr CF. Rowe DB. Mizuno Y. Mori H. Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson's disease. Brain. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- 109.Pandiyan P. Zheng L. Ishihara S. Reed J. Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 110.Parker WD., Jr. Boyson SJ. Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 111.Pentcheva-Hoang T. Egen JG. Wojnoonski K. Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 112.Perez N. Karumuthil–Melethil S. Li R. Prabhakar BS. Holterman MJ. Vasu C. Preferential costimulation by CD80 results in IL-10-dependent TGF-beta1(+) -adaptive regulatory T cell generation. J Immunology. 2008;180:6566–6576. doi: 10.4049/jimmunol.180.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perier C. Bove J. Wu DC. Dehay B. Choi DK. Jackson–Lewis V. Rathke–Hartlieb S. Bouillet P. Strasser A. Schulz JB. Przedborski S. Vila M. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson's disease. Proc Natl Acad Sci USA. 2007;104:8161–8166. doi: 10.1073/pnas.0609874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perier C. Tieu K. Guegan C. Caspersen C. Jackson–Lewis V. Carelli V. Martinuzzi A. Hirano M. Przedborski S. Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ponomarev ED. Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 116.Qin L. Wu X. Block ML. Liu Y. Breese GR. Hong JS. Knapp DJ. Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Revuelta M. Venero JL. Machado A. Cano J. Serotonin hyperinnervation in the adult rat ventral mesencephalon following unilateral transection of the medial forebrain bundle. Correlation with reactive microglial and astroglial populations. Neuroscience. 1999;91:567–577. doi: 10.1016/s0306-4522(98)00624-1. [DOI] [PubMed] [Google Scholar]
- 118.Reynolds AD. Banerjee R. Liu J. Gendelman HE. Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. J Leuk Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 119.Reynolds AD. Glanzer JG. Kadiu I. Ricardo–Dukelow M. Chaudhuri A. Ciborowski P. Cerny R. Gelman B. Thomas MP. Mosley RL. Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson's disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 120.Roncarolo MG. Gregori S. Battaglia M. Bacchetta R. Fleischhauer K. Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 121.Ross CA. Poirier MA. Protein aggregation and neurodegenerative disease. Nature Med 10. 2004;Suppl:S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 122.Sakaguchi S. Sakaguchi N. Asano M. Itoh M. Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 123.Sakaguchi S. Sakaguchi N. Shimizu J. Yamazaki S. Sakihama T. Itoh M. Kuniyasu Y. Nomura T. Toda M. Takahashi T. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 124.Schapira AH. Mitochondrial complex I deficiency in Parkinson's disease. Adv Neurol. 1993;60:288–291. [PubMed] [Google Scholar]
- 125.Schiefer J. Kampe K. Dodt HU. Zieglgansberger W. Kreutzberg GW. Microglial motility in the rat facial nucleus following peripheral axotomy. J Neurocytol. 1999;28:439–453. doi: 10.1023/a:1007048903862. [DOI] [PubMed] [Google Scholar]
- 126.Schiess M. Nonsteroidal anti-inflammatory drugs protect against Parkinson neurodegeneration: can an NSAID a day keep Parkinson disease away? Archives of Neurology. 2003;60:1043–1044. doi: 10.1001/archneur.60.8.1043. [DOI] [PubMed] [Google Scholar]
- 127.Schneider H. Downey J. Smith A. Zinselmeyer BH. Rush C. Brewer JM. Wei B. Hogg N. Garside P. Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 128.Shevach EM. DiPaolo RA. Andersson J. Zhao DM. Stephens GL. Thornton AM. The lifestyle of naturally occurring CD4+CD25+Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 129.Stokes AH. Hastings TG. Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 130.Su X. Maguire–Zeiss KA. Giuliano R. Prifti L. Venkatesh K. Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sugama S. Cho BP. Degiorgio LA. Shimizu Y. Kim SS. Kim YS. Shin DH. Volpe BT. Reis DJ. Cho S. Joh TH. Temporal and sequential analysis of microglia in the substantia nigra following medial forebrain bundle axotomy in rat. Neuroscience. 2003;116:925–933. doi: 10.1016/s0306-4522(02)00572-9. [DOI] [PubMed] [Google Scholar]
- 132.Sugama S. Yang L. Cho BP. DeGiorgio LA. Lorenzl S. Albers DS. Beal MF. Volpe BT. Joh TH. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- 133.Sulzer D. Zecca L. Intraneuronal dopamine-quinone synthesis: A review. Neurotox Res. 2000;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 134.Suri–Payer E. Amar AZ. Thornton AM. Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 135.Suzumura A. Sawada M. Takayanagi T. Production of interleukin-12 and expression of its receptors by murine microglia. Brain Res. 1998;787:139–142. doi: 10.1016/s0006-8993(97)01166-9. [DOI] [PubMed] [Google Scholar]
- 136.Tadokoro CE. Shakhar G. Shen S. Ding Y. Lino AC. Maraver A. Lafaille JJ. Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taher YA. van Esch BC. Hofman GA. Henricks PA. van Oosterhout AJ. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180:5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 138.Takeuchi H. Mizuno T. Zhang G. Wang J. Kawanokuchi J. Kuno R. Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- 139.Tan J. Town T. Mori T. Wu Y. Saxe M. Crawford F. Mullan M. CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J Neurosci. 2000;20:7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang Q. Adams JY. Tooley AJ. Bi M. Fife BT. Serra P. Santamaria P. Locksley RM. Krummel MF. Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nature Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tansey MG. McCoy MK. Frank–Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 143.Teismann P. Ferger B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson's disease. Synapse. 2001;39:167–174. doi: 10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 144.Teismann P. Tieu K. Choi DK. Wu DC. Naini A. Hunot S. Vila M. Jackson–Lewis V. Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomas MP. Chartrand K. Reynolds A. Vitvitsky V. Banerjee R. Gendelman HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: Relevance for the pathogenesis of Parkinson's disease. J Neurochem. 2007;100:503–519. doi: 10.1111/j.1471-4159.2006.04315.x. [DOI] [PubMed] [Google Scholar]
- 146.Thornton AM. Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 147.Tivol EA. Borriello F. Schweitzer AN. Lynch WP. Bluestone JA. Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 148.Ton TG.Heckbert SR.Longstreth WT. Jr., Rossing MA.Kukull WA.Franklin GM.Swanson PD.Smith–Weller T.Checkoway H.Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease Movement Disord 21964–969.2006 [DOI] [PubMed] [Google Scholar]
- 149.Tsirka SE. Clinical implications of the involvement of tPA in neuronal cell death. J Mol Med. 1997;75:341–347. doi: 10.1007/s001090050119. [DOI] [PubMed] [Google Scholar]
- 150.Tyurina YY. Kapralov AA. Jiang J. Borisenko GG. Potapovich AI. Sorokin A. Kochanek PM. Graham SH. Schor NF. Kagan VE. Oxidation and cytotoxicity of 6-OHDA are mediated by reactive intermediates of COX-2 overexpressed in PC12 cells. Brain Res. 2006;1093:71–82. doi: 10.1016/j.brainres.2005.10.105. [DOI] [PubMed] [Google Scholar]
- 151.van Loo G. Saelens X. van Gurp M. MacFarlane M. Martin SJ. Vandenabeele P. The role of mitochondrial factors in apoptosis: A Russian roulette with more than one bullet. Cell Death Diff. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 152.Vane JR. Botting RM. The mechanism of action of aspirin. Thrombosis Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 153.Vasu C. Dogan RN. Holterman MJ. Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol. 2003;170:5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 154.Vijitruth R. Liu M. Choi DY. Nguyen XV. Hunter RL. Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. J Neuroinflamm. 2006;3:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vila M. Jackson–Lewis V. Vukosavic S. Djaldetti R. Liberatore G. Offen D. Korsmeyer SJ. Przedborski S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci USA. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vila M. Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nature Rev Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 157.Viswanath V. Wu Y. Boonplueang R. Chen S. Stevenson FF. Yantiri F. Yang L. Beal MF. Andersen JK. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci. 2001;21:9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Walunas TL. Lenschow DJ. Bakker CY. Linsley PS. Freeman GJ. Green JM. Thompson CB. Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 159.Wang T. Pei Z. Zhang W. Liu B. Langenbach R. Lee C. Wilson B. Reece JM. Miller DS. Hong JS. MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005;19:1134–1136. doi: 10.1096/fj.04-2457fje. [DOI] [PubMed] [Google Scholar]
- 160.Wang XJ. Yan ZQ. Lu GQ. Stuart S. Chen SD. Parkinson disease IgG and C5a-induced synergistic dopaminergic neurotoxicity: role of microglia. Neurochem Internatl. 2007;50:39–50. doi: 10.1016/j.neuint.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 161.Warner TT. Schapira AH. Genetic and environmental factors in the cause of Parkinson's disease. Ann Neurol 53 Suppl. 2003;3:S16–23; discussion S23–15. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- 162.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xystrakis E. Boswell SE. Hawrylowicz CM. T regulatory cells and the control of allergic disease. Expert Opin Biol Ther. 2006;6:121–133. doi: 10.1517/14712598.6.2.121. [DOI] [PubMed] [Google Scholar]
- 164.Yamada T. McGeer PL. McGeer EG. Lewy bodies in Parkinson's disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84:100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- 165.Yamada T. MPLMEG. Relationship of complement-activated oligodendrocytes to reactive microglia and neuronal pathology in neurodegenerative disease. Dementia. 1991;2:71–77. [Google Scholar]
- 166.Yi-qun Z. Lorre K. de Boer M. Ceuppens JL. B7-blocking agents, alone or in combination with cyclosporin A, induce antigen-specific anergy of human memory T cells. J Immunol. 1997;158:4734–4740. [PubMed] [Google Scholar]
- 167.Zhang W. Wang T. Pei Z. Miller DS. Wu X. Block ML. Wilson B. Zhang W. Zhou Y. Hong JS. Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 168.Zheng Y. Manzotti CN. Burke F. Dussably L. Qureshi O. Walker LS. Sansom DM. Acquisition of suppressive function by activated human CD4+CD25− T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol. 2008;181:1683–1691. doi: 10.4049/jimmunol.181.3.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]