Introduction
The Epithelial Sodium (Na+) Channel (ENaC) plays a critical role in blood pressure regulation by controlling renal salt and water reabsorption. Channel over-activity can lead to severe hypertension and under-activity to salt wasting and hypotension1. In addition to their role in salt/water homeostasis, recent studies suggest ENaC proteins, and their relatives Acid Sensing Ion Channel (ASIC) proteins, may play a more ubiquitous role in cardiovascular regulation than previously considered. Recent evidence suggests ENaC/ASIC proteins may act as mechanosensors and chemosensors in the cardiovascular system. ENaC/ASIC proteins are expressed in mechano- and chemosensing tissues such as vascular smooth muscle cells, carotid body glomus cells and sensory neurons innervating arterial baroreceptors, heart, and skeletal muscle. Disruption of ENaC/ASIC channels alters myogenic constriction, arterial chemoreceptor and baroreceptor responses, and acid-induced responses in heart and skeletal muscle. This brief review will summarize the evidence supporting a role for ENaC and ASIC proteins in diverse systems of cardiovascular mechano- and chemosensing. Together, these studies suggest ENaC/ASIC proteins contribute to cardiovascular homeostasis by mediating neural and local regulatory mechanisms.
The DEG/ENaC/ASIC Family
ENaC and ASIC proteins are members of a protein family termed the Degenerin/ENaC/ASIC (DEG/ENaC/ASIC) family. Members of this family are expressed in a wide range of species (nematode Caenorhabditis elegans, Drosophila, and mammals) and participate in diverse biological functions including neurodegeneration, acid sensation, taste, learning and memory, proprioception, Na+/water transport and mechanosensation. All members of the DEG/ENaC/ASIC family share a highly conserved structure: intracellular N- and C-termini and two membrane-spanning domains separated by a large extracellular domain. Most DEG/ENaC/ASIC proteins form amiloride sensitive, non-voltage gated cation channels1,2.
C. elegans Degenerins
Members were first identified in the nematode where a chemically induced mutation caused a subset of neurons to swell and lyse. This phenotype led to the first name of the family, Deg, short for degeneration. Subsequently, other C. elegans degenerin genes expressed in neurons and muscle have been identified following genetic screens for proteins involved in touch responsiveness and proprioception, responses dependent on mechanotransduction1,2. These data provided the initial genetic link between the DEG/ENaC/ASIC channels and mechanotransduction.
Mammalian ENaC and ASIC proteins
In vertebrates, there are at least two subgroups of DEG/ENaC/ASIC proteins: ENaC and ASIC. Gain-of-function and loss-of-function mutations in ENaC channels are manifested in two diseases, Liddle’s Disease and Pseudohypoaldosteronism Type I (PHA), respectively. In Liddle’s Disease, certain mutations disrupt normal channel degradation resulting in increased channel density, excessive salt/water retention and severe hypertension. In PHA, under-active channels produce salt wasting and hypotension. At least five different ENaC proteins have been identified in mammals (αENaC, βENaC, γENaC, δENaC, εENaC). αENaC, βENaC and γENaC proteins form a heteromultimeric channel critical in Na+ and water transport in the renal, colon and lung epithelial1,2. The δENaC and εENaC subunits can substitute for αENaC or interact with αβγENaC channels. Expression of the δENaC subunit is limited to brain, pancreas, testes, ovary and retinal cells3–5. Expression of the εENaC subunit is limited to the brain, skeletal muscle, kidney and urinary bladder in Xenopus6. ENaC channels are constitutively active, non-voltage gated and highly sensitive to amiloride (αβγENaC IC50 < 100nM; αδβγENaC IC50 = 1 µM; δβγENaC IC50 = 2.6 µM) and its lipophylic analog benzamil1. While αENaC protein is required to form the fully functional channel characteristic of epithelial Na+ channel in renal epithelia, β and γENaC can form a Na+ conducting ion channel in the absence of αENaC7.
ASIC and ENaC proteins are closely related. ASIC proteins (ASIC1, ASIC2, ASIC3, ASIC4) can form homo and heteromultimeric channels that generally conduct Na+1. Although ASIC channels are also sensitive to amiloride, they tend to require higher doses than ENaC (10–100 fold). A specific inhibitor for ASIC1a is available (psalmotoxin), however, specific inhibitors for other ASIC channels are not available. A drop in extracellular pH gates most ASIC channels1. Until recently, ASICs were only identified in neuronal and neuro-epithelial tissue where they may contribute to acid-taste, acid-sensation, learning and mechanosensation, however, recent evidence suggests ASICs are also expressed in vascular smooth muscle8.
Evidence of Mechanosensitivity and Chemosensitivity of ENaC/ASIC Channels in Isolated Systems and Epithelial Tissues
Mechanosensitivity
Early investigations into the mechanosensitivity of ENaC in heterologous and endogenous expression systems demonstrated αENaC and αβγENaC channels could be activated by the application of negative hydrostatic pressure9–12. Contrasting results were found in the Xenopus oocyte expression system in response to osmotic induced swelling and shrinking13,14. However, using the oocyte expression system, ENaC can be activated by a different mechanical stimulus, shear stress. In the cortical distal tubule, shear stress may be the appropriate stimulus to mechanically gate ENaC channels15,16. The reasons underlying the conflicting results in isolated expression systems is unknown, however, it may reflect several factors including 1) specificity of mechanical gating of αβγENaC to the stimulus (i.e. shear stress vs. osmotic stretch), 2) presence of inhibitory substances such as ATP12 and 3) importance of the appropriate combination of intracellular and extracellular proteins necessary to gate the channel in response to stretch or strain. These findings suggest that under certain conditions, mechanical forces can gate isolated ENaC channels. Currently, the mechanosensitivity of ASIC channels has not been addressed.
Chemosensitivity
Direct evidence of the chemosensitivity of homo and heteromeric ASIC channels is derived from studies in isolated expression systems, where extracellular acidosis (EC50 pH range 3.5 –7.0) gates the channels with the different channels having varying pH sensitivities. Although αβγENaC channels are not gated by pH, the presence of the δENaC subunit confers pH sensitivity with and EC50 of pH 6.1 for δβγENaC and 6.5 for δαβγENaC channels1,17. These findings suggest ASIC channels, and ENaC channels containing δENaC, can be gated by protons. Because protons are thought to signal chemoreflex responses initiated in carotid chemoreceptors and peripheral chemoreceptors, ENaC/ASIC proteins are considered candidates for these receptors.
Contribution of Neuron and Vascular Smooth Muscle ENaC/ASIC Proteins to Cardiovascular Homeostasis
In addition to their role in salt and water reabsorption, evidence suggests ENaC/ASIC proteins contribute to cardiovascular homeostasis by functioning as 1) mechanoreceptors in arterial baroreceptor neurons and vascular smooth muscle cells (VSMC), and 2) acid sensors in arterial chemosensors (carotid body glomus cells), myocardial tissue and skeletal muscle. In this section, we will discuss evidence implicating ENaC/ASIC proteins as mechanosensors and chemosensors in cardiovascular tissue.
If ENaC/ASIC proteins are to be considered as mechanosensors or chemosensors, then at least two criteria must be met. First, ENaC/ASIC proteins must be expressed at the site of mechanotransduction or chemoreception. Second, inhibition or disruption of ENaC/ASIC activity should inhibit the mechanosensitive or chemosensitive response. Since ENaC null mice are very ill or die shortly after birth, genetic evidence for ENaC involvement in mechanotransduction is lacking18–20. ASIC null mice thrive and have provided evidence for their involvement in mechano- and chemoreception. As an alternative to ENaC null mice, selective ENaC inhibitors, such as amiloride and benzamil, have been useful tools to determine ENaC involvement since αβγENaC can be blocked by as little as 100 nM1,21. Thus, low doses of amiloride can discern the importance of ENaC channels from other transporters and ion channels.
Role in Neural Cardiovascular Mechanosensation
ENaC and ASIC molecules are expressed in specific sensory neuron populations in the dorsal root, trigeminal and nodose ganglia and the afferent nerve terminals innervating certain somatic and visceral receptors1,2,22–25. One of these sites includes arterial baroreceptor nerve endings located in the aortic arch and carotid sinus. Arterial baroreceptors discharge in response to pressure-induced vessel wall stretch and play an important role in the beat-to-beat control of the cardiovascular system. Four lines of evidence suggest ENaC/ASIC channels participate in arterial baroreceptor activation. First, baroreceptor neurons express at least βENaC, γENaC, and ASIC2 molecules22,26. Second, ENaC inhibition prevents mechanically activated membrane depolarization and Ca2+ transients in baroreceptor neurons22,27. Third, ENaC inhibition blocks pressure-induced changes in carotid baroreceptor activity and reflex induced changes in systemic blood pressure. Fourth, ASIC2 null mice have reduced spontaneous baroreflex sensitivity28. Taken together these findings suggest at least βENaC, γENaC and ASIC2 are expressed in baroreceptor neurons where they may mediate mechanically initiated responses in vitro and in-vivo. It is unknown if βENaC, γENaC and ASIC2 associate to form a homogenous population of heteromeric channels (i.e. all channels are comprised of βENaC, γENaC and ASIC2) or if the subunits associate to form multiple heteromeric channels (i.e., channels are comprised of βENaC/γENaC, γENaC/ASIC or βENaC/ASIC channels), or homomeric channels (i.e. βENaC, γENaC or ASIC only). While ENaC/ASIC proteins are expressed in non-baroreceptor neurons in sensory ganglia, it is unknown if ENaC/ASIC proteins act as mechanoreceptors in non-baroreceptor cardiovascular mechanoreceptors, such as cardiopulmonary receptors.
Local Control of Vascular Resistance: Pressure-Induced Vasoconstriction
In addition to neural mechanisms, ENaC/ASIC proteins may contribute to cardiovascular homeostasis by participating in local mechanisms regulating vascular resistance. Pressure-induced, or myogenic, constriction is an inherent response of certain vessels that allows resistance arteries to adjust tone in response to luminal pressure; vessels constrict to increases and dilate to decreases in pressure. The response is initiated by vessel wall stretch and thus activated by a mechanical stimulus. The response may play a critical role in preventing the transmission of pressure waves to small, fragile microvasculature, particularly in the renal and cerebral circulations, and thus may protect against hypertension-induced injury29,30.
The importance of ENaC/ASIC proteins in pressure-induced vasoconstriction has been examined in cerebral and renal arteries. VSMCs express βENaC, γENaC and ASIC231–34. In these VSMCs, ENaC/ASIC2 proteins are expressed at or near the cell surface, the predicted site of mechanotransduction of a VSMC stretch into a cellular signaling event (Figure 1A). Pharmacological inhibition of ENaC with amiloride or benzamil inhibits pressure-mediated constriction (at submicromolar and low micromolar concentrations) in middle cerebral artery, renal interlobar, and renal afferent arterioles31,32,35,36. In a follow-up study, Jernigan et al. used gene specific silencing approaches, siRNA and dominant-negatives, to determine the importance of βENaC and γENaC in pressure induced constriction (Figure 1B). Both approaches inhibited protein expression and pressure-induced vasoconstriction in isolated renal interlobar segments. Constriction in response to the α-adrenergic receptor phenylephrine was not altered following ENaC inhibition or gene silencing, suggesting vasoconstriction per se was not altered following ENaC inhibition32,33. While these findings suggest certain ENaC proteins may mediate pressure-induced constriction, the role of ASIC proteins has not been thoroughly examined. However, preliminary studies suggest pressure-induced constriction is absent in cerebral vessels of ASIC2 heterozygous null mice34. When we consider 1) ASIC2, βENaC, and γENaC are expressed in similar VSMC populations, 2) ASIC2 biochemically interacts with γENaC in other systems, and 3) loss of ASIC2, βENaC, and γENaC produces the same phenotype (loss of pressure-induced constriction), the speculation that these proteins form a heteromultimeric channel seems reasonable22,31–34,37,38.
Figure 1.
VSMC ENaC. A. Localization of βENaC (left panel) and smooth muscle α-actin (middle panel) in a single VSMC dissociated from mouse renal arteries. Yellow coloring in the merged image (right panel) suggests co-localization of βENaC and the cytoskeleton. B. Suppression of βENaC/γENaC expression in isolated mouse renal interlobar artery segments inhibits vasoconstriction (% myogenic tone) to increases in intraluminal pressure.
Lack of Direct Electrophysiological Evidence of ENaC/ASIC Channels in VSMCs
Direct electrophysiological evidence of ENaC/ASIC channels in VSMCs is not available, however, one report of an epithelial-like Na+ current in VSMCs was found39. Similar to αβγENaC, the channel reported in VSMCs is non-voltage gated and has a 10 pS conductance and high Na+:K+ selectivity. Unlike αβγENaC, the channel is insensitive to amiloride (100 µM). While the amiloride characteristics of this channel are not consistent with the reported amiloride sensitivity of αβγENaC and βγENaC channels in heterologous expression systems, this finding supports the potential presence of an ENaC-like Na+ channel in VSMCs. It is not clear why there is so little electrophysiological evidence of ENaC, however, one possibility is investigators have not looked for them. Another possibility is the channels are electrically silent until gated by mechanical stimuli40,41.
Can βENaC and γENaC Form a Channel in the Absence of αENaC?
In VSMCs and sensory neurons, βENaC and γENaC appear to be the predominant ENaC proteins expressed, while αENaC is rare. Since αENaC is required to generate the fully functional, constitutively active ENaC channel typically found in epithelial tissue, are βENaC and γENaC capable of forming a channel in the absence of αENaC? Evidence from Rossier’s laboratory suggests αENaC is not required for β and γENaC to form a channel7. Bonny et al. demonstrated oocytes expressing βENaC and γENaC generate amiloride sensitive currents in the absence of αENaC, when provided a longer incubation period (~6 days). Channels formed by βENaC and γENaC have a greater selectivity for Na+ and conduct less current. Additionally, βγENaC channels have a 10 fold higher Ki for amiloride (~2 µM in βγENaC vs. 0.2µM in αβγENaC). Thus, channels formed by βγENaC are not the same as channels formed by αβγENaC. The finding by Jernigan and Drummond that approximately 40% of myogenic constrictor responses are blocked with 1 µM amiloride is consistent with the amiloride Ki for βγENaC channels32.
Compared to αβγENaC channels, trafficking of βγENaC channels to the surface membrane in Xenopus oocytes is delayed, which results in protein localization in the intracellular compartment. This may be the basis for lack of current generated by βγENaC in heterologous expression systems7. In freshly dissociated VSMCs, trafficking of β and γENaC does not appear impaired as they are expressed at or near the cell surface (Figure 1)32,33. The mechanism(s) mediating membrane localization of βENaC and γENaC in the absence of αENaC is unknown, however there are a few possible explanations. First, VSMCs may express another protein that associates with and stabilizes βENaC and γENaC. Second, another pore forming subunit may interact with βENaC and γENaC, such as δENaC, or an ASIC protein. Third, αENaC may be expressed in VSMCs, but we are unable to detect it and the small amount expressed is sufficient to stabilize the channel. Lastly, the presence of proteins within the dense extracellular matrix of blood vessels may help stabilize βγENaC channels that reach the membrane. Regardless of the mechanism, in the absence of detectable levels of αENaC, βENaC and γENaC appear to traffic to the cell surface of VSMC in-vivo and remain there following enzymatic dissociation.
How Do ENaC/ASIC Proteins Transduce Mechanical Stimuli?
While the studies presented in this review demonstrate ENaC/ASIC proteins play a significant role in the mechano-dependent responses, it is not entirely clear how ENaC/ASIC proteins transducer mechanical stimuli. A universal, or “all-purpose” mechanotransducer model has been developed in the nematode for related degenerin proteins1. In this model, the mechanosensor is a large heteromultimeric channel complex consisting of five basic components: 1) extracellular matrix proteins 2) extracellular linking proteins, 3) pore forming channels, 4) intracellular linking proteins and 5) cytoskeleton proteins (Figure 2). Nematode members of the DEG/ENaC/ASIC family form the ion-conducting unit of the complex. The application of a mechanical force is transduced through the extracellular matrix to gate the channel. Thus, the interaction between the pore forming proteins and the extracellular matrix is critical to channel gating. The cytoskeleton may also participate in transduction of the applied force and along with other extracellular proteins, may also stabilize the pore at the cell surface. We speculate that a similar model also applies to ENaC/ASIC proteins in mammalian mechanosensors. When the channel is gated open, Na+ and possibly Ca2+, entry leads to membrane depolarization and subsequent activation of downstream signaling events leading to smooth muscle cell contraction or neuronal action potential generation.
Figure 2.
Proposed model of a mammalian mechanosensor. This model is based on the mechanotransducer model established in the nematode. The mechanosensing apparatus may be a large heteromultimeric complex. ENaC/ASIC proteins form the ion-transducing core of the mechanotransducer, which are anchored to the extracellular matrix and cytoskeleton by associated linking proteins. The application of a mechanical stimulus, such as strain allows influx of Na+/Ca2+.
Role in Cardiovascular Chemosensation
In addition to their role in mechanosensing, ENaC/ASIC proteins may contribute to cardiovascular homeostasis via chemosensing mechanisms. A well-established characteristic of ASIC channels is their activation by drops in extracellular pH1, which has made them very attractive candidates for chemosensing processes in arterial chemoreceptors and muscle metaboreceptors in skeletal and cardiac tissue.
Several lines of evidence support a potential role for ASIC channels in arterial chemoreceptors. First, carotid body glomus cells express ASIC1 and ASIC3, and to a lesser extent ASIC242. Second, carotid body glomus cells have pH-gated currents that resemble ASIC channels and are partially amiloride sensitive42. Third, cardiovascular responses to chemoreceptor stimulation with carotid artery occlusion are attenuated in ASIC3 and ASIC1/ASIC3 double null mice43. Together these findings suggest ASIC proteins may participate in pH sensing in arterial chemoreceptors.
Some investigators have suggested ASIC proteins may be chemotransducers in muscle tissue that signal ischemic pain25,44–47. In cardiac tissue, ischemia induced extracellular acidosis is part of the signaling event leading to the sensation of pain. Activation of cardiac sympathetic afferents leads to sympathoinhibitory/vagal stimulatory effect to reduce cardiac work48. The first study to address the role of ASIC proteins in ischemic pain demonstrated that cardiac sensory neurons have substantial extracellular-acid evoked Na+ currents that resemble ASIC currents and are sensitive to amiloride44. Follow-up studies suggest an important role for ASIC3 because 1) ASIC3 is highly sensitive to lactic acid, a mediator of ischemic pain, and 2) acid gated current in cardiac sensory neurons are nearly identical to acid gated currents of ASIC3 homomultimers49,50. Studies of cardiovascular responses to cardiac ischemia in genetically modified mice are needed to confirm the importance of ASIC proteins in sensing cardiac ischemia.
A similar role for ASIC3, as well as other ASIC proteins, in sensing changes in skeletal muscle pH has been proposed25,46,47. Similar to cardiac tissue, fine sensory afferents in skeletal muscle are activated with lactic acid, a by-product of anaerobic muscle activity. Activation of these skeletal muscle afferents contributes to the cardiovascular and respiratory response to exercise. There are three lines of evidence supporting involvement of ASIC channels in this response. First ASIC proteins are localized in populations of small nociceptive neurons innervating skeletal muscle25,51. In particular, ASIC3 is expressed in fine nerve endings innervating skeletal muscle blood arterioles25. Additionally, amiloride inhibits increases in blood pressure and heart rate to muscle contraction and intramuscular injection of lactic acid46,47. Although these findings support a role for ASIC proteins as pH sensors in muscle tissue and potential mediators of ischemic pain, involvement of ENaC channels cannot be ruled out because δENaC can confer pH sensitivity to ENaC channels and ENaC proteins can interact with ASIC proteins to form channels. Future studies on ASIC/ENaC null mice are needed to elucidate the importance of specific ASIC (and possibly ENaC) proteins in muscle ischemic responses.
It is important to make a cautionary note: ENaC/ASIC channels are probably not the only cardiovascular mechano- and chemosensors. This protein family probably represents one of multiple signaling mechanisms for mechano- and pH- sensing. Other ion channels are also involved, such as members of the 2-pore K+ channels and transient receptor potential channel (TRP) families52.
Aldosterone Regulation and Hypertension-Related Organ Injury
Results of recent clinical trials, Randomized Aldactone Evaluation Study (RALES) and Eplerenone Heart Failure Efficacy and Survival Study (EPHESUS) demonstrate the protective effect of aldosterone inhibition on cardiovascular function53,54. However, mechanisms of this protection are still unclear. One might consider, in the context of this review, a possible role for ENaC proteins. Aldosterone stimulates ENaC activity in epithelial tissue, yet its effect on vascular/neuronal ENaC expression is unknown1. Although sensory neurons and VSMCs might be expected to respond to aldosterone in a manner similar to epithelial tissue, this may not necessarily be true. Based upon published studies and preliminary studies from our laboratory, we suspect that aldosterone may be a negative regulator of ENaC expression in sensory neurons and VSMCs,
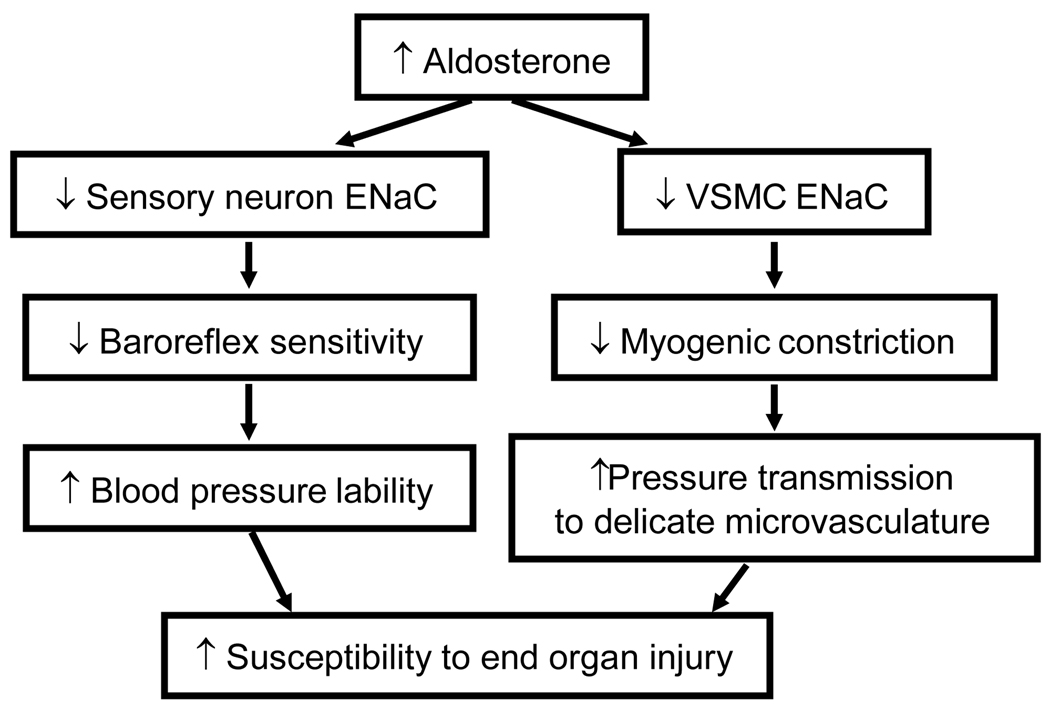
Preliminary data from our laboratory suggest that aldosterone (1 nM-100 µM) inhibits expression of β/γENaC in cultured sensory neurons. The effect is blocked by the aldosterone antagonists spironolactone and RU752. In addition, indirect evidence suggests aldosterone inhibits ENaC in sensory neurons and VSMCs. Several investigations have linked elevated plasma aldosterone to reduced arterial baroreflex sensitivity and inhibition of myogenic tone in cerebral vessels. The studies discussed in this review suggest baroreflex and myogenic control are mediated by ENaC55–57. Thus, aldosterone may suppress baroreflex and myogenic responses by inhibiting ENaC expression. These factors increase blood pressure lability and pressure transmission to the delicate microvasculature, which may result in susceptibility to pressure-related end organ injury (Figure 3). We speculate part of the cardio-protective effect of aldosterone antagonism in clinical trials may be due to its stimulatory effect on ENaC expression in sensory neurons and VSMCs, which may augment 1) baroreflex sensitivity and preventing swings in systemic pressure and 2) myogenic responsiveness and prevent transmission of system pressure to the microvasculature.
Figure 3.
Possible mechanism of aldosterone action on non-epithelial targets. Aldosterone inhibition of ENaC in neurons and VSMCs may suppress baroreflex sensitivity and myogenic constriction. These actions increase blood pressure lability and pressure transmission to delicate microvessels in brain and kidney and, thus, increase susceptibility to end-organ injury. Protection against injury may contribute to the beneficial effect of aldosterone antagonism in clinical trials (RALES, EPHESUS).
How might aldosterone inhibit ENaC expression? Biochemical evidence suggests aldosterone is capable of activating at least one negative regulatory pathway, Epidermal Growth Factor Receptor (EGFR) - Mitogen Activated Protein Kinase (MAPK) pathway58. Recent studies suggest aldosterone-mineralocorticoid binding leads to transactivation of the EGFR, which acts as a “brake” that prevents over-activation of ENaC in epithelial tissue59. We speculate this negative pathway is favored in VSMCs and sensory neurons which results net inhibition of ENaC expression. Thus, aldosterone mediated transactivation of EGFR is one mechanism that could mediate aldosterone inhibition of ENaCs in VSMCs and sensory neurons.
Perspectives
A growing body of evidence suggests ENaC/ASIC proteins play a more diverse role in cardiovascular homeostasis than previously recognized. Certain ENaC and ASIC proteins may also influence cardiovascular homeostasis by acting as mechanosensors that mediate arterial baroreflex responses and local control of vascular resistance. ASIC proteins may influence cardiovascular homeostasis by acting as chemosensors, detecting changes in arterial pH to mediate arterial chemoreflex and ischemic responses in cardiac and skeletal muscle, and mechanosensors in baroreceptors. This is a very exciting time for this area of research; we are just beginning to understand the importance of ENaC/ASIC proteins as sensors in cardiovascular mechano- and chemoreception. How these channels 1) interact with neighboring proteins to signal mechanical and chemical stimuli, 2) contribute to the neural and local cardiovascular reflex responses, and 3) contribute to the progression of cardiovascular disease, such as hypertension, remain to be determined.
Acknowledgments
Sources of Fundings. This work was supported by NIH HL071603-02, HL086996-01 and HL51971.
References
- 1.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 2.Syntichaki P, Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol Rev. 2004;84:1097–1153. doi: 10.1152/physrev.00043.2003. [DOI] [PubMed] [Google Scholar]
- 3.Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. Delta-subunit confers novel biophysical features to alpha beta gamma-human epithelial sodium channel (ENaC) via a physical interaction. J Biol Chem. 2006;281:8233–8241. doi: 10.1074/jbc.M512293200. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride- sensitive Na+ channel. J Biol Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- 5.Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol. 2002;283:C126–C134. doi: 10.1152/ajpcell.00457.2001. [DOI] [PubMed] [Google Scholar]
- 6.Babini E, Geisler HS, Siba M, Grunder S. A new subunit of the epithelial Na+ channel identifies regions involved in Na+ self-inhibition. J Biol Chem. 2003;278:28418–28426. doi: 10.1074/jbc.M301315200. [DOI] [PubMed] [Google Scholar]
- 7.Bonny O, Chraibi A, Loffing J, Jaeger NF, Grunder S, Horisberger JD, Rossier BC. Functional expression of a pseudohypoaldosteronism type I mutated epithelial Na+ channel lacking the pore-forming region of its alpha subunit. J Clin Invest. 1999;104:967–974. doi: 10.1172/JCI6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awayda MS, Ismailov II, Berdiev BK, Benos DJ. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am J Physiol. 1995;268:C1450–C1459. doi: 10.1152/ajpcell.1995.268.6.C1450. [DOI] [PubMed] [Google Scholar]
- 10.Kizer N, Guo X-L, Hruska K. Reconstitution of stretch-activated cation channels by expression of the -subunit of the epithelial sodium channel cloned from osteoblasts. Proceedings of the National Academy of Sciences, USA. 1997;94:1013–1018. doi: 10.1073/pnas.94.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. J Gen Physiol. 1996;107:35–45. doi: 10.1085/jgp.107.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol. 2002;282:F501–F505. doi: 10.1152/ajprenal.00147.2001. [DOI] [PubMed] [Google Scholar]
- 13.Awayda MS, Subramanyam M. Regulation of the epithelial Na+ channel by membrane tension. J Gen Physiol. 1998;112:97–111. doi: 10.1085/jgp.112.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji H-L, Fuller CM, Benos DJ. Osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes. American Journal of Physiology. 1998;275:C1182–C1190. doi: 10.1152/ajpcell.1998.275.5.C1182. [DOI] [PubMed] [Google Scholar]
- 15.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem. 2004;279:4120–4126. doi: 10.1074/jbc.M311783200. [DOI] [PubMed] [Google Scholar]
- 16.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 17.Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H(+)-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- 18.Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B. Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest. 1998;102:1634–1640. doi: 10.1172/JCI3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha- ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 20.McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, McCray PB, Jr, Stokes JB, Welsh MJ, Williamson RA. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci U S A. 1999;96:1727–1731. doi: 10.1073/pnas.96.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- 22.Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron. 1998;21:1435–1441. doi: 10.1016/s0896-6273(00)80661-3. [DOI] [PubMed] [Google Scholar]
- 23.Drummond HA, Abboud FM, Welsh MJ. Localization of beta and gamma subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res. 2000;884:1–12. doi: 10.1016/s0006-8993(00)02831-6. [DOI] [PubMed] [Google Scholar]
- 24.Fricke B, Lints R, Stewart G, Drummond H, Dodt G, Driscoll M, von During M. Epithelial Na+ channels and stomatin are expressed in rat trigeminal mechanosensory neurons. Cell Tissue Res. 2000;299:327–334. doi: 10.1007/s004419900153. [DOI] [PubMed] [Google Scholar]
- 25.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Expression and localization of acid-sensing ion channels in mouse nodose ganglia. Faseb J. 2006;20:A775. [Google Scholar]
- 27.Snitsarev V, Whiteis CA, Abboud FM, Chapleau MW. Mechanosensory transduction of vagal and baroreceptor afferents revealed by study of isolated nodose neurons in culture. Auton Neurosci. 2002;98:59–63. doi: 10.1016/s1566-0702(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 28.Sabharwal R, Stauss HM, Lazartigues E, Whiteis CA, Davisson RL, Price MP, Welsh MJ, Abboud FM, Chapleau MW. Abnormalities in baroreflex sensitivity and autonomic control in conscious ASIC2−/− mice. Faseb J. 2006;20:A1186. [Google Scholar]
- 29.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 30.Smeda JS, Payne GW. Alterations in autoregulatory and myogenic function in the cerebrovasculature of Dahl salt-sensitive rats. Stroke. 2003;34:1484–1490. doi: 10.1161/01.STR.0000073842.18224.AA. [DOI] [PubMed] [Google Scholar]
- 31.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension. 2004;44:643–648. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- 32.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol. 2005;289:F891–F901. doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- 33.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous beta and gammaENaC. Am J Physiol Renal Physiol. 2006;291:F1184–F1191. doi: 10.1152/ajprenal.00177.2006. [DOI] [PubMed] [Google Scholar]
- 34.Gannon KP, Galmiche L, Drummond HA. ASIC2 protein is required for pressure-induced constriction in mouse middle cerebral artery. Am J Physiol Heart Circ Physiol. 2008 Feb 22; doi: 10.1152/ajpheart.01380.2007. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 35.Oyabe A, Masumoto N, Ueta K, Nakayama K. Amiloride-sensitive pressure-induced myogenic contraction in rat cerebral artery. Fundam Clin Pharmacol. 2000;14:369–377. doi: 10.1111/j.1472-8206.2000.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 36.Guan ZCA, Inscho E. Effect of ENaC blockade on the myogenic response of rat juxtamedullary afferent arterioles. Faseb J. 2007;21 doi: 10.1161/HYPERTENSIONAHA.109.137992. 595.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric Assembly of Acid-sensitive Ion Channel and Epithelial Sodium Channel Subunits. J Biol Chem. 2007;282:25548–25559. doi: 10.1074/jbc.M703825200. [DOI] [PubMed] [Google Scholar]
- 38.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 39.Van Renterghem C, Lazdunski M. A new non-voltage-dependent, epithelial-like Na+ channel in vascular smooth muscle cells. Pflugers Arch. 1991;419:401–408. doi: 10.1007/BF00371123. [DOI] [PubMed] [Google Scholar]
- 40.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 42.Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-Sensing Ion Channels Contribute to Transduction of Extracellular Acidosis in Rat Carotid Body Glomus Cells. Circ Res. 2007;101:1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- 43.Sabharwal R, Chapleau MW, Price MP, Welsh MJ, Abboud FM. Molecular Mechanisms of Baro- and Chemoreceptor Activation: Evidence that ASIC1 and ASIC3 Contribute to Chemoreceptor Activation. Hypertension. 2005;46:832. [Google Scholar]
- 44.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol. 2004;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- 47.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581:1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guyton AC, Hall JE. Textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
- 49.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 50.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 52.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 53.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 54.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 55.Wang W. Chronic administration of aldosterone depresses baroreceptor reflex function in the dog. Hypertension. 1994;24:571–575. doi: 10.1161/01.hyp.24.5.571. [DOI] [PubMed] [Google Scholar]
- 56.Yee KM, Struthers AD. Aldosterone blunts the baroreflex response in man. Clin Sci (Lond) 1998;95:687–692. doi: 10.1042/cs0950687. [DOI] [PubMed] [Google Scholar]
- 57.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73:198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gekle M, Freudinger R, Mildenberger S, Silbernagl S. Rapid actions of aldosterone on cells from renal epithelium: the possible role of EGF-receptor signaling. Steroids. 2002;67:499–504. doi: 10.1016/s0039-128x(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 59.Grossmann C, Freudinger R, Mildenberger S, Krug AW, Gekle M. Evidence for epidermal growth factor receptor as negative-feedback control in aldosterone-induced Na+ reabsorption. Am J Physiol Renal Physiol. 2004;286:F1226–F1231. doi: 10.1152/ajprenal.00378.2003. [DOI] [PubMed] [Google Scholar]