Abstract
Loss of Cdc2 activity following Cyclin B degradation is necessary, but not sufficient, for mitotic exit. Proteins phosphorylated by Cdc2 and downstream mitotic kinases must also be dephosphorylated. We report here that protein phosphatase-1 (PP1) is the major catalyst of mitotic phosphoprotein dephosphorylation. Suppression of PP1 during early mitosis is maintained through the dual inhibition of PP1 by Cdc2 phosphorylation and the binding of Inhibitor-1 (I1), which is facilitated by PKA-mediated I1 phosphorylation. As Cdc2 levels drop following Cyclin B degradation, autodephosphorylation of PP1 at the site of Cdc2 phosphorylation (T320) allows partial PP1 activation. This promotes PP1-regulated dephosphorylation of I1 at its activating site (T35), dissociation of the I1-PP1 complex, and full PP1 activation to promote mitotic exit. Thus, Cdc2 both phosphorylates multiple mitotic substrates and inhibits their PP1-mediated dephosphorylation.
Introduction
Exit from mitosis requires destruction of Cyclin B protein, Cdc2 kinase inactivation, and dephosphorylation of mitotic phosphoproteins. The identity and regulation of the vertebrate phosphatase(s) responsible for this dephosphorylation have not been established1.
In yeast, mitotic phosphoprotein dephosphorylation is catalyzed by the phosphatase Cdc14. Prior to M phase exit, Cdc14 is sequestered within the nucleolus through association with its inhibitor, Net1. Cdc2-mediated Net1 phosphorylation promotes Cdc14 release from the nucleolus to allow dephosphorylation of mitotic phosphoproteins; nucleolar exclusion is then maintained by the mitotic exit network (MEN)2–4. Although Cdc14 homologs exist in vertebrates, they do not seem to play a similar role in these cells, though yeast and vertebrate Cdc14 proteins share a conserved role in cytokinesis.5
In Aspergillus, Drosophila, and S Pombe, genetic analyses have suggested a role for PP1 in controlling mitotic exit6–9. In attempting to purify a phosphatase from interphase Xenopus egg extracts able to dephosphorylate mitotic phosphoproteins, Che et al concluded that the predominant phosphatase was neither PP1 nor protein phosphatase 2A (PP2A), but they noted that this activity might differ from the phosphatase acting at mitotic exit10. Skoufias et al reported a requirement for okadaic acid (OA)-inhibitable phosphatase(s) in dephosphorylating Cdc2 substrates in human cells11. Finally, calcineurin is required for the specialized exit from M phase triggered by Ca2+ in the cytostatic factor (CSF)-arrested egg12, 13. It was also noted that an additional unidentified phosphatase activity was required for full dephosphorylation of M phase phosphoproteins and release from CSF arrest12, 13.
In studying the early embryonic mitotic cycles in Xenopus, we have found that PP1 dephosphorylates mitotic phosphoproteins required for mitotic exit. PP1 is not similarly required for exit from CSF arrest. Furthermore, we have identified a regulatory loop that controls PP1 to promote the timely dephosphorylation of mitotic substrates. As reported previously, PP1 can be inhibited by Cdc2-mediated phosphorylation at T32014, 15. We show that PP1 auto-dephosphorylates T320, but that this activity is inhibited during M phase by the association of PP1 with its inhibitor, I1. Activation of I1 through PKA-mediated phosphorylation of T35 is enhanced in M phase, both because PKA activity is elevated and the rate of T35 dephosphorylation is low. When Cyclin B is destroyed at mitotic exit, Cdc2 activity drops, allowing PP1 auto-dephosphorylation to predominate, promoting partial PP1 activation. PP1-regulated dephosphorylation of T35 then inactivates I1, allowing complete PP1 activation, dephosphorylation of mitotic phosphoproteins, and M phase exit.
Results
Okadaic acid-inhibitable phosphatases are required for M phase exit
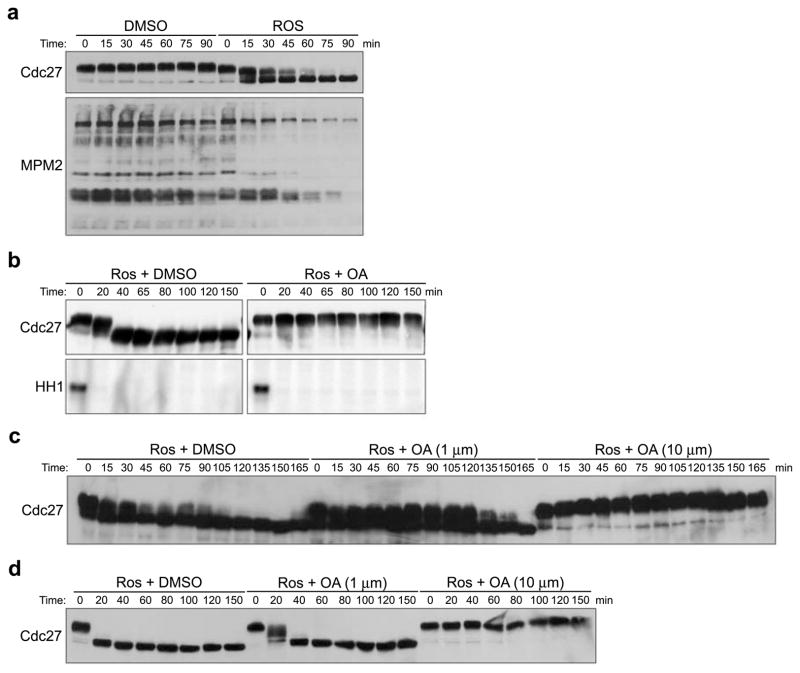
To characterize phosphatases involved in dephosphorylation of mitotic phosphoproteins, without having to account for possible effects of phosphatases in controlling the APC and Cyclin degradation, we supplemented interphase egg extracts with recombinant, non-degradable Cyclin B to drive mitotic entry, and then inhibited Cdc2 using the Cdk inhibitor, Roscovitine (Ros). This inhibition resulted in marked dephosphorylation of M phase substrates, including the APC subunit, Cdc27, and multiple mitotic phosphoproteins recognized by the MPM-2 antibody (Fig. 1A). This dephosphorylation was inhibited by OA, consistent with the involvement of PP1 or PP2A-like phosphatases (Fig. 1B)11, 12. Similar results were obtained when we treated CSF-arrested extracts with Ros (S. Fig. 1). OA treatment did not affect Cdc2 kinase activity (Fig. 1B and S. Fig. 1).
Figure 1. Dephosphorylation of mitotic phosphoproteins by okadaic acid-sensitive phosphatase activity.
A. Mitotic extracts were supplemented with DMSO or Roscovitine (Ros, 0.28mM). Aliquots were taken at the indicated times and immunoblotted with anti-Cdc27 or MPM2 antibodies.
B. Ros (0.28mM) was added to mitotic extracts that had been pre-treated with DMSO or OA (10μM). Aliquots were withdrawn at the indicated times to immunoblot for Cdc27 and to measure Cdc2 kinase activity using Histone H1 (HH1) as an exogenous substrate. Full scan of Cdc27 blot and HH1 kinase assay is shown in S. Fig. 5A.
C and D. Ros (0.28mM) was added to mitotic extracts (A) or CSF extracts (B) in the presence of either DMSO or different concentrations of OA. Samples were taken at the indicated times and blotted with anti-Cdc27 antibody.
PP1 is required for dephosphorylation of substrates at mitotic, but not meiotic exit
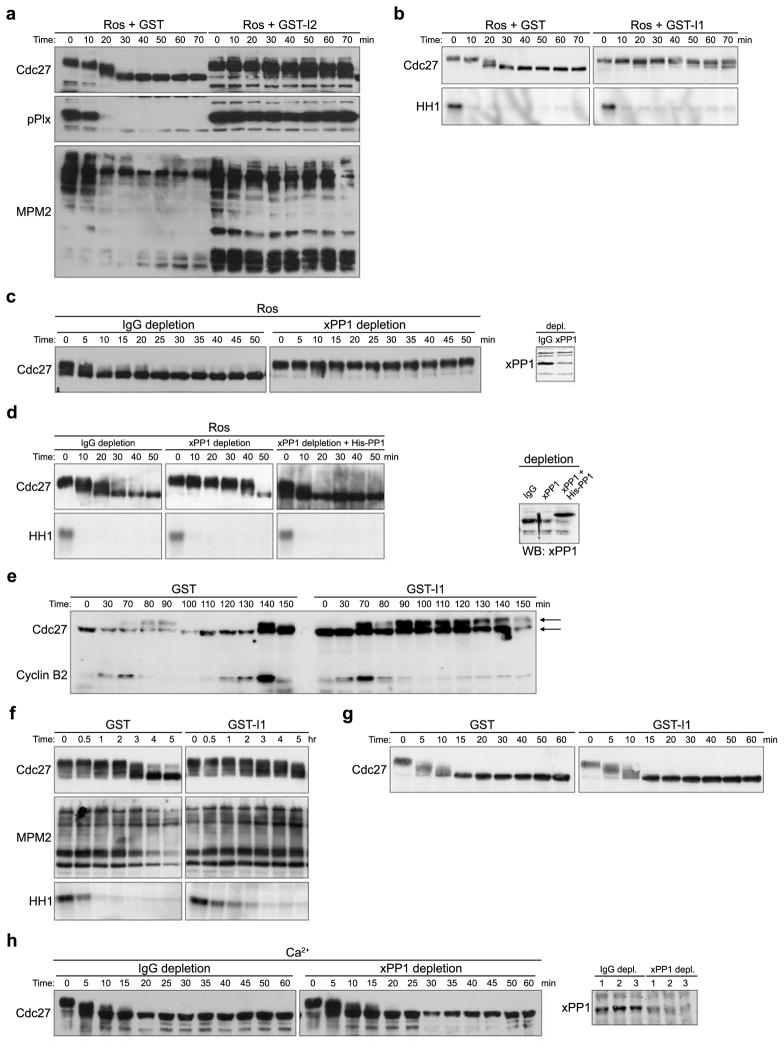
As shown in Fig. 1C and 1D, the phosphatase promoting M phase exit was sensitive to 10μM, but not 1μM OA, consistent with the responsible phosphatase being PP1-like, rather than PP2A-like16. We added a PP1-specific inhibitor (either I1 or I2) to Ros-treated extracts and monitored Cdc27 and MPM2 epitope dephosphorylation. As shown in Fig. 2A and 2B, both I1 and I2 inhibited dephosphorylation of mitotic phosphoproteins (but not Cdc2 kinase activity). We then immunodepleted PP1 from either mitotic or CSF-arrested meiotic extracts prior to Ros addition and found that PP1 depletion prevented Cdc27 dephosphorylation in mitotic extracts and largely prevented dephosphorylation in the CSF-arrested extract, without affecting Cdc2 kinase activity (Fig. 2C and 2D). Adding recombinant PP1 back to the depleted extract restored Cdc27 dephosphorylation (Fig. 2D). Cdc27 exhibited partial dephosphorylation even in the presence of PP1-specific inhibitors or after PP1 depletion, reflecting either incomplete PP1 inhibition/depletion or the possible involvement of additional phosphatases.
Figure 2. PP1 is required for the dephosphorylation of substrates at mitotic, but not meiotic exit.
A. Ros (0.28mM) was added to CSF extracts in the presence of GST or GST-I2. Aliquots were withdrawn at the indicated times and immunoblotted with anti-Cdc27, anti-pPlx, or MPM2 antibody. Full scans of Cdc27 and pPlx blots are shown in S. Fig. 5B.
B. Ros (0.28mM) was added to CSF extracts in the presence of GST or GST-I1. Aliquots were withdrawn at the indicted times for Cdc27 immunoblotting and HH1 kinase assays.
C. Left: PP1 was immunodepleted from mitotic extracts using anti-Xenopus PP1 antibodies. Rabbit IgG was used for mock depletion. Control or PP1-depleted extracts were treated with Ros (0.28mM). Aliquots were immunoblotted with anti-Cdc27 antibody. Right: Control and PP1-depleted extracts were immunoblotted with PP1 antibodies.
D. Left: Same as Figure 2C except that CSF extract was used for PP1 depletion. PP1 depleted extract was supplemented with buffer or recombinant His-PP1 (0.5 μM). In addition to Cdc27 immunoblotting, HH1 kinase activity was assayed. Right: Extracts were also immunoblotted with PP1 antibodies. Full scan of PP1 blot is shown in S. Fig. 5C.
E. GST or thio-phosphorylated GST-I1 was added to cycling extracts and aliquots were withdrawn at the indicated times and immunoblotted with anti-Cdc27 and anti-Cyclin B2 antibodies. Arrows indicate gel mobilities of Cdc27. Full scan of Cdc27 and Cyclin B2 blots are shown in S. Fig. 5D.
F. HeLa cells were arrested in Mitosis with Nocodazole for 17 hours, and then allowed to resume cell cycle in fresh DMEM for 1 hour. Cell lysates were made and supplemented with GST or GST-I1. Aliquots were taken to immunoblot with MPM2 antibody and to measure HH1 kinase activity.
G. Ca2+ was added to CSF extracts in the presence of GST or GST-I1. Aliquots were immunoblotted with anti-Cdc27 antibody.
H. Left: PP1 antibodies were used for PP1 immunodepletion and rabbit IgG was used for control depletion. Calcium was added to the depleted CSF extracts. Aliquots were taken at the indicated times and blotted with anti-Cdc27 antibody. Right: Aliquots of depleted extracts were blotted with anti-PP1 antibody.
Although Ros ought to have mimicked the shut off of Cdc2 upon Cyclin degradation, to confirm that PP1 was involved in the dephosphorylation of Cdc2 substrates following physiological Cyclin degradation, we added I1 to cycling extracts of Xenopus eggs (that oscillate between S phase and M phase) immediately prior to mitosis. As shown in Fig 2E, this largely prevented Cdc27 dephosphorylation. The timing of I1 addition was critical because if added too early, it could inhibit mitotic entry, perhaps reflecting the requirement for PP1 in promoting Cdc25 activation17, 18.
To investigate further the physiological relevance of PP1-mediated M phase substrate dephosphorylation, we examined the effect of PP1 inhibition in HeLa cell lysates. Cell lysates prepared from mitotically-arrested HeLa cells exited mitosis spontaneously (through Cyclin B degradation, S. Fig. 2A); both I1 and I2 largely prevented dephosphorylation of M phase phosphoproteins (Fig. 2F and S. Fig. 2B). Cdc2 kinase assays confirmed that the delayed mitotic substrate dephosphorylation was due to PP1 inhibition and not sustained Cdc2 kinase activity. We did not examine mitotic exit following knock-down of PP1 in intact HeLa cells as PP1 is required for mitotic entry17.
Release from CSF arrest requires calcineurin to promote M phase substrate dephosphorylation12, 13. As reported, Cdc27 dephosphorylation following Ca2+ addition to CSF extracts was markedly delayed by the calcineurin inhibitor, cyclosporine A (S. Fig. 2C). It has also been reported that an additional phosphatase cooperates with calcineurin to achieve full dephosphorylation of meiotic phosphoproteins12, 13. Since neither I1 addition nor PP1 depletion altered Cdc27 dephosphorylation following Ca2+ addition to CSF-arrested extracts, PP1 is unlikely to be the additional phosphatase required for CSF release(Fig. 2G and 2H).
Cdc2 regulation of PP1 prevents premature substrate dephosphorylation in mitosis
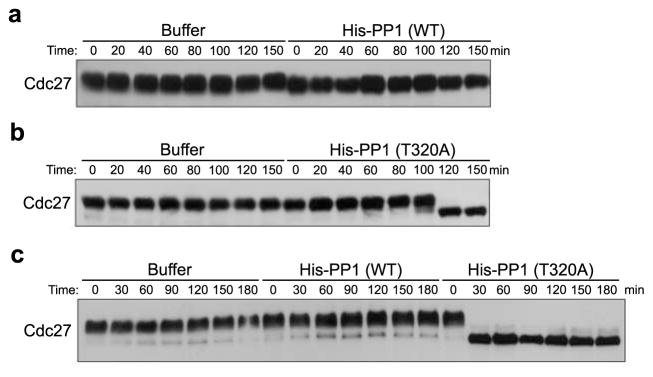
Because PP1 seemed to be required for dephosphorylation of M phase phosphoproteins in the mitotic (though not meiotic) cycle, we thought excess PP1 might drive M phase substrate dephosphorylation even in the absence of Ros. However, excess PP1 (added to levels 8-fold greater than those found endogenously (0.5 μM)), did not drive Cdc27 dephosphorylation in mitotic (or meiotic) extracts (Fig. 3A). The PP1 added was active and able to dephosphorylate Cdc25 at a known PP1 site (S. Fig. 3). These data suggested that PP1 might be turned off in M phase to prevent premature dephosphorylation of M phase phosphoproteins. This was interesting in that Skoufias et al11 suggested that Cdc2 activity could inhibit dephosphorylation of Cdc2/Cyclin B substrates.
Figure 3. Cdc2 regulation of PP1 prevents premature substrate dephosphorylation in mitosis.
A. Mitotic extracts were supplemented with rabbit His-PP1 catalytic subunit (4 μM) or buffer control. Aliquots were withdrawn at the indicated time points for Cdc27 immunoblotting.
B. Mitotic extracts were supplemented with rabbit His-PP1T320A (4 μM) or buffer control. Aliquots were taken at the indicated time points for Cdc27 immunoblotting.
C. CSF extracts were supplemented with rabbit His-PP1 wild type (WT), rabbit His-PP1T320A (4 μM) or buffer control. Samples were withdrawn at the indicated time points for Cdc27 immunoblotting.
It is known that Cdc2 can inhibit PP1 and that PP1 phosphorylation at T320 peaks in mitosis in mammalian cells14. However, the physiological consequences of altering this phosphorylation have not been reported. Accordingly, we mutated T320 to A, and added this recombinant PP1 to mitotic egg extracts. As shown in Fig. 3B and 3C, when added at levels 8-fold greater than that found endogenously, the mutant, but not the WT PP1 promoted Cdc27 dephosphorylation, suggesting that PP1 inhibition by Cdc2 can be overcome by mutation of T320. However, at lower levels (~2–4 fold that found endogenously) this mutant protein alone was insufficient to drive dephosphorylation of Cdc2 substrates (Fig. 4K and 4L), raising the possibility that another cell cycle-regulated activity might physiologically control PP1.
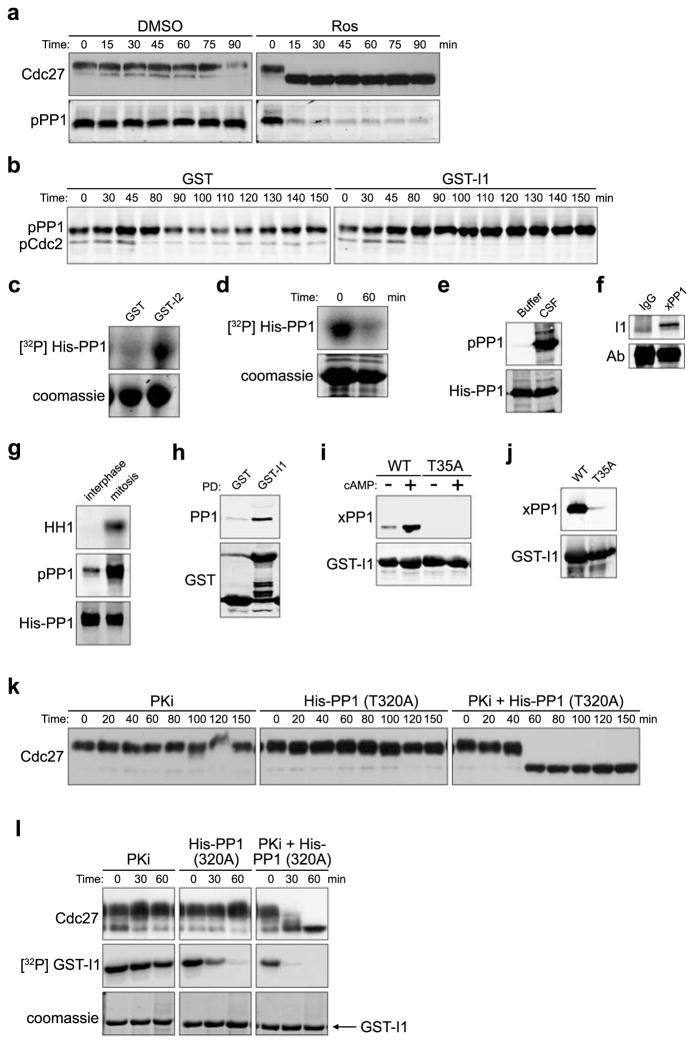
Figure 4. PP1 autodephosphorylation and I1 dephosphorylation control PP1-regulated Cdc2 substrate dephosphorylation.
A. CSF extracts treated with DMSO or Ros (0.28 mM) were immunoblotted for Cdc27 and PP1pT320.
B. GST or thio-phosphorylated GST-I1 was added to cycling extracts and samples were immunoblotted with Cdc2pY15 (mitotic entry coincides with Y15 dephosphorylation) and PP1pT320 antibodies. Full scan in S. Fig. 5F.
C. Ni-NTA agarose-bound His-PP1 was phosphorylated by Cdc2/Cyclin B in the presence of GST or GST-I2 and γ32P ATP for 30 min. His-PP1 phosphorylation was detected by SDS-PAGE/phosphorimager. Full scan in S. Fig. 5G.
D. Ni-NTA agarose-bound His-PP1 was pre-phosphorylated with high concentrations of Cdc2/Cyclin B and γ32P ATP for 30min. Ros (0.28mM) was then added and reactions were either stopped with sample buffer immediately or were incubated in buffer for an additional 60 min before resolution by SDS-PAGE/phosphorimager.
E. His-PP1 incubated with buffer or CSF extracts for 30 min, was retrieved and immunoblotted with anti-PP1pT320 or anti-His antibody. Full scan in S. Fig. 5H.
F. Protein A Sepharose-bound Xenopus PP1 antibody or control IgG were incubated with mitotic extracts for 1hr. Immunoprecipitates were blotted with anti-I1 antibody. Full scan in S. Fig. 5I.
G. His-PP1 protein was dipped into interphase or mitotic extracts for 10 min, washed and immunoblotted with anti-PP1pT320 or anti-His antibody. HH1 phosphorylation is shown.
H. GST or GST-I1 incubated with nocodazole-arrested HeLa lysates were retrieved and immunoblotted for GST or PP1.
I. Glutathione Sepharose-bound GST-I1 (WT) or GST-I1T35A incubated in interphase extracts +/− cAMP for 1 hour were washed and immunoblotted with anti-Xenopus PP1 or anti-GST antibodies. Full scan in S. Fig. 5J.
J. Glutathione Sepharose-bound GST-I1 (WT) or GST-I1T35A were incubated in mitotic extracts for 1 hour, washed, and immunoblotted with anti-Xenopus PP1 or anti-GST antibodies.
K. CSF extracts incubated with PKA specific inhibitor (PKi) H89 (0.2 mM), rabbit His-PP1T320A (2 μM) or PKI together with His-PP1T320 were immunoblotted with anti-Cdc27 antibody.
L. Glutathione Sepharose-bound GST-I1 was prephosphorylated by PKA and γ32P ATP, washed and added to CSF extracts. Extracts were treated as in 4K except GST-I1 was also retrieved and visualized by SDS-PAGE/phosphorimager.
PP1 autodephosphorylation and I1 phosphorylation cooperatively control PP1-mediated Cdc2 substrate dephosphorylation
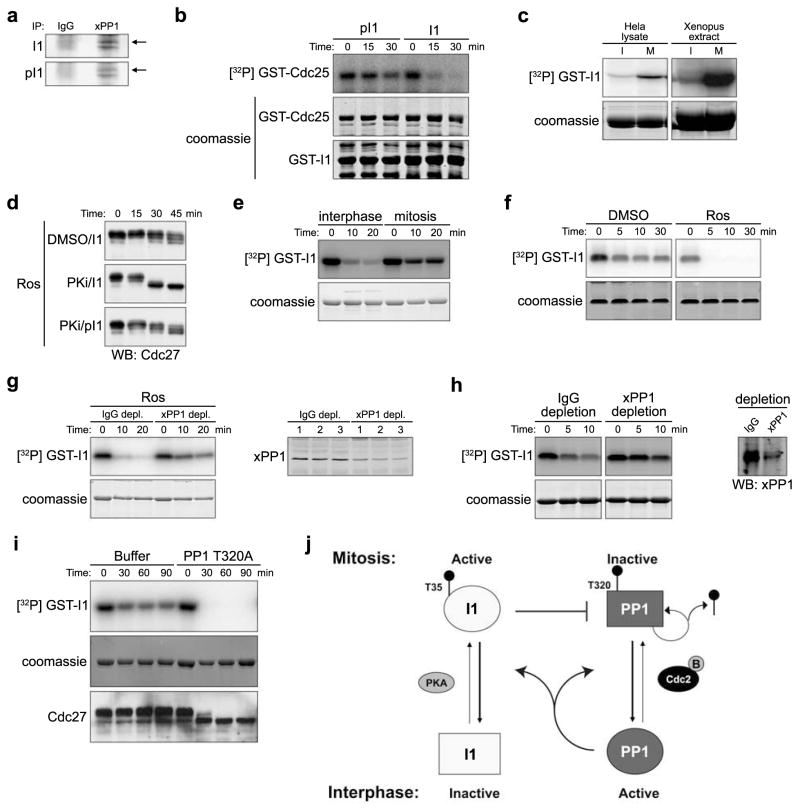
Consistent with the idea that PP1-mediated dephosphorylation of mitotic phosphoproteins could be inhibited by Cdc2 phosphorylation of T320, when we added Ros to mitotic egg extract, Cdc27 dephosphorylation and PP1 T320 dephosphorylation were coincident (Fig. 4A). To determine how T320 dephosphorylation might be regulated, we monitored PP1 T320 phosphorylation throughout the cell cycle in a cycling extract. In the extract shown in Fig. 4B, mitosis occurred at 80 min and interphase resumed at ~100 min. T320 of PP1 was noticeably dephosphorylated by 100 min, consistent with phosphorylation of this site inhibiting PP1 function (Fig. 4B, left panel; some interphase phosphorylation may have resulted from the constitutively active Cdk2 in egg extracts). When these experiments were repeated and extracts were supplemented with I1 immediately prior to mitotic entry, T320 dephosphorylation was abrogated (Fig. 4B, right panel), suggesting that PP1 might auto-dephosphorylate T320. Indeed, PP1 could be more robustly phosphorylated in vitro by recombinant Cdc2 when I2 was included in the reaction (Fig. 4C). More importantly, pre-phosphorylated purified PP1 was able to auto-dephosphorylate in vitro in the absence of any co-factors (Fig. 4D).
If PP1 could auto-dephosphorylate to relieve its Cdc2-mediated inhibition, we reasoned that there must be an additional factor restraining PP1 activity during M phase. Specifically, since I1 is controlled by phosphorylation, we speculated that cell cycle regulation of I1 phosphorylation might contribute to control of M phase exit19. PP1 phosphorylated at T320 in CSF extracts could be co-immunoprecipitated with endogenous I1 (Fig. 4E and 4F). Moreover, mitotic PP1 exhibited much higher phosphorylation than interphase PP1 (Fig. 4G). As in Xenopus egg extracts, I1 interacted with PP1 in mitotic HeLa cell lysate (Fig. 4H).
Protein kinase A (PKA) phosphorylates I1 and this phosphorylation is required for I1 activity20. If I1-mediated phosphorylation contributes to M phase restraint of PP1, activation of PKA should strengthen I1-PP1 interactions. Accordingly, the PKA activator, 8-bromo cyclic AMP, enhanced binding of PP1 to I1 (Fig 4I). Moreover, WT I1 bound more strongly to PP1 than T35A mutant I1 lacking the site of PKA phosphorylation; T35A mutant binding could not be enhanced by 8-bromo cyclic AMP (Fig. 4I and 4J). When CSF extracts were treated with the PKA-specific inhibitor PKI, the T320A PP1 mutant, or both, PKI synergized with low levels of the T320A mutant PP1 (4-fold endogenous), allowing dephosphorylation of Cdc2 substrates, even without Ros addition (Fig. 4K). Moreover, Cdc2 substrate dephosphorylation correlated with I1 dephosphorylation (Fig. 4L). Thus, inhibiting I1 activation and preventing Cdc2-mediated PP1 inhibition could swing the Cdc2/PP1 balance in favor of substrate dephosphorylation.
Cell cycle regulation of I1
This foregoing suggested that phosphorylation/dephosphorylation of I1 must be under cell cycle control. To assess this, we first examined phosphorylation of mitotic I1. As shown in Fig. 5A, I1 co-precipitated with PP1 was recognized by anti-phospho-T35 I1 antibody. PKA-mediated I1 T35 phosphorylation was, as reported, required for I1’s PP1 inhibitory activity (Fig. 5B). Moreover, PKA immunoprecipitated from mitotic egg extract or HeLa cell lysates phosphorylated I1 much more robustly than PKA from interphase extracts/lysates (Fig. 5C), consistent with a previous report that PKA activity peaks in mitosis21. Equal amounts of PKA were immunoprecipitated from interphase and mitotic extracts (S Fig. 4A). In addition, I1 lost significant activity when PKA was inhibited with PKI (Fig. 5D). Conversely, thio-phosphorylated I1 largely inhibited PP1 (and consequently Cdc27 dephosphorylation) even in the presence of PKI, indicating that I1 is a key mitotic target of PKA in preventing dephosphorylation of M phase substrates.
Figure 5. I1 Cell cycle regulation.
A. Protein A Sepharose-bound Xenopus PP1 antibody or control IgG were incubated in mitotic extracts for 1hr. Immunoprecipitates resolved by SDS-PAGE were immunoblotted for I1 or p-I1 (pT35).
B. Glutathione Sepharose-bound GST-Cdc25 pre-phosphorylated with Chk1 and γ32P ATP were dipped into phosphatase buffer in the presence of His-PP1 and either GST-I1 or T35 thio-phosphorylated GST-I1. Aliquots were resolved by SDS-PAGE/phosphorimager.
C. PKA was immunoprecipitated from interphase (I) or mitotic (M) HeLa cell lysate (Left) or Xenopus egg extracts (Right). Immunoprecipitates were incubated with GST-I1 and γ32P ATP for 20 min and resolved by SDS-PAGE/phosphorimager.
D. CSF extracts pretreated with DMSO or PKi (0.2 mM) for 15 min were supplemented with GST-I1 that had been either pre-thiophosphorylated or not. Samples were then immunoblotted for Cdc27.
E. Glutathione Sepharose-bound GST-I1 was prephosphorylated by PKA and γ32P ATP and dipped in interphase or mitotic extracts, retrieved at the indicated times and resolved by SDS-PAGE/phosphorimager.
F. Glutathione Sepharose-bound GST-I1 was prephosphorylated as in 5E and then dipped in CSF extracts or Ros-pretreated CSF extracts and processed as in 5E.
G.(Left): Glutathione Sepharose-bound GST-I1 was prephosphorylated as in 5E and dipped into control or PP1 depleted CSF extracts in the presence of Ros. Aliquots were processed as in 5E. (Right): Aliquots of control or PP1-depleted extracts were immunoblotted for PP1.
H. (Left) Anti-PP1 or IgG-depleted CSF extracts were treated with Ca2+ for 20 min. Glutathione Sepharose-bound GST-I1 prephosphorylated as in 5E was dipped into the Ca2+ treated extracts. Aliquots were resolved by SDS-PAGE/phosphorimager. (Right) IgG- or PP1-depleted extracts were immunoblotted for PP1.
I. Glutathione Sepharose –bound GST-I1 prephosphorylated as in 5E was dipped into CSF extracts supplemented with Buffer or His-PP1T320A (4 μM), and processed as in 5E. Samples were also immunoblotted for Cdc27.
J. PP1 is inhibited both by Cdc2/Cyclin B and by phosphorylated I1 in mitosis. During mitotic exit, Cdc2 inactivation allows PP1 auto-dephosphorylation on T320 to prevail, and at the same time, active PP1 dephosphorylates I1 at T35, resulting in full activation of PP1.
We next sought to identify the I1-directed phosphatase. As shown in Fig. 5E, I1 radiolabeled in the presence of γ32P ATP and PKA was dephosphorylated in interphase, but not mitotic egg extract, suggesting that the I1-directed phosphatase might be cell cycle regulated (T35 is the only PKA site on I1, S. Fig. 4B). When we treated CSF extracts with Ros, we detected I1 dephosphorylation, suggesting that I1 might be controlled by a Cdc2-inhibited phosphatase (Fig. 5F). Importantly, I1 dephosphorylation was inhibited in PP1-depleted extracts (Fig. 5G). Similar results were obtained in CSF extracts supplemented with calcium (Fig. 5H). Finally, PP1 T320A mutant addition alone (to 8-fold endogenous PP1) dramatically promoted I1 dephosphorylation and this correlated directly with the gel mobility downshift of Cdc27 (Fig. 6I).
Discussion
In addition to Cyclin B destruction, mitotic exit requires dephosphorylation of mitotic phosphoproteins11, 22. We show here that the appropriately timed PP1-mediated dephosphorylation of these substrates results from the combined effects of Cdc2-mediated PP1 T320 phosphorylation, PP1 autodephosphorylation, and PKA-controlled I1-PP1 binding (Fig. 5J).
Control of PP1 activity
In that I1 prevented PP1 dephosphorylation in cycling extracts and I2 enhanced the weak in vitro phosphorylation of PP1 by Cdc2/Cyclin B, it appears that PP1 catalytic activity is required for T320 dephosphorylation. Indeed, prephosphorylated PP1 lost phosphorylation after incubation in the absence of any co-factor, substantiating the notion that PP1 can auto-dephosphorylate.
Although PP1 is off during M phase to ensure accumulation of mitotic phosphoproteins, some PP1 activity is required for Cdc25 activation to promote mitotic entry7. Upon entry into mitosis, PP1 activity can be suppressed through T320 phosphorylation and binding of I1, which is enhanced by the M phase peak in PKA-mediated T35 phosphorylation. Unlike in cardiac myocytes where PP2A and calcineurin appear to control I1 dephosphorylation or renal medulla, where PP2A regulates this dephosphorylation23, 24, PP1 appears to control I1 dephosphorylation in the embryonic mitotic cycles. Though we have shown that PP1 is required for T35 dephosphorylation, based on previous in vitro studies20, PP1 may not directly dephosphorylate T35 of I1. In theory, PP1 might act through its ability to dephosphorylate other sites in I1 that influence the phosphorylation/dephosphorylation of T35 or PP1 might regulate another phosphatase to promote I1 dephosphorylation25.
Though exogenous I2 could control PP1 in M phase extracts, it is not known if I2 is normally present in these extracts and/or if I2 is also cell cycle regulated. I2 does not require phosphorylation for activation and we have not seen cell cycle oscillations in stability or activity of exogenously added I2.
Additional phosphatases in control of M phase exit
As cells enter mitosis, the phosphorylation of mitotic proteins is precisely timed and spatially regulated. The same is likely true for dephosphorylation of mitotic substrates by PP1, whose regulation by subcellular location remains to be determined. Proteins phosphorylated at mitosis by enzymes other than Cdc2 may be subject to distinct regulation, either by PP1 or other phosphatases.
Although targeting subunits typically confer substrate recognition by PP1, substrate recognition can also occur via direct docking of PP118, 26. It will be interesting to determine if recognition of mitotic phosphoproteins occurs via a uniform mechanism or if different substrates are recognized by different targeting subunits/direct docking. Since cell cycle regulation of PP1 appears to be at the level of the catalytic subunit (thorough phosphorylation and I1 binding), there may be no need to invoke targeting subunit regulation to explain cell cycle-dependent dephosphorylation of mitotic phosphoproteins.
We saw no effect of PP1 depletion on Ca2+ –induced release from CSF arrest. Therefore, PP1 is unlikely to be the phosphatase that cooperates with calcineurin at meiotic exit. Because OA can inhibit full dephosphorylation of meiotic phosphoproteins, and PP1/PP2A represent >95% of the OA-sensitive phosphatase activity in egg extracts, PP2A may be involved.
Following PP1 depletion or inhibition, we still observed partial Cdc27 dephosphorylation at M phase exit. Thus, it remains possible that additional phosphatases contribute to mitotic substrate dephosphorylation. In yeast, Cdc14 is the major phosphatase catalyzing M phase substrate dephosphorylation. Interestingly, negative feedback loops also link Cdc2 and Cdc1427. Even within the same species different phosphatases might be utilized under different circumstances. Moreover, more than one phosphatase may cooperate in substrate dephosphorylation at mitosis, either within a single cell type or in a manner dependent on cell type or developmental stage. Our analysis demonstrates that PP1 makes a major contribution to M phase substrate dephosphorylation in vertebrate mitotic divisions and that a tightly regulated feedback loop ensures that dephosphorylation will occur in a timely manner, well-coordinated with the destruction of Cyclin B.
Methods
Cloning and protein expression
Xenopus PP1 cDNA was cloned from an oocyte cDNA library using the following primers: forward-GCCGAATTCATGGGGGACGGAGAAAAACTAAA and reverse-GGCTCTAGATCATTTGGACTGTTTGTTTTTG. It was then cloned into pMAL-c2X in frame with maltose-binding protein (MBP) or pGEX-KG in frame with GST. Rabbit His-PP1 was a gift from Dr. David Armstrong (NIEHS).
His-tagged proteins or GST-fusion proteins were expressed in bacteria and purified by using Ni-NTA Agarose (Invitrogen) or glutathione-Sepharose (Amersham) beads respectively. All eluted proteins were dialyzed into XB buffer (100 mM KCl, 50 mM sucrose, 10 mM Hepes, pH 7.7, 1 mM MgCl2, and 0.1 mM CaCl2, 0.5mM EGTA).
Antibodies
Rabbit polyclonal anti-xPP1 was raised against MBP-xPP1 protein and affinity purified using GST-xPP1. Anti-Cdc27 (BD Biosciences, Franklin Lakes, NJ), anti-MPM2 (Upstate, Lake Placid, NY), anti-pCdc2 (Upstate), anti-pPP1T320 (Abcam, Cambridge, MA), anti-Cyclin B1 (Santa Cruz, CA), anti-PKA (Abcam), and anti-pPlk1 (Abcam) antibodies were purchased. Anti-Cyclin B2 antibody was as reported28.
Xenopus Egg Extracts and immunodepletion
Xenopus cycling extracts, interphase extracts, and CSF extracts were prepared as described previously29. Mitotic extracts were prepared by adding non-degradable Cyclin B1 to interphase extracts.
For immunodepletion of PP1, CSF or mitotic extracts were incubated with purified polyclonal anti-xPP1 antibody coupled to protein A Sepharose beads for 30 min at 4 °C. Following incubation, the beads were removed, and the supernatants were treated again for another two consecutive depletions. The same amount of control rabbit IgG (Jackson Immuno Research) was used for mock depletion.
Tissue Culture
HeLa cells were treated with 40 ng/mL of Nocodazole for 17 hours to arrest in mitosis. The cells were then washed with PBS twice and cultured in fresh DMEM for another 1 hour. The cells were finally lysed in hypotonic buffer (20 mM HEPES pH 7.5, 5 mM KCl, 1.5 mM MgCl2 and 1 mM DTT) with dounce and cellular supernatants were collected after centrifuge.
Dephosphorylation assays
To generate prephosphorylated GST-Cdc25 or GST-I1, Glutathione-Sepharose beads coupled with GST fusion proteins were incubated in kinase buffer (10 mM Tris-HCl, pH 7.2, 0.1mM ATP, 2μCi γ-32P ATP, 10mM MgCl2, 1mM DTT, pH7.2) with Chk1 or PKA (New England Biolabs, Ipswich, MA) for 30min at room temperature, then washed. Glutathione-Sepharose beads bound to prephosphorylated proteins were then dipped into phosphatase buffer (10 mM Tris-HCl, pH 7.2, 10mM MgCl2, 1mM DTT) with His-PP1 or the indicated extracts. Aliquots were retrieved, washed with PBS plus 300 mM NaCl and 0.1% Triton, eluted by SDS sample buffer and subjected to SDS-PAGE. The phosphorylation was detected by phosphorimager (Molecular Dynamics).
Supplementary Material
Acknowledgments
This work was supported by RO1 GM67225 to SK and DA10044 to ACN.
References
- 1.Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 2.Azzam R, et al. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 2004;305:516–519. doi: 10.1126/science.1099402. [DOI] [PubMed] [Google Scholar]
- 3.Amon A. A decade of Cdc14--a personal perspective. Delivered on 9 July 2007 at the 32nd FEBS Congress in Vienna, Austria. FEBS J. 2008;275:5774–5784. doi: 10.1111/j.1742-4658.2008.06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Queralt E, Uhlmann F. Cdk-counteracting phosphatases unlock mitotic exit. Curr Opin Cell Biol. 2008;20:661–668. doi: 10.1016/j.ceb.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trautmann S, McCollum D. Cell cycle: new functions for Cdc14 family phosphatases. Curr Biol. 2002;12:R733–735. doi: 10.1016/s0960-9822(02)01250-2. [DOI] [PubMed] [Google Scholar]
- 6.Axton JM, Dombradi V, Cohen PT, Glover DM. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990;63:33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- 7.Bailis JM, Roeder GS. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell. 2000;101:211–221. doi: 10.1016/S0092-8674(00)80831-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, et al. Multiple protein phosphatases are required for mitosis in Drosophila. Curr Biol. 2007;17:293–303. doi: 10.1016/j.cub.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 9.Doonan JH, Morris NR. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989;57:987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- 10.Che S, et al. A phosphatase activity in Xenopus oocyte extracts preferentially dephosphorylates the MPM-2 epitope. FEBS Lett. 1998;424:225–233. doi: 10.1016/s0014-5793(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 11.Skoufias DA, Indorato RL, Lacroix F, Panopoulos A, Margolis RL. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J Cell Biol. 2007;179:671–685. doi: 10.1083/jcb.200704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449:341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- 14.Kwon YG, Lee SY, Choi Y, Greengard P, Nairn AC. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc Natl Acad Sci U S A. 1997;94:2168–2173. doi: 10.1073/pnas.94.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohadwala M, et al. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc Natl Acad Sci U S A. 1994;91:6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol. 2007;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis SS, et al. A role for PP1 in the Cdc2/Cyclin B-mediated positive feedback activation of Cdc25. Mol Biol Cell. 2006;17:1779–1789. doi: 10.1091/mbc.E05-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis SS, et al. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. EMBO J. 2003;22:5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder GL, et al. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kotani S, et al. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- 22.Stegmeier F, Amon A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- 23.El-Armouche A, et al. Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun. 2006;346:700–706. doi: 10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi E, Nishi A, Higashi H, Ito Y, Kato H. Phosphorylation of protein phosphatase-1 inhibitors, inhibitor-1 and DARPP-32, in renal medulla. Eur J Pharmacol. 2000;408:107–116. doi: 10.1016/s0014-2999(00)00767-6. [DOI] [PubMed] [Google Scholar]
- 25.Bibb JA, et al. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5. J Biol Chem. 2001;276:14490–14497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi M, Villa-Moruzzi E. Binding of phosphatase-1 delta to the retinoblastoma protein pRb involves domains that include substrate recognition residues and a pRB binding motif. Biochem Biophys Res Commun. 2001;280:1–3. doi: 10.1006/bbrc.2000.4067. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe BA, McDonald WH, Yates JR, 3rd, Gould KL. Phospho-regulation of the Cdc14/Clp1 phosphatase delays late mitotic events in S. pombe. Dev Cell. 2006;11:423–430. doi: 10.1016/j.devcel.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Casaletto JB, et al. Inhibition of the anaphase-promoting complex by the Xnf7 ubiquitin ligase. J Cell Biol. 2005;169:61–71. doi: 10.1083/jcb.200411056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.