Abstract
In only 5 simple steps and 48% overall yield from the natural trioxane artemisinin, the thermally and hydrolytically stable trioxane fluoroanilide 4b has been prepared. Upon one oral dose of only 6.8 mg/kg of monomeric trioxane 4b combined with 20 mg/kg of mefloquine hydrochloride, all of the malaria-infected mice lived until at least day 30 post infection. Of the five mice in this surviving group, four (80%) were completely cured (no parasites in their blood), and one mouse had 4% blood parasitemia. Importantly, the efficacy of this ACT chemotherapy using monomeric trioxane 4b plus mefloquine hydrochloride is considerably better than the efficacy under the same conditions using the popular trioxane drug artemether plus mefloquine hydrochloride.
Introduction
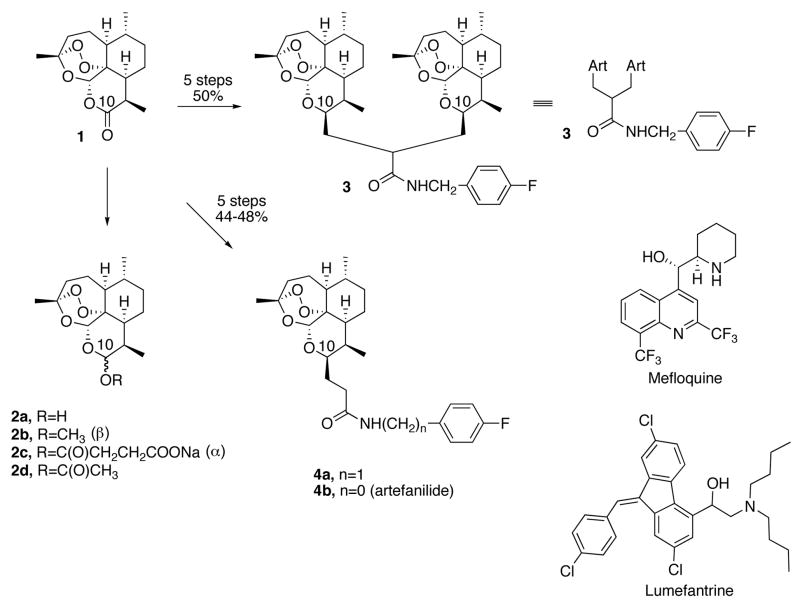
Resistance of malaria parasites especially to chloroquine is now widespread, seriously compromising the efficacy of this antimalarial drug that has been so widely and successfully used during the past 60 years.1 Today, as recommended by the World Health Organization (WHO)2 and as adopted by most countries where malaria is endemic, artemisinin (1) combination therapy (ACTa) is popular.3–7 Typically, natural trioxane 1 derivatives like artemether (2b) or sodium artesunate (2c) are used. One leading ACT drug for chemotherapy of people sick with malaria is a fixed 1:6 combination of the trioxane 2b with the amino-alcohol lumefantrine.8 Typically, a six-dose adult regimen requires a total of approximately 320 mg of trioxane 2b and 1920 mg of lumefantrine. A second ACT drug requires a three-dose adult regimen totaling 600 mg of trioxane 2c and 750 mg of the quinoline antimalarial mefloquine. Patient compliance with adhering to a repeated dose regimen, however, is often a serious problem. Therefore, a single dose oral cure is highly desirable. Toward this goal, we have recently reported a “proof of principle” advance in malaria chemotherapy: a single 144 mg/kg oral dose cure of malaria-infected mice by a new trioxane dimer sulfone carbamate.9 We have now prepared and tested the in vivo antimalarial efficacies of the fluorobenzyl amide trioxane dimer 3, of the corresponding trioxane monomer fluorobenzyl amide 4a, and of the trioxane monomer fluoroanilide 4b (artefanilide). Monomeric trioxane fluoroanilide 4b is the most efficacious; using only one single-digit mg/kg oral dose of this new trioxane monomer combined with a 3-fold higher amount of mefloquine hydrochloride prolonged survival of malaria-infected mice until at least day 30. The total amount of fluoroanilide 4b and mefloquine hydrochloride needed to achieve this single oral dose high efficacy compares favorably with the amounts of the antimalarial trioxane drugs 2b and 2c plus amine currently used clinically in repeated oral dose human ACT chemotherapy.8
Results and Discussion
Chemistry
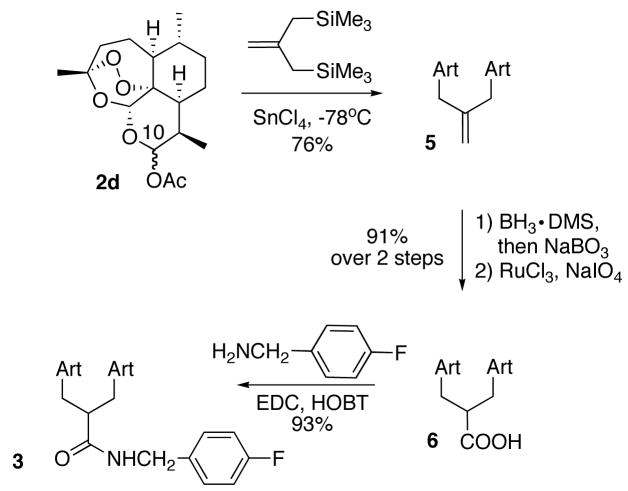
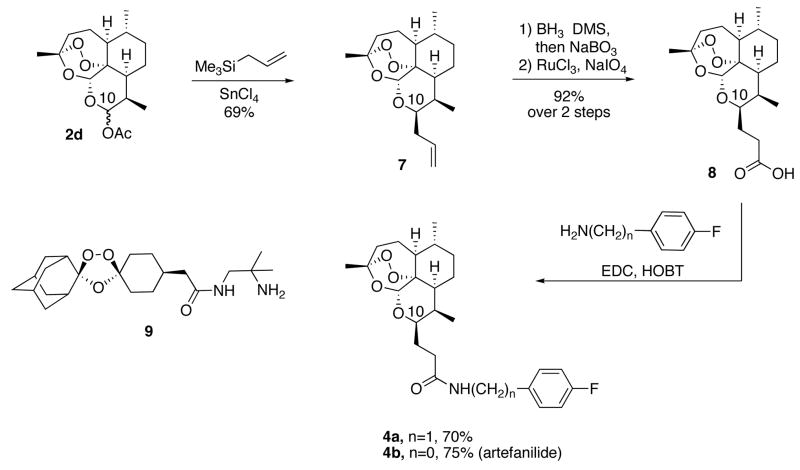
Monomeric trioxane dihydroartemisin C-10 acetate (2d) has been converted into dimeric trioxane 5 and then into dimer carboxylic acid 6 (Scheme 2).10 Facile amidation produced trioxane dimer fluorobenzyl amide 3. Also, C-10 acetate 2d has been converted into C-10-allyl derivative 7 and then, via hydroboration followed by oxidation, into monomeric trioxane carboxylic acid 8 (Scheme 3).11 Monomeric trioxane carboxylic acid 8 can be transformed in one step directly into a diverse library of monomeric trioxane amides.11 For example, one-step amidation of carboxylic acid 8 produced trioxane fluorobenzyl amide 4a, a monomeric version of dimer fluorobenzyl amide 3 (Scheme 3). In the same way, monomer trioxane fluoroanilide 4b was prepared directly and in good yield from carboxylic acid 8 (Scheme 3). The overall yield of 4b is approximately 48% from natural trioxane 1, and scale up to multigram or even kilogram amounts is expected to be straightforward. Fluoroanilide 4b is stable as a solid in the absence of solvent for at least 7 days at 60 °C and for at least 1 day at 70 °C. Because amides 3, 4a, and 4b are also C-10 non-acetal trioxanes, they are all more hydrolytically stable than the C-10 acetal trioxane drugs 2b and 2c.
Scheme 2.
Scheme 3.
Biology
Each trioxane 2b, 3, 4a, and 4b (0.90 mg) was dissolved in 0.11 mL of 7:3 Tween 80:ethanol and then diluted with 1.10 mL of water for oral administration to 5-week old C57BL/6J male mice (from the Jackson Laboratory) weighing about 22 grams that were infected intraperitoneally on day 0 with the Plasmodium berghei, ANKA strain (2 × 107 parasitized erythrocytes).12 Each of 5 mice in a group was treated orally 24 hours post-infection with a single dose of 0.20 mL (0.20 mL/1.21 mL × 0.9 mg = 0.15 mg) of diluted compound solution, corresponding to a dose of 6.8 mg/kg, combined with 20 mg/kg of mefloquine hydrochloride. Determining blood parasitemia levels as well as monitoring the duration of animal survival compared to survival time of animals receiving no drug are both widely accepted as measures of a drug’s efficacy in antimalarial drug development. Three days after infection, an average of 16 % blood parasitemia was observed in the control (no drug) group. Animals receiving no drug died on days 6–7 post infection. A widely accepted yardstick of cure (i.e. 100% efficacy) is survival of animals to day 30 post infection, with no detectable malaria parasites in the animal’s blood at that time. Average survival results are summarized in Table 1, including single oral doses of 13 mg/kg of trioxane combined with 13 mg/kg of mefloquine hydrochloride. The clinically used monomeric water-soluble trioxane drug 2c and the synthetic trioxolane peroxide drug development candidate OZ277 (9) maleate are included as monotherapy reference compounds.
Table 1.
Antimalarial Efficacy Using a Single Oral Dose of Trioxane Combined with Mefloquine Hydrochloride in Plasmodium berghei-infected Mice
| Oral Dose |
||||
|---|---|---|---|---|
| Trioxane | Trioxane (mg/kg) | Mefloquine Hydrochloride (mg/kg) | Average survival (days) after infection | % Suppression of parasitemia (on day 3 post infection) |
| 2b | 6,8 | 20 | 19.8 (16,17,21,22,23)a | 99.8 |
| 2b | 13 | 13 | 19.6 (16,17,21,22,22)a | 99.7 |
| 3 | 6.8 | 20 | 27.4 (22,25,30,30,30)a | 99.5 |
| 3 | 13 | 13 | 24.6 (17,21,25,30,30)a | 99.9 |
| 4a | 6.8 | 20 | 27.8 (22,27,30,30,30)a | 99.9 |
| 4a | 13 | 13 | 26.4 (21,23,26,30,30)a | 99.6 |
| 4b | 6.8 | 20 | 30 | 99.0 |
| 4b | 13 | 13 | 30 | 99.6 |
| 4b | 72 | 0 | 24.4 (21,23,24,27,27)a | 99.0 |
| Controls | ||||
| vehicle (no drug) | 0 | 0 | 6.4 (6,6,6,7,7)a | 0 |
| 2c | 30 | 0 | 7.6b | -c |
| 9 | 30 | 0 | 10.7b | 99.95b |
| Mefloquine | 0 | 20 | 17.6 (16,16,17,19,20)a | 99.6 |
Actual mouse survival until day.
Data from supporting information in ref. 13 using a single oral dose of 30 mg/kg.
Dash indicates “not measured”.
It is clear from the data in Table 1 that all three of the new trioxane fluorinated amides 3, 4a, and 4b plus mefloquine hydrochloride prolonged average survival time much more effectively than monotherapy using trioxolane 9, which is in phase II clinical trials.13 A non-fluorinated version of dimeric trioxane fluorobenzyl amide 3 was much less antimalarially efficacious than fluorobenzyl amide 3 (data not shown). It is also apparent from the data in Table 1 that the monomeric trioxane fluoroanilide 4b, at a single oral dose of only 6.8 mg/kg plus 20 mg/kg of mefloquine hydrochloride, was the most efficacious at prolonging survival. Of the five mice in this 30-day surviving group, four (80%) were completely cured (no parasites in their blood) on day 30 post infection, and one mouse had 4% blood parasitemia. This trioxane fluoroanilide 4b caused a 99.0% suppression of parasitemia on day 3 post infection. A single oral dose of 13 mg/kg of trioxane fluoroanilide 4b combined with 13 mg/kg of mefloquine hydrochloride also prolonged survival of all of the mice until day 30, with four mice cured and one mouse having 2% blood parasitemia. Reinfection of the four cured mice on day 32 after the original infection caused an extended survival time, approximately four days longer than the average survival time of a newly infected control group; the mechanism(s) for this protective effect of trioxane fluoroanilide 4b against malaria reinfection is not clear at this time. In monotherapy control experiments, a single high oral dose (72 mg/kg) of the trioxane fluoroanilide 4b prolonged average survival until day 24.4, and a single oral dose (20 mg/kg) of mefloquine hydrochloride alone prolonged average survival to day 17.6. In an ACT control experiment, 6.8 mg/kg of the popular trioxane drug 2b combined with 20 mg/kg of mefloquine hydrochloride prolonged average survival to only day 19.8 (Table 1). Neither overt toxicity nor behavioral change attributable to trioxane drug administration was observed in any of the malaria-infected animals cured by trioxane fluoroanilide 4b plus mefloquine hydrochloride combination. The water-soluble monomeric trioxane antimalarial drug 2c and the trioxolane antimalarial drug candidate 9, although able to lower parasitemia levels considerably by day 3 post infection, were not efficacious in prolonging the mouse average survival time beyond day 11 when used as monotherapy at a dose of 30 mg/kg.
In conclusion, a single-digit oral dose of any one of the three new trioxane fluorinated amides 3, 4a, and 4b combined with mefloquine hydrochloride is considerably more antimalarially efficacious than the popular ACT trioxane drug 2b combined with mefloquine hydrochloride.14,15 Monomer trioxane fluoroanilide 4b stands out as being the most powerful antimalarial in this series of semi-synthetic trioxane fluorophenyl amides. Preclinical drug development is continuing on trioxane lead optimization and on use of other amine combination partners.
Experimental
High-pressure liquid chromatography (HPLC) was performed on a Rainin HPLX system equipped with two 25-mL pump heads and a Rainin Dynamax UV-C dualbeam variable wavelength detector set at 254 using a Phenomenex Luna 5 μ C18 250 × 10 mm column. The purity of analogs 3, 4a and 4b was ≥98% based on HPLC analysis.
Synthesis of Trioxane Dimer Fluorobenzyl Amide 3
To a solution of acid 610 (600 mg, 0.967 mmol) in CH2Cl2 (10 mL) were added N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (222 mg, 1.16 mmol) and 1-hydroxybenzotriazole (157 mg, 1.16 mmol) and it was stirred for 1 h at rt. To the reaction were added 4-fluorobenzylamine (0.33 mL, 2.9 mmol) and the solution was stirred for 5 h. It was quenched with water (3 mL). Layers were separated and the aqueous layer was extracted with EtOAc (2 × 4 mL). The combined organic solution was dried (MgSO4) and concentrated. The residue was purified by flash column chromatography (elution with EtOAc:hexanes = 1:3) to provide 3 (651 mg, 93%) as a white solid: [α]D24 = +82.1 (c=1.55, CHCl3); mp= 110°C; IR (thin film) 3312, 2939, 1669, 1510, 1377, 1221, 1052, 1012, 735 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32 (m, 2H), 6.97 (m, 2H), 6.23 (t, J=5.6 Hz, 1H), 5.27 (s, 1H), 5.20 (s, 1H), 4.41 (s, 1H), 4.39 (s, 1H), 4.09 (m, 2H), 2.76 (dq, J=13.2, 7.2 Hz, 1H), 2.66 (dq, J=13.6, 6.4 Hz, 1H), 2.54 (octet, J=4.0 Hz, 1H), 2.31 (m, 2H), 2.18 (m, 1H), 2.01-1.95 (m, 3H), 1.92-1.18 (m, 24H including s at 1.35 and 1.26), 0.98-0.79 (m, 14H including d at 0.95 with J=5.6 Hz, 0.93 with J=6.0 Hz, 0.85 with J=7.6 Hz, and 0.82 with J=7.2 Hz); 13C NMR (100 MHz, CDCl3) δ 175.8, 160.8, 134.3, 129.8, 129.7, 115.3, 115.1, 103.4, 102.9, 100.8, 88.6, 88.4, 81.2, 81.1, 76.4, 73.7, 52.5, 52.4, 44.7, 44.5, 44.3, 43.3, 37.4, 37.2, 36.5, 34.5, 33.3, 32.9, 30.2, 29.9, 26.2, 26.0, 24.9, 24.8, 24.6, 24.5, 20.2, 13.5, 13.0; 19F NMR (282 MHz, CDCl3) δ −115.7; HRMS (FAB) calculated for C41H59FNO9 [(M+H)+] 728.4174, found 728.4177.
Synthesis of Trioxane Monomer Fluorobenzyl Amide 4a
Into a flame dried 5 mL RBF was charged acid 811 (75 mg, 0.22 mmol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (67 mg, 0.35 mmol), and 1-hydroxybenzotriazole (35 mg, 0.26 mmol). Dichloromethane (2.5 mL) was then added and the mixture was stirred for an hour at which time, 4-fluorobenzylamine (95 μL, 0.84 mmol) was added by syringe. The reaction was allowed to stir at room temperature for 3 hours. It was then quenched with 1N HCl, extracted with dichloromethane (3 × 5 mL), washed with aqueous NaHCO3 and brine, dried over magnesium sulfate and evaporated. The crude product was purified by preparative thin layer chromatography (silica gel, 100% diethyl ether) to afford 4a as an amorphous, white solid (69 mg, 0.15 mmol, 70%): IR (thin film) 3321, 2947, 2875, 1648, 1546, 1510, 1453, 1378, 1223, 1127, 1095, 1052, 1011, 940, 879, 822, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.25 (m, 2H), 7.00 (m, 2H), 6.08 (br. s, 1H), 5.27 (s, 1H), 4.40 (m, 2H), 4.09 (m, 1H), 2.69 (m, 1H), 2.46 (m, 1H), 2.33 (m, 2H), 2.04-1.77 (m, 5H), 1.64-1.55 (m, 2H), 1.48-1.20 (m, 7H, including singlet at 1.36), 0.99-0.92 (m, 4H), 0.85 (d, 3H, J=6.0 Hz); 13C NMR (100 MHz, CDCl3) δ 172.97, 163.40, 160.96, 134.27, 129.54, 115.41, 103.28, 88.91, 81.14, 75.75, 52.44, 44.45, 43.01, 37.43, 36.58, 34.64, 34.48, 30.22, 26.11, 25.15, 24.89, 24.68, 20.12, 13.00; [α]D22= +72 (c=0.97, CHCl3); HRMS (FAB) m/z calcd for C25H34FNO5Na (M+Na)+ 470.2313, found 470.2300.
Synthesis of Trioxane Monomer Fluoroanilide 4b
Into a flame dried 5 mL RBF was charged carboxylic acid 811 (55 mg, 0.16 mmol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (50 mg, 0.26 mmol), and 1-hydroxybenzotriazole (30 mg, 0.19 mmol). Dichloromethane (2.5 mL) was then added and the mixture was stirred for an hour at which time, 4-fluoroaniline (60 μL, 0.61 mmol) was added by syringe. The reaction was allowed to stir at room temperature for 3 hours. It was then quenched with 1N HCl, extracted with dichloromethane (3 ×5 mL), washed with aqueous NaHCO3 and brine, dried over magnesium sulfate and evaporated. The crude product was purified by flash column chromatography (silica gel, 30% ethyl acetate/hexanes) to afford 4b (artefanilide) as an amorphous, white solid (51 mg, 0.12 mmol, 75%): IR (thin film) 3313, 2939, 2874, 1663, 1614, 1543, 1509, 1451, 1406, 1377, 1212, 1124, 1091, 1055, 1012, 876, 835, 754 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.07 (br. s, 1H), 7.50 (m, 2H), 6.96 (m, 2H), 5.34 (s, 1H), 4.14 (m, 1H), 2.73 (m, 1H), 2.60 (m, 1H), 2.46 (m, 1H), 2.31 (m, 1H), 2.02-1.78 (m, 5H), 1.65-1.55 (m, 2H), 1.47-1.20 (m, 7H, including singlet at 1.35), 0.97-0.93 (m, 4H), 0.87 (d, 3H, J=7.6 Hz); 13C NMR (100 MHz, CDCl3) δ 171.33, 157.89, 134.17, 121.63, 115.33, 103.42, 88.79, 81.08, 76.12, 52.30, 44.31, 37.34, 36.42, 35.62, 34.32, 30.85, 30.13, 25.98, 24.82, 24.55, 20.09, 13.04; [α]D22= +60 (c=0.47, CHCl3); HRMS (FAB) m/z calcd for C24H33FNO5 (M+H)+ 434.2343, found 434.2335.
Supplementary Material
Scheme 1.
Acknowledgments
We thank Nirbhay Kumar (JHU) for a gift of the P. berghei malaria parasites, the NIH (AI 34885 to GHP), the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation for financial support (to GHP and to JOL).
Abbreviations
- ACT
artemisinin combination therapy
- DMSO
dimethyl sulfoxide
- DMS
dimethyl sulfide
- EDC
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- HOBT
1-hydroxybenzotriazole
Footnotes
In honor of the 100th anniversary of the Division of Medicinal Chemistry
Supporting Information Available: 1H, 13C NMR spectra for all of the new trioxanes. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Olliaro PL, Boland PB. Clinical public health implications of antimalarial drug resistance. In: Rosenthal PJ, editor. Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Humana Press; Totowa, NJ: 2001. pp. 65–83. [Google Scholar]
- 2.Guidelines for the Treatment of Malaria. World Health Organization; Switzerland: 2006. [Google Scholar]
- 3.Ashley EA, White NJ. Artemisinin-based combinations. Curr Opin Infect Dis. 2005;18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- 4.(a) Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]; (b) Guthmann JP, Cohuet S, Rigutto C, Fortes F, Saraiva N, Kiguli J, Kyomuhendo J, Francis M, Noel F, Mulemba M, Balkan S. High efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. Am J Trop Med Hyg. 2006;75:143–145. [PubMed] [Google Scholar]
- 5.Myint HY, Ashley EA, Day NPJ, Nosten F, White NJ. Efficacy and safety of dihydroartemisinin-piperaquine. Trans R Soc Trop Med Hyg. 2007;101:858–866. doi: 10.1016/j.trstmh.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Sirima SB, Tiono AB, Gansane A, Diarra A, Ouedraogo A, Konate AT, Kiechel JR, Morgan CC, Olliaro PL, Taylor WRJ. Malaria Journal. 2009;8:48. doi: 10.1186/1475-2875-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Pilla Varotti F, Botelho ACC, Andrade AA, de Paula RC, Fagundes EMS, Valverde A, Mayer LMU, Mendonca JS, de Souza MVN, Boechat N, Krettli AU. Synthesis, antimalarial activity, and intracellular targets of MEFAS, a new hybrid compound derived from mefloquine and artesunate. Antimicrob Agents Chemother. 2008;52:3868–3874. doi: 10.1128/AAC.00510-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagara I, Diallo AD, Kone M, Coulibaly M, Diawara SI, Guindo O, Maiga H, Niambele MB, Sissoko M, Dicko A, Djimde A, Doumbo OK. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 9.Rosenthal AS, Chen X, Liu JO, West DC, Hergenrother PJ, Shapiro TA, Posner GH. Malaria-infected mice are cured by a single oral dose of new dimeric trioxane sulfones which are also selectively and powerfully cytotoxic to cancer cells. J Med Chem. 2009;52:1198–1203. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posner GH, Paik I-H, Sur S, McRiner AJ, Borstnik K, Xie S, Shapiro TA. Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high stability and efficacy. J Med Chem. 2003;46:1060–1065. doi: 10.1021/jm020461q. [DOI] [PubMed] [Google Scholar]
- 11.Jung M, Lee S, Ham J, Lee K, Kim H, Kim SK. Antitumor activity of novel deoxoartesmisinin monomers, dimers, and trimer. J Med Chem. 2003;46:987–994. doi: 10.1021/jm020119d. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Chong CR, Shi l, Yoshimoto T, Sullivan DJ, Jr, Lin JO. Inhibitors of Plasmodium falciparum methionine aminopeptidase 1b possess antimalarial activity. Proc Natl Acad Sci U S A. 2006;103:14548–14553. doi: 10.1073/pnas.0604101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FCK, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H, McIntosh K, Padmanilayam M, Santo TJ, Scheurer C, Scorneaux B, Tang Y, Urwyler H, Wittlin S, Charman WN. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 14.Sagara I, Rulisa S, Mbacham W, Adam I, Sissoko K, Maiga H, Traore OB, Dara N, Dicko YT, Dicko A, Djimde A, Jansen FH, Doumbo OK. Efficacy and safety of a fixed dose artesunate-sulphamethoxypyrazine-pyrimethamine compared to artemether- lumefantrine for the treatment of uncomplicated falciparum malaria across Africa: a randomized multi-centre trial. Malaria Journal. 2009;8:63. doi: 10.1186/1475-2875-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam A, Ahmed T, Batra V, Paliwal J. Pharmacokinetics and Pharmacodynamics of Endoperoxide Antimalarials. Curr Drug Metab. 2009;10:289–306. doi: 10.2174/138920009787846323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.