Abstract
Steroid receptors (SRs) are hormone-activated transcription factors important for a wide variety of cellular functions. Posttranslational modifications of SRs, including phosphorylation, ubiquitination, acetylation, and sumoylation regulate their expression and function. The remarkable number of phosphorylation sites in these receptors and the wide variety of kinases shown to modulate phosphorylation influence the integration between cell-signaling pathways and SR action. These phosphorylation sites have been identified in all of the functional domains with the majority being located within the amino-terminal portions of the receptors. The regulation of function is receptor specific, site specific, and often dependent on the cellular context. Numerous roles for site-specific phosphorylation have been elucidated including sensitivity of hormone response, DNA binding, expression, stability, subcellular localization, dimerization, and protein-protein interactions that can determine the regulation of specific target genes. This review summarizes the current knowledge regarding receptor site-specific phosphorylation and regulation of function. As functional assays become more sophisticated, it is likely that additional roles for phosphorylation in receptor function will be identified.
Keywords: steroid receptor, phosphorylation, kinase, transcription
Introduction
Steroid receptors (SRs) are hormone-activated transcription factors whose expression and function are highly regulated by posttranslational modifications including phosphorylation, ubiquitination and sumoylation. Steroid receptors and their associated cofactors can be phosphorylated on multiple sites by a wide range of kinases, which regulate various functions such as protein stability, hormone sensitivity, DNA binding, subcellular localization, and protein interactions. These functions can determine the timing, specificity, and extent of steroid receptor target gene regulation. The specific response of steroid receptors to their cognate ligands is largely influenced by the cellular context including the levels of active kinases and phosphatases in addition to the expression levels of coregulators and other receptor-interacting proteins. Thus, at least some aspects of tissue specific actions of SRs are controlled by cell signaling pathways.
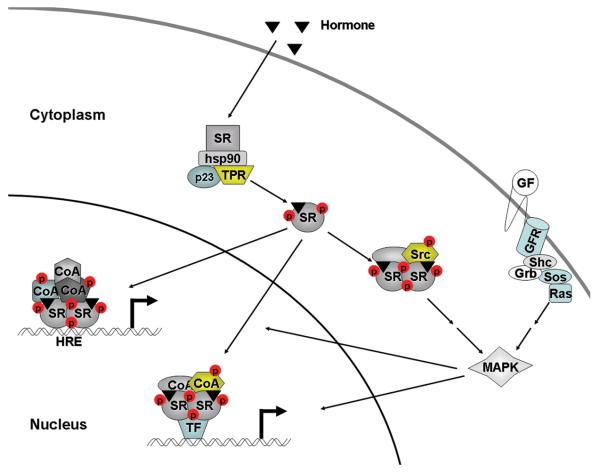
Steroid receptor function requires a series of complex molecular interactions in order to facilitate target gene induction. In the absence of hormone, SRs exist as monomers that are bound to chaperone complexes which include heat shock protein 90 (Hsp90), p23, and tetratricopeptide repeat-containing cochaperones such as protein phosphatase 5 (PP5) (1) (Figure 1). These receptor complexes are dynamic, and SRs can shuttle between the cytoplasm and nucleus; the relative subcellular distribution in the absence of ligand is receptor specific. Glucocorticoid receptor (GR) and androgen receptor (AR) are mainly cytoplasmic while progesterone receptor (PR) and estrogen receptor (ER) are predominantly nuclear. In response to hormone, SRs dissociate from the chaperone complexes and translocate to the nucleus. SRs exhibit an increase in receptor phosphorylation and, in the classical pathway, form homodimers that bind to response elements on DNA, and recruit a series of coactivator complexes that modify chromatin to facilitate transcription of target genes (Figure 1). SRs also interact with other transcription factors to bind DNA indirectly by tethering to regulate target gene expression (2). More recent studies have revealed that SRs also activate cell signaling pathways independent of their transcriptional function. Typically, a small fraction of the cytoplasmic SR upon binding hormone will associate with Src tyrosine kinase (Src) and other proteins causing activation of Src and downstream signaling pathways (2). Whether receptor dimers or receptor monomers are required for this activity has not been determined. Finally, SRs can be ubiquitinated, exported to the cytoplasm, and undergo proteosome-mediated degradation which can limit SR action.
Figure 1. Steroid receptor action.
In the absence of hormone, steroid receptor (SR) monomers are associated with chaperone complexes that include heat shock protein 90 (hsp90), p23, and cochaperones containing tetratricopeptide repeats (TPR). Hormone binding activates SRs by inducing conformational changes, dissociation of the chaperone complex, nuclear translocation, dimerization, binding to hormone response elements (HRE), and recruitment of a series of coactivators (CoA) to regulate target gene transcription. SRs can bind directly to HREs or indirectly by interacting with other transcription factors (TF) by tethering. Site-specific phosphorylation (P) of receptors increases subsequent to hormone binding, with some increases occurring rapidly, and others with delayed kinetics. In some cases, upon hormone binding, receptors will interact with Src tyrosine kinase (Src) outside the nucleus, thus activating Src and downstream kinases including p42/p44 MAPK. Alternatively, rapid signaling through growth factors (GF) and associated growth factor receptors (GFR) can activate kinase pathways that can enhance phosphorylation of SRs and converge upon and activate target genes.
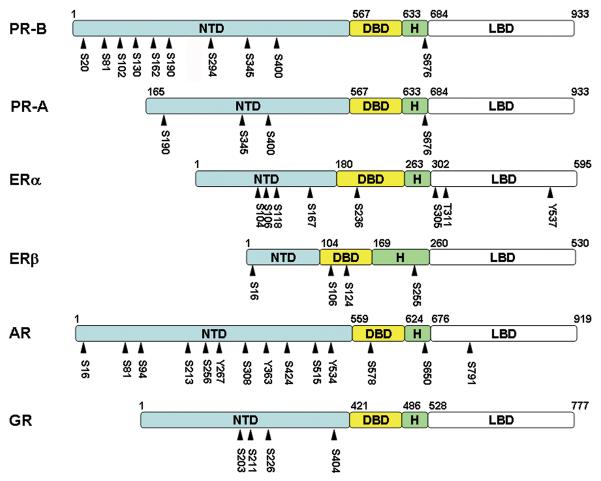
Steroid receptors share a common structure (Figure 2). The carboxyl-terminal ligand binding domain (LBD) is linked by a hinge region (H) to a highly conserved DNA-binding domain (DBD). The LBD contains a hormone-dependent coactivator interface called activation function 2 (AF2). The amino-terminal domain (NTD) contains a hormone independent coactivator interface, AF1. It is the least conserved domain and contains little intrinsic structure. Binding to DNA or interacting with other proteins induces a more ordered structure (3-5). The NTD contains the majority of phosphorylation sites, many of which contain serine-proline motifs (Ser-Pro) which can be recognized by the peptidyl prolyl isomerase, Pin1 (6). Thus, in addition to inherent change of charge, phosphorylation of these sites can result in the isomerization and subsequent alteration of receptor structure.
Figure 2. Domain structures and phosphorylation site locations of human steroid receptors.
The numbers of the amino acids found at the boundaries in the individual receptors between the NTD (amino-terminal domain), DBD (DNA binding domain), H (hinge region), and LBD (ligand binding domain) are indicated. Phosphorylation sites are indicated by the arrowheads. PR, progesterone receptor; ER, estrogen receptor; AR, androgen receptor; GR, glucocorticoid receptor; S, serine; T, threonine; Y, tyrosine. A summary of the locations of phosphorylation sites can be found at Phosphosite (http://www.phosphosite.org) (79).
The goal of this review is to summarize what is known about how the phosphorylation of specific sites within SRs can influence function for PR, ER, AR, and GR. This aspect of site-specific phosphorylation has been studied extensively in cell lines using a wide variety of techniques. Many human and rodent cell lines have been used to study the effects of site-specific phosphorylation on receptor protein stability, nuclear localization, hormone sensitivity, DNA binding, and transcription activity. Initial studies depended upon reporter plasmids to measure transcriptional activity and more recent studies have included analyses of the regulation of endogenous target genes. Although the majority of identified phosphorylation sites are serines (Ser), a few threonines (Thr) and tyrosines (Tyr) also have been identified. SR mutants have been made to block or mimic phosphorylation by residue substitution. Typically, Ala is substituted for Ser or Thr and phenylalanine (Phe) is substituted for Tyr to prevent phosphorylation. Although some studies have utilized glycine (Gly) for Ser or Thr, or Ala or other amino acids for Tyr, these substitutions may cause larger structural changes leading to changes in activity that are not strictly dependent upon phosphorylation. Aspartic acid (Asp) or glutamic acid (Glu) substitutions for Ser or Thr mimic phosphorylation if the role of the phosphorylation is to increase the negative charge. When phosphorylation plays a role in the secondary structure, an Asp or Glu substitution often is insufficient to mimic phosphorylation. There are no suitable substitutions to mimic a phosphorylated Tyr.
Estrogen Receptor
Site-specific SR phosphorylation has been studied extensively in estrogen receptor α (ERα), and to a lesser extent in ERβ (Figure 2). The role of ERα Ser118 phosphorylation and the regulation of this phosphorylation site has been studied most extensively, in part because phosphorylation of this site causes a reduction in mobility on SDS-PAGE gels (7), and thus the level of phosphorylation at this site could be detected prior to the development of site specific phosphoantibodies. This site contains a Ser-Pro motif, is phosphorylated in response to hormone, and has been shown to be a substrate for many kinases including p42/p44 MAPK and CDK7 (8-11). Early studies in COS1 cells demonstrated that the ERα Ser104/106/118Ala triple mutant had only 50-55% of wild type activity measured using an estrogen response element (ERE) reporter (12). The ERα Ser118 phosphorylation is involved in ligand-dependent interaction with transcription factor IIH (TFIIH)/CDK7 and the Ser118Glu phospho-mimic had increased E2-mediated activity compared to wild type (8). Furthermore, the Ser118 site has been shown to be important for the direct binding to and activation/repression of a subset of endogenous ERα target genes (13). In addition to target gene induction, Ser118 has been reported to be important for both ligand-dependent dimerization of ERα and ERα-mediated RNA splicing (14, 15). ERα also can be activated in a hormone independent manner by activation of cell signaling pathways. Epidermal growth factor (EGF) induces ligand independent activity of ERα measured using an ERE containing reporter; the Ser118Ala mutant was not activated by EGF demonstrating that phosphorylation of the site is required for this form of hormone independent activation in some cell types (16). Alanine substitutions at Ser104/106 and Ser118 reduce the interaction between ERα and several coactivators in the absence of ligand, but do not alter the E2-mediated response in HeLa cells (17). This effect may be due to the specific cellular context since studies in the breast cancer cell line, MDA-MB231, revealed a role for Ser104/106 and Ser118 phosphorylation in ligand-dependent ERα activity (18). Most of the functional studies of Ser104/106 have also included the Ser118 site (12). However, Rogatsky et al demonstrated that potentiation of ERα activity by the CyclinA/CDK2 complex in human osteosarcoma cells required Ser104/106, but not Ser118 (19).
Unlike the majority of phosphorylation of sites in ERα and other receptors, Ser167 does not reside in a Ser-Pro motif. It has been reported to be a substrate for casein kinase II, pp90Rsk1 kinase, and Akt (20-22) indicating that it can integrate signals from a number of pathways. Castano et al have shown that the ERα Ser167Ala mutant exhibits a 70% decrease in estradiol (E2) mediated activity in yeast. This mutant bound ligand at wild type levels, but exhibited a 10 fold decrease in DNA binding to EREs. Furthermore, they suggested that ligand-bound ERα undergoes a conformational change that exposes the Ser167 thus making this residue available for phosphorylation by casein kinase II (23). Joel et al demonstrated that the ERα Ser118/167Ala double mutant had reduced transcriptional activity following E2 treatment that was more substantial than either single mutant alone indicating the importance of both sites for maximal ER function (24). Further, activation of cell-signaling cascades by sarcoma (Src) kinase has been shown to be required for optimal estradiol dependent and tamoxifen dependent (partial ERα agonist) activation of an ERE containing reporter and mutation of either Ser118 or Ser167 blocks this potentiation (25). These studies also revealed that although overall binding of the Ser167Ala mutant was lower on the ER target gene promoter, pS2, it was still enriched by E2 treatment, but was not potentiated by active Src kinase when compared to wild type (25). Functional studies have shown that Ser167 phosphorylation is essential for ligand-independent, rapid-signaling induced ERα transcriptional activity in several cell lines (9, 26). The Ser118 site also has been shown to be required for ligand-independent activation induced by AKT and mitogen activated protein kinase (MAPK), but not by Jun N-terminal kinase (9, 10).
Functional studies have also been carried out on Ser236 in the DNA-binding domain. This site is phosphorylated by Protein Kinase A (PKA). The Ser236Glu phospho-mimic prevented DNA binding by inhibiting the dimerization of ER which resulted in reduced activity following E2 or OHT (hydroxyl tamoxifen) treatment with or without PKA. On the other hand, blocking phosphorylation of this site by substituting an alanine has little effect on either dimerization or DNA binding (27). The authors conclude that PKA-mediated phosphorylation of ERα Ser236 inhibits dimerization in the absence of ligand and that this is partially overcome by estrogens. Other actions of PKA must compensate in part, since activation of PKA induces some ERα activity in the absence of hormone (28, 29).
Phosphorylation of ERα Ser305, located within the LBD, is important for ERα interaction with the coactivators, steroid receptor coactivator 1 (SRC-1) and neurotrophin receptor interacting factor (NRIF) (30, 31). Ser305 phosphorylation by protein kinase A (PKA) induced the recruitment of RNA polymerase II to the prolactin transcription locus, an effect that is enhanced following tamoxifen treatment (31). It also has been suggested that ERα Ser305 phosphorylation is coupled to ERα acetylation. The ERα Ser305Asp phospho-mimic blocked Lys303 acetylation and the mutant exhibited an enhanced transcriptional response compared to wild type. This increase in activity was similar to that seen with the Lys303Arg acetylation mutant, thus providing a role for Ser305 phosphorylation in negative regulation of ERα acetylation (32). ERα Ser118 cooperates with Ser305 in p21 activated kinase (PAK)-signaling. PAK1-signalling dependent activation of ER Ser305 phosphorylation leads to enhanced Ser118 phosphorylation in tamoxifen resistance, perhaps due to conformation changes (33-35).
Two additional sites have been identified in the LBD. ERα Thr311 is phosphorylated by E2 treatment and by a kinase associated with or activated by p38α-SAPK2α (36, 37); substituting Ala for Thr311 did not affect E2 binding of the receptor but compromised its interaction with coactivators SRC-1 and SRC-2 in ERα negative Ishikawa cells (37). Furthermore, suppression of p38 SAPK activity or substitution of Ala for Thr311 inhibited E2-induced nuclear localization and subsequent transcriptional activation by ligand or MEKK1 activation.
In addition to Ser-Pro site phosphorylation, tyrosine phosphorylation plays a role in ERα action. The ERα Tyr537, located within the ligand-binding domain is basally phosphorylated in MCF7 cells and is a substrate for src family kinases (38). A variety of amino acid substitutions have been made at this site and the function of the mutants examined. The Phe substitution should most closely mimic the nonphosphorylated form; there is no adequate mimic for a phosphorylated Tyr. An ERα Tyr537Phe mutant exhibits a 25 – 30% decrease in activity in yeast despite having DNA and hormone binding affinities that were similar to with type (39). The Try537Phe mutant had decreased receptor stability and slightly different E2 binding kinetics, which suggests that Tyr537 phosphorylation is important for optimal ligand binding conformation of ER. Substitutions of polar residues for Tyr537 increase hormone independent activation of ERα. The ERα Tyr537Asn phosphorylation mutant exhibits strong constitutive activity but no ligand-mediated induction on ERE and pS2 promoters in both COS1 and MDA-MB-231 cells (40). The ERα Tyr537Asn mutant also exhibited increased ligand-independent and -dependent interaction with SRC-1 compared to wild type (40). Potentiation of the AF1 region of ERα by SRC1/JNK is Ser118 independent and appears to require Tyr537 phosphorylation (10). This study also demonstrates that amino acid substitutions can dramatically affect the experimental outcome when assessing the importance of a particular phosphorylation site. Substitution of Ser for Tyr resulted in increased SRC-1-mediated ligand-independent activity compared to wild type whereas Phe substitution resulted in a dramatic decrease in ligand-independent activity. In contrast, arginine substitution at this site had no effect (10). Clearly, some of these changes in function are not simply a consequence of phosphorylation or lack of phosphorylation.
Finally, functional studies also have been done to assess site-specific phosphorylation in ERβ. Activation of AKT targets Ser255, located in the hinge region of ERβ, and inhibits ERβ activity (41). Analysis of the Ser255Ala mutant revealed an increased interaction between ERβ and CREB-binding protein (CBP) rendering the mutant transcriptionally more responsive to CBP coactivation, suggesting that phosphorylation of this site inhibits this interaction. ERβ function has also been studied in the context of the mouse ERβ. The mouse homologues of ERβ Ser106/Ser124, mERβ Ser104/Ser124, are important for the physical interaction of mERβ with SRC1 and subsequent transcriptional activation (42). The mERβ Ser106Ala mutant exhibited increased stability and decreased ubiquitination suggesting that E6-AP ubiquitin ligase recruitment to ERβ is dependent on Ser106 phosphorylation thus providing a link between ubiquitination and phosphorylation.
Progesterone receptor
In most species, PR is expressed as two isoforms, PR-A and PR-B, which are derived from a single gene by alternative transcription from 2 different promoters. PR-A is identical to PR-B except that it lacks some of the initial N-terminal amino acids (164 in the case of human PR) (Figure 2). Early studies involving the chicken progesterone receptor (cPR) identified four phosphorylation sites (43, 44). All four of these sites are Ser-Pro motifs in the region common to PR-A and PR-B. Ser211 and Ser260 are located in the amino terminus, are basally phosphorylated, and exhibit an increased phosphorylation in response to progesterone treatment (43, 45). The remaining sites, Ser367 (amino terminus) and Ser530 (hinge region), are phosphorylated primarily in response to hormone treatment (43-46). The cPR-A Ser530Ala mutant bound ligand with wild type affinity but exhibited a reduction in transcriptional activity measured using reporter assays at low levels of hormone but not at saturating levels of hormone suggesting that the site is important for response to suboptimal levels of hormone (46). Phosphorylation of Ser211 is required for the hormone-dependent reduction in mobility of cPR-A on SDS-PAGE gels. In addition, substitution of an Ala for this Ser resulted in a 20% to 70% reduction in activity that was promoter and cell type context specific (45). This phosphorylation appeared to be more important for induction of a reporter containing tandem PR response elements with no additional transcription factor binding sites than for one that also included a region of the TK gene suggesting that it may play a role in interactions between PR dimers.
Ten phosphorylation sites have been described in human PR (Figure 2). Most of these are located in the amino terminus and are Ser-Pro motifs. Of these, five are unique to the PR-B specific region. Few studies have demonstrated functional roles for site-specific phosphorylation of human PR. Early studies provide some evidence that both Ser190 and Ser676 can mediate hormone dependent PR transcriptional activity (47). A transiently expressed PR-A Ser190Ala mutant exhibits a 20 to 80% reduction in activity depending on the cell type and promoter context with the greatest effect seen on tandem response elements in promoters with no additional transcription factor binding sites. Likewise, PR Ser676Ala, which is located in the hinge region, exhibited a reduction in transcriptional activity (47). However, both mutants were expressed at somewhat lower levels than wild type PR, which may have contributed to the observed reduction in activity. Nonetheless, these were the first hints of a functional role for human PR site-specific phosphorylation.
Phosphorylation of PR Ser294 has been studied extensively. Both the PR-A and PR-B isoforms contain Ser294, but there is a very strong preferential hormone-dependent phosphorylation of Ser294 in PR-B compared to PR-A suggesting a distinct conformation of the N-terminal domain of PRA may prevent this phosphorylation (48). Lange et al showed that PR-B Ser294Ala stably expressed in T47D-Y cells was resistant to ligand-dependent downregulation compared to wild type and that there was reduced ubiquitination of the mutant, consistent with reduced 26S proteasome mediated degradation (49). However, the Ser294Ala mutant of PR-A also is more stable than wild type PR-A despite negligible phosphorylation of wild type PR-A. This suggests that the Ala substitution may play an additional role in determining stability. In addition, Ser294 phosphorylation has been shown to be important for EGF induced nuclear translocation, proliferation, and EGF stimulated ligand-dependent PR transactivation on both progesterone response element (PRE)-containing reporters and endogenous genes (50). Interestingly, one study has suggested a possible link between PR Ser294 phosphorylation and sumoylation. Phosphorylation of PR at Ser294 was reported to prevent PR sumoylation which, in turn, de-represses PR transcriptional activity. Ser294 phosphorylation mutants failed to undergo ligand-dependent downregulation and were heavily sumoylated compared to wild type (51). However, a recent study challenged this result and suggested that, in fact, PR Ser294 phosphorylation and sumoylation were uncoupled (52) and that there are additional roles for sumoylation in regulating overall PR activity. Phosphorylation of Ser294 also has been implicated in aspects of rapid signaling leading to activation of kinases. Although the Ser294Ala mutant was capable of activating p42/p44 MAPK in response to hormone, activation of Stat3 was eliminated (53). Ser294 is the best characterized PR phosphorylation site and appears to have an important role in many aspects of PR function.
Functional roles for Ser345 and Ser400 phosphorylation also have been described. Rapid signaling mediates PR-B Ser345 phosphorylation requiring activated Src. Ser345 phosphorylation is, in turn required for induction of EGFR and p21, two genes whose hormone dependent induction relies on PR tethering to SP1 at the SP1 sites within the promoters (54). Finally, a functional role for PR S400 phosphorylation in ligand-independent transcriptional activity has been elucidated. This study revealed that cyclin dependent kinase 2 (CDK-2) in the absence of progestins can phosphorylate PR-B Ser400. Although expression of a constitutively active CDK-2 induced nuclear translocation, PR-B Ser400Ala mutants failed to translocate under these conditions. Interestingly, expression of the constitutively active CDK-2 also induced hormone independent activation of WT PR measured using a PR responsive luciferase reporter whereas the basal activity of the Ser400Ala mutant was unaffected (55). Thus, although much more work is needed to assess the importance of site-specific PR phosphorylation, it is apparent that this process has important roles in many aspects of PR function.
Androgen Receptor
Like PR, the majority of identified phosphorylation sites for AR are located in the N-terminal domain (Figure 2). Although many of the NTD sites have been verified in vivo, assigning function to them has been difficult. Studies of transcriptional activation by AR of several androgen response elements containing promoters carried out in various cell lines has provided little information as to the function of NTD site-specific phosphorylation as many phosphorylation mutants have exhibited no aberrant activation characteristics when compared to wild type (56-60). However, greater differences in activity can be detected when specific signaling pathways are activated or inhibited. For example, overexpression of cyclin D3/CDK11p58 inhibits AR activity. The kinase phosphorylates Ser308; substitution of an alanine elevates hormone dependent activity modestly and prevents cyclin D3/CDK11p58 mediated repression of AR transcriptional activity (61).
Several studies have suggested that AKT mediated phosphorylation of Ser213 and Ser791 (numbered based on an AR length of 919 amino acids) reduces AR activity. Both sites are phosphorylated by the P13K/Akt signaling pathways (62-64). Mutation of the AR Ser213 to alanine caused AR to be resistant to AKT mediated suppression of activity in DU145 cells (64, 65). Palazzolo et al found that substituting alanines for both of the sites also prevented AKT mediated inhibition of AR transcriptional activity. Surprisingly, substitution of aspartic acids at either site blocked hormone binding and, therefore, ligand dependent AR protein stabilization, ligand-mediated translocation, and AR transcriptional activity (66).
The remaining sites in the AR NTD also have been shown to have important functional roles. When a fragment of the androgen receptor (amino acids 507-660) is expressed, Ser515 and Ser578 are phosphorylated in response to EGF treatment (67). The AR Ser515Ala mutation exhibited a more severe phenotype than the Ser578Ala and the double mutant displayed little to no activity; furthermore, EGF treatment had no effect on the activity of this mutant. A Ser578Ala substitution results in increased nuclear localization of AR in the absence of ligand but eliminates AR transcriptional response to EGF. AR Ser578Ala also exhibits increased binding to Ku-70/80 regulatory subunits of DNA-dependent protein kinase in addition to nuclear retention of the AR in association with hyperphosphorylation at Ser515 (67). Finally, the Ser515Ala mutant is not phosphorylated on Ser650, which is located in the hinge region of AR (68). One possible explanation for this is that phosphorylation at Ser515 mediates a conformational change of the AR thus making the Ser650 phospho site either more available to phosphatases or less accessible to kinases. Attempting to assign function to the AR Ser650 phosphorylation site itself has produced conflicting reports. Early studies have suggested that blocking phosphorylation of this site resulted in 30% reduced activity with an MMTV-Luc reporter in CV1 cells (56). This was in direct contrast to later reports examining the function of Ser650 phosphorylation on AR activity in various cell lines using various reporters in which no phenotype was detected (59, 68). However, careful examination of these studies revealed that the original observation by Zhuo et al was evident at only high concentrations of receptor (56). Wong et al also found that at high concentrations of receptor the Ser650Ala mutant is less active than wild type (68). AR Ser650 phosphorylation also plays an important role in nuclear export of AR in response to stress kinase signaling (69). A recent report has shown that protein phosphatase 1 (PP1) inhibition increases phosphorylation at AR Ser650 which causes a marked increase in nuclear export of AR which is not observed for the Ser650Ala mutant (70). This study suggests that PP1 plays a critical role in regulating AR protein stability and nuclear localization through dephosphorylation of AR at Ser650.
In addition to Ser-Pro motifs, several tyrosine phosphorylation sites are present in the NTD of AR. A number of candidate sites have been identified in AR isolated from cells overexpressing Src. Based on the overall level of tyrosine phosphorylation in AR, substituting Phe for Tyr534 reduced the Tyr phosphorylation most substantially suggesting that this is a major site under these conditions (71). The Tyr534Phe mutant also exhibited reduced activity and DNA binding at low doses of ligand and defective nuclear translocation in response to various stimuli (71). Finally, Tyr534Phe mutant expression caused growth inhibition in both cell lines and tumor xenografts containing the Tyr534Phe mutant grew more slowly than tumors expressing WT AR in castrated mice, thereby demonstrating a role for Tyr534 phosphorylation in prostate cancer cell growth under androgen-depleted conditions (71). Two additional tyrosine phosphorylation sites have been identified in cells treated with heregulin or transfected with constitutively active Ack (Cdc42 associated kinase) (72). Mutation of these sites, Tyr267 and Tyr363 to phenylalanine (Tyr267Phe and Tyr363Phe, respectively), reduced Ack-induced reporter activation and recruitment to the enhancer, thus demonstrating the importance of these sites in AR basal and ligand-dependent activity as well as in potentiation of AR activity by kinase signaling (72). In addition, substituting Phe at both of these sites also reduced tumor growth of Ack-driven tumor xenografts in castrated nude mice (72).
Glucocorticoid Receptor
The phosphorylation sites that have been identified in human GR are contained within the N-terminus of GR (Figure 2). Ser203, Ser211, and Ser226 of hGR were identified first and all show enhanced phosphorylation in response to Dexamethasone (Dex) (73). Comparison of gene regulation in U2OS cells stably expressing WT, Ser226Ala, or Ser211Ala mutants revealed that the effects of these phosphorylations are gene specific. Phosphorylation of Ser211 enhances GR interaction with MED14 (vitamin D receptor interacting protein 150), and genes that are dependent on MED14 are not induced as well by the Ser211Ala whereas some MED14 independent genes showed reduced Dex dependent induction only at lower hormone levels (74). Analysis of the major GR phosphorylation sites also has revealed a role for site-specific phosphorylation in cell survival. Activated GR induces apoptosis in some cell types. GR Ser211 phosphorylation has been implicated in the activation of GR target gene transcription and apoptosis in glucocorticoid-resistant human lymphoblastic cells (75). In addition, expression of a Ser211Ala mutant can prevent phosphorylation of this site by p38 MAPK in response to glucocorticoid treatment preventing or mediating apoptosis (76). In contrast, the Ser226Ala mutant is more active than the wild type on these genes. Phosphorylation of Ser226 has been associated with nuclear export (77). Activation of the JNK pathway results in phosphorylation of Ser226 and subsequent enhancement of GR nuclear export after withdrawal of ligand. In addition, UV radiation can facilitate nuclear export of GR and block GR activity in COS7 cells whereas the Ser226Ala mutant is retained in the nucleus and maintains transcriptional activity (77).
Ser404 has been identified as a target for Glycogen Synthase Kinase 3 β, an enzyme that inhibits GR activity. The phosphorylation has been shown to be important for ligand-dependent nuclear export of GR. In addition, substituting Ala for Ser404 results in increased stability and gene-specific changes in ligand-dependent GR target gene regulation. Finally, phosphorylation of hGR Ser404 protects against dexamethasone stimulated apoptosis in U2OS cells (78).
Conclusion
Initial studies of receptor phosphorylation focused on sites that were either basally phosphorylated or phosphorylated in response to hormone. Assigning functions to specific phosphorylation sites within steroid receptors has been a daunting task. The studies to date suggest that the roles of individual sites are context specific and many factors including receptor expression levels, dose of the ligand or other stimulus, duration of the stimulus, target gene measured and cellular context can play a role in the overall SR activity. Nonetheless, the studies outlined in this report support the idea that specific phosphorylation events within steroid receptors can influence receptor localization, stability, dimerization, and transcriptional activity. In addition, a growing body of evidence suggests that certain phosphorylation events can regulate other post translational modifications including ubiquitination and sumoylation. More recent studies have identified a number of sites that are phosphorylated only when specific kinases are activated. Functional analyses of phosphorylation site mutants under conditions where these signaling pathways are activated or the kinases are overexpressed yield some of the most striking differences between the activities of wild type and Ala or Phe substitutions for Ser/Thr or Tyr. This suggests that the phosphorylations are particularly important in responding to a variety of cellular signals. The relative importance of these sites and the contribution of these signaling pathways in vivo remain to be determined. Using more physiologically relevant models including mice expressing modified receptors may help to address these questions.
Acknowledgements
Support for this work was provided by NIH grant R01 CA57539. In addition, Robert Ward was supported by a post-doctoral fellowship from the Ruth L. Kirschstein National Research Service Award, F32 CA130430.
Grant support: NIH R01 CA57539 and F32 CA130430
References
- 1.Picard D. Chaperoning steroid hormone action. Trends Endocrinol Metab. 2006;17:229–235. doi: 10.1016/j.tem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Volk DE, Li J, Lee JC, Gorenstein DG, Thompson EB. TATA box binding protein induces structure in the recombinant glucocorticoid receptor AF1 domain. Proc Natl Acad Sci U S A. 2004;101:16425–16430. doi: 10.1073/pnas.0407160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R, Betney R, Li J, Thompson EB, McEwan IJ. Induced alpha-helix structure in AF1 of the androgen receptor upon binding transcription factor TFIIF. Biochemistry. 2004;43:3008–3013. doi: 10.1021/bi035934p. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Lee JC, Bolen DW, Thompson EB. The conformation of the glucocorticoid receptor af1/tau1 domain induced by osmolyte binds co-regulatory proteins. J Biol Chem. 2001;276:18146–18152. doi: 10.1074/jbc.M100825200. [DOI] [PubMed] [Google Scholar]
- 6.Yi P, Wu RC, Sandquist J, Wong J, Tsai SY, Tsai MJ, Means AR, O'Malley BW. Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1) Mol Cell Biol. 2005;25:9687–9699. doi: 10.1128/MCB.25.21.9687-9699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joel PB, Traish AM, Lannigan DA. Estradiol and phorbol ester cause phosphorylation of serine 118 in the human estrogen receptor. Mol Endocrinol. 1995;9:1041–1052. doi: 10.1210/mend.9.8.7476978. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Riedl T, Washbrook E, Pace PE, Coombes RC, Egly JM, Ali S. Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell. 2000;6:127–137. [PubMed] [Google Scholar]
- 9.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 10.Feng W, Webb P, Nguyen P, Liu X, Li J, Karin M, Kushner PJ. Potentiation of estrogen receptor activation function 1 (AF-1) by Src/JNK through a serine 118-independent pathway. Mol Endocrinol. 2001;15:32–45. doi: 10.1210/mend.15.1.0590. [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 12.Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994;269:4458–4466. [PubMed] [Google Scholar]
- 13.Cheng J, Zhang C, Shapiro DJ. A functional serine 118 phosphorylation site in estrogen receptor-alpha is required for down-regulation of gene expression by 17beta-estradiol and 4-hydroxytamoxifen. Endocrinology. 2007;148:4634–4641. doi: 10.1210/en.2007-0148. [DOI] [PubMed] [Google Scholar]
- 14.Masuhiro Y, Mezaki Y, Sakari M, Takeyama K, Yoshida T, Inoue K, Yanagisawa J, Hanazawa S, O'Malley B,W, Kato S. Splicing potentiation by growth factor signals via estrogen receptor phosphorylation. Proc Natl Acad Sci U S A. 2005;102:8126–8131. doi: 10.1073/pnas.0503197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheeler CQ, Singleton DW, Khan SA. Mutation of serines 104, 106, and 118 inhibits dimerization of the human estrogen receptor in yeast. Endocr Res. 2003;29:237–255. doi: 10.1081/erc-120022321. [DOI] [PubMed] [Google Scholar]
- 16.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. Embo J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 17.Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-alpha: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- 18.Medunjanin S, Hermani A, De Servi B, Grisouard J, Rincke G, Mayer D. Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor alpha and is involved in the regulation of receptor activity. J Biol Chem. 2005;280:33006–33014. doi: 10.1074/jbc.M506758200. [DOI] [PubMed] [Google Scholar]
- 19.Rogatsky I, Trowbridge JM, Garabedian MJ. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–22302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SF, Obourn JD, Jaffe H, Notides AC. Phosphorylation of the human estrogen receptor by mitogen-activated protein kinase and casein kinase II: consequence on DNA binding. J Steroid Biochem Mol Biol. 1995;55:163–172. doi: 10.1016/0960-0760(95)00177-2. [DOI] [PubMed] [Google Scholar]
- 21.Clark DE, Poteet-Smith CE, Smith JA, Lannigan DA. Rsk2 allosterically activates estrogen receptor alpha by docking to the hormone-binding domain. Embo J. 2001;20:3484–3494. doi: 10.1093/emboj/20.13.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilgelm A, Lian Z, Wang H, Beauparlant SL, Klein-Szanto A, Ellenson LH, Di Cristofano A. Akt-mediated phosphorylation and activation of estrogen receptor alpha is required for endometrial neoplastic transformation in Pten+/− mice. Cancer Res. 2006;66:3375–3380. doi: 10.1158/0008-5472.CAN-05-4019. [DOI] [PubMed] [Google Scholar]
- 23.Castano E, Vorojeikina DP, Notides AC. Phosphorylation of serine-167 on the human oestrogen receptor is important for oestrogen response element binding and transcriptional activation. Biochem J. 1997;326(Pt 1):149–157. doi: 10.1042/bj3260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joel PB, Smith J, Sturgill TW, Fisher TL, Blenis J, Lannigan DA. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18:1978–1984. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah YM, Rowan BG. The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor (alpha) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol. 2005;19:732–748. doi: 10.1210/me.2004-0298. [DOI] [PubMed] [Google Scholar]
- 26.Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 27.Chen D, Pace PE, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–1015. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman KM, Dutertre M, El-Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-alpha (ER alpha)- and ER beta-dependent gene expression by cAMP signaling pathway(s) J Biol Chem. 2003;278:12834–12845. doi: 10.1074/jbc.M212312200. [DOI] [PubMed] [Google Scholar]
- 29.Tsai HW, Katzenellenbogen JA, Katzenellenbogen BS, Shupnik MA. Protein kinase A activation of estrogen receptor alpha transcription does not require proteasome activity and protects the receptor from ligand-mediated degradation. Endocrinology. 2004;145:2730–2738. doi: 10.1210/en.2003-1470. [DOI] [PubMed] [Google Scholar]
- 30.Talukder AH, Li DQ, Manavathi B, Kumar R. Serine 28 phosphorylation of NRIF3 confers its co-activator function for estrogen receptor-alpha transactivation. Oncogene. 2008;27:5233–5242. doi: 10.1038/onc.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zwart W, Griekspoor A, Berno V, Lakeman K, Jalink K, Mancini M, Neefjes J, Michalides R. PKA-induced resistance to tamoxifen is associated with an altered orientation of ERalpha towards co-activator SRC-1. Embo J. 2007;26:3534–3544. doi: 10.1038/sj.emboj.7601791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64:9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 33.Rayala SK, Molli PR, Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 2006;66:5985–5988. doi: 10.1158/0008-5472.CAN-06-0978. [DOI] [PubMed] [Google Scholar]
- 34.Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, Aldaz CM, Khan S, Kumar R. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006;66:1694–1701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 35.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. Embo J. 2002;21:5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Dhaheri MH, Rowan BG. Application of phosphorylation site-specific antibodies to measure nuclear receptor signaling: characterization of novel phosphoantibodies for estrogen receptor alpha. Nucl Recept Signal. 2006;4:e007. doi: 10.1621/nrs.04007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22:5835–5845. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold SF, Obourn JD, Jaffe H, Notides AC. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol Endocrinol. 1995;9:24–33. doi: 10.1210/mend.9.1.7539106. [DOI] [PubMed] [Google Scholar]
- 39.Yudt MR, Vorojeikina D, Zhong L, Skafar DF, Sasson S, Gasiewicz TA, Notides AC. Function of estrogen receptor tyrosine 537 in hormone binding, DNA binding, and transactivation. Biochemistry. 1999;38:14146–14156. doi: 10.1021/bi9911132. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay GB, Tremblay A, Labrie F, Giguere V. Ligand-independent activation of the estrogen receptors alpha and beta by mutations of a conserved tyrosine can be abolished by antiestrogens. Cancer Res. 1998;58:877–881. [PubMed] [Google Scholar]
- 41.Sanchez M, Sauve K, Picard N, Tremblay A. The hormonal response of estrogen receptor beta is decreased by the phosphatidylinositol 3-kinase/Akt pathway via a phosphorylation-dependent release of CREB-binding protein. J Biol Chem. 2007;282:4830–4840. doi: 10.1074/jbc.M607908200. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 43.Denner LA, Schrader WT, O'Malley BW, Weigel NL. Hormonal regulation and identification of chicken progesterone receptor phosphorylation sites. J Biol Chem. 1990;265:16548–16555. [PubMed] [Google Scholar]
- 44.Poletti A, Weigel NL. Identification of a hormone-dependent phosphorylation site adjacent to the DNA-binding domain of the chicken progesterone receptor. Mol Endocrinol. 1993;7:241–246. doi: 10.1210/mend.7.2.8469237. [DOI] [PubMed] [Google Scholar]
- 45.Bai W, Weigel NL. Phosphorylation of Ser211 in the chicken progesterone receptor modulates its transcriptional activity. J Biol Chem. 1996;271:12801–12806. doi: 10.1074/jbc.271.22.12801. [DOI] [PubMed] [Google Scholar]
- 46.Bai W, Tullos S, Weigel NL. Phosphorylation of Ser530 facilitates hormone-dependent transcriptional activation of the chicken progesterone receptor. Mol Endocrinol. 1994;8:1465–1473. doi: 10.1210/mend.8.11.7877616. [DOI] [PubMed] [Google Scholar]
- 47.Takimoto GS, Hovland AR, Tasset DM, Melville MY, Tung L, Horwitz KB. Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J Biol Chem. 1996;271:13308–13316. doi: 10.1074/jbc.271.23.13308. [DOI] [PubMed] [Google Scholar]
- 48.Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 49.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Hafiz H, Dudevoir ML, Horwitz KB. Mechanisms underlying the control of progesterone receptor transcriptional activity by SUMOylation. J Biol Chem. 2009;284:9099–9108. doi: 10.1074/jbc.M805226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proietti CJ, Rosemblit C, Beguelin W, Rivas MA, Diaz Flaque MC, Charreau EH, Schillaci R, Elizalde PV. Activation of Stat3 by heregulin/ErbB-2 through the co-option of progesterone receptor signaling drives breast cancer growth. Mol Cell Biol. 2009;29:1249–1265. doi: 10.1128/MCB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–10557. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou ZX, Kemppainen JA, Wilson EM. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Z, Becklin RR, Desiderio DM, Dalton JT. Identification of a novel phosphorylation site in human androgen receptor by mass spectrometry. Biochem Biophys Res Commun. 2001;284:836–844. doi: 10.1006/bbrc.2001.5030. [DOI] [PubMed] [Google Scholar]
- 58.Yang CS, Vitto MJ, Busby SA, Garcia BA, Kesler CT, Gioeli D, Shabanowitz J, Hunt DF, Rundell K, Brautigan DL, Paschal BM. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol Cell Biol. 2005;25:1298–1308. doi: 10.1128/MCB.25.4.1298-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Weber MJ. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277:29304–29314. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 60.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci U S A. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zong H, Chi Y, Wang Y, Yang Y, Zhang L, Chen H, Jiang J, Li Z, Hong Y, Wang H, Yun X, Gu J. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Mol Cell Biol. 2007;27:7125–7142. doi: 10.1128/MCB.01753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 63.Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280:40916–40924. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- 64.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci U S A. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin HK, Hu YC, Yang L, Altuwaijri S, Chen YT, Kang HY, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 66.Palazzolo I, Burnett BG, Young JE, Brenne PL, La Spada AR, Fischbeck KH, Howell BW, Pennuto M. Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet. 2007;16:1593–1603. doi: 10.1093/hmg/ddm109. [DOI] [PubMed] [Google Scholar]
- 67.Ponguta LA, Gregory CW, French FS, Wilson EM. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J Biol Chem. 2008;283:20989–21001. doi: 10.1074/jbc.M802392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong HY, Burghoorn JA, Van Leeuwen M, De Ruiter PE, Schippers E, Blok LJ, Li KW, Dekker HL, De Jong L, Trapman J, Grootegoed JA, Brinkmann AO. Phosphorylation of androgen receptor isoforms. Biochem J. 2004;383:267–276. doi: 10.1042/BJ20040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gioeli D, Black BE, Gordon V, Spencer A, Kesler CT, Eblen ST, Paschal BM, Weber MJ. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–515. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- 70.Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009 doi: 10.1074/jbc.M109.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, Qiu Y. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 72.Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, Earp HS, Whang YE. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, Rogatsky I, Logan SK, Garabedian MJ. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol. 2008;22:1754–1766. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller AL, Garza AS, Johnson BH, Thompson EB. Pathway interactions between MAPKs, mTOR, PKA, and the glucocorticoid receptor in lymphoid cells. Cancer Cell Int. 2007;7:3. doi: 10.1186/1475-2867-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 77.Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16:2382–2392. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- 78.Galliher-Beckley AJ, Williams JG, Collins JB, Cidlowski JA. Glycogen synthase kinase 3beta-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Mol Cell Biol. 2008;28:7309–7322. doi: 10.1128/MCB.00808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cell_Signaling_Technology http://phosphosite.org/ (revised 2007)