Abstract
In this experiment, the correlation between antigenemia and specific antibody responses in Toxoplasma gondii-infected rabbits was assessed. We injected 1,000 T. gondii tachyzoites (RH) subcutaneously into 5 rabbits. Parasitemia, circulating antigens, and IgM and IgG antibody titers in blood were tested by ELISA and immunoblot. For detection of parasitemia, mice were injected with blood from rabbits infected with T. gondii and mice died between days 2 and 10 post-infection (PI). Circulating antigens were detected early on day 2 PI, and the titers increased from day 4 PI and peaked on day 12 PI. Anti-Toxoplasma IgM antibody titers increased on day 6 PI and peaked on days 14-16 PI. IgG was detected from day 10 PI, and the titers increased continuously during the experiment. The antigenic protein patterns differed during the infection period, and the number of bands increased with ongoing infection by the immunoblot analysis. These result indicated that Toxoplasma circulating antigens during acute toxoplasmosis are closely related to the presence of parasites in blood. Also, the circulating antigen levels were closely correlated with IgM titers, but not with IgG titers. Therefore, co-detection of circulating antigens with IgM antibodies may improve the reliability of the diagnosis of acute toxoplasmosis.
Keywords: Toxoplasma gondii, rabbit, antigenemia, parasitemia, antibody
Toxoplasmosis is usually diagnosed using serological tests with increases in specific IgG and IgM antibodies. However, these tests are not reliable in immunocompromised patients, nor is it possible to differentiate clearly between the acute and latent forms of toxoplasmosis [1,2]. Because a relatively high percentage of individuals have antibodies without a recognized parasite infection, the early detection of Toxoplasma gondii antigen in the serum or other body fluids of patients would help to diagnose correctly and prevent late complications. For detecting Toxoplasma antigen, several laboratory procedures are available. Direct detection procedures, such as microscopic examination, immune histology, or cell culture are reliable, but they are either insensitive or time-consuming [1,2]. PCR is highly sensitive and specific, although heme, heparin, and other poorly characterized substances have been reported to decrease the efficiency of PCR [3]. ELISA is considered to be a highly sensitive, practical method for detecting the parasite antigen [2]. Many reports have discussed titrating serum antibodies in hosts after Toxoplasma infection, however, little information is available on the correlations among parasitemia, circulating antigens, and antibody titers in T. gondii-infected hosts.
To assess the sequential changes in parasite antigen and antibody responses in blood, 5 New Zealand white rabbits weighing 2-3 kg were infected with 1,000 tachyzoites of the RH strain of T. gondii subcutaneously. Then, blood samples were drawn from an ear vein of each rabbit every other day for 20 days. To check parasitemia in the rabbits, 0.5 ml of heparinized blood from each rabbit was injected intraperitoneally into 4 mice, and their survival was monitored for 20 days after infection.
The ELISA for detecting circulating antigens was performed in microtitration trays [4,5]. To obtain mouse anti-Toxoplasma antisera, mice were infected with 20 brain cysts of avirulent Me49 strain of T. gondii orally. The mice were then sacrificed at 6 months after infection, and the sera were precipitated with saturated ammonium sulfate solution, resuspended in 0.01 M phosphate buffered saline. Mouse anti-Toxoplasma antisera were diluted with 0.1 M carbonate-bicarbonate buffer (pH 9.6, 10 µg/ml). Then, 100 µl were pipetted into 96-well microtiter plates (Nunc, Roskilde, Denmark) and incubated at 4℃ overnight. The plates were washed with PBS containing 0.05% Tween 20 (PBS/Tween 20), to which 0.1 ml of rabbit serum diluted 1 : 50 with PBS/Tween 20 containing 0.1% bovine serum albumin was added. Toxoplasma lysate antigen (TLA) was prepared as a control. The plates were incubated at room temperature (RT) for 2 hr, and then 0.1 ml sample serum from the infected rabbit was added. After washing, 150 µl of horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Sigma Chemical Co., St. Louis, Missouri, USA) diluted 1 : 3,000 were added to each well, and then the plates were incubated for 2 hr at RT. Subsequently, the plates were washed with PBS/Tween 20, and 150 µl of o-phenylenediamine dihydrochloride (Sigma) were pipetted into the wells, and the plates were incubated for 20 min at RT. Then, 8 N H2SO4 was added to stop the reaction, and the absorbance was determined at 490 nm with an automatic ELISA reader. Titers higher than the mean plus 3 SD from serum of uninfected rabbits were considered a positive level.
To titrate the antibodies using ELISA, 96-well microtiter plates were coated with TLA (10 µg/ml) and incubated overnight at 4℃. After blocking, serum samples from infected rabbits were diluted 1 : 100 in PBS/Tween20 containing 0.1% BSA, and 100 µl of diluted serum from infected rabbits were added to each well. After 2 hr, HRP-conjugated goat anti-rabbit IgM (1 : 2,000 dilution) (Southern Biotechnology Associates, Birmingham, Alabama, USA) and HRP-conjugated goat anti-rabbit IgG (1 : 8,000 dilution) (Sigma) were applied. After washing, freshly prepared o-phenylenediamine dihydrochloride was added, and the reaction was stopped by adding 4 N H2SO4. The optical density at 490 nm was read using an automatic ELISA plate reader (SPECTRA) (Molecular Devices, Sunnyvale, California, USA). Statistical differences in ELISA titers were determined using the Kruskal-Wallis test. Differences between groups were considered significant at P < 0.05.
For immunoblotting, TLA was heated with sample buffer at 100℃ for 4 min, separated on 12% acrylamide separating gels under reducing conditions, and then transferred electrophoretically to nitrocellulose sheets (Schleicher & Schuell BioScience Inc., Dassel Germany) at a constant voltage of 50 V for 1 hr at 4℃. The nitrocellulose sheets were incubated for 2 hr with 5% nonfat powdered milk in PBS. Strips were cut and incubated with serum from the rabbits (diluted 1 : 100 in 1% BSA/PBS) for 2 hr. After 3 washes with PBS, the strips were incubated for 2 hr in HRP-conjugated goat anti-rabbit immunoglobulin (Sigma) diluted 1 : 5,000 in 1% BSA/PBS. After washing, the strips were incubated with 4-chloro-1-naphthol solution for 2 hr at RT. The reaction was stopped by rinsing with PBS.
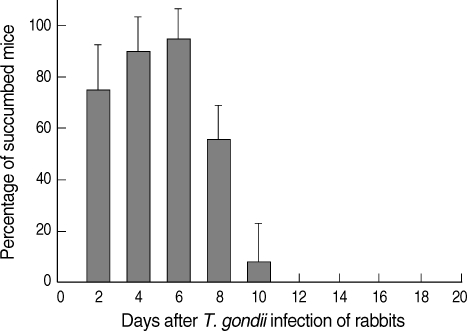
Two rabbit died on 8 to 10 days after T. gondii infection, while the other 3 rabbits survived until the end of the experiment. For the determination of parasitemia, 4 mice of each group were inoculated intraperitoneally with 0.5 ml of infected rabbit blood. As shown in Fig. 1, the rabbits developed parasitemia beginning on day 2 post-infection (PI), and this peaked between days 4 and 6 PI (90 ± 13% to 95 ± 11%). Mice did not die after day 12 PI and it means that T. gondii tachyzoites were not contained any more in the rabbit blood.
Fig. 1.
Mouse inoculation test for detecting parasitemia in rabbits infected with the RH strain of T. gondii. Four mice of each group were injected with 500 µl of heparinized blood from rabbits intraperitoneally, and mouse survival was monitored for 20 days after the infection.
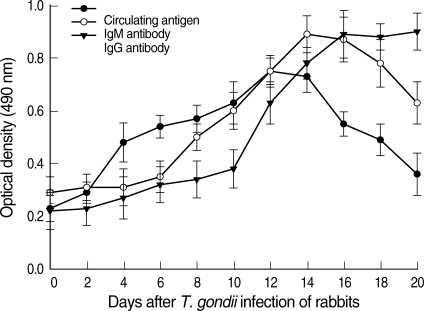
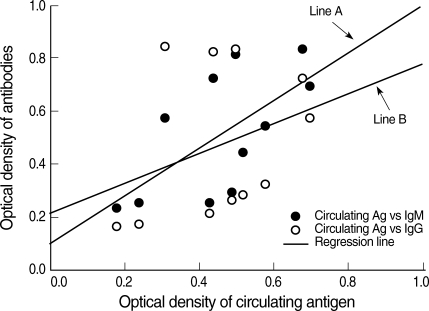
Toxoplasma circulating antigens in infected rabbits were observed on day 2 PI, and the antigen titers were increased significantly from day 4 PI compared to the uninfected rabbits (0.18 ± 0.05) and peaked on day 12 PI (Fig. 2). The positive circulating antigen titers appeared between days 4 and 18 PI (0.43 ± 0.07 and 0.44 ± 0.06). The specific anti-Toxoplasma IgM antibodies were detected on day 6 PI. Subsequently, the IgM titers increased significantly compared with those of uninfected controls (0.24 ± 0.06) and peaked on day 14 PI (0.84 ± 0.07). The IgG titers increased significantly from day 10 PI (0.33 ± 0.07) compared to uninfected rabbits (0.17 ± 0.07), and the titers increased continuously during the experiment. The correlation equation between the optical density of IgM antibodies and circulating antigens was Y = 0.64X + 0.120 (Line A, correlation coefficient 0.6478, P = 0.0313). The correlation equation between the optical density of IgG antibodies and circulating antigens was Y = 0.39X + 0.215 (Line B, correlation coefficient 0.3391, P = 0.3076) (Fig. 3).
Fig. 2.
Kinetics of the Toxoplasma circulating antigen (●), specific IgM (○), and IgG antibody (▼) titers in T. gondii-infected rabbit sera. The circulating antigen and anti-Toxoplasma IgM and IgG antibody titers were determined by ELISA. Each point represents the mean ± SD of 3 or 5 rabbits.
Fig. 3.
Correlation between the circulating antigen and IgM (Line A) or IgG (Line B) antibody titers of sera from rabbits infected with T. gondii. The circulating antigen and antibody titers were determined by ELISA.
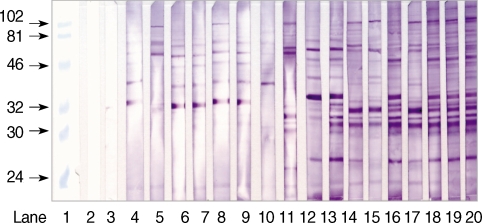
The serum from rabbits infected with T. gondii revealed different antigenic patterns according to the infection period on immunoblot analysis (Fig. 4). The main reactivity against TLA was at 30-32, 38, 58-65, and 76 kDa until day 10 PI. From day 12 through 16 PI, western blot analysis of the rabbit serum with tachyzoite antigen identified immunoreactive proteins at 20-21, 25-26, 30-32, 35, 64, and 76 kDa. The immunoblots after day 18 PI showed intense bands at 26, 30-31, 32, 36, 58, and 89 kDa. All antigenic protein bands were intensified according to the increased IgG antibodies.
Fig. 4.
Immunoblot patterns of sera from rabbits infected with 1,000 tachyzoites of the RH strain of T. gondii. Lane 1, molecular weight standard (kDa); lane 2, serum from normal rabbits; lane 3, rabbit serum on day 2 PI; lane 4, day 4 PI; lanes 5 and 6, day 6 PI; lanes 7 and 8, day 8 PI; lanes 9 and 10, day 10 PI; lanes 11 and 12, day 12 PI; lanes 13 and 14, day 14 PI; lanes 15 and 16, day 16 PI; lanes 17 and 18, day 18 PI; lanes 19 and 20, day 20 PI.
In most cases, the diagnosis of acute toxoplasmosis is confirmed by serologic tests. However, the production of specific antibodies in the host may be delayed or impaired, especially in congenital toxoplasmosis or in immunocompromised individuals. An alternative serologic test is detection of parasite components and metabolites, circulating antigens which are present in blood during the acute phase of infection. The circulating antigens have been found in both experimental and human toxoplasmosis [5-10]. In this study, circulating antigens of T. gondii began to be detected on day 2 PI, and the antigen titers increased on day 4 and peaked on day 12 PI. The appearance of positive circulating antigens after T. gondii infection in rabbits were similar with the previous reports [5,9,10]. Interestingly, the detection time of circulating antigens in rabbits coincided with the parasitemia period. The persistence of antigenemia and its consequent detection in serum is probably related to active proliferation of T. gondii [8].
In the serodiagnosis of acute toxoplasmosis, titration of anti-Toxoplasma IgM antibodies is important and informative. However, these serological methods may be inconclusive or unreliable in patients with underlying diseases that cause suppressed antibody responses or receiving immunosuppressive therapy. In addition, IgM antibodies do not form in all cases of acute toxoplasmosis [2]. In our study, IgM antibodies increased significantly from day 8 PI and peaked on days 12-14 PI. Also, serum IgM titers were closely related to the time of appearance of circulating antigens which appeared in rabbit blood. By contrast, the time at which IgG appeared was not significantly related to the circulating antigens.
Other researchers reported that specific antigenic bands were detected from 7-13 days after tachyzoite infection and major antigens were 20, 28, 30, 35, 45, 63, and 77 kDa on the immunoblot [11-14]. In this study, 8 major bands (20-21, 25-26, 30-32, 35-36, 38, 58-65, 76, and 89 kDa) and several minor bands were identified reacting with T. gondii-infected rabbit sera. The 30 kDa protein, which is the major antigen in T. gondii, presented strongly from day 14 after infection. The results seen in our infected rabbits were consistent with the previous results [13]. The number of major antigen bands increased with elevated IgM or IgG antibody titers. Although there were some differences in minor antigen bands, specific major bands from rabbit blood were similar compared with the published data. The differences in antigen bands may be affected by hosts, routes of infection, and the number of injected parasites [12,13].
Circulating antigens of Toxoplasma can be detected easily by immunoenzymatic methods like ELISA. A single serum sample is used for the detection of both antibody levels and circulating antigens of T. gondii in diagnosing active toxoplasmosis. Our results show that the circulating antigens are closely correlated with the IgM antibody titers and parasitemia during acute toxoplasmosis, therefore, co-detection of circulating antigens with IgM antibody may improve the reliability of the diagnosis of acute toxoplasmosis.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Science and Engineering Foundation through the Infection Signaling Network Research Center at Chungnam National University (R13-2007-020-01000-0), and National Research Foundation of Korea Grant funded by the Korean Government (2009-00-68706).
References
- 1.Ajioka JW, Soldati D. Toxoplasma: Molecular and Cellular Biology. Norfolk, UK: Horizon Bioscience; 2007. pp. 37–58. [Google Scholar]
- 2.Shaapan RM, El-Nawawi FA, Tawfik MA. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet Parasitol. 2008;153:359–362. doi: 10.1016/j.vetpar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Yokota M, Tatsumi N, Nathalang O, Yamada T, Tsuda I. Effects of heparin on polymerase chain reaction for blood white cells. J Clin Lab Anal. 1999;13:133–140. doi: 10.1002/(SICI)1098-2825(1999)13:3<133::AID-JCLA8>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voller A, Bartlett A, Bidwell DE. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978;31:507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Lu S, Lou D, Lin A, Zeng X, Ding Z, Wen L, Ohta N, Wang J, Fu C. Evaluation of a rapid ELISA technique for detection of circulating antigens of Toxoplasma gondii. Microbiol Immunol. 2008;52:180–187. doi: 10.1111/j.1348-0421.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Hafid J, Tran Manh Sung R, Raberin H, Akono ZY, Pozzetto B, Jana M. Detection of circulating antigens of Toxoplasma gondii in human infection. Am J Trop Med Hyg. 1995;52:336–339. doi: 10.4269/ajtmh.1995.52.336. [DOI] [PubMed] [Google Scholar]
- 7.Hassan MM, Mansour SA, Atta M, Shalaby MM, Seksaka MA, Awad A. The importance of detecting circulating Toxoplasma antigens in human cases. J Egypt Soc Parasitol. 1997;27:27–34. [PubMed] [Google Scholar]
- 8.Susanto L, Muljono R. Preparation of Toxoplasma gondii RH strain antigen, antigen analysis and antigen detection in sera: a review. Southeast Asian J Trop Med Public Health. 2001;32(suppl 2):195–201. [PubMed] [Google Scholar]
- 9.Ise Y, Iida T, Sato K, Suzuki T, Shimada K, Nishioka K. Detection of circulating antigens in sera of rabbits infected with Toxoplasma gondii. Infect Immun. 1985;48:269–272. doi: 10.1128/iai.48.1.269-272.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acebes MV, Diez B, Garcia-Rodriguez JA, Viens P, Cisterna R. Detection of circulating antigens in the diagnosis of acute toxoplasmosis. Am J Trop Med Hyg. 1994;51:506–511. [PubMed] [Google Scholar]
- 11.Chardès T, Bourguin I, Mevelec MN, Dubremetz JF, Bout D. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infect Immun. 1990;58:1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makioka A, Suzuki Y, Kobayashi A. Recognition of tachyzoite and bradyzoite antigens of Toxoplasma gondii by infected hosts. Infect Immun. 1991;59:2763–2766. doi: 10.1128/iai.59.8.2763-2766.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazabonne P, Bessieres MH, Seguela JP. Kinetics study and characterisation of target excreted/secreted antigens of immunoglobulin G, M, A and E antibodies from mice infected with different strains of Toxoplasma gondii. Parasitol Res. 1994;80:58–63. doi: 10.1007/BF00932625. [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Kim KY, Kang MS, Shin DW. Detection of Toxoplasma antigens and antibodies in mice infected with different strains of Toxoplasma gondii. Korean J Parasitol. 1995;33:201–210. doi: 10.3347/kjp.1995.33.3.201. [DOI] [PubMed] [Google Scholar]