Abstract
Virus-like particles (VLPs) can be exploited as platforms to increase the immunogenicity of poorly immunogenic antigens, including self-proteins. We have developed VLP-based vaccines that target two domains of the HIV coreceptor CCR5 that are involved in HIV binding. These vaccines induce anti-CCR5 antibodies that bind to native CCR5 and inhibit SIV infection in vitro. Given the role of mucosal surfaces in HIV transmission and replication, we also asked whether an aerosolized, VLP-based pulmonary vaccine targeting CCR5 could induce a robust mucosal response in addition to a systemic response. In rats, both intramuscular and pulmonary immunization induced high titer IgG and IgA against the vaccine in the serum, but only aerosol vaccination induced IgA antibodies at local mucosal sites. An intramuscular prime followed by an aerosol boost resulted in strong serum and mucosal antibody responses. These results show that VLP-based vaccines targeting CCR5 induce high-titer systemic antibodies, and can elicit both local and systemic mucosal response when administered via an aerosol. Vaccination against a self-molecule that is critically involved during HIV transmission and pathogenesis is an alternative to targeting the virus itself. More generally, our results provide a general method for inducing broad systemic and mucosal antibody responses using VLP-based immunogens.
Keywords: VLP, CCR5, HIV vaccine, aerosol, mucosal immunity
1. Introduction
Many viral structural proteins can self-assemble into virus-like particles (VLPs) that resemble infectious virus, but lack a viral genome and are therefore non-infectious. Because of their repetitive, multivalent structures, VLPs are highly immunogenic and make excellent vaccines for the virus from which they were derived; the human papillomavirus (HPV) and hepatitis B virus (HBV) vaccines are two examples of VLP-based vaccines. VLPs can also be adapted as platforms for display of antigens that are either not normally or poorly immunogenic. Heterologous antigens displayed in a highly dense, multivalent format on the surface of VLPs are extremely immunogenic; VLP-displayed antigens can induce high titer antibody responses at low doses and in the absence of exogenous adjuvants [1]. VLP display can even be used to induce antibody responses against self-antigens, essentially abrogating the mechanisms of B cell tolerance [2-4]. This observation has led to the development of a new class of vaccines that target self-molecules involved in a variety of chronic diseases, including Alzheimer’s Disease [5, 6], hypertension [7, 8] and rheumatoid arthritis [3, 9].
As an alternative strategy to conventional HIV vaccines, we have been interested in using VLP display technology to target CCR5, a self-molecule that is critically involved in HIV acquisition. During infection, HIV uses chemokine coreceptors in addition to its primary receptor, CD4, to gain entry into cells [10-12]. Although HIV can use several coreceptors, CCR5 is the most physiologically important. In the early stages of infection, the virus strains isolated are exclusively CCR5-tropic, suggesting a possible selective advantage for these viruses during transmission or during the early stages of disease [13]. Furthermore, individuals harboring a homozygous genetic mutation of the CCR5 allele (termed Delta-32) are resistant to HIV infection, and infected heterozygous individuals (who express lower levels of CCR5) progress more slowly to AIDS [14-16]. In 2008 the first small molecule CCR5 inhibitor, Maraviroc (Pfizer), was clinically approved. Maraviroc binds to CCR5 and changes its conformation so that it is not recognized by the coreceptor binding sites present on the HIV envelope glycoprotein, gp120. HIV infected patients receiving Maraviroc monotherapy have dramatically decreased viral loads, often to undetectable levels [17-19]. These data, in addition to the effects of the Delta-32 mutation on HIV infection, indicate that a reduction in the availability of functional CCR5 on target cells profoundly affects viral pathogenesis.
Unlike most viral vaccine targets, which mutate rapidly during the course of infection, CCR5 is a cellular protein and therefore genetically stable. We hypothesized that a vaccine that targeted CCR5 - either by limiting its expression on the cell surface or by blocking virus-receptor interactions - could block viral replication and affect viral pathogenesis. A number of vaccine strategies targeting CCR5 have been tested, including the use of a recombinant Flock House Virus that contains a CCR5 epitope [20], a CCR5-HSP70 fusion protein immunogen [21, 22], a DNA vaccine consisting of human CCR5 fused to tetanus toxoid [23], and CCR5 peptide conjugate vaccines [24, 25], among others. Our laboratory has developed several VLP-based vaccines to induce anti-CCR5 antibodies in which CCR5 epitopes are conjugated to the surface of pre-formed VLPs. We have previously shown that a papillomavirus (PV) VLP-based vaccine targeting the N-terminal extracellular domain of macaque CCR5 induced antibodies that bound to native CCR5 and blocked HIV infection in vitro [26]. Although this initial study was complicated somewhat by the low fitness of the challenge SHIV virus, prophylactic vaccination of macaques with the VLP-CCR5 vaccine reduced viral loads and time to clearance in pig-tailed macaques infected with a CCR5-tropic SHIV [27]. These data, and similar data from Misumi and colleagues [24], suggest that prophylactic vaccination against CCR5 may play a role in controlling viral replication in a SHIV/macaque model. In this study, we have developed second generation vaccines based on CCR5-derived peptides conjugated to bacteriophage VLPs. These vaccines target multiple domains of CCR5.
Most current vaccines are administered by intramuscular (IM) or subcutaneous injection. While these routes of immunization are extremely effective for the induction of systemic immunity, they generally result in poor mucosal immune responses. Most infectious pathogens, including HIV, enter the body and infect target cells at mucosal surfaces, so an ideal vaccine against HIV would induce both systemic and mucosal immune responses. Both the genital and gastrointestinal mucosa play crucial roles in the establishment of HIV infection, either as a site of transmission (at the vaginal or rectal mucosa) or as an important and critical site of viral replication and amplification seeding the bloodstream (in the gastrointestinal mucosa) [28].
We have been interested in examining the ability of VLP-based immunogens to induce mucosal immune responses. In particular, we have investigated the effectiveness of pulmonary vaccination using aerosolized VLP-vaccines in inducing broad immune responses. Aerosol delivery to the lung has a number of advantages. First, the lower respiratory tract contains abundant antigen-presenting cells, predominantly pulmonary macrophages and dendritic cells, which play important roles in priming adaptive immune responses. Second, although the mucosal immune system is, by and large, compartmentalized, pulmonary vaccination results not only in local mucosal responses in the lung, but also can give rise to strong mucosal responses in the genital/vaginal mucosa [29]. Third, previous studies have shown that mucosal immunization can in turn induce systemic immunity, which could eliminate the need for an intramuscular immunization [30].
In this study, we compared the immune responses induced by VLP-based vaccines targeting macaque CCR5 upon intramuscular and pulmonary immunizations. Both routes of immunization resulted in high-titer antibody responses against the vaccine preparation, and anti-CCR5 antibodies were effective at blocking SIV infection. However, only aerosol exposure led to the induction of local mucosal antibody responses.
2. Materials and Methods
2.1 CCR5-VLP preparation
A 21 amino acid peptide (designated EC1) representing the N-terminal 21 amino acids (MDYQVSSPTYDIDYYTSEPC; sulfated at Y10 and Y14) of pig-tailed macaque CCR5 (ptCCR5) was synthesized by American Peptide (Sunnyvale, CA), and then directly linked to Qß bacteriophage using a bifunctional cross-linker (SMPH, Pierce Endogen, IL), as described previously (4). A second peptide representing the second extracellular loop (ECL2) of ptCCR5 was synthesized by Celtek Peptides (Nashville, TN). The ECL2 peptide (DRSQREGLHYTG) is a cyclic peptide spanning amino acids 168 - 177 of ptCCR5 in which the Arg and Thr residues are linked through an Asp-Gly dipeptide spacer. Both peptides are shown in Figure 1. As with the EC1 peptide, the ECL2 peptide was linked to Qß bacteriophage via SMPH.
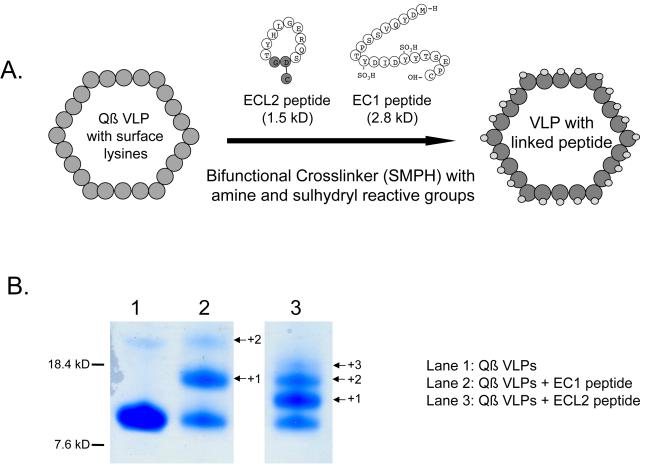
Figure 1.
Generating the CCR5 vaccines. A) EC1 and ECL2 peptides were linked to Qß VLPs through the use of a bifunctional crosslinker (SMPH). SMPH crosslinks surface lysines on Qß VLPs to a cysteine located at the C-terminus of the EC1 peptide or the base of the cyclized ECL2 peptide. Non-CCR5 derived amino acids are highlighted in grey. Numerous copies of peptide can be attached per coat protein, resulting in peptide presentation in a dense and repetitive array on the VLP surface. B) Polyacrylamide gel analysis of denatured Qß VLPs (lane 1), EC1-conjugated Qß VLPs (lane 2), and ECL2-conjugated Qß VLPs (lane 3). Qß VLPs are comprised of a single protein subunit, coat protein, which migrates with a mobility corresponding to its molecular weight, ~14000 Daltons. Conjugation of the EC1 peptide results in higher molecular weight species, representing individual coat protein subunits modified with 1 (+1), 2 (+2), or 3 (+3) copies of the peptide.
2.2 Animal inoculations
Intramuscular Immunizations
6-8 week-old female rats (Harlan Sprague Dawley, Indianapolis, IN) were inoculated with 15 μg of Qβ-EC1 VLPs in incomplete Freund’s adjuvant (IFA). 6-8 week-old female C57Bl/6 mice were inoculated with either 10 μg of Qβ-EC1 or Qβ-ECL2 VLPs, or 5μg of each VLP preparation, in incomplete Freund’s adjuvant (IFA). Inoculations were administered intramuscularly as shown in Table 1. Serum samples (approximately 0.1-0.2 mL) were collected one week following the 1st and 2nd immunization, and every week following the 3rd immunization (in rats) until sacrifice.
Table 1.
Experimental design
| Species | Inoculum | Delivery & Dose | Vaccination Schedule | Sacrificed @ |
|---|---|---|---|---|
| Mice | Qβ-EC1 | Intramuscular + IFA (10 ug) | weeks 0, 2 | 27 weeks |
| Mice | Qβ-ECL2 | Intramuscular + IFA (10 ug) | weeks 0, 2 | 27 weeks |
| Rats | Qβ | IM prime (15ug), aerosol boost (100ug) | weeks 0 (IM), 2, 6,15 (A) | 6 weeks |
| Rats | Qβ-EC1 | IM prime (15ug), aerosol boost (100ug) | weeks 0 (IM), 2, 6,15 (A) | 6 weeks |
| Rats | Qβ-EC1 | IM + IFA (15ug) | weeks 0, 2 | 6 weeks |
| Rats | Qβ-EC1 | Aerosol only (100ug) | weeks 0, 2 | 6 weeks |
| Rats | Qβ-EC1 | Aerosol + CTB adjuvant (100ug) | weeks 0, 2 | 6 weeks |
Aerosol Immunizations
Qß-EC1 VLP preparations, both prior to and after nebulization, were visualized by electron microscopy. VLPs were adsorbed to carbon-coated grids, stained with 1% uranyl acetate, and then were examined with a Philips electron microscope model EM400RT at magnification x36,000. Groups of rats were each exposed to 0.1 mg of Qβ or Qβ-EC1 nebulized VLPs (for a total of 0.3 mg in 5mL of phosphate-buffered saline (PBS) in a nose-only exposure chamber (InTox, Albuquerque, NM). The chamber incorporated an aerosol pathway that provides individual supply and exhaust routes in order to ensure uniform delivery of the test atmosphere. Compressed air was used for both nebulization air and dilution air to ensure adequate air supply. The chamber pressure was maintained just below zero during the dosing. Doses were determined by sampling the nose-only chamber and quantifying the aerosol concentration and using the following equation: Dose = (Aerosol concentration x Respiratory minute volume x Exposure time) / Body weight. Aerosol particle size was determined using a laser diffraction particle size analyzer (Sympatec, Germany). The median diameters of the aerosols were between 0.8 and 1.2 microns, thus ensuring predominant respiratory deposition in rodents[31]. Rats were acclimated to aerosol exposure restraint tubes prior to the initial exposure. Shown in Table 1, two groups (Qß-immunized rats and Qß-EC1 immunized rats) received an IM prime as their first inoculation, with three subsequent aerosol boosts. Two additional groups (Qß-EC1 immunized rats) received only aerosol inoculations, either with or without Cholera Toxin B (CTB) adjuvant. Serum samples were collected as described above unless otherwise indicated (IM prime groups). Animals were housed three per cage in autoclaved, ventilated cages (Tecniplast, Phoenixville, PA) containing autoclaved Tek-Fresh bedding (Harlan). The animals had ad libitum access to irradiated chow (Harlan) and autoclaved water. All animal care and experimental protocols were in accordance with the National Institutes of Health and University of New Mexico School of Medicine guidelines.
2.3 Quantifying antibody responses
Sera and, if appropriate, feces, uterine washes and bronchial-alveolar lavage fluid (BAL) were tested for antibodies specific for the CCR5-EC1 and ECL2 peptides and Qβ bacteriophage VLPs by ELISA. Briefly, Immulon II ELISA plates (Dynex Technologies, Chantilly, VA) were coated overnight at 4°C with either 0.5 μg of bovine serum albumin (BSA)-conjugated EC1 or ECL2 peptide or 0.5 μg Qβ VLPs per well. Wells were then blocked with 50 μL of PBS with 0.5% milk (w/v) per well for 2 h at room temperature. An initial 1:40 dilution of serum was serially diluted 4-fold and applied to wells for 2.5 h at room temperature. All dilutions were done in 0.5% milk (w/v) in PBS unless otherwise noted. Reactivity to target peptides was determined by using horseradish peroxidase (HRP)-labeled goat anti-mouse or anti-rat IgG (Jackson Immunoresearch, Bar Harbor, ME) at a dilution of 1:2000 and incubated for 1 h at room temperature. Upon development, the optical density at 405nm (OD405) was determined using a Thermo Max microplate reader (ThermoLab Systems, Fisher Scientific, Pittsburgh, PA). Absorbancies greater than twice the background were considered positive. ELISAs for IgA in sera were conducted as above, incorporating the following changes: sera were diluted 1:10 in PBS with 0.5% BSA, and presence of antibodies was detected using HRP-labeled goat anti-rat IgA (Open Biosystems, Huntsville, AL). For ELISA analysis of BAL fluids, feces and uterine washes, samples were diluted 1:1 in PBS.
2.4 ELISPOT
96-well ELISpot plates were activated with 70% ethanol according to manufacturer’s instructions (Millipore, Billerica, MA) and coated overnight at 4°C with 0.5 μg Qβ VLPs or BSA-EC1 peptide. Lungs were harvested, perfused and processed for lymphocyte isolation as previously described [32]. 4×105 cells per well were plated in complete RPMI and incubated overnight at 37°C. Wells were washed three times with 0.5% fetal calf serum (FCS) in PBS and the appropriate HRP-labeled goat anti-rat secondary (IgG or IgA) antibody was added at a 1:1000 dilution. Following a 2 h incubation at room temperature, wells were washed as described above and developed with TMB substrate (MABtech, Mariemont, OH) until spots appeared. Spots were quantitated using an AID ViruSpot/EliSpot Reader (Cell Technology, Inc., Columbia, MD).
2.5 Flow cytometry
Binding to CCR5 was tested by incubating pooled sera from immunized animals with 293T cells that were transiently transfected (or mock-transfected) with a pig-tailed macaque CCR5 expression vector (obtained from the NIH AIDS reference and reagent program). Two days after transfection, CCR5-expressing 293T cells were detached from the plate using 0.5mM EDTA and then washed three times in FACS buffer (0.5% BSA in PBS). Cells were incubated with Protein G-purified IgG isolated from the pooled sera of animals (rats or mice) immunized with Qß VLPs, Qß-EC1, or Qß-ECL2. Approximately 105 cells were resuspended in 100 μL of staining buffer and then incubated with 10-20 μL of purified IgG (at a concentration of 1 mg/mL) followed by a fluorescein isothiocyanate (FITC)-labeled goat anti-mouse or FITC-labeled goat anti-rat IgG (Jackson Immunoresearch, West Grove, PA). As a positive control, cells were incubated with a phycoerythrin (PE)-labeled anti-human CCR5 monoclonal antibody (3A9; BD Biosciences, San Jose, CA). Flow cytometric analysis was performed on a FACS Calibur by using the Cell Quest software package (BD Biosciences, San Jose, CA). Specific IgG binding was measured relative to mock-transfected cells.
2.6 SIV inhibition assay
SIVmac251 inhibition was measured using MAGI-ptCCR5 indicator cells [33] pretreated with heat-inactivated sera from immunized mice for 30 minutes at 37 degrees and then infected with approximately 100 infectious SIVmac251 particles (obtained from the NIH AIDS Research and Reference Reagent Program). Two days after infection, infected cells were scored by counting the number of blue cells in each well. Inhibition of SIV infection was determined by comparing the number of blue (infected) nuclei in the presence of antibody versus the number of blue nuclei in the absence of sera.
3. Results
3.1 Vaccine Preparation
Antigens displayed at high density on the surface of VLPs are highly immunogenic. We have used the VLP-display approach to develop vaccines targeting the HIV/SIV coreceptor CCR5. Because of their role in HIV binding to CCR5 [34, 35], we targeted the N-terminal extracellular domain of CCR5, also referred to as extracellular domain 1 (EC1), and a domain from extracellular loop 2 (ECL2). For the EC1 vaccine, a 21 amino acid peptide corresponding to the amino region of EC1 of pig-tailed macaque (pt) CCR5 was synthesized. The tyrosines at positions 10 and 14 of the peptide were sulfated to reflect the fact that sulfation of these residues in native CCR5 is thought to be important in HIV binding [36]. The ECL2 peptide is a cyclic peptide spanning amino acids 168 - 177 of ptCCR5 in which the Arg and Thr residues are linked through an Asp-Gly dipeptide spacer. This peptide was originally identified as an immunogen capable of inducing anti-CCR5 antibodies by Misumi and colleagues [37]. The EC1 and ECL2 peptides were chemically conjugated to VLPs derived from an RNA bacteriophage, Qß, using a bifunctional crosslinker, SMPH, that allowed us to link the C-terminal cysteine on the peptides to exposed surface lysine residues on the coat protein of Qß (Fig. 1a). The extent of conjugation was determined by analysis of denatured particles by gel electrophoresis (Fig. 1b). Peptide-modified Qß coat protein displays a mobility shift relative to unmodified Qß coat protein. The degree of the mobility shift reflects the addition of one, two, or three peptides per coat protein molecule. As is shown in Fig. 1b, the majority of coat protein has been modified with peptide. We estimate that the average Qß-ECL2 vaccine preparation contains 1.5 copies of peptide per coat protein and the Qß-EC1 preparation contains greater than 0.5 copies of peptide per coat protein, resulting in a total of ~270 (ECL2) or >90 (EC1) peptides being presented on the VLP surface. In either case, our earlier studies have indicated that these densities of peptide on the surface of the VLPs are sufficient to induce a strong antibody response [4, 38].
3.2 VLP-based CCR5 vaccines are immunogenic
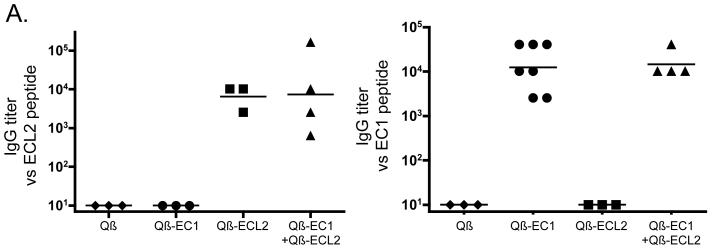
We determined whether Qß-EC1 and Qß-ECL2 could elicit anti-CCR5 antibody responses upon intramuscular (IM) immunization. Groups of three to seven mice were immunized with Qβ-EC1 and/or Qß-ECL2 as shown in Table 1. Briefly, animals were given two doses of 10 μg total inoculum (mice immunized concurrently with Qß-EC1 and Qß - ECL2 received 5 μg of each), at a two-week interval and then sera were collected weekly and analyzed for antibodies specific to the EC1 and ECL2 peptides by end-point dilution ELISA (Fig. 2a). Immunization with either Qß-EC1 or Qß -ECL2 elicited high-titer (geometric mean titer = ~104) IgG antibodies specific to the peptide of interest, but not against the other peptide. Animals immunized with Qß VLPs alone did not produce antibodies that recognized either of the CCR5 peptides. Simultaneous immunization with Qß-EC1 and Qß-ECL2 elicited high-titer IgG antibodies against both CCR5 peptides.
Figure 2.
Antibody responses to Qß-CCR5 vaccines in mice. A) IgG antibody responses in C57Bl/6 mice immunized three times at 2 week intervals with 5 μg wild-type Qß VLPs, 10 μg Qß conjugated to the CCR5 extracellular region 1 peptide (Qß-EC1), 10 μg Qß conjugated to the cyclic ECL2 peptide (Qß-ECL2), or a mixture of 5 μg Qß-EC1 plus 5 μg Qß-ECL2. This figure shows end-point dilution IgG ELISA titers for sera taken two weeks after the final immunization against peptides representing the CCR5 ECL2 (left panel) or EC1 (right panel). Each data point represents the antibody titer from an individual mouse. Lines represent the geometric mean titer for each group. B) Qβ-EC1 and Qß-ECL2 antibodies bind to native CCR5 in vitro. 293T cells were mock-transfected (solid line) or transfected with an expression vector encoding pig-tailed macaque CCR5 (dashed line). Two days after transfection, cells were incubated with a PE-labeled monoclonal antibody (3A9) that binds to the EC1 domain of CCR5 (top left), secondary antibody alone (left middle), pooled protein G-purified IgG from Qβ-immunized mice (bottom left), protein G-purified IgG from Qβ-EC1 immunized mice (top right), and protein G-purified IgG from Qβ-ECL2 immunized mice (bottom right), and then antibody binding was assessed by flow cytometry. Geometric mean fluorescence values are shown below each panel.
3.3 Anti-CCR5 antibodies bind native CCR5 in vitro and inhibit SIV infection
Although these data indicated that the two CCR5 peptide-conjugated VLP vaccines elicited antibodies that recognized ptCCR5 peptides, it was possible that these antibodies might not recognize the EC domains in their native conformation on membrane-associated ptCCR5. To examine this question, the ability of anti-EC region antibodies to bind to membrane-associated ptCCR5 was assessed by flow cytometry. IgG was purified from the pooled sera of Qß-EC1 or Qß-ECL2 intramuscularly immunized mice and incubated with ptCCR5- or mock-transfected 293T cells. As a positive control, some cells were incubated with 3A9, a monoclonal antibody that binds to the EC1 domain of CCR5. As a negative control, transfected cells were incubated with purified IgG from the sera of mice immunized with Qß VLPs alone. As shown in the right panels of Fig. 2b, IgG from the sera of Qß-EC1 and Qß-ECL2 immunized mice specifically bound to CCR5-expressing cells relative to mock-transfected cells, indicating binding of IgG to CCR5. There was no binding detected in IgG purified from the sera isolated from Qß-immunized mice (Fig. 2b, bottom left). Thus, both Qß-EC1 and Qß-ECL2 vaccines elicited antibodies that recognize the conformation of native CCR5 expressed on 293T cells.
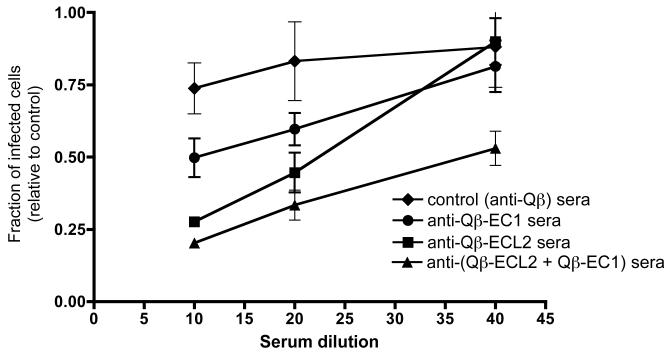
To assess the ability of induced sera to inhibit SIV infection, we used a single cycle infectivity assay utilizing the MAGI-ptCCR5 indicator cell line (previously described by [33]) to determine whether antibodies elicited from our CCR5 vaccines could inhibit SIV infection in vitro. Cells were incubated with increasing dilutions of heat-inactivated sera from mice immunized intramuscularly with Qß-EC1, Qß-ECL2, both Qß-EC1 and Qß-ECL2, or Qß VLPs alone and then infected with the CCR5-tropic strain SIVmac251. As shown in Fig. 3, sera from animals immunized with either Qß-EC1 or Qß-ECL2 inhibited SIVmac251 infection. Although anti-ECL2 and anti-EC1 antibodies were somewhat weakly inhibitory individually, in combination they displayed 50% inhibition at a 1:40 serum dilution. Although this inhibition titer is not particularly high, it is important to recognize that inhibition of virus infection by blocking the receptor is a much more stringent task than blocking the virus; every potential target cell expresses multiple copies of CCR5.
Figure 3.
Inhibition of SIVmac251 infection. SIVmac251 inhibition was measured using MAGI-ptCCR5 indicator cells pretreated with sera from immunized mice for 30 minutes at 37 degrees and then infected with approximately 100 infectious SIVmac251 particles. Two days after infection, infected cells were scored by counting the number of blue cells in each well. Inhibition of SIV infection was determined by comparing the number of blue (infected) nuclei in the presence of purified IgG versus the number of blue nuclei in the absence of IgG. Data represents the average of two different experiments; error bars show standard error of the mean.
3.4 Pulmonary immunization induces systemic antibodies against CCR5
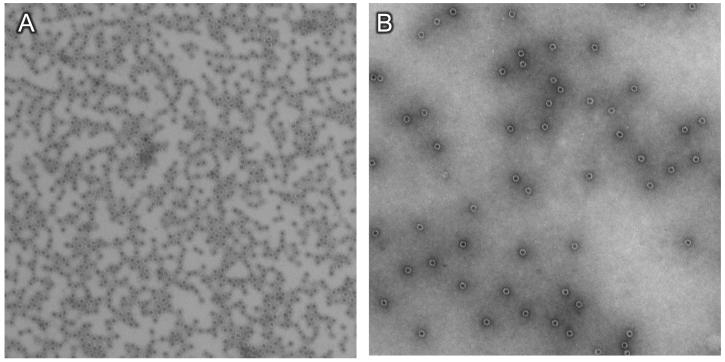
To see whether our VLP-based vaccine could also induce antibody responses upon mucosal immunization, we translated our intramuscular Qß-EC1 vaccine into an aerosolized vaccine for pulmonary administration. We first wanted to determine whether Qß-EC1 VLPs remained intact following the aerosolization process. VLPs were nebulized, recovered, and concentrated, and then visualized by electron microscopy. The nebulized and recovered VLPs had similar morphology to un-nebulized VLPs (Fig. 4).
Figure 4.
Qβ-EC1 virus-like particles survive nebulization. Qß-EC1 was nebulized and then captured and reconcentrated by filter centrifugation. Particles were then adsorbed to carbon-coated grids, were stained with 1% uranyl acetate, and were examined with a Philips electron microscope model EM 400RT at magnification x36,000. Shown is Qß-EC1 prior to (A) or post-nebulization (B).
Pulmonary immunizations were carried out in to 6-8 week-old female rats. We used rats because they have larger tidal volumes than mice, thereby inspired particles are better able to reach the lower respiratory tracts in this species [39]. Particles that reach the lower respiratory tracts are more difficult to expel, and are in a milieu rich in antigen-presenting cells ideal for initiating an immune response throughout mucosal tissues. Since the induction of anti-CCR5 antibodies in the genital tract was also a primary aim, female rats were chosen so that uterine washes could be collected (along with sera) throughout the study, and the uterus later recovered and analyzed.
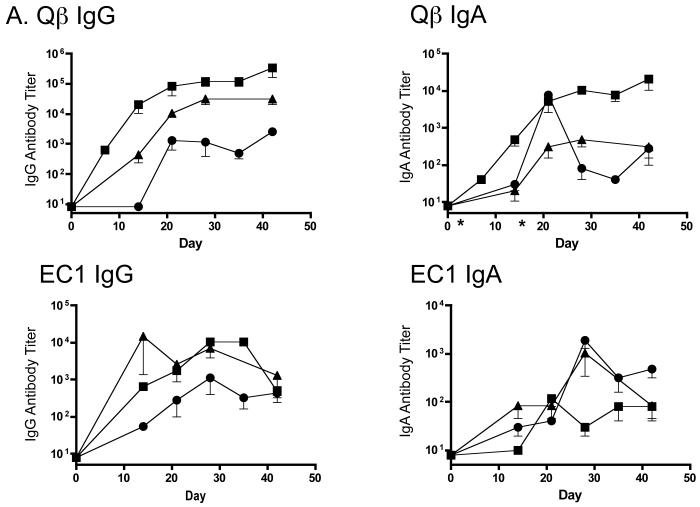
First, we assessed the antibody responses in rats immunized via the pulmonary route. Rats received aerosol immunizations of 100 μg Qß-EC1 twice at a two-week interval. One group of rats was immunized with Qß-EC1 formulated with 25 μg of cholera toxin B (CTB). CTB is a non-toxic and recombinantly produced cholera toxin B-subunit that has been reported to have mucosal adjuvant properties. Sera was collected every week until necropsy and IgG and IgA antibodies against Qβ and EC1 were quantitated by end-point dilution ELISA and compared to the antibody responses elicited by intramuscular (IM) immunization. As shown in Fig. 5a, aerosol administration of Qß-EC1 elicited anti-EC1 and anti-Qß IgG and IgA antibodies that were comparable to those elicited when the vaccine was delivered intramuscularly. Coadministration of CTB adjuvant had slightly enhanced IgG titers, but elicited no appreciable difference in IgA titers. Rats given IM immunizations had the highest titers against the VLP platform, but had similar anti-EC1 antibody levels as those measured in rats receiving the aerosolized vaccine.
Figure 5.
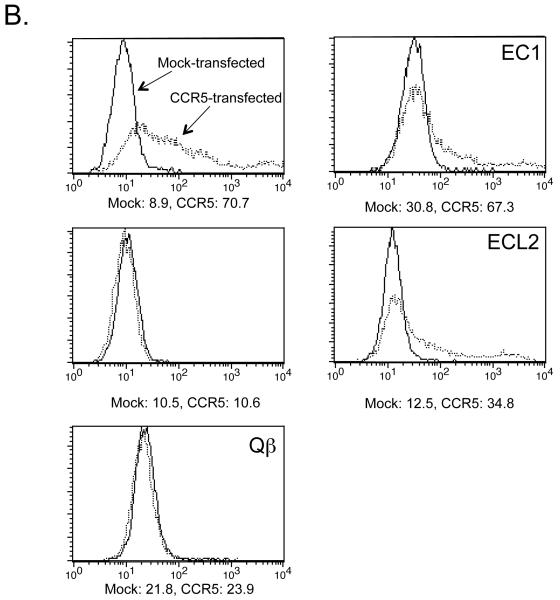
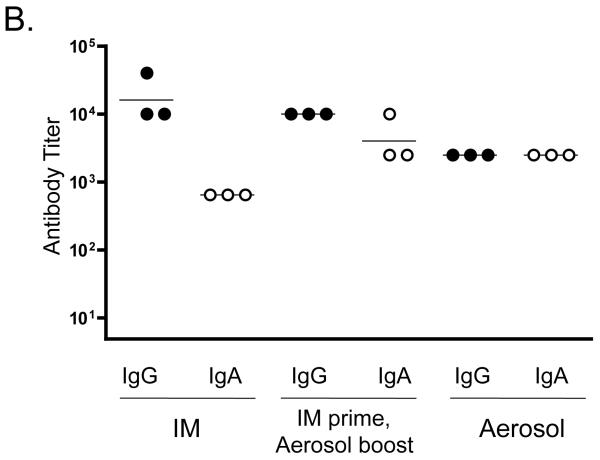
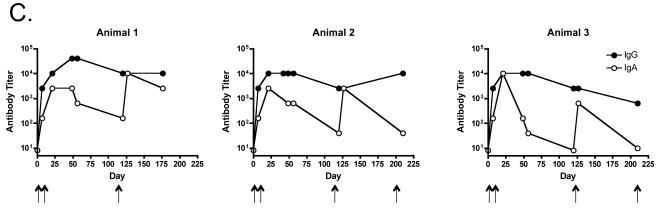
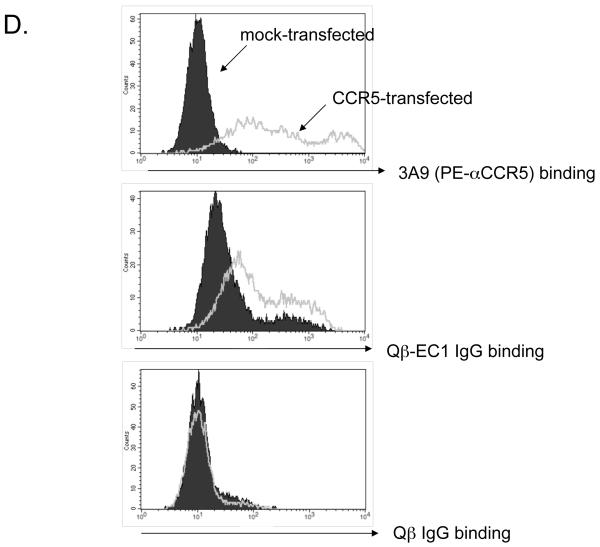
Systemic IgG and IgA responses in rats immunized with Qβ-EC1 VLPs. A) Groups of three rats were immunized intramuscularly (squares) or via the pulmonary route with (triangles) or without (circles) CTB adjuvant. Immunizations were carried out on days 0 and 14, sera were collected at the time points indicated, and antibody levels were determined by end-point dilution ELISA. Shown in the top panel are anti-Qβ IgG (left) and anti-Qβ IgA (right) antibody titers, and on the bottom panel anti-EC1 IgG (left) and anti-EC1 IgA (right) antibody titers. The data shows the geometric mean titer of three immunized rats and error bars represent SEM. B) Comparison of IgG and IgA serum anti-EC1 titers in rats one week following a second inoculation with Qβ-EC1. Rats received two intramuscular inoculations, two inoculations via aerosol, or an intramuscular prime followed by an aerosol boost. C) Kinetics of serum anti-EC1 responses in individual rats receiving an IM prime followed by aerosol boosts of Qβ-EC1. Rats were immunized on weeks 0, 2, 6, and 15 (arrows), sera collected at the time points indicated and IgG (closed circles) and IgA (open circles) titers were determined by ELISA. D) Immunization of rats via pulmonary route induces antibodies that bind native CCR5. 293T cells were mock-transfected (filled) or transfected with an expression vector encoding pig-tailed macaque CCR5 (line). Two days after transfection, cells were incubated with a PE-labeled monoclonal antibody 3A9 (top), pooled protein G-purified IgG from Qβ-EC1 immunized rats (middle), and pooled protein G-purified IgG from Qβ-immunized rats (bottom), and then antibody binding was assessed by flow cytometry.
We also wanted to determine whether systemic antibody responses could be increased by “priming” rats with an initial IM immunization followed by an aerosol boost. Groups of rats were immunized with two IM injections, two aerosol exposures, or an IM prime followed by an aerosol boost. One week following the second immunization, sera was collected and IgG and IgA titers against the EC1 peptide were measured (Fig. 5b). Rats receiving two IM immunizations had the highest serum-associated IgG levels against EC1, but had lower IgA levels. In contrast, rats receiving two aerosol immunizations had lower IgG levels, but higher IgA antibodies. Rats receiving the prime/boost regimen had an intermediate response that achieved a balance between the two other immunization strategies. The IM prime group displayed a 10-fold increase in IgA titers as compared to rats given only IM immunizations; there was also a moderate increase in IgA titer compared to the group receiving the aerosolized vaccine. Moreover, the level of IgG elicited by the IM group was not lower in the IM prime group, and was slightly higher than that seen in animals receiving only pulmonary immunizations. To investigate the longevity of the immune response and the efficacy of a boost in evoking memory, we continued collecting sera from the prime/boost group over 27 weeks. Animals in this group were given additional aerosol boosts at weeks 6 and 15, and then sacrificed 27 weeks after the initial immunization. IgG titers against EC1 were sustained, and remained above 103 for 12 weeks following the final boost in all three animals (Fig. 5c). Serum anti-EC1 IgA antibodies diminished more rapidly, perhaps reflecting the shorter half-life of this molecule. However, aerosol boosts increased IgA antibody levels to peak titers (104) in 2 out of 3 animals.
We once again confirmed via flow cytometry that antibodies induced by pulmonary immunization could specifically bind membrane-bound CCR5. Using the same experimental methods described above, we purified IgG from rat sera and incubated it with ptCCR5- or mock-transfected 293T cells. As shown in Fig. 5d, IgG purified from the sera of animals receiving aerosolized immunizations of Qβ-EC1 bound to cells expressing CCR5 relative to mock-transfected cells. As expected, there was no binding detected using IgG purified from the sera isolated from rats immunized with Qß alone.
3.5 Pulmonary immunization enhances local antibody secretion in mucosal tissues
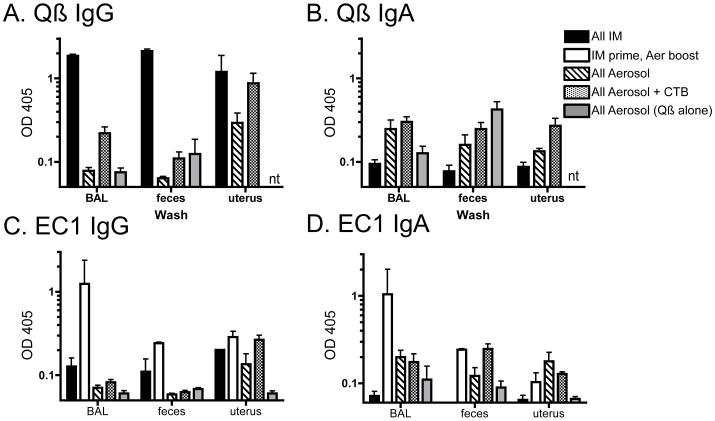
Pulmonary administration of our Qβ-EC1 vaccine was successful in inducing both IgG and IgA antibodies in the blood, so we next asked whether antibodies specific to Qβ and EC1 were also present in mucosal secretions of animals immunized by the various strategies described previously. As an additional control, one group of rats was immunized with Qß VLPs alone, via the IM prime/aerosol boost regimen. At necropsy, we collected bronchi-alveolar lavage fluid (BAL), and we also collected washes from the uterus and feces of the immunized rats. These samples were diluted 1:1 in PBS with 0.5% BSA, and then anti-Qβ and -EC1 IgG and IgA antibodies were measured by ELISA (note: we did not test the anti-Qß antibody responses of the prime/boost group because of a limited amount of material). As was observed in the sera, rats given the IM vaccine alone and the IM prime/aerosol boost regimen had the highest IgG levels in all three mucosal sites tested (Fig. 6a, c). Rats immunized via the aerosol route alone had relatively high IgG titers in uterine washes, but surprisingly low IgG levels in BAL fluid. However, only those animals immunized with the aerosolized vaccine secreted IgA at the mucosal sites tested (Fig. 6b, d)--essentially no IgA response was induced by IM immunization. Co-administration of CTB adjuvant with the aerosolized vaccine somewhat boosted secreted IgG levels relative to aerosol alone, but did not have a pronounced impact on IgA levels in secretions. In general, the IM prime/aerosol boost regimen induced the strongest anti-EC1 antibody response, particularly in the lung.
Figure 6.
Anti-Qβ and -EC1 titers in mucosal washes of immunized rats. Bronchi-alveolar lavage fluid, feces, and uterine lavage samples were collected at necropsy, diluted 1:1 in PBS, and anti -Qβ and -EC1 IgG and IgA antibodies levels were measured by ELISA. Rats were immunized with Qβ-EC1 via the intramuscular route (black), the pulmonary route with or without CTB adjuvant (dotted or lined fill, respectively), or given an IM-prime followed by aerosol boosts (no fill). As a negative control, a fifth group was given an IM prime followed by aerosol boosts of QB VLPs alone (grey). Shown are (A) anti-Qβ IgG, (B) Anti-Qβ IgA, (C) anti-EC1 IgG, and (D) anti-EC1 IgA.
3.6 CCR5-specific B cells in the lung following pulmonary immunization
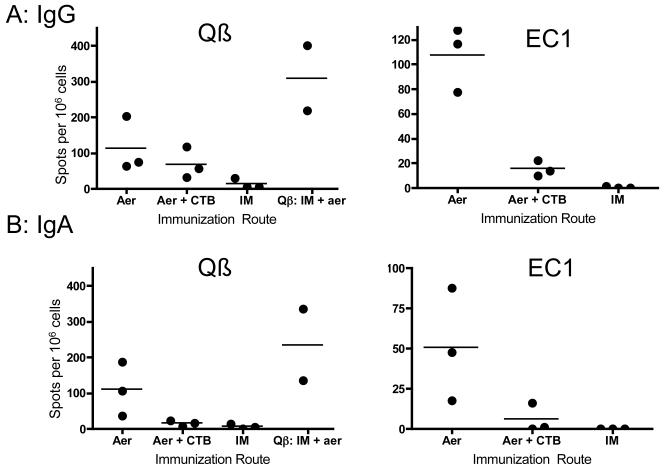
After determining that pulmonary immunization could confer systemic immunity as well as mucosal immunity in remote tissues, we assessed the local immune response more quantitatively by determining the number of antibody-specific B cells in the lung by ELISPOT. Lungs were collected from animals receiving only aerosol immunizations or only IM immunizations of Qβ-EC1, and processed as previously described for lymphocyte collection [32]. ELISPOT analysis was performed on plated B cells and the number of IgG- and IgA- secreting B cells specific for either Qβ or EC1 quantified. As a control, some animals were immunized with Qβ VLPs alone. Consistent with what was observed in mucosal washes, only animals receiving the aerosolized vaccine had detectable Qβ- and EC1-specific B cells. Animals receiving an IM administration of the vaccine had few Qβ-specific IgG secreting B cells in the lung, and no EC1-specific B cells. Coadministration of CTB did not enhance the frequency of EC1-specific B cells, and, if anything, seemed to have a negative effect (Fig. 7), in contrast to the neutral and/or positive effects observed in the sera and mucosal washes. Rats given the inhaled Qβ-EC1 vaccine without adjuvant had nearly equal amounts of IgA secreting B cells against both Qβ and EC1, and comparable numbers of IgG-secreting B cells as well, indicating an immune response against both platform and target.
Figure 7.
Aerosol exposure, but not intramuscular immunization, results in vaccine specific B cells in the lung. Lymphocytes were isolated from lungs of rats receiving intramuscular (IM) or pulmonary (Aer) deliveries of QB-EC1 either with or without CTB adjuvant. Cells were analyzed by ELISPOT for IgG (A) and IgA (B) secreting QB- and EC1-specific B-cells. As a positive control, one group received an IM prime followed by aerosol boosts of QB VLPs alone (panels A and B).
4. Discussion
Development of a prophylactic HIV vaccine is an important component in battling the worldwide HIV epidemic, but the path towards this goal has been fraught with difficulties. Viral sequence diversity and antigenic variation are major and perhaps insurmountable barriers in the development of vaccines based on the induction of humoral and/or cellular immunity against the virus. As an alternative strategy to conventional HIV vaccines, we have been interested developing a vaccine that targets CCR5, a self-molecule that is critically involved in HIV acquisition, and which is not subject to antigenic variation. However, because CCR5 is a self-protein, the ability to initiate an antibody response against the molecule is seemingly limited by the mechanisms of B cell tolerance, which normally prevent the induction of antibody responses against self-molecules. In spite of this, we and others have shown that by arraying self-molecules at high density on the surface of virus-like particles (VLPs) we can completely abrogate these tolerance mechanisms and induce high titer IgG antibodies against diverse self-antigens [40, 41]. Our laboratory has taken advantage of these findings to develop several VLP-based vaccines that elicit anti-CCR5 antibodies. Previously, we developed a papillomavirus (PV) VLP-based vaccine targeting the N-terminal extracellular domain of macaque CCR5 that induced anti-CCR5 antibodies in macaques that bind to native CCR5 and blocked HIV infection in vitro. Moreover, prophylactic vaccination of macaques with this vaccine reduced viral loads and time to clearance in pig-tailed macaques infected intravenously with a CCR5-tropic SHIV [27].
Here, we show that bacteriophage VLP-based vaccines that target two extracellular domains of macaque CCR5 that are involved in SIV/HIV binding induce similarly high titer anti-CCR5 antibodies in rodents. In the current study, the question of immune tolerance was not addressed, as we targeted macaque CCR5 in rodent models. However we, and others, have shown that the ability to induce antibody responses in the face of immune tolerance mechanisms is a general characteristic of VLP display [40, 41]. Moreover, several laboratories have previously or are currently working on approaches to induce anti-CCR5 antibodies. These include Lucia Lopalco’s laboratory, which has developed a recombinant Flock House virus that presents a peptide derived from CCR5 ECL1 [20] and Tom Lehner’s laboratory, which has developed a CCR5-HSP70 fusion protein immunogen [21, 22], among others. Three macaque challenge experiments have been reported; our own and the previously mentioned Misumi study [24], in which vaccinated macaques were challenged with a SHIV isolate and which some degree of viral inhibition was reported, and studies by Wahren and colleagues, in which DNA vaccination with a construct containing human CCR5 fused to tetanus toxoid failed to protect macaques from SIVsm challenge [23].
HIV, like many pathogens, most frequently establishes infection at mucosal surfaces. As such, the efficient induction of mucosal immunity by vaccination, and particularly the induction of local immunoglobulin production, including secretory IgA (SIgA), will likely play an important role in future HIV-1 vaccine approaches [42, 43]. The genital and gastrointestinal mucosa play crucial roles in the establishment of HIV infection, either as a site of transmission or as an important site of early viral replication and amplification. Although antibodies against CCR5 would presumably not act as classical neutralizing antibodies, it is possible that they could block interactions between virus and the cells that are targeted early in infection, specifically resident activated memory T cells or Langerhans cells (LCs). LCs have been proposed to facilitate HIV infection by capturing virus, migrating to regional lymph nodes, and then transferring virions to susceptible T cells. Although LCs can interact with HIV via a variety of different surface receptors, it has been shown that antibodies that bind CCR5 can partially block the uptake of HIV by LCs [44]. Low level CCR5-reactive antibodies (with virus inhibitory activity in vitro) have been detected in the seronegative partners of HIV-infected individuals, suggesting that anti-CCR5 antibodies may play a role in protection from natural HIV infection [45].
Numerous mucosal vaccination strategies have been investigated, including administration of vaccines to oral, genital, rectal, and respiratory mucosal surfaces. The mucosal immune system is largely compartmentalized, and the expression of mucosal IgA typically occurs primarily at the site of vaccination [29]. Thus, the choice of a mucosal vaccination route needs to be tailored to the specific target. The respiratory tract is a particularly attractive site for immunization. Not only does immunization of the respiratory tract stimulate a local mucosal antibody response, but it can also elicit a strong genital mucosal immune response [29]. Moreover, administration of vaccines via aerosol to the nasal and bronchial lymphoid tissues is less invasive than other approaches and may facilitate vaccine implementation.
A number of different studies in mice have shown that intranasal vaccination with VLPs or VLP-based immunogens successfully results in local (in the lung) mucosal and systemic antibody responses [20, 46, 47]. One advantage of aerosol over intranasal immunization is that aerosol delivery allows for VLP deposition in the lower respiratory tract. The trachea, lungs, and mediastinal lymph nodes act as the major immune inductive sites, allowing for a robust systemic humoral response. The upper respiratory tract, represented by the nasal-associated lymphoid tissue (NALT), although directly stimulated upon intranasal immunization, in some cases appears to play a negligible role in the induction of a mucosal immune response at distal sites [46, 48, 49], although at least one report has shown that nasal immunization can result in induction of IgA in the genital tract [50]. Immunization of the lower respiratory tract is more effective at inducing genital antibody responses in mice [48]. Moreover, immunization of the lower respiratory tract may be particularly important in inducing mucosal immunity in humans. For example, a comparative study of intranasal versus aerosol immunization of humans with HPV16 VLPs demonstrated that nasal immunization resulted in weak systemic and mucosal anti-VLP antibody responses. In contrast, bronchial aerosolization of HPV16 VLPs resulted in significantly higher antibody levels and rates of seroconversion [51]. Consequently, if the lower respiratory tract is not reached for antigen presentation during intranasal immunization, it could account for reduced levels of mucosal, but not systemic, VLP-specific humoral responses. In this study, we provide the first indication that a VLP-based vaccine targeting a heterologous antigen can be aerosolized to effectively induce IgG and IgA antibodies in both local and distal mucosal tissues. The presence of Qβ—specific IgG antibody in distant mucosal tissues following aerosol immunization suggests that our vaccine was likely able to reach the lower respiratory tract.
We found that Qβ-EC1 VLPs were able to survive nebulization and delivery in a rat model, and that immunization via a pulmonary route was effective at generating both anti-EC1 IgG and IgA antibodies in the sera, the lung, the genital tract, and, to a lesser extent, the gastrointestinal (GI) tract. Presence of IgA in the uterine washes is most likely due to the upregulation of CCR10 on antibody producing cells, which allows for migration from the respiratory tract-draining lymph node to the genital tract [52]. Pulmonary immunization does not however typically result in upregulation of CCR9, which directs homing to the intestine [53, 54]. While this could explain the decreased amounts of IgG antibody found in the feces, the levels of IgA seen in both the uterine wash and the feces is somewhat surprising. It is possible that some of the inoculum reached the GI tract through animal inspiration during the aerosol exposure.
In accordance with the secreted antibody levels observed in the sera and mucosal tissues following pulmonary immunization, rats receiving the aerosolized vaccine were also able to maintain high levels of Qβ-EC1 specific B cells in the lung, as well as detectable levels in the spleen and uterus 55 days following final exposure (data not shown). Similar to our study, Bessa et al. recently compared subcutaneous and intranasal deliveries of Qβ VLPs in mice and found that while both routes were effective at generating specific IgG in the serum, only the intranasal route of vaccination yielded Qβ-specific IgA in BAL; levels of mucosal IgG were also generally higher in these mice as compared to the s.c. group [47]. Bessa also showed that the numbers of IgG antibody-forming cells in the mesenteric lymph node were higher in mice receiving i.n. immunization of Qβ VLPs, which parallels our finding that aerosol immunization yields higher numbers of Qβ VLP- and EC1-specific B cells in the lung itself.
Unexpectedly, use of the mucosal adjuvant CTB did not appear to enhance immune responses at mucosal sites. Indeed, in some cases, CTB actually dampened antibody levels when compared to the group receiving the aerosolized vaccine without adjuvant. It is possible that the CTB was delivered to different mucosal compartments than the VLPs, or simply that CTB is not compatible with the nebulization process and thus not an appropriate adjuvant for aerosol delivery. Previously, Nardelli et al looked at the ability of mucosal adjuvants to increase both serum-specific IgG and mucosal IgA titers following “aerosol-like” vaccination. In this protocol, mice were anesthetized and immunized with HPV VLPs and either heat labile enterotoxin (HLT) or CpG oligodeoxynucleotides, following priming with influenza peptides. HLT proved a potent adjuvant and was able to increase VLP-specific IgG in the serum by more than 10-fold [49]. Furthermore, use of HLT was able to restore IgG and IgA titers in both sera and mucosa that were previously obtained following vaccination with HPV VLPs and CTB adjuvant (without influenza priming). In contrast to our results, these prior experiments with CTB were successful, however titers were higher when HLT was used. The success of this adjuvant could be due to its ability to induce both Th1 and Th2 responses, as well as SIgA and serum IgG and IgA responses [55]. Recent studies have shown that when CTB is used (along with whole cholera toxin [CT]) as a vector, it can give rise to either mucosal immunity or induce peripheral anti-inflammatory tolerance to chemically or genetically linked foreign antigens administered mucosally. More specifically, CTB seems to steer the immune response towards Th2-only immunity or tolerance, while CT favored a broad Th1 + Th2 + CTL immunity [56]. The use of HLT instead of CTB may enhance the IgA titers induced by VLP-based immunogens.
Use of the VLP-based approach for vaccine delivery to the mucosa-associated lymphoid tissue (MALT) has widespread applicability, with relevance to virtually any pathogen infecting at a mucosal surface. A vaccine that elicits both IgA and IgG antibodies at the site of infection prior to exposure could be a powerful deterrent to viral entry and subsequent infection. The innate immunogenic properties of VLPs provide a useful mechanism to generate antibody responses against poorly antigenic molecules both systemically and in the respiratory tract. The simultaneous flexibility and stability of VLPs offer further advantages with regard to mucosal vaccine design. The very type of VLP used for a particular vaccine can be catered to both its destination within the mucosa/environment and to the conjugate antigen, if applicable. For example, VLP size, surface chemistry, and tolerance for antigen insertion or conjugation are all factors in vaccine design that can be readily manipulated for optimal efficacy. Translating this technology for non-invasive aerosolized delivery, with the result of an enhanced mucosal immune response, significantly broadens its applicability.
Acknowledgements
We thank Dave Peabody for providing us with Qß coat protein expression constructs, C. Rick Lyons for use of the aerosol exposure chambers, and Susana Pang for assistance with electron microscopy. SIVmac251 was obtained from the NIH Research and Reference Reagent Program. This study was supported by the NIH (grant R01 AI065240 to B.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. BC is a coinventor on a US government-owned patent: virus-like particles for the induction of autoantibodies (US patent 6,719,978).
REFERENCES
- [1].Spohn G, Bachmann MF. Exploiting viral properties for the rational design of modern vaccines. Expert Rev Vaccines. 2008 Feb;7(1):43–54. doi: 10.1586/14760584.7.1.43. [DOI] [PubMed] [Google Scholar]
- [2].Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol. 2008 May 1;180(9):5816–25. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001 Aug;108(3):415–23. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002;169(11):6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- [5].Ambuhl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007 Jan;25(1):63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- [6].Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006 Sep 11;24(3739):6321–31. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- [7].Li Q, Cao C, Chackerian B, Schiller J, Gordon M, Ugen KE, et al. Overcoming antigen masking of anti-amyloidbeta antibodies reveals breaking of B cell tolerance by virus-like particles in amyloidbeta immunized amyloid precursor protein transgenic mice. BMC Neurosci. 2004 Jun 8;5(1):21. doi: 10.1186/1471-2202-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zamora E, Handisurya A, Shafti-Keramat S, Borchelt D, Rudow G, Conant K, et al. Papillomavirus-like particles are an effective platform for amyloid-beta immunization in rabbits and transgenic mice. J Immunol. 2006 Aug 15;177(4):2662–70. doi: 10.4049/jimmunol.177.4.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spohn G, Guler R, Johansen P, Keller I, Jacobs M, Beck M, et al. A virus-like particle-based vaccine selectively targeting soluble TNF-alpha protects from arthritis without inducing reactivation of latent tuberculosis. J Immunol. 2007 Jun 1;178(11):7450–7. doi: 10.4049/jimmunol.178.11.7450. [DOI] [PubMed] [Google Scholar]
- [10].Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- [11].Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85(7):1149–58. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- [12].Deng HK, Unutmaz D, KewalRamani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997 Jul 17;388(6639):296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- [13].Li S, Juarez J, Alali M, Dwyer D, Collman R, Cunningham A, et al. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999 Dec;73(12):9741–55. doi: 10.1128/jvi.73.12.9741-9755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- [15].Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- [16].Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science. 1998;279:389–93. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- [17].Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005 Nov;11(11):1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- [18].Hunt JS, Romanelli F. Maraviroc, a CCR5 coreceptor antagonist that blocks entry of human immunodeficiency virus type 1. Pharmacotherapy. 2009 Mar;29(3):295–304. doi: 10.1592/phco.29.3.295. [DOI] [PubMed] [Google Scholar]
- [19].Yost R, Pasquale TR, Sahloff EG. Maraviroc: A coreceptor CCR5 antagonist for management of HIV infection. Am J Health Syst Pharm. 2009 Apr 15;66(8):715–26. doi: 10.2146/ajhp080206. [DOI] [PubMed] [Google Scholar]
- [20].Barassi C, Soprana E, Pastori C, Longhi R, Buratti E, Lillo F, et al. Induction of murine mucosal CCR5-reactive antibodies as an anti-human immunodeficiency virus strategy. J Virol. 2005 Jun;79(11):6848–58. doi: 10.1128/JVI.79.11.6848-6858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bogers WM, Bergmeier LA, Oostermeijer H, ten Haaft P, Wang Y, Kelly CG, et al. CCR5 targeted SIV vaccination strategy preventing or inhibiting SIV infection. Vaccine. 2004 Aug 13;22(2324):2974–84. doi: 10.1016/j.vaccine.2004.02.050. [DOI] [PubMed] [Google Scholar]
- [22].Bogers WM, Bergmeier LA, Ma J, Oostermeijer H, Wang Y, Kelly CG, et al. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. Aids. 2004 Jan 2;18(1):25–36. doi: 10.1097/00002030-200401020-00003. [DOI] [PubMed] [Google Scholar]
- [23].Zuber B, Hinkula J, Vodros D, Lundholm P, Nilsson C, Morner A, et al. Induction of immune responses and break of tolerance by DNA against the HIV-1 coreceptor CCR5 but no protection from SIVsm challenge. Virology. 2000 Dec 20;278(2):400–11. doi: 10.1006/viro.2000.0633. [DOI] [PubMed] [Google Scholar]
- [24].Misumi S, Nakayama D, Kusaba M, Iiboshi T, Mukai R, Tachibana K, et al. Effects of immunization with CCR5-based cycloimmunogen on simian/HIVSF162P3 challenge. J Immunol. 2006 Jan 1;176(1):463–71. doi: 10.4049/jimmunol.176.1.463. [DOI] [PubMed] [Google Scholar]
- [25].Chain BM, Noursadeghi M, Gardener M, Tsang J, Wright E. HIV blocking antibodies following immunisation with chimaeric peptides coding a short N-terminal sequence of the CCR5 receptor. Vaccine. 2008 Oct 23;26(45):5752–9. doi: 10.1016/j.vaccine.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci USA. 1999;96:2373–8. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chackerian B, Briglio L, Albert PS, Lowy DR, Schiller JT. Induction of autoantibodies to CCR5 in macaques and subsequent effects upon challenge with an R5-tropic simian/human immunodeficiency virus. J Virol. 2004 Apr;78(8):4037–47. doi: 10.1128/JVI.78.8.4037-4047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Veazey R, Lackner A. The mucosal immune system and HIV-1 infection. AIDS Rev. 2003 Oct-Dec;5(4):245–52. [PubMed] [Google Scholar]
- [29].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005 Apr;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [30].Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003 Jan;9(1):99–103. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- [31].Roy CJ, Hale M, Hartings JM, Pitt L, Duniho S. Impact of inhalation exposure modality and particle size on the respiratory deposition of ricin in BALB/c mice. Inhal Toxicol. 2003 May;15(6):619–38. doi: 10.1080/08958370390205092. [DOI] [PubMed] [Google Scholar]
- [32].Swanson KA, Zheng Y, Heidler KM, Mizobuchi T, Wilkes DS. CDllc+ cells modulate pulmonary immune responses by production of indoleamine 2,3-dioxygenase. Am J Respir Cell Mol Biol. 2004 Mar;30(3):311–8. doi: 10.1165/rcmb.2003-0268OC. [DOI] [PubMed] [Google Scholar]
- [33].Kimata JT, Gosink JJ, KewalRamani VN, Rudensey LM, Littman DR, Overbaugh J. Coreceptor specificity of temporal variants of simian immunodeficiency virus Mne. J Virol. 1999 Feb;73(2):1655–60. doi: 10.1128/jvi.73.2.1655-1660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Edinger AL, Amedee A, Miller K, Doranz BJ, Endres M, Sharron M, et al. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):4005–10. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, et al. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186(8):1373–81. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999 Mar 5;96(5):667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- [37].Misumi S, Nakajima R, Takamune N, Shoji S. A cyclic dodecapeptide-multiple-antigen peptide conjugate from the undecapeptidyl arch (from Arg(168) to Cys(178)) of extracellular loop 2 in CCR5 as a novel human immunodeficiency virus type 1 vaccine. J Virol. 2001 Dec;75(23):11614–20. doi: 10.1128/JVI.75.23.11614-11620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on Virus-like Particles of RNA phage MS2. Journal of Molecular Biology. 2008 doi: 10.1016/j.jmb.2008.04.049. doi:10.1016/j.jmb/2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Parent RA. Comparative Biology of the Normal Lung. CRC Press; Boca Raton: 1992. [Google Scholar]
- [40].Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007 Jun;6(3):381–90. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- [41].Jennings GT, Bachmann MF. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol. 2009;49:303–26. doi: 10.1146/annurev-pharmtox-061008-103129. [DOI] [PubMed] [Google Scholar]
- [42].Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000 Nov 1;165(9):5170–6. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- [43].Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997 Nov;3(11):1250–7. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- [44].Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007 Feb;26(2):257–70. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lopalco L, Barassi C, Pastori C, Longhi R, Burastero SE, Tambussi G, et al. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 In vitro. J Immunol. 2000;164(6):3426–33. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- [46].Balmelli C, Demotz S, Acha-Orbea H, De Grandi P, Nardelli-Haefliger D. Trachea, lung, and tracheobronchial lymph nodes are the major sites where antigen-presenting cells are detected after nasal vaccination of mice with human papillomavirus type 16 virus-like particles. J Virol. 2002 Dec;76(24):12596–602. doi: 10.1128/JVI.76.24.12596-12602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol. 2008 Jan;38(1):114–26. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- [48].Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998 Oct;72(10):8220–9. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Revaz V, Zurbriggen R, Moser C, Schiller JT, Ponci F, Bobst M, et al. Humoral and cellular immune responses to airway immunization of mice with human papillomavirus type 16 virus-like particles and mucosal adjuvants. Antiviral Res. 2007 Oct;76(1):75–85. doi: 10.1016/j.antiviral.2007.05.005. [DOI] [PubMed] [Google Scholar]
- [50].Johansson EL, Wassen L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun. 2001 Dec;69(12):7481–6. doi: 10.1128/IAI.69.12.7481-7486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005 May 25;23(28):3634–41. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- [52].Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005 Aug;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- [53].Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- [54].Bowman EP, Kuklin NA, Youngman KR, Lazarus NH, Kunkel EJ, Pan J, et al. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med. 2002 Jan 21;195(2):269–75. doi: 10.1084/jem.20010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005 Mar 7;23(15):1804–13. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- [56].Sanchez J, Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol Life Sci. 2008 May;65(9):1347–60. doi: 10.1007/s00018-008-7496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]