Abstract
As prostate cancer and aberrant changes in reactive oxygen species (ROS) become more common with aging, ROS signaling may play an important role in the development and progression of this malignancy. Increased ROS, otherwise known as oxidative stress, is a result of either increased ROS generation or a loss of antioxidant defense mechanisms. Oxidative stress is associated with several pathological conditions including inflammation and infection. ROS are products of normal cellular metabolism and play vital roles in stimulation of signaling pathways in response to changing intra- and extracellular environmental conditions. Chronic increases in ROS over time are known to induce somatic mutations and neoplastic transformation. In this review we summarize the causes for increased ROS generation and its potential role in etiology and progression of prostate cancer.
Keywords: Reactive oxygen species, oxidative stress, prostate cancer, aging, mitochondrial DNA mutation
1. Introduction
Prostate cancer is the most frequently diagnosed non-cutaneous malignancy in males, statistics from the American Cancer Society project 186,000 new cases and 28,000 deaths in US for the year 2008 [1]. This is a multi-focal, filed-type disease which forms solid tumors of glandular origin. Androgens play an important role in the differentiation, development and normal functioning of the prostate and therefore likely have a role in developing prostate carcinogenesis. Conventional therapies produce a high rate of cure for patients with localized prostate cancer, but there is no cure once the disease has spread beyond the prostate. Traditionally, treatment of prostate cancer was based on the deprivation of androgens to the developing tumor [2]. Though initially successful, this form of therapy fails in advanced stages of the disease, as the cells develop the ability to sustain growth and proliferation even in the absence of androgens, thus acquiring androgen independence [3]. Although several molecular alterations are known to be involved in the acquisition of androgen independence, the precise mechanism of this phenomenon is poorly understood. Molecular genetic changes in androgen independent prostate cancer cells result in a shift from paracrine to autocrine regulation driven by growth factors and cytokines [4–6].
Prostate cancer cells that proliferate in the absence of androgens typically have an aggressive phenotype. Though multiple factors and signaling pathways have been implicated in the development of aggressive prostate cancer [7,8], the trigger for initiation of malignancy is still a topic of debate. Prostate cancer is mainly a disease of aging, with most cases occurring in men over the age of 55. Therefore, progressive inherent or acquired changes in cellular metabolism occurring over the years may play a very important role in the development of this disease. Many factors like diet, environmental carcinogens, and other inflammatory diseases have been linked to an increased risk of prostate cancer.
Hydroxyl radicals, peroxides and superoxides are ROS that are generated during everyday metabolic processes in a normal cell. ROS, generated either endogenously (mitochondria, metabolic process, inflammation etc.) or from external sources [9], play a vital role in regulating several biologic phenomena. While increased ROS generation has traditionally been associated with tissue injury or DNA damage which are general manifestations of pathological conditions associated with infection, aging, mitochondrial DNA mutations and cellular proliferation; new and exciting information points to an essential role for increased ROS generation in several cellular processes associated with neoplastic transformation and aberrant growth and proliferation [10,11]. Processes associated with proliferation, apoptosis, and senescence may be a result of the activation of signaling pathways in response to intracellular changes in ROS levels [12]. Thus, excessive production of ROS or inadequacy in a normal cell’s antioxidant defense system (or both) can cause the cell to experience oxidative stress and the increased ROS may play a broader role in cellular processes associated with initiation and development of many cancers including Prostate Cancer.
Over the last decade association between prostate cancer risk and oxidative stress has been recognized, and epidemiological, experimental and clinical studies have unequivocally proven a role for oxidative stress in the development and progression of this disease. Differences in prostate cancer incidence among various races, environment, diet, life style, genetic constitution and hormone of an individual/community are some of the contributing risk factors for occurrence of prostate cancer [13–15]. Though recent studies have indicated that oxidative stress is higher in the epithelium of prostate cancer patients than men without the disease, the association of ROS mediated oxidative stress and prostate cancer risk remains to be elucidated. Theories abound regarding their role in initiation of prostate cancer, and include but are not limited to, failure of antioxidant defense mechanism (due to persistent oxidative stress that leads to inherited and acquired defects in the defense system), mtDNA mutations, chronic inflammation, defective DNA repair mechanism and apoptosis etc., finally leading to the development of prostate cancer. Thus, many of the factors that are associated with prostate cancer like aging, imbalance of androgens, antioxidant system, dietary fat, and pre malignant conditions like high grade prostate intraepithelial neoplasia etc. may be linked to oxidative stress. In recent years several anti-oxidant trails have been conducted against prostate cancer, but the usefulness of such therapies needs extensive research before put into practice [16].
In this article, we reviewed literature pertaining to the role of ROS generation in prostate cancer, and the cellular effects of oxidative stress (Fig. 1). In addition, we will also discuss the relationship between prostate cancer susceptibility and oxidative stress in relation to antioxidant defense system, metabolic switch, mtDNA mutation, inflammation and regulation of androgens. This review is aimed at providing an overview about the role of ROS in promoting Prostate Cancer.
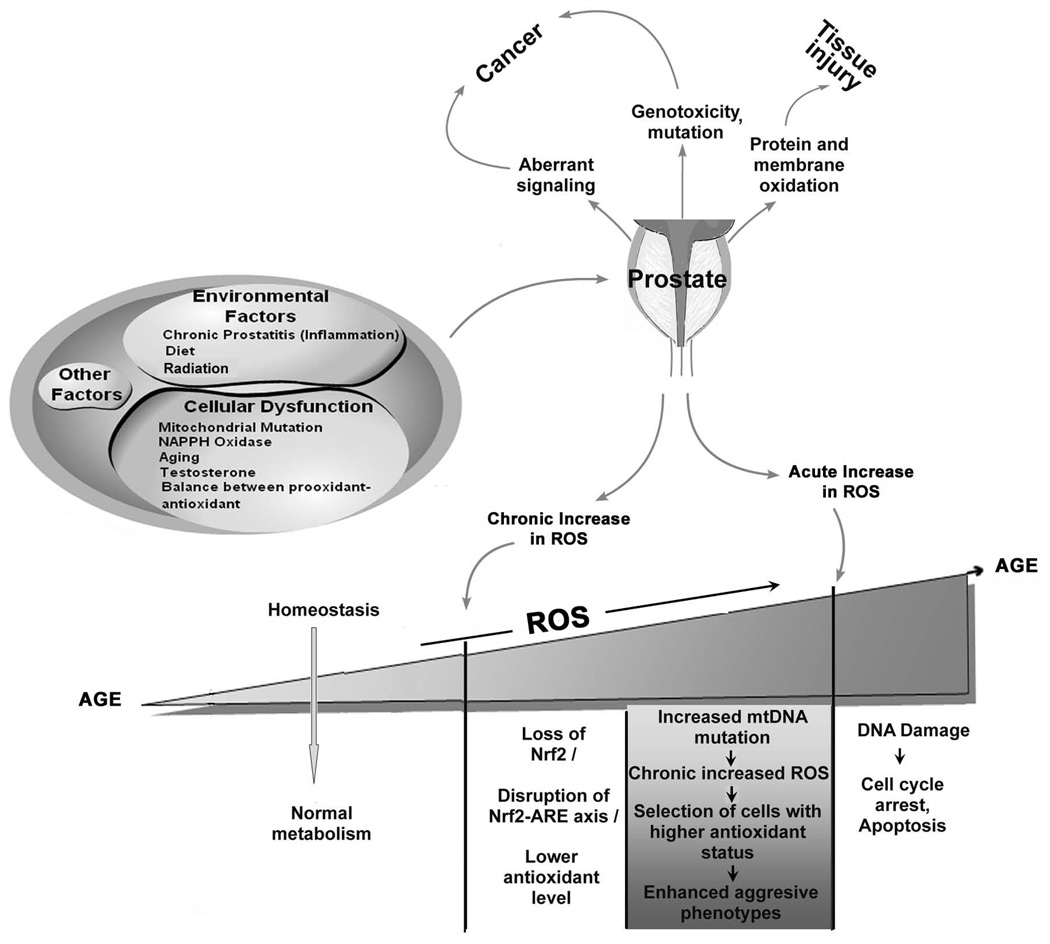
Fig. 1. Mechanisms of ROS production, and cellular response to ROS in prostate cells.
Many factors both intrinsic to the cells and to external environment can lead to higher ROS production in the prostate. Increased ROS levels can lead to prostate dysfunction which in turn leads to more ROS production. An enzymatic or non-enzymatic antioxidant defense system counteracts and regulates ROS level to maintain physiological homeostasis. Lowering ROS level below the homeostatic point may interrupt proliferation and host defense system, while accumulative ROS in prostate can alter normal functioning of the prostate leading to low antioxidant level [by disrupting Nrf2-antioxidant response element axis(ARE)], increase mtDNA mutation and aggressive phenotypes, and caused DNA damage.
2. Antioxidant therapy in prostate cancer: Where are we?
In 1981, a landmark study by Doll and Peto estimated that a higher percentage of cancer deaths in USA could be attributed to dietary factors, and proposed that antioxidant present in diet could deactivate formation of free radicals inside the cell [17]. After this discovery, a set of projects on cancer prevention were funded by NCI on a large scale, including clinical trials to test the role of dietary antioxidants in cancer prevention. Among the available antioxidant vitamins, Vitamin E was of the greatest interest to researchers. But collective data from all the different clinical trials, like Alpha-Tocopherol, Beta-carotene prevention (ATBC), Heart Outcome Prevention Evaluation-The ongoing outcomes (HOPE-TOO), Prostate, Lung, Colorectal and ovarian (PLCO), and Selenium and Vitamin E Cancer Prevention Trial (SELECT) was a complete disappointment due to the conclusion that the overall risk for prostate cancer were unaffected by supplemental dietary antioxidants. These studies did not provide strong support for population-wide implementation of high dose antioxidant supplementation for the prevention of prostate cancer [18–20]. However, the data obtained from these studies were beneficial to some subgroups-such as smokers, as 50mg of vitamin E daily had a statistically significant 32% lower prostate cancer incidence and a statistically significant 41% lower prostate cancer mortality than those assigned to receive placebo [21]. In this context, our study with prostate cancer cell lines indicated that ROS production rather than accumulation, plays an important role in prostate cancer phenotypic behavior and therefore, the use of an antioxidant may not be of a higher benefit as antioxidants can only neutralize the accumulated ROS inside the cells [22]. Thus, treatment stratergies aimed at reducing ROS production, rather than ROS neutralization, might offer an effective means against prostate cancer in particular and in other malignancies in general.
3. Antioxidant-prooxidant paradox in prostate cancer
Prostate cancer is commonly associated with a shift in the antioxidant-prooxidant balance towards increased oxidative stress. Previous studies highlighted the altered prooxidant-antioxidant status in prostatic tissue of man, rat and also in cell lines, where the imbalance between these antagonist played a major role in the initiation of prostate carcinogenesis [23]. However, there is very little idea about the cause of this imbalance. Androgens are considered to be the most powerful candidates that regulate ROS balance in the prostate, though the mechanistic relation between androgen status and redox homeostasis in the prostate is not proven [24]. Tam et al. [25] in this context indicated that replacement of androgens reduced the oxidative stress level by down-regulating NADPH Oxidase (Nox) expression, thereby bringing the antioxidant level to normalcy. Besides androgen, the transcription factor erythroid 2p45 (NF-E2)-related factor 2 (Nrf2) mediates the expression of key protective enzymes through the antioxidant-response element (ARE) in prostate cancer [26,27]. Recent studies suggested that Nrf2 and several of its target genes are significantly downregulated in human prostate cancer and as a result (Fig. 1), cells were continually exposed to increased oxidative stress and may have resulted in their progression to metastatic disease [28]. Another major component involved in the maintenance of redox balance in the cell is the Glutathione oxidation-reduction system. Somatic mutations, causing inactivation of the Glutathione S-Transferase gene (GSTP1) have been identified in almost all the prostate cancer cases examined by Nelson and colleagues [29]. Therefore, the sensitive balance between the oxidant and anti-oxidant components of the cells and their regulatory mechanisms seem to play a major role in developing a malignant state in prostate tissue.
4. Metabolic switch and mitochondrial DNA mutations in prostate cancer
Mitochondrial DNA mutations are very frequent in cancer, and the accompanying mitochondrial dysfunction and altered metabolism may contribute to tumor pathogenesis and metastasis [30–32]. In the case of a normal prostate, higher concentration of zinc present in the tissue causes a block in Krebs cycle and accumulation of citrate in the prostatic fluid. Thus, normal prostate glandular epithelial cells have low respiration causing low terminal oxidation, energy inefficient and presumably generate less ROS [33,34]. Unlike in the normal prostate, malignant transformation is associated with an early metabolic switch leading to decreased zinc accumulation, and increased citrate oxidation [35]. Thus malignant prostate is energy efficient, capable of higher rate of respiration and therefore, generates more ROS (Fig. 2).
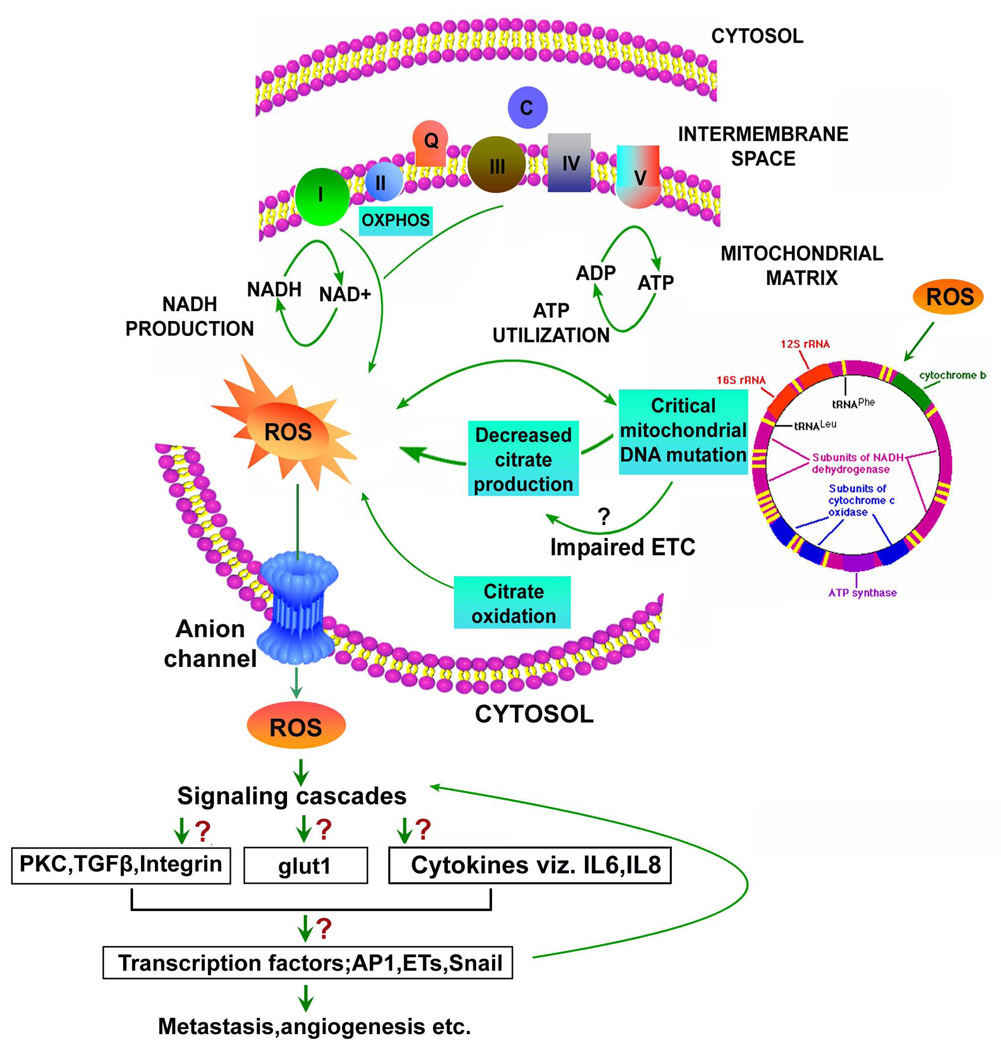
Fig. 2. Metabolic switch and mitochondrial DNA (mtDNA) mutation accelerate ROS generation in prostate cancer.
Alterations in metabolism from high citrate to low citrate production, and truncated oxidative phosphorylation (OXPHOS) to complete OXPHOS status during the malignant transformation of prostate lead to complete citrate oxidation, and more ROS generation in prostate cancer cells. Similarly, homoplasmic mtDNA point mutations and mtDNA instability with time and age cause mitochondrial hyper mutagenesis. This event causes enormous amount of ROS generation, and we hypothesize that it might lead to impaired electron transport chain (ETC) resulting in decreased citrate production, which in consequence generates more ROS in prostate cancer cells. Once enhanced ROS generation is started, subsequent activation of signaling pathways and redox-sensitive transcription factors like HIF-1α, Ets, Snail has been shown to play a major role in progression and metastasis of the cancer cells.
4.1 Mitochondrial mutations and Oxidative Stress
The role of mitochondria in prostate cancer progression has attracted much attention in the last decade when several reports highlighted a possible link between metabolic alteration and mitochondrial DNA (mtDNA) mutation in prostate cancer [30,36,37]. Given the fact that the mitochondria are a major source of ROS generation, it would not be surprising for altered mitochondria bioenergetics and mutations to the mtDNA to underlie the development of prostate cancer. Studies by many investigators showed significant changes in the nuclear encoded mitochondrial subunit IV of malignant prostate compared to the normal prostate and not much difference in the mitochondrial encoded subunit I and II [37,38]. Jessie and colleagues in 2001 were the first to conclude that more deletions occur in the mtDNA of older malignant prostates compared to younger malignant prostates, and suggested that the increase in oxidative stress with time caused an increase in accumulation of mutations in mtDNA [36]. With the advancement of research involving mitochondrial, Chen et al. in 2002 and 2003 [38,39] reported that homoplasmic point mutations and mtDNA instability occurred at a high frequency in prostate cancer, and the process of mitochondrial hyper mutagenesis is mediated by cellular oxidative stress causing a burst of multiple mtDNA mutations in prostate tumor. Above all, the studies of Petros and colleagues in 2005 [37] reported that prostate cancer has a significantly higher frequency of functionally important Cytochrome Oxidase subunit I mutations, and have minimal effect on the genetic fitness of the mutant mtDNA. Thus, mtDNA appears to have a great role in pathogenecity of prostate cancer.
4.2 Relationship between mitochondrial mutations and changes in cellular metabolism
Is there is a link between metabolic shift, mtDNA mutation and oxidative stress? With increasing age, occurrence of mtDNA mutations and metabolic alteration has been demonstrated, but their relationship remains unclear. Based on previous studies, we can hypothesize that malignant transformation of the prostate gland is initiated by ROS mediated mtDNA mutations, which might result in genetic or epigenetic alterations in the nucleus. This mutation further affects regulatory pathways which may ultimately lead to down-regulation of genes such as Zip1 (Zinc uptake transporter) and cause the metabolic switch [30,40]. It might also be possible that mtDNA mutation could lead to impairment of electron transport chain, resulting in decreased citrate production, which in consequence generates more ROS in prostate cancer cells(Fig. 2).
Of particular interest is the difference in the spatial distribution of zinc and citrate in normal and malignant prostate. In the normal prostate, high zinc and high citrate levels are associated with the lateral lobe of the peripheral zone and regulated by testosterone, where as in the case of a malignant prostate, low zinc and low citrate levels are associated with the central zone [40]. Therefore, if any correlation exists between the occurrence of mtDNA mutations and metabolic switch, then the pattern of mtDNA mutations found in the central zone of the prostate tumor should differ from those found in the peripheral zone. Moreover, in general if the mtDNA mutation is associated with high ROS generation due to a defect in oxidative phosphorylation, the cells would also oxidize less pyruvate and NADH as part of the respiratory chain, resulting in excessive lactate production. Thus, if mitochondrial ROS production is essential for a solid tumor like prostate, then anaerobic glycolysis should be the common feature, as was postulated by Warburg more than 70 years ago [41] and could also explain why solid tumors have a higher rate of glycolysis. Based on these discussions potential role of mtDNA mutations and oxidative stress in the pathogenesis of prostate cancer requires greater attention.
5. NADPH Oxidase: an emerging candidate in prostate cancer
The NAD(P)H dependent reduction of molecular oxygen is responsible for the generation of ROS in a cell, in the form of superoxide anion (O2−), which is then dismutaed to form peroxide (H2O2) [42]. Phagocytic cells generate higher amount of ROS using NADPH Oxidases (Nox, Fig. 3) as part of their armory of microbicidal mechanisms. Recent reports also indicate their presence in some of the non-phagocytic tissue like fetal kidney, thyroid, prostate, colon etc. [43,44]. NAD(P)H oxidase is associated with the generation of a respiratory burst in phagocytes and consists of gp91phox and p67phox (plasma membrane bound catalytic protein subunit) and p22 phox (cytochrome b558). The active assembly complex also includes p67phox and p47phox (two cytosolic protein components) and a small GTPase Rac [44, Fig. 3a]. Homologues of gp91phox have been identified and named as Nox (for NAD(P)H oxidase) proteins in non-phagocytic cells [45], providing an explanation for non-phagocytic cell NAD(P)H oxidase activity. To date the Nox family consists of five members (Nox 1–5). These oxidases are believed to play a role in a variety of signaling events, including cell growth, cell survival and death, however, the exact functional role of these oxidases has largely remained unexplored.
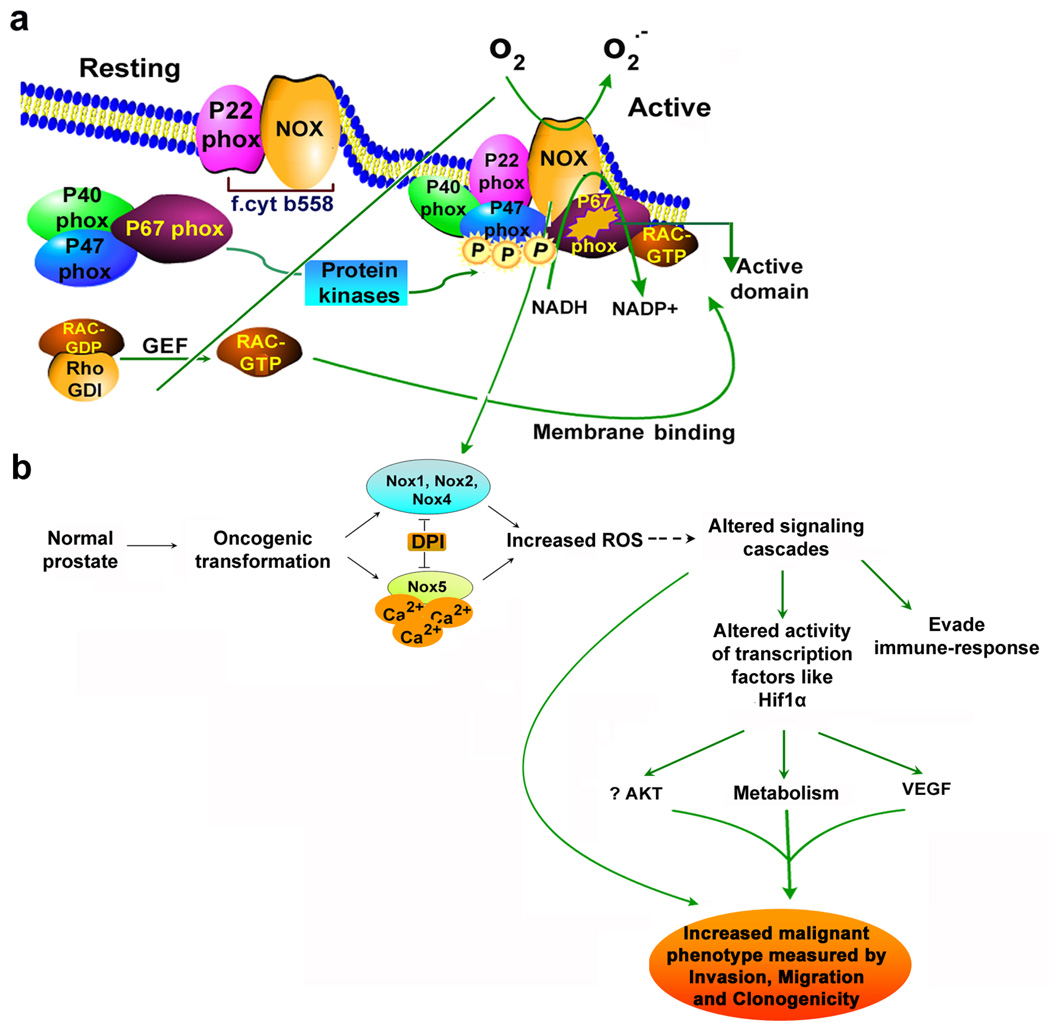
Fig. 3. Activation of reactive oxygen species (ROS) generation by assembly of phox regulatory protein.
(a). Activation of Nox enzyme (equivalent to gp91phox component of phagocytes) system results in assembly of cytosolic regulatory proteins (p40phox, p47phox and p67phox) with flavocytochrome b558 (f.cyt b558; comprised of membrane associated catalytic subunit Nox plus p22phox). These trigger nucleotide exchange protein that activates the GTPase RAC. Protein kinases catalyse many phosphorylation events and allowing p47phox binding to lipid along with p67 phox and p40phox. Activation of exchange factors triggers GTP binding, resulting in conformational change in RAC that promote dissociation from RhoGDI, and promote RAC-GTP binding to p67phox, helping to assemble the active complex. (b). schematic illustrating the role of NAD(P)H oxidase system in prostate cancer cells. In case of prostate cancer cells, NAD(P)H oxidase1 (Nox1), Nox2, Nox3 and Nox4 are similar in size to gp91phox, while Nox5 consists of an additional amino-terminal calcium binding domain, and independent of p22phox requirement for their activity. We hypothesize that increased ROS generation as a result of activation of NAD(P)H oxidase system(s) in prostate cancer cells mediates several signaling pathways critical for growth and could potentially regulate various phenotypic features of cancer cells. Dashed lines indicate possible mechanisms of action.
Studies by others and by us [22,45] have shown that the aggressive growth, proliferation and metastatic ability of prostate cancer cells may be a manifestation of high levels of intracellular ROS generated in these cells. Prostate cancer cells generate substantial amount of ROS and Nox enzymes which are not only an important source of ROS generation in the case of prostate cancer, but are also very critical for growth and maintenance of malignant phenotype in these cells [46,47]. Recent studies suggest that Nox1 triggers an angiogenic switch and converts tumors from dormant to aggressive growth, while Nox4 has been indicated to be active in melanoma and pancreatic cells [48]. Both Nox1 and Nox5 are reported in prostate cancer tissue/ cells, while our studies identified various isoform of Nox including Nox4, Nox2 and Nox5 (Fig. 3b) in prostate cancer lines which are absent in normal prostate cell lines. Ectopic expression of Nox1 in prostate cancer cells enhances growth, tumorigenicity and angiogenicity [49], whereas down regulation of Nox5 causes growth arrest and apoptosis [47]. Our findings of the expression of various isoforms of NADPH oxidases and the apparent connection between ROS generation by Nox system and tumorogenic potential suggested that this pathway might play a critical role in tumor modulation [22]. In addition, a recent study showed that castration resulted in dramatic increases of three ROS generating NAD(P)H oxidases including Nox1, Nox2 and Nox4 [25]. Thus increased Nox expression driven ROS generation in prostate cancer could lead to the generation of a malignant phenotype by modulating various signaling cascades and may prove to be an effective target for therapeutic intervention.
6. Aging, oxidative stress and prostate cancer
Aging is associated with many metabolic disorders and also increased incidence of various cancers [50,51]. Prostate cancer is a major age related malignancy. Many theories have been formulated to explain the molecular and biochemical aspect of aging, but Harman in 1956 proposed “free radical theory of aging” in which he suggested that accumulation of damage to biomolecules caused by free radicals play a major role in human aging [52,53]. With the advancement of technology, many researchers supported the above theory and concluded that accumulation of somatic mutations in mtDNA is a major contributor to human aging since mitochondria are the major source of intracellular ROS generation and is therefore vulnerable to oxidative damage leading to progressive decline in respiratory function over time [54,55]. We believe that cellular oxidative stress increases with age and the increase in mitochondrial mutations can lead to further increase in ROS generation due to defective oxidative phosphorylation and electron transport. Thus, it is possible that increase in ROS leads to a self perpetuating cycle with an ever increasing oxidative challenge placed on the cells. Therefore, mtDNA mutations/ deletions not only act as a marker for aging but may also explain increased incidence of prostate cancer with advancing age.
6.1 Changes in Androgen Receptor Activity with Age
Most of the cells in the prostate tumor express the androgen receptor and respond to androgens at an early stage, which facilitate their growth. Age related changes in the levels of androgens and ratios of other androgenic hormones, and changes in the balance between auto/paracrine growth stimulatory factors [56] like insulin growth factor (IGF), epidermal growth factor (EGF), nerve growth factor (NGF) and growth inhibitory factors like transforming growth factors β (TGFβ), IGF binding proteins (IGFBPs) are implicated for abnormal prostatic growth [57–59]. Although, most of cells in the prostate tumor will eventually become hormone refractory upon traditional treatment procedures involving hormonal withdrawal, they develop other means of androgen receptor activation. Interestingly, physiological stimulation of androgen receptor has been shown to increase ROS production [60,61]. Since aging is associated with a decrease in intracellular antioxidant levels and activities of free radical scavenging enzymes, and androgen stimulation in prostate cancer cells causes a shift in the prooxidant-antioxidant balance, it can be speculated that the level of androgen stimulation existing in prostate cancer cells is a by-product of mtDNA mutations and aging.
In addition to the accumulation of oxidative stress with advancing age, recent studies shed light on changes in insulin-like growth factor 2 (Igf2) imprinting with age and its relevance to Prostate Cancer [62]. This study did not identify any correlation between Igf2 and oxidative stress, cautioning that aging as we know may be a result of not only persistent oxidative stress, but it also due to a combination of multiple factors totally unrelated to oxidative stress.
7. Steroid Hormones in ROS Generation and Incidence of Prostate Cancer
Prostate development, maturation and normal function depends on the activity of the androgens testosterone and its derivative dihydrotestosterone (DHT). DHT, synthesized from testosterone in the prostate by 5α-reductase [63], has a more potent effect due to its higher affinity to the Androgen Receptor (AR). The AR in turn, binds to Androgen Receptor Elements (ARE) present in the promoter regions of many genes involved in cellular proliferation [64]. Traditionally, initial stages of prostate cancer were controlled by Androgen deprivation therapy; however, aberrant AR activity, in prostate tumors finally leads to the development of a highly malignant state of disease unresponsive to androgen control [65].
Many studies have dwelt on the increased oxidative damage in cells due to ROS as a result of abnormal and increased androgen stimulation of androgen sensitive prostate cancer cells [66,67]. Though studies have not pointed out a potential mechanism for the increased levels of ROS after androgen stimulation, as discussed above, changes in the balance of pro-oxidant and anti-oxidant molecules in a cell may play an important role. Intracellular redox balance is largely a result of cyclic reduction and oxidation of Glutathione both in the cytoplasm and mitochondria of a cell [68]. Glutathione, synthesized in the cytosol and imported into the mitochondria, plays an important role in the protection of mitochondria from the deleterious effects of ROS generated as a result of electron transport [69]. Studies by Ripple et al., [23] suggest that stimulation of prostate cells by addition of androgens increases the activity of γ-Glutamyl transpeptidase, an enzyme responsible for Glutathione recycling in cells, by metabolizing glutathione back to amino acids. Recent research has also thrown light on GSH Peroxidase enzyme which catalyzes the neutralization of peroxide via Glutathione redox system. Circulating levels of GSH Peroxidase in the plasma as well as in the prostate tissue are markedly decreased in prostate cancer biopsy specimens from patients [70,71]. Therefore, increased loss of glutathione may be a prime reason for the shift in intracellular environment to a pro-oxidant state leading to multiple changes in gene expression [72] eventually evolving into a malignant state.
7.1 Role of Estrogens and Estrogenic Compounds in Increased ROS in Prostate
Even though Testosterone is the predominant hormone responsible for the regulation of prostate gland growth and functioning, recent discovery of the presence of estrogen receptors in the prostate has brought its role in prostate cancer progression to prominence [73,74]. Estradiol can be synthesized from testosterone in the prostate epithelial cells or taken up from general circulation. Certain Isoflavonoids can also have weak estrogenic effects [75] and have been observed to cause significant infiltration of Neutrophils and lymphocytes in the prostate lobes of rats fed with dietary Isoflavonoids. Chronic administration of DHT and estradiol to rats induces the expression of pro-inflammatory cytokines within the prostate [76]. These inflammatory infiltrates have been identified to be a major source ROS production and the incidental oxidative injury to the prostate epithelium has been suggested to be the cause for the formation of Proliferative Inflammatory Atrophy (PIA) [77]. These lesions generally form the basis for enhanced epithelial cell proliferation, regeneration and give rise to Prostatic Intraepithelial Neoplasia (PIN), and progressively to prostate cancer. It is generally believed that estrogen alone is not enough to cause malignancy, but abnormal estrogen receptor α (ERα) signaling in conjunction with elevated levels of testosterone have been shown to induce prostate hyperplasia and prostate cancer in mice [78].
8. Hypoxia and ROS
Extensive cell proliferation coupled with unorganized vasculature present in a tumor result in a low oxygen environment (Hypoxia) forcing the cells to shift to anaerobic glycolysis for their energy requirements [79,80]. Tumor cells have the ability to overcome low oxygen tension due to concomitant activation and stabilization of Hypoxia Inducible factor (HIF-1). Studies in many systems have shown an increase in intracellular ROS production when exposed to hypoxic environment [81] and mostly originating from the mitochondria [82]. Studies by Bourdeau-Heller and Oberley [83] suggests that long term exposure of prostate cancer cells to hypoxia results in modulation of ROS levels and energy metabolism, to ensure cell survival and growth. Other studies have shown that many signaling pathways are activated as a result of increased ROS levels under hypoxia that finally result in the increased expression of HIF-1 and angiogenic factor VEGF in prostate cancer cells [84]. In addition to the synthesis of HIF-1, the redox status of a cell has a direct impact on the maintenance of HIF-1 in a conformationally active state [85] and probably in its final degradation through the ubiquitin pathway also [86]. Studies in our laboratory (unpublished results) implicate p38 MAPK as an early factor in hypoxic response of androgen dependent prostate cancer cells. Hypoxia-reoxygenation eventually leads to androgen independent increased survival in these cells which may be speculated as a result of higher levels of ROS found in these cells upon hypoxia exposure.
For more detailed information regarding the role of ROS in HIF-1 signaling, readers are referred to a recent review by Galanis et al. [87].
9. Chronic Prostatitis, Inflammatory Response and ROS
9.1 Bacterial and non-bacterial Prostatitis
Prostatitis is a manifestation characterized by painful inflammation of the prostate. Even though the reason for the occurrence of Prostatitis is much in debate, two classes of Prostatitis have been recognized, Bacterial and non-bacterial Prostatitis [88]. Prostatitis is often associated with symptoms that range from voiding discomfort to adverse sexual function [89]. Epidemiological studies suggest that on an average about 11 to 16% of men in the United States have been or are diagnosed with Prostatitis [90], out of which only a few (5 to 10% of total cases) are of bacterial origin [91].
Bacterial Prostatitis is thought to be caused by a retrograde transfer of infection from the lower urinary tract to the prostate. This belief is strengthened by the isolation of gram negative enteric bacteria commonly associated with Urinary Tract Infections from the affected prostate [90]. However, a wide spectrum of organisms have also identified to be involved in Prostatitis; from Enterobacteria like Escherichia coli, Enterococcus fecalis, and Proteus mirabilis to Chlamydia spp. and Ureaplasma spp. [92]. This has strengthened the belief of possible contiguous spread from other sources including the bladder, bowels, blood or the lymph to prostate [93]. Antimicrobial therapy targeted against specific infectious agents can cure acute cases of bacterial Prostatitis. However, chronic cases can be distinguished by persistent occurrence of bacteria (Chronic Prostatitis) in prostatic fluid even after therapy [94]. In addition to bacterial infections, certain non-bacterial causes have also been identified in chronic Prostatitis [95]. These include but are not limited to elevated prostate pressures as a result of voiding dysfunction, bladder neck hypertrophy, and in some cases emotional disorders.
9.2 Prostatitis, Reactive Oxygen Species generation and Prostate Cancer
Chronic Prostatitis either bacterial or non-bacterial leads to stromal or epithelial cell damage causing inflammation in a majority of cases [96]. Inflammatory cells, particularly macrophages that are attracted to the site of inflammation can be found in the Expressed Prostatic Secretions (EPS) characterizing Inflammatory Chronic Prostatitis. Non-specific immune defense, mediated by inflammatory cells, activated as a result of Chronic Prostatitis has been labeled as the primary cause for a rapid increase in the amount of Hydroxyl radicals, superoxides and peroxides in prostate tissue [97]. The continual exposure of prostate tissue to the source of inflammation can lead to a dramatic increase in ROS, causing changes in protein structure and function, somatic genetic alterations and post translational DNA modifications [98]. These changes can lead to further tissue damage resulting in enhanced epithelial cell proliferation to compensate for the tissue damage and can therefore induce prostatic neoplasia [99]. Chronic inflammation, therefore, can induce a tissue microenvironment comprising higher levels of mutagenic ROS and this process has been implicated in the formation of many tumors including breast adenocarcinomas [100]. Studies by Stanick et al., [101] identified an increase in PSA levels in patients with chronic Prostatitis and epidemiological studies refer to a small increase in the risk of prostate cancer in men with a history of Prostatitis [102].
10. Summary and Future Directions
Evidence from epidemiological, experimental and clinical studies suggest that prostate cancer cells are exposed to increased oxidative stress. Environmental factors like diet, inflammation, and changes in cellular functions pertaining to NAD(P)H Oxidase, androgen signaling, mtDNA mutations, aging, and redox imbalance are possible mechanisms that contribute to increase ROS generation (Fig. 1). This increased ROS may further stimulate cell proliferation, cause somatic DNA mutations and promote genetic instability, cell cycle arrest, senescence, and in cancer cells can cause increased angiogenesis, and motility. Studies in our laboratory have identified a potential role for increased ROS generation for the development of an aggressive phenotype in Prostate Cancer cell lines. A lingering question that has not been addressed to date is when is the exposure to elevated ROS result in increased predisposition to promotion of a malignant phenotype and when does ROS result is cell damage, apoptosis and cell death. Our central hypothesis is that chronic exposure to moderate to high levels of ROS promotes malignant phenotype, while acute exposure to high levels of ROS promotes cell death and irreversible damage. Based on this hypothesis we propose that conditions associated with chronic exposure of elevated ROS in prostate (Fig 1) would promote prostate cancer in general.
Potential role for ROS in the regulation of cellular process controlling malignant transformation holds a lot of promise in understanding etiology and progression of cancer in general and prostate cancer in particular, as this may open doors for the development of novel therapeutics for cancer prevention and treatment. In the case of prostate, besides acting as a DNA damaging agent, moderately elevated levels of ROS may act as secondary messengers and can control various signaling pathways which are essential for the maintenance of oncogenic phenotype by virtue of activating many transcription factors like HIF-1α, Snail, Ets etc. in prostate cancer. Identification of pathway(s) that channels these signaling cascades to the transcription factors may provide novel targets for treatment options. Studies in our laboratory are focused on the role of Focal Adhesion Kinase (FAK) and Mitogen Activated Protein Kinase (MAPK) signaling pathways [103] that may play an essential role in the development of aggressive androgen independent prostate cancer through ROS mediated signaling. Another potential avenue for future studies may be the sources of ROS generation in cancer cells, including NADPH oxidase (Nox) enzyme(s) which appear to be an exciting player on the ROS mediated biological scene in prostate cancer. Since prostate cancer cells are under inherent oxidative stress, a strategy that can take advantage of this inherent higher oxidative stress may also provide an advance in salvage therapy. ROS play an increasingly important role not only in malignant transformation, but also in progression and aggressive phenotype of Prostate Cancer. As such, strategies designed to utilize ROS-mediated signaling events may offer promise in the prevention and potential treatment of Prostate Cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
None Decalred
References
- 1.Cancer Facts and Figures. American Cancer Society. 2008 URL: http://www.cancer.org/docroot/STT/stt_0.asp.
- 2.Huggins C, Hodges CV. Hodges, Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 3.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67:10455–10465. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs JT. The biology of hormone refractory prostate cancer Why does it develop? Urol. Clin. North Am. 1999;26:263–273. doi: 10.1016/s0094-0143(05)70066-5. [DOI] [PubMed] [Google Scholar]
- 5.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 6.Pilat MJ, Kamradt JM, Pienta KJ. Hormone resistance in prostate cancer. Cancer Metastasis Rev. 17:373–381. doi: 10.1023/a:1006166511344. [DOI] [PubMed] [Google Scholar]
- 7.McPhaul MJ. Mechanisms of prostate cancer progression to androgen independence. Best Pract. Res. Clin. Endocrinol. Metab. 2008;22:373–388. doi: 10.1016/j.beem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Kreisberg JI, GhoshPm PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer, Curr. Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 9.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;22:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid. Redox. Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 11.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner NE, Klotz LH. Diet, androgens, oxidative stress and prostate cancer susceptibility. Cancer Metastasis Rev. 1999;17:325–330. doi: 10.1023/a:1006118628183. [DOI] [PubMed] [Google Scholar]
- 14.Fair WR, Fleshner NE, Heston W. Cancer of the prostate: a nutritional disease? Urology. 1997;50:840–848. doi: 10.1016/S0090-4295(97)00339-7. [DOI] [PubMed] [Google Scholar]
- 15.Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann. Intern. Med. 1993;118:793–803. doi: 10.7326/0003-4819-118-10-199305150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 17.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 19.Fleshner NE, Klotz LH. Diet, androgens, oxidative stress and prostate cancer susceptibility. Cancer Metastasis Rev. 1998–1999;17:325–330. doi: 10.1023/a:1006118628183. [DOI] [PubMed] [Google Scholar]
- 20.Kirsh VA, Hayes RB, Mayne ST, Chatterjee N, Subar AF, Dixon LB, et al. Supplemental and dietary vitamin E, β -carotene, and vitamin C intakes and prostate cancer risk. J. Natl. Cancer Inst. 2006;98:245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J. Natl. Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 22.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 23.Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J. Natl. Cancer Inst. 1997;89:40–48. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Wilding G. Endocrine control of prostate cancer. Cancer Surv. 1995;23:43–62. [PubMed] [Google Scholar]
- 25.Tam NN, Gao Y, Leung YK, Ho SM. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am. J. Pathol. 2003;163:2513–2522. doi: 10.1016/S0002-9440(10)63606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 27.Zhu M, Fahl WE. Functional characterization of transcription regulators that interact with the electrophile response element. Biochem. Biophys. Res. Commun. 2001;289:212–219. doi: 10.1006/bbrc.2001.5944. [DOI] [PubMed] [Google Scholar]
- 28.Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- 29.Nelson WG, de Marzo AM, deWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J. Urol. 2004;172:S6–S11. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- 30.Dakubo GD, Parr RL, Costello LC, Franklin RB, Thayer RE. Altered metabolism and mitochondrial genome in prostate cancer. J. Clin. Pathol. 2006;59:10–16. doi: 10.1136/jcp.2005.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma M, Naviaux RK, Tanaka M, Kumar D, Franceschi C, Singh KK. Meeting report: mitochondrial DNA and cancer epidemiology. Cancer Res. 2007;67:437–439. doi: 10.1158/0008-5472.CAN-06-4119. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 33.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J. Biol. Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Juang HH. Modulation of mitochondrial aconitase on the bioenergy of human prostate carcinoma cells. Mol. Genet. Metab. 2004;81:244–252. doi: 10.1016/j.ymgme.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Jessie BC, Sun CQ, Irons HR, Marshall FF, Wallace DC, Petros JA. Accumulation of mitochondrial DNA deletions in the malignant prostate of patients of different ages. Exp. Gerontol. 2001;37:169–174. doi: 10.1016/s0531-5565(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 37.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer, Proc. Natl. Acad. Sci. (USA) 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JZ, Gokden N, Greene GF, Green B, Kadlubar FF. Simultaneous generation of multiple mitochondrial DNA mutations in human prostate tumors suggests mitochondrial hyper-mutagenesis. Carcinogenesis. 2003;24:1481–1487. doi: 10.1093/carcin/bgg102. [DOI] [PubMed] [Google Scholar]
- 39.Chen JZ, Gokden N, Greene GF, Green B, Mukunyadzi P, Kadlubar FF. Extensive somatic mitochondrial mutations in primary prostate cancer using laser capture microdissection. Cancer Res. 2002;62:6470–6474. [PubMed] [Google Scholar]
- 40.Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J. Biol. Chem. 1999;274:17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- 41.Warburg O. London: Constable; 1930. The Metabolism of Tumors. [Google Scholar]
- 42.Clark RA. Activation of the neutrophil respiratory burst oxidase. J. Infect Dis. 1999;179 Suppl 2:S309–S317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 43.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 44.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 45.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 46.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 47.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am. J. Physiol. Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 48.Block K, Ricono JM, Lee DY, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)Hoxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid.Redox. Signal. 2006;8:1497–1508. doi: 10.1089/ars.2006.8.1497. [DOI] [PubMed] [Google Scholar]
- 49.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc.Natl. Acad. Sci. (USA) 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei YH, Lu CY, Lee HC, Pang CY, Ma YS. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann. N. Y. Acad. Sci. 1998;854:155–170. doi: 10.1111/j.1749-6632.1998.tb09899.x. [DOI] [PubMed] [Google Scholar]
- 51.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 52.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 53.Harman D. The aging process. Proc.Natl. Acad. Sci. (USA) 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chomyn A, Attardi G. mtDNA mutations in aging and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:519–529. doi: 10.1016/s0006-291x(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 55.Miquel J. An integrated theory of aging as the result of mitochondrial-DNA mutation in differentiated cells. Arch. Gerontol. Geriatr. 1991;12:99–117. doi: 10.1016/0167-4943(91)90022-i. [DOI] [PubMed] [Google Scholar]
- 56.Untergasser G, Rumpold H, Hermann M, Dirnhofer S, Jilg G, Berger P. Proliferative disorders of the aging human prostate: involvement of protein hormones and their receptors. Exp. Gerontol. 1999;34:275–287. doi: 10.1016/s0531-5565(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 57.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J. Biol. Chem. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 58.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J. Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 59.Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-1 and the IGF binding proteins (IGFBP-1, 2 and 3) decrease with age in healthy adults and is increased in acromegalic patients. Clin. Endocrinol. Oxf. 1994;41:85–91. doi: 10.1111/j.1365-2265.1994.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 60.Mehraein-Ghomi F, Lee E, Church DR, Thompson TA, Basu HS, Wilding G. JunD mediates androgen-induced oxidative stress in androgen dependent LNCaP human prostate cancer cells. Prostate. 2008;68:924–934. doi: 10.1002/pros.20737. [DOI] [PubMed] [Google Scholar]
- 61.Shigemura K, Sung SY, Kubo H, Arnold RS, Fujisawa M, Gotoh A, Zhau HE, Chung LW. Reactive oxygen species mediate androgen receptor- and serum starvation-elicited downstream signaling of ADAM9 expression in human prostate cancer cells. Prostate. 2007;67:722–731. doi: 10.1002/pros.20565. [DOI] [PubMed] [Google Scholar]
- 62.Fu VX, Dobosy JR, Desotelle JA, Almassi N, Ewald JA, Srinivasan R, Berres M, Svaren J, Weindruch R, Jarrard DF. Aging and cancer-related loss of insulin-like growth factor 2 imprinting in the mouse and human prostate. Cancer Res. 2008;68:6797–6802. doi: 10.1158/0008-5472.CAN-08-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson IM, Coltman CA, Brawley OW, Ryan A. Chemoprevention of prostate cancer. Semin. Urol. 1995;13:122–129. [PubMed] [Google Scholar]
- 64.Wong YC, Wang YZ. Growth factors and epithelial-stromal interactions in prostate cancer development. Int. Rev. Cytol. 2000;199:65–116. doi: 10.1016/s0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- 65.Asirvatham AJ, Schmidt M, Gao B, Chaudhary J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology. 2006;147:257–271. doi: 10.1210/en.2005-0942. [DOI] [PubMed] [Google Scholar]
- 66.Pathak S, Singh R, Verschoyle RD, Greaves P, Farmer PB, Steward WP, Mellon JK, Gescher AJ, Sharma RA. Androgen manipulation alters oxidative DNA adduct levels in androgen-sensitive prostate cancer cells grown in vitro and in vivo. Cancer Lett. 2008;261:74–83. doi: 10.1016/j.canlet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Miyake H, Hara I, Kamidono S, Eto H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J. Urol. 2004;171:1533–1536. doi: 10.1097/01.ju.0000116617.32728.ca. [DOI] [PubMed] [Google Scholar]
- 68.Fernández-Checa JC, Kaplowitz N, García-Ruiz C, Colell A, Miranda M, Marí M, Ardite E, Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 69.Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 70.Kotrikadze N, Alibegashvili M, Zibzibadze M, Abashidze N, Chigogidze T, Managadze L, Artsivadze K. Activity and content of antioxidant enzymes in prostate tumors. Exp. Oncol. 2008;30:244–247. [PubMed] [Google Scholar]
- 71.Zachara BA, Szewczyk-Golec K, Tyloch J, Wolski Z, Szylberg T, Stepien S, Kwiatkowski S, Bloch-Boguslawska E, Wasowicz W. Blood and tissue selenium concentrations and glutathione peroxidase activities in patients with prostate cancer and benign prostate hyperplasia. Neoplasma. 2005;52:248–254. [PubMed] [Google Scholar]
- 72.Meyer M, Schreck R, Muller JM, Baeuerle PA. Redox control of gene expression by eukaryotic transcription factors NF-kB, AP-1 and SRF/TCF. In: Pasquier C, Oliver RY, Auclair C, Packer L, editors. Oxidative stress, cell activation and viral infection. Birkhauser Verlag: Boston; 1994. pp. 217–235. [Google Scholar]
- 73.Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, Koji T. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 2003;88:1333–1340. doi: 10.1210/jc.2002-021015. [DOI] [PubMed] [Google Scholar]
- 74.Linja MJ, Savinainen KJ, Tammela TL, Isola JJ, Visakorpi T. Expression of ERalpha and ERbeta in prostate cancer. Prostate. 2003;55:180–186. doi: 10.1002/pros.10242. [DOI] [PubMed] [Google Scholar]
- 75.Kwon SM, Kim SI, Chun DC, Cho NH, Chung BC, Park BW, Hong SJ. Development of rat prostatitis model by oral administration of isoflavone and its characteristics. Yonsei. Med. J. 2001;42:395–404. doi: 10.3349/ymj.2001.42.4.395. [DOI] [PubMed] [Google Scholar]
- 76.Harris MT, Feldberg RS, Lau KM, Lazarus NH, Cochrane DE. Expression of proinflammatory genes during estrogen-induced inflammation of the rat prostate. Prostate. 2000;44:19–25. doi: 10.1002/1097-0045(20000615)44:1<19::aid-pros3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 77.Ho E, Boileau TW, Bray TM. Dietary influences on endocrine-inflammatory interactions in prostate cancer development. Arch. Biochem. Biophys. 2004;428:109–117. doi: 10.1016/j.abb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512–1520. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 79.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat. Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 80.Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia signalling controls metabolic demand. Curr. Opin. Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 83.Bourdeau-Heller J, Oberley TD. Prostate carcinoma cells selected by long-term exposure to reduced oxygen tension show remarkable biochemical plasticity via modulation of superoxide, HIF-1alpha levels, and energy metabolism. J. Cell Physiol. 2007;212:744–752. doi: 10.1002/jcp.21069. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis. 2007;28:28–37. doi: 10.1093/carcin/bgl085. [DOI] [PubMed] [Google Scholar]
- 85.BelAiba RS, Djordjevic T, Bonello S, Flügel D, Hess J, Kietzmann T, Görlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol. Chem. 2004;385:249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 86.Wartenberg M, Hoffmann E, Schwindt H, Grünheck F, Petros J, Arnold JR, Hescheler J, Sauer H. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett. 2005;579:4541–4549. doi: 10.1016/j.febslet.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 87.Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266:12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 88.Krieger JN, Nyberg L., Jr NIH consensus definition and classification of Prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 89.Sadeghi-Nejad H, Seftel A. Sexual dysfunction and Prostatitis. Curr. Urol. Rep. 2006;7:479–484. doi: 10.1007/s11934-006-0058-1. [DOI] [PubMed] [Google Scholar]
- 90.Nickel JC, Moon T. Chronic bacterial prostatitis: an evolving clinical enigma. Urology. 2005;66:2–8. doi: 10.1016/j.urology.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 91.McNaughton-Collins M, Joyce GF, Wise T, Pontari MA. Prostatitis. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. US Department of Health and Human Services, Public Health Service, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. NIH Publication No. 07-5512. Washington DC: US Government Publishing Office; 2007. [Google Scholar]
- 92.Krieger JN, Riley DE. Prostatitis: what is the role of infection,Int. J. Antimicrob. Agents. 2002;19:475–479. doi: 10.1016/s0924-8579(02)00086-9. [DOI] [PubMed] [Google Scholar]
- 93.Terai, Ishitoya S, Mitsumori K, Ogawa O. Molecular epidemiological evidence for ascending urethral infection in acute bacterial Prostatitis. J. Urol. 2000;164:1945–1947. [PubMed] [Google Scholar]
- 94.Taylor BC, Noorbaloochi S, McNaughton-Collins M, Saigal CS, Sohn MW, Pontari MA, Litwin MS, Wilt TJ. Urologic Diseases in America Project, Excessive antibiotic use in men with Prostatitis. Am. J. Med. 2008;121:444–449. doi: 10.1016/j.amjmed.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Potts J, Payne RE. Prostatitis: Infection, neuromuscular disorder, or pain syndrome? Proper patient classification is key. Cleve.Clin. J. Med. 2007;74 Suppl 3:S63–S71. doi: 10.3949/ccjm.74.suppl_3.s63. [DOI] [PubMed] [Google Scholar]
- 96.de Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat.Rev. Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Espey MG, Miranda KM, Thomas DD, Xavier S, Citrin D, Vitek MP, Wink DA. A chemical perspective on the interplay between NO, reactive oxygen species, and reactive nitrogen oxide species. Ann. N. Y. Acad. Sci. 2002;962:195–206. doi: 10.1111/j.1749-6632.2002.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 98.Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic. Biol. Med. 2002;33:192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 99.Naber KG, Weidner W. Chronic prostatitis-an infectious disease? J. Antimicrob. Chemother. 2000;46:157–161. doi: 10.1093/jac/46.2.157. [DOI] [PubMed] [Google Scholar]
- 100.Scholl SM, Pallud C, Beuvon F, Hacene K, Stanley ER, Rohrschneider L, Tang R, Pouillart P, Lidereau R. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J. Natl. Cancer Inst. 1994;86:120–126. doi: 10.1093/jnci/86.2.120. [DOI] [PubMed] [Google Scholar]
- 101.Stancik W, Lüftenegger W, Klimpfinger M, Müller MM, Hoeltl W. Effect of NIH-IV prostatitis on free and free-to-total PSA. Eur. Urol. 2004;46:760–764. doi: 10.1016/j.eururo.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 102.Dennis LK, Lynch CF, Torner jc. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 103.Maroni PD, Koul S, Meacham RB, Koul HK. Mitogen Activated Protein kinase signal transduction pathways in the prostate. Cell Commun. Signal. 2004;2:5. doi: 10.1186/1478-811X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]