Abstract
The phosphoinositide (PI) cycle, discovered over 50 years ago by Mabel and Lowell Hokin, describes a series of biochemical reactions that occur on the inner leaflet of the plasma membrane of cells in response to receptor activation by extracellular stimuli. Studies from our laboratory have shown that the retina and rod outer segments (ROSs) have active PI metabolism. Biochemical studies revealed that the ROSs contain the enzymes necessary for phosphorylation of phosphoinositides. We showed that light stimulates various components of the PI cycle in the vertebrate ROS, including diacylglycerol kinase, PI synthetase, phosphatidylinositol phosphate kinase, phospholipase C, and phosphoinositide 3-kinase (PI3K). This article describes recent studies on the PI3K-generated PI lipid second messengers in the control and regulation of PI-binding proteins in the vertebrate retina.
THE PI CYCLE
In the early 1950s, Hokin and Hokin (1, 2) discovered that addition of acetylcholine to brain slices stimulated the incorporation of phosphate and inositol but not glycerol into lipids; the major products of this incorporation were phosphatidylinositol (PI) and phosphatidic acid. Subsequent studies defined the reactions of the PI cycle and showed that the initial event was receptor-meditated activation of a phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PI-4,5-P2) to 1,2-diacylglycerol (DG) and inositol 1,4,5-trisphosphate (IP3). This increase in lipid synthesis reported by the Hokins was a recovery reaction that rapidly replenished PI separate from de novo PI synthesis. The role of 1,4,5-IP3 was established by Streb et al. (3) in their classic paper that showed elevations in IP3 caused intracellular release of bound calcium. Subsequently, 1,2-DG was found to stimulate protein kinase C (PKC), a serine/threonine kinase that phosphorylates a number of cellular proteins (4). Activation of the PLC/PKC cascade affects a variety of cellular events, including secretion, phagocytosis, smooth muscle contraction, proliferation, neurotransmission, and metabolism [see reviews by Rhee (5), Rhee and Choi (6), and Berridge (7)]. In 1989, Auger et al. (8) discovered the receptor-mediated conversion of PI-4,5-P2 to phosphatidylinositol 3,4,5-trisphosphate (PI-3,4,5-P3) in platelet-derived growth factor (PDGF)-stimulated smooth muscle cells and PI to phosphatidylinositol 3-phosphate (PI-3-P) in yeast. Subsequent studies showed that phosphorylation of the D3-position of the inositol ring by phosphoinositide 3-kinase (PI3K) can be stimulated by several extracellular molecules, including PDGF, insulin, insulin-like growth factor-1 (IGF-1), and nerve growth factor [see reviews by Vanhaesebroeck and Waterfield (9), and Datta et al. (10)]. The formation of all of these phosphoinositides has been demonstrated in mammalian cells [reviewed by Rameh and Cantley(11)] and we have shown their formation (except for PI-3-P) in intact rod outer segment membranes (ROSs) prepared from fresh bovine retinas (12–15).
ACTIVATION OF PHOSPHOINOSITIDE SIGNALING PATHWAYS
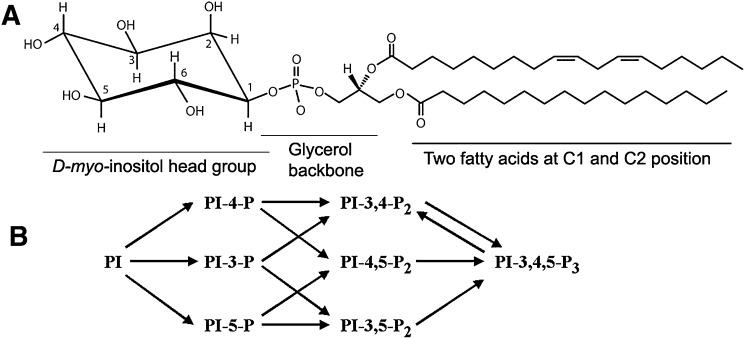
PIs, as components of phospholipids in the cell membrane, contain a D-myo-inositol head group, a glycerol backbone, and two fatty acids at the C1 and C2 acyl positions of glycerol (Fig. 1A). Phosphorylation of multiple free hydroxyls in the inositol head group generates several phosphorylated PI derivatives. Differential phosphorylation at the 3, 4, and 5 positions allows for the generation of seven distinct phosphoinositides (Fig. 1B). The intracellular levels of the phosphoinositides are controlled by PI-specific kinases and phosphatases that can rapidly convert one phosphoinositide into another (16). PI signals regulate signal transduction, cytoskeletal assembly, membrane binding, and fusion that are spatially restricted to specific membrane domains (16). The classic work of Grado and Ballou (17) characterized phosphatidylinositol 4-phosphate (PI-4-P) and PI-4,5-P2 as D4 phosphoinositides. The discovery that phosphorylation at the D3 hydroxyl of the inositol head group (18) led to the generation of PI-3-P, phosphatidylinositol 3,4-bisphosphate (PI-3,4-P2), and PI-3,4,5-P3, which are referred to as D3 phosphoinositides, increased this diversity. The formation of all of these phosphoinositides has been demonstrated in mammalian cells (11).
Fig. 1.
A: Phosphatidylinositol (PI). B: Naturally occurring PIs. Phosphatidylinositol contain a D-myo-inositol head group, a glycerol backbone, and two fatty acids at the C1 and C2 acyl positions of glycerol (A). Phosphorylation of multiple free hydroxyls in the inositol head group generates several phosphorylated PI derivatives. Differential phosphorylation at the 3, 4, and 5 positions allows for the generation of seven distinct phosphoinositides (B). PI-4-P, phosphatidylinositol 4-phosphate; PI3-P, phosphatidylinositol 3-phosphate; PI-5-P, phosphatidylinositol 5-phosphate; PI-3,4-P2, phosphatidylinositol 3,4-bisphosphate; PI-4-5-P2, phosphatidylinositol 4,5-bisphosphate; PI-3-5-P2, phosphatidylinositol 3,5-bisphosphate; PI-3,4,5-P3, phosphatidylinositol 3,4,5-trisphosphate.
PI3K
The PI3K pathway is highly conserved among different species, including Drosophilia melangaster, Caenorhabditis elegans, and mammals (9, 19). Studies in Drosophila have established the involvement of this pathway in the regulation of cell size and number (20–22). Genetic studies in C. elegans have linked this pathway to regulation of dauer formation. The dauer phenotype is a larval state characterized by developmental arrest and reduced metabolic rate triggered by adverse environmental conditions, including nutrient deprivation and overcrowding. Genetic dissection of the genes involved in this pathway led to the identification of the daf (dauer affected) genes (23, 24), some of which are homologs of the mammalian components of the insulin-PI3K signaling pathway.
PI3K belongs to the large family of PI3K-related kinases or PIKK. Other members of the family include mammalian target of rapamycin (mTOR), ataxia-telangiectasia mutated, ataxia-telangiectasia mutated and RAD3 related, and DNA-dependent protein kinase. All possess the characteristic PI3K-homologus kinase domain and a highly conserved carboxy-terminal tail (25). However, only PI3K is known to have an endogenous lipid substrate. Mammalian cells carry at least eight different genes with significant homology and yeast contains only one PI3K gene (26). The PI3K enzymes are broadly divided into classes I, II, and III, depending upon their substrate specificity (27, 28) (Table 1). The class I PI3K phosphorylates PI-4,5-P2 to produce PI-3,4,5-P3 and class III enzymes produce PI-3-P from PI (16, 29). The activity of class II PI3K is debatable and probably involved in the production of both PI-3,4-P2 and PI-3-P (26). Existing data suggest that class II and III PI3K may be involved in vesicular trafficking (30, 31). The class I PI3K is the most characterized and best understood enzyme (29). Class I PI3K enzymes are heterodimers composed of a catalytic subunit and an adaptor regulatory subunit (32). Class I catalytic subunits share significant homology and have an apparent molecular weight of p110 kDa and thus are referred to as p110 subunits (32). There are four class I PI3Kp110 genes known in mammals; these are named Pik3ca, Pik3cb, Pik3cg, and Pik3cd and are referred to as PI3Kα, β, γ, and δ (33). Pik3ca and Pik3cb genes are ubiquitously expressed; Pik3cg and Pik3cd genes are specifically found in leukocytes with the exception of Pik3cg, which was recently detected in the cardiovascular system (34). The protein products of these genes have the highest homology at the N-terminal end, and all have a GTPase Ras domain, a C2-lipid binding domain, the phosphatidylinositol kinase domain, and a catalytic domain (35).
TABLE 1.
PI3K family members
| Class | Catalytic Subunit | Regulatory Subunit | Activator | Phosphoinositide Products |
|---|---|---|---|---|
| 1a | p110α | p85 | RTK, RAS | PI-3-P |
| p110β | PI-3, 4-P2 | |||
| p110δ | PI-3,4,5-P3 | |||
| 1b | p110γ | p101 | PI-3-P | |
| PI-3, 4-P2 | ||||
| PI-3,4,5-P3 | ||||
| II | PI3KC2 α | RTK, integrins | PI-3-P | |
| PI3KC2 β | PI-3, 4-P2 | |||
| PI3KC2γ | ||||
| III | VSP34p | PI-3-P |
In mammals, the PI3K catalytic subunits (p110α, p110β, and p110δ) are bound to any of five distinct regulatory subunits (p85α, p85β, p55γ, p55α, and p50α, collectively referred to as “p85s”) (36). These p85 adapters result from three genes: Pik3r1, Pik3r2, and Pik3r3 (35). The Pik3r1 can be expressed in splice variants that encode p85α, p55α, and p50α. The adapters p85α and p85β are ubiquitously expressed (35), whereas p50α and p55α are present in fat, muscle, liver, and brain (37, 38), and p55γ is mainly expressed in the brain (39). All members of the p85 family contain a p110-binding region that interacts with a specific domain present at the N-terminal ends of the class IA p110 catalytic domains (40).
Phosphorylation of cell surface receptors can be stimulated by several extracellular molecules, including PDGF, insulin, IGF-1, and nerve growth factor (9, 10). The adaptor subunit of these enzymes contains an Src homology (SH)2-domain that mediates recruitment to phosphotyrosine resides on the cytoplasmic domain of receptors, which results in the activation of the PI3K (29). Class II enzymes preferentially phosphorylate PI and PI-4-P, but not PI-4,5-P2, in vitro and contain a C2 domain that can mediate membrane interactions (11, 29). Evidence also indicates that class 1A PI3Kβ is activated by both G-protein-coupled receptor (GPCR) and tyrosine kinase receptors (41). The N-terminal p85-binding motif is absent in class IB PI3K and PI3Kγ, but interacts with the p101 (42) and p84/87 adaptor for regulation (43, 44). The GPCR can activate PI3Kγ and is regulated by free Gβγ subunits of heterotrimeric G proteins, mainly the subtype Gi (34). Class II comprises three members, PI3KC2α, β, and γ, which are characterized by a carboxy-terminal phospholipid-binding domain. Although no regulatory subunit has been identified, class II enzymes are predominantly membrane-bound and activated by membrane receptors including receptor tyrosine kinases and integrins (35). Class III enzymes, which phosphorylate only PI, are heterodimers of a catalytic subunit associated with the serine/threonine protein kinase adaptor subunit that is required for membrane recruitment (16, 29). The class III kinase, VSP34p, is responsible for producing the majority of the cellular PI-3-P and is involved in protein trafficking through the lysosome.
The regulatory p85 subunit contains an SH3 domain capable of binding to proline-rich sequences, a region of homology to the breakpoint cluster region gene product, a p110 binding domain, and two SH2 domains (N and C terminus). The regulatory subunit maintains the p110 catalytic subunit in a low-activity state in quiescent cells and mediates activation by direct interaction with phosphotyrosine residues of activated growth-factor receptors or adaptor proteins (29). The activated PI3K converts the plasma membrane lipid PI-4,5-P2 to PI-3,4,5-P3. The termination of PI3K signaling by the degradation of PI-3,4,5-P3 can be mediated by at least two different types of phosphatases. The SH2-containing phosphatases, SHIP1 and SHIP2, dephosphorylate the 5-position of the inositol ring to produce PI-3,4-P2 (45). Although this dephosphorylation impairs some signaling downstream of PI3K, PI-3,4-P2 can also mediate PI3K-dependent responses, and may mediate events that are independent of those stimulated by PI-3,4,5-P3. Loss of SHIP2 causes a dramatic increase in insulin sensitivity, suggesting that this phosphatase critically regulates PI3K signaling downstream of insulin (45). In contrast, the phosphatase PTEN (phosphatase and tensin homolog) dephosphorylates the 3-position of PI-3,4,5-P3 to produce PI-4,5-P2 (46). The loss of PTEN protein function has been found in a large fraction of advanced cancers, suggesting that uncontrolled signaling through PI3K may contribute to metastatic cancers (47).
Class 1A PI3Kα has been extensively studied in the retina and photoreceptors (14, 15, 48–52). The retinal transducin βγ subunits can stimulate G-protein-regulated PI3K, presumably PI3Kγ or class 1A PI3Kβ (53). Class III PI3K-generated PI-3-P was found to be involved in the vesicular membrane targeting of rhodopsin (54). Biochemical and functional studies related to other class of PI3K or different isoforms have yet to be performed on the retina and photoreceptors.
ACTIVATION OF CLASS IA PI3K
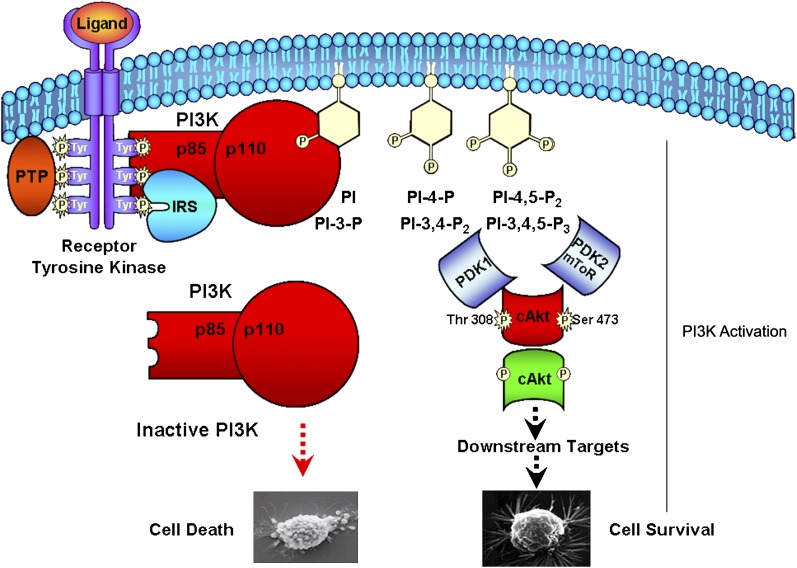
The growth factor-stimulated PI3K pathway is described in Fig. 2. PI3K activity increases in response to PDGF binding to its receptor, in a large part because the class IA p85/p110 complex is translocated from the cytosol to the plasma membrane by the direct binding of the p85 SH2 domain to tyrosine-phosphorylated sites on the receptor (55, 56). Insulin receptor (IR) activation stimulates intrinsic tyrosine kinase activity that leads to autophosphorylation. Unlike the epidermal growth factor and PDGF receptors, the phosphorylated IR does not usually directly associate with SH2 proteins (57). Rather, the activated IR usually phosphorylates insulin receptor substrate (IRS)-1, a principal substrate of the IR, on multiple tyrosine residues, which, in turn, recognizes and binds to the SH2 domain of PI3K (58). With few exceptions (59), PI3K is activated when phosphorylated IRS-1 binds to the SH2 domain in its p85α regulatory subunit, which establishes a direct molecular connection between circulating insulin and cellular PI3K (60). Insulin, IGF-1, IGF-2, brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), ciliary neurotrophic factor (CNTF), erythropoietin (EPO), and PDGF can activate the PI3K pathway in the retina. Insulin and IGF-1 can stimulate PI3K in the photoreceptors (52, 61). PDGF can stimulate PI3K in ganglion (62, 63) and retinal pigmented epithelium (RPE) cells (63), whereas bFGF activates PI3K in Muller glial cells (64). EPO and BDNF can activate PI3K in ganglion cells (65, 66). CNTF protects the murine retina from constant light via activation of PI3K in vivo (67). We (68) and others (52) have shown that IR activation does not induce the activation of IRS-1 but does induce IRS-2 activation in the retina. We have also found that the IR can directly interact with the p85 subunit of PI3K (50). It should be mentioned that IRS-2 knockout mice lose up to 50% of their photoreceptors by 2 weeks of age, resulting from increased apoptosis (69).
Fig. 2.
Signaling pathway downstream of PI3K affect cell death and cell survival. Activation of growth factor receptor protein tyrosine kinases results in autophosphorylation on tyrosine residues and transphosphorylation of adaptor proteins such as IRS. PI3K can also be stimulated by integrin-dependent cell adhesion and by G-protein coupled receptors (not shown). PI3K is brought to the membrane and activated by directly binding to phosphotyrosine residues of growth factor receptors or adapters. The lipid products of PI3K, PI-3,4-P2, or PI-3,4,5-P3, recruit a subset of signaling proteins with pleckstrin homology (PH) domains to the membrane where they are activated. The proteins include protein serine-threonine kinases, protein tyrosine kinases, exchange factors, and adaptor proteins (downstream effectors). Ultimately these proteins initiate complex sets of events that control protein synthesis, actin polymerization, cell survival, and cell entry. PI3K, phosphoinositide 3-kinase; IRS, insulin receptor substrate; PH, pleckstrin homology.
PI3K REGULATED DOWNSTREAM EFFECTORS
The serine/threonine protein kinase B/Akt is a key mediator of signal transduction processes downstream of PI3K. Upon growth factor stimulation, PI3K is the key enzyme catalyzing the transfer of phosphate from ATP to the D-3 position of the inositol ring of membrane-localized phosphoinositides, thereby generating 3′-phosphorylated phosphoinositides such as PI-3-P, PI-3,4-P2 and PI-3,4,5-P3. This lipid second messenger recruits Akt to the membrane by engaging its pleckstrin homology (PH) domain (70–75). Once localized in the membrane, Akt is phosphorylated on two sites of the protein: the activation loop (Thr 308) and the hydrophobic motif (Ser 473) (76, 77). Phosphorylation at the activation domain is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) (78) and the kinase that mediates phosphorylation at the hydrophobic motif is catalyzed by mTOR (79). Akt is fully activated when it is phosphorylated on both sites, after which it dissociates from the membrane and phosphorylates many substrates in the cytoplasm and nucleus (80–83) and thereby plays an important role in the regulation of metabolism, apoptosis, cell cycle, and transcription of various genes (84, 85).
The PI3K-generated phosphoinositides are able to recruit phospholipid binding proteins such as PDK1 and Akt. PDK1 phosphorylates Akt, and the activated Akt phosphorylates multiple proteins on serine and threonine residues (86). These substrates include glycogen synthase kinase 3 (GSK3), ribosomal protein S6 kinase (p70S6K), Bcl-2XL-antagonist causing cell death, IkB kinase, endothelial nitric oxide synthase, mTOR, eukaryotic translation initiation factor 4E binding protein (4E-BP), forkhead transcriptional factor, and caspase 9. Through phosphorylation of these targets, Akt carries out its role as a key regulator of a variety of critical cell functions including glucose metabolism, cell proliferation, and survival.
Insulin stimulates protein synthesis via two major effects: the rapid activation of existing components of the translational apparatus and the longer term increase in the capacity of the cell or tissue for protein synthesis, which includes an increase in ribosome number (87). The rapid activation of protein synthesis by insulin is mediated primarily through the GSK3β inactivation mediated by activated Akt. Insulin, by inhibiting GSK3β, stimulates the dephosphorylation and activation of eukaryotic initiation factor (eIF)2B, a protein required for recycling of eIF2, itself necessary for all cytoplasmic translation initiation events. Insulin-activated Akt also phosphorylates the tuberous sclerosis TSC1–TSC2 complex to relieve its inhibitory action on mTOR, a key controller of translation initiation and elongation. The cap-binding factor eIF4E can be sequestered in inactive complexes by 4E-BP1. By activating mTOR, insulin induces phosphorylation of 4E-BP1 and its release from eIF4E, allowing eIF4E to form initiation factor complexes. It also induces dephosphorylation and activation of eukaryotic translation elongation factor 2 to accelerate elongation. Insulin inactivates eukaryotic translation elongation factor 2 kinase by increasing its phosphorylation at several mTOR-regulated sites. Insulin also stimulates synthesis of ribosomal proteins by promoting recruitment of their mRNAs into polyribosomes.
p70S6K is one of the downstream effectors of PI3K (88–92). To date, PI3K (93, 94), PDK1, Akt (95–98), PKC (99, 100), the Rho family of small G proteins, and mTOR (101–106) are thought to be the upstream effectors of S6K1 phosphorylation. Many growth factors, including insulin, activate p70S6K in a PI3K-dependent manner. In addition, amino acids can activate p70S6K in a PI3K- independent manner. For example, branched-chain amino acids such as leucine are sufficient to activate mTOR, resulting in an increase in p70S6K phosphorylation and thereby activating it. mTOR is also in a pathway downstream of Akt. Akt is typically activated upon growth factor (such as insulin, IGF-1) stimulation of a cell. Akt then activates mTOR (by inhibiting the TSC complex), leading to p70S6K activation. Insulin promotes rat retinal neuronal cell survival in a p70S6K-dependent manner and this pathway is completely inhibited by the PI3K inhibitor LY294002. The mechanism by which p70S6K supports insulin- stimulated retinal cell survival remains uncertain. It has been suggested that internal ribosomal initiation of mRNA translation, a step that is affected by rapamycin and p70S6K, is critical for survival of cells under transient apoptotic stress (107). Postmitotic neurons require protein synthesis for survival (108) so it is possible that insulin stimulates protein synthesis via a rapamycin-dependent mechanism. In support of this hypothesis are the data that show stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa (109). Cone cell death in retinitis pigmentosa could, at least in part, be a result of the starvation of cones. We recently observed that cone photoreceptor-specific deletion of p85 subunit of PI3K resulted in age-related cone degeneration (unpublished observations). These studies highlight the importance of protein synthesis in cone cell survival.
PROTEIN TYROSINE PHOSPHATASE-1B AND PI3K PATHWAY
The extent of tyrosyl phosphorylation on a given protein is controlled by the reciprocal action of protein tyrosine kinase and protein tyrosine phosphatase (PTP) activities. Specific PTPs, including Leukocyte Antigen-Related (LAR), SHP-2, and PTP1B, have been implicated in the regulation of normal IR signaling (110–123). Of these PTPs, PTP1B has received significant attention because it is an abundant enzyme expressed in all insulin-sensitive tissues (124, 125). PTP1B is an abundant and widely expressed nonreceptor tyrosine phosphatase, which is thought to be a key negative regulator of insulin signaling (126, 127). It was previously shown that PTP1B overexpression results in the inhibition of IR and IRS-1 signaling (117, 122, 128). Furthermore, introduction of anti-PTP1B antibodies into cells enhances IR signaling (129). Global deletion of PTP1B in mice results in increased systemic insulin sensitivity, enhanced glucose uptake into skeletal muscle, and improved glucose tolerance (130, 131). Increased and prolonged tyrosine phosphorylation of the IR was also found in mice lacking PTP1B (130, 131). The increased insulin sensitivity is due to the absence of PTP1B and results from failure to dephosphorylate the IR (130, 131). The IR, PI3K, and Akt pathway was found to be downregulated in diabetic retinopathy (132). Recently, we reported that diabetes downregulates IR activation and increases the activity of PTP1B (133).
Akt phosphorylates substrates at the consensus motif RXRXXS/T. PTP1B contains this motif (RYRDVS50), which can be phosphorylated by Akt on Ser50 (134). Insulin stimulation can cause a significant increase in phosphorylation of PTP1B that can be blocked by pretreatment of cells with wortmannin or cotransfection of a dominant inhibitory Akt mutant. Phosphorylated PTP1B has an impaired ability to dephosphorylate the IR (134). These studies suggested that PTP1B is a novel substrate for Akt and that phosphorylation of PTP1B by Akt at Ser50 may negatively modulate its phosphatase activity, creating a positive feedback mechanism for IR/PI3K signaling. Currently, we do not know whether such a mechanism exists in the retina; however, Akt2 knockout mice have increased susceptibility to light-induced photoreceptor degeneration (135). It is possible that failure to deactivate PTP1B by Akt2 may result in the degeneration phenotype.
PI3K SIGNALING IN DIABETIC RETINOPATHY
PI3K and Akt are downstream effectors of insulin signaling (136) as well as important signaling molecules in the regulation of glycogen metabolism in myocytes, lipocytes, and hepatocytes (137, 138). Uncoupling of insulin signaling at PI3K-Akt in these cell types in response to high glucose concentrations has been implicated in the pathogenesis of insulin resistance and Type II diabetes (139). However, PI3K-Akt also plays an important role in endothelial cells by regulating angiogenesis (140), proliferation (141), microvascular permeability (142), survival (86), cellular transformation, and embryonic differentiation (143). It has been shown previously that compromised muscle PI3K signaling contributes to symptoms of impaired insulin response and hyperlipidemia associated with human type 2 diabetes (144). The PI3K/Akt pathway plays an important role in diabetic retinopathy. The diabetic rat retina has a loss of PI3K, Akt-1 and Akt-3, mTOR, and p70S6K activities, but has greater GSK3β activity than in the wild-type retina (145). These studies clearly highlight the importance of this pathway in diabetic retinopathy.
We recently reported that in insulin-lacking cultures, photoreceptors from wild-type rat retinas exhibited an abnormal morphology with a wide axon cone and disorganization of the actin and tubulin cytoskeleton (146). Photoreceptors from IR knockout mouse retinas also exhibited a similar abnormal morphology (146). A novel finding in this study was that addition of docosahexaenoic acid (DHA), a photoreceptor trophic factor, restored normal axonal outgrowth in insulin-lacking cultures (146). These data suggest that IR-signaling pathways regulate actin and tubulin cytoskeletal organization in photoreceptors; they also imply that insulin and DHA activate at least partially overlapping signaling pathways that are essential for the development of normal photoreceptors. We further reported that high glucose and high insulin levels affected the cytoskeletal organization of the developing photoreceptors, and DHA prevented the abnormal development of these photoreceptors in culture (146). Further, the combination of insulin and DHA protects the Cytochalasin D mediated disruption of actin cytoskeleton of the developing photoreceptors (146). Phosphoinositides are the key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane (147). In photoreceptors, we previously reported that the IR regulates PI3K and Akt activation (49, 50, 148). Further, we recently reported that 3′-phosphoinostides generated through light-induced tyrosine phosphorylation of IR regulates the reorganization of actin cytoskeleton (61). The results from our current study also suggest that IR activation may regulate the actin cytoskeletal organization. Dysfunction of the actin cytoskeleton is a key event in the pathogenesis of diabetic nephropathy (149), diabetic neuropathy (150, 151), and diabetic cardiomyopathy (152, 153). Our findings that high glucose and high insulin concentrations induced severe morphological changes in actin cytoskeleton, altering growth cone structure, are consistent with reports showing impaired axonal growth and aberrant dystrophic structures in dorsal root ganglion neurons from diabetic animals and in neurons cultured under high glucose conditions (154, 155).
LIGHT-INDUCED ACTIVATION OF PI3K PATHWAY IN ROD PHOTORECEPTOR CELLS
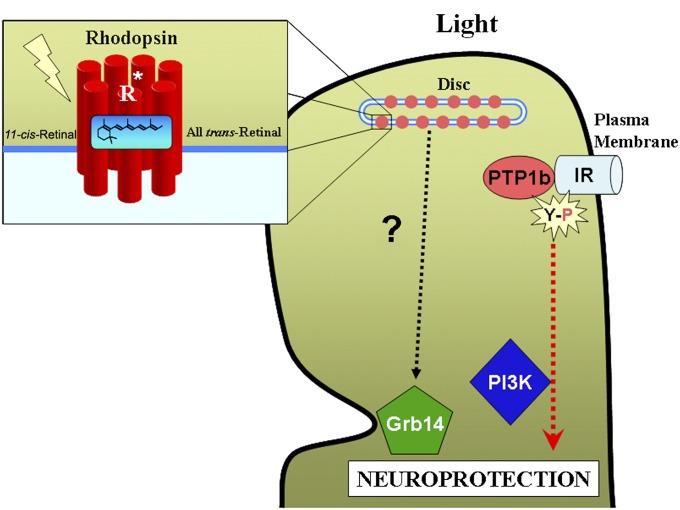
We were the first to discover that light-induced tyrosine phosphorylation of the IR in rod photoreceptors leads to the activation of PI3K (Fig. 3). This novel pathway is independent of insulin secretion but dependent on photoreceptor neurons (50). We subsequently found that this pathway is mediated through the photobleaching of GPCR rhodopsin but is independent of transducin activation (49). Compared with the liver IR, retinal IR has a higher basal level of autophosphorylation (52), which is light-dependent (50). These observations led us to hypothesize that retinal IR phosphorylation could be modulated by a soluble factor(s) in the retina. To identify the regulators of IR, yeast two-hybrid screening of a bovine retinal cDNA library constructed with the cytoplasmic domain of retinal IR (68) identified growth factor receptor-bound protein 14 (Grb14) (156, 157), which binds to various tyrosine kinase receptors including IR (158–161). Grb14 is localized predominantly in the rod inner segment, nuclear layer, and synapse in dark-adapted rods, whereas Grb14 is redistributed throughout the entire cell, including the outer segment, in the light-adapted rods (162). The translocation of Grb14 requires photoactivation of rhodopsin, but not signaling through the phototransduction cascade, and is not based on direct Grb14-rhodopsin interactions (162). Previously, we hypothesized that Grb14 protects light-dependent IR activation in rod photoreceptors against dephosphorylation by PTP1B (49). We failed to observe light-dependent IR activation in Grb14 knockout mouse retinas, supporting our hypothesis (162). Our studies suggest that Grb14 translocates to ROSs after photobleaching of rhodopsin and protects IR phosphorylation in rod photoreceptor cells. These studies demonstrate that Grb14 can undergo subcellular redistribution upon illumination and suggest that rhodopsin photoexcitation may trigger signaling events alternative to the classic transducin activation.
Fig. 3.
Light-induced activation of PI3K. Grb14, an upstream regulator of IR, requires photobleaching of rhodopsin for membrane targeting. Grb14 protects light-dependent IR activation in rod photoreceptors against dephosphorylation by PTP1B. Our studies suggest that Grb14 translocates to photoreceptor outer segments after photobleaching of rhodopsin and protects IR phosphorylation in rod photoreceptor cells. Light-activated IR is subsequently associated with PI3K, a cell survival factor, and, thus, regulates the downstream survival pathway. R*, photoactivated rhodopsin; Grb14, growth factor receptor-bound protein 14; IR, insulin receptor; PTP1B, protein tyrosine phosphatase; Y-P, phosphotyrosine.
The molecular mechanism behind the light-dependent translocation of Grb14 is not clearly understood. Identification of binding partner(s) of Grb14 may provide additional clues as to how this protein is translocated within rod photoreceptor cells. Grb14 has several functional domains that may potentially interact with a variety of proteins involved in intracellular signaling. It may also be important to investigate the nature of the interaction of Grb14 with motor proteins or other components of the polarized transport machinery. However, it should be recognized that the entire Grb14 translocation phenomenon could be potentially explained by a combination of the light-dependent changes in the affinity of Grb14 for binding sites outside ROS and intracellular diffusion.
Our results also suggest that a cross-talk exists between phototransduction and IR/PI3K signal transduction pathway. This cross-talk phenomenon has been shown for other GPCRs and many tyrosine kinase cascades are regulated by GPCRs (163, 164). Examples include mitogen-activated protein kinase (MAPK) cascade, such as extracellular-regulated kinases, and stress-activated protein kinases (164). The binding of PYK2, a nonreceptor protein tyrosine kinase, to N-terminal domain-interacting receptors is activated by GPCRs (165). The N-terminal domain-interacting receptor proteins are the human homologs of the Drosophila retinal degeneration B protein, a protein implicated in the Drosophila visual transduction pathway (165). These studies, along with our observations, suggest that photobleaching of rhodopsin may activate more than one signaling pathway. GPCR can activate the PI3Kγ and is regulated by the free Gβγ subunits of heterotrimeric G proteins, mainly the subtype Gi (35). Evidence also indicates that class 1A PI3Kβ is activated both by GPCR and tyrosine kinase receptors (41); however, it has not been shown that class 1A PI3Kα is regulated through GPCR, and our studies clearly suggest that rhodopsin regulates the activation of class 1A PI3Kα in rod photoreceptors.
We found that Akt, the downstream effector of PI3K, is also activated by light adaptation (61). We further found that the membrane binding of Akt1 to ROS is IR/PI3K-dependent; reduced binding of Akt1 to ROS membranes was observed in the photoreceptor-specific IR knockout mice (61). Membrane binding of Akt1 is mediated through its PH domain. Fluorescently-tagged protein domains (e.g., PH domains) as PI sensors have been used by a number of independent laboratories (166–170). We applied this approach to an in vivo model of transgenic frogs and found light-dependent generation of 3′-phosphoinositides specifically in photoreceptor cells using the Akt1-AH domain as the cellular probe (61). We expressed various green fluorescent protein/Akt1-PH domain fusion proteins in rod photoreceptors of transgenic Xenopus laevis under the control of the Xenopus opsin promoter and found light-induced trafficking and binding of Akt occurs (61). The light-dependent binding of this probe coupled with its in vitro binding affinity for 3′-phosphoinositides strongly suggests that PI-3,4-P2 is generated in photoreceptor membranes in response to light exposure (61). Identification and quantitation of these PI species in response to light in vivo would further advance our understanding of the role of individual phosphoinositides in the regulation of specific phospholipid-binding proteins.
The functional consequence of light-dependent activation of PI3K in photoreceptor cells is not known. PI3K activity in rods is regulated by light, suggesting that it may function shedding of photoreceptor tips (171), biogenesis of new ROS membranes through addition of newly synthesized membranes at the base of the ROS (172), or light adaptation (173). Alternatively, it is possible that light-induced PI3K activity may mediate an innate self-protection mechanism. Receptor activation of PI3K has been shown to protect some neuronal cell types, such as cerebellar granular neurons (174) and PC-12 cells (175), from stress-induced neurodegeneration.
It has been well established that PI3K/Akt is crucial in mediating cell survival. Because bright light stimulation can cause the death of rod and cone photoreceptor cells (176, 177), activation of the PI3K/Akt pathway by relatively modest light levels may serve to prevent death of photoreceptors. Supporting this hypothesis are the data that show deletion of Akt2, one of the isoforms of Akt, results in stress-induced photoreceptor degeneration (135). Furthermore, rod-specific IR knockout mice have reduced PI3K and Akt recruitment to the membranes, suggesting that the IR regulates the downstream PI3K and Akt survival signal in rod photoreceptors (148). The IR knockout mice exhibited no detectable phenotype when they were raised in dim cyclic light. However, reduced IR expression in rod photoreceptors significantly decreased retinal function and caused the loss of photoreceptors in mice exposed to bright light stress (148).
The PI3K/Akt pathway is highly conserved throughout evolution and regulates cell size and survival and cell cycle progression. Very recently, PI3K/Akt was found to regulate purine nucleotide biosynthesis (178). Entry into S phase requires an abundant supply of purine nucleotides. PI3K/Akt can regulate the early steps of de novo synthesis by modulating phosphoribosylpyrophosphate production by the nonoxidative pentose phosphate pathway and the late steps by modulating activity of the bifunctional enzyme aminoimidazole-carboxamide ribonucleotide transformylase IMP cyclohydrolase (178). These studies defined a new mechanism in which the PI3K/Akt cassette functions as a master regulator of cellular metabolism.
PI3K KNOCKOUT PHENOTYPES
Mice lacking p85α are viable but are hypoglycemic and exhibit increased insulin sensitivity (179, 180). This phenotype is due to the upregulation of the other Pik3r1 splice variants, p50α and p55α, in fat and muscle (179, 180). Mice lacking the Pik3r1 gene, which abolishes all splice variants (p85α, p50α and p55α), exhibit perinatal lethality, and loss of this deletion causes a significant reduction in the expression and activity of class 1A PI3K catalytic subunits (181). Mice lacking p50α/p55α or p85β exhibit increased insulin signaling (182). Also, mice lacking p85β are viable and show hypoinsulinemia, hypoglycemia, and improved insulin sensitivity (183, 184). These studies suggest both positive and negative roles of the p85 regulatory subunits in insulin signaling. The p85 proteins are essential for p110 stabilization but conversely, free p85s exert negative effects on PI3K signaling (185). Under physiological conditions, p85 is more abundant than the catalytic p110 subunit, producing a competition between p85 monomers and p85-p110 dimers. PI-3,4,5-P3 production is inhibited by monomeric p85, either by binding to phosphorylated IRS proteins (185) or by altering subcellular localization of p110/p85 dimers (186). These findings suggest that p85 family members have a complex role in regulating PI3K-mediated insulin signaling, which probably involves p110-independent activities.
The p85 regulatory subunit of PI3K downregulates IRS-1 signaling via the formation of a sequestration complex (187). In response to IGF-1, but not to PDGF signaling, p85α localizes to discrete foci in the Chinese hamster ovary (CHO)-K1 cells, which contain the IRS-1 adaptor molecule, and the foci formation requires the binding of p85 to IRS-1 (187). Compared with the p85-p110 dimer, monomeric p85 is preferentially localized to these foci, which are not sites of PI-3,4,5-P3 production (187). Ultrastructural studies reveal that p85-IRS-1 foci are cytosolic protein complexes devoid of a membrane (187). These studies suggest a mechanism of signal downregulation of IRS-1 that is mediated by monomeric p85 through the formation of a sequestration complex between p85 and IRS-1 (187). Modulation of PI3K-Akt signaling components in the membrane raft microdomains have been reported (188–190). Consistent with these reports, we have also demonstrated the localization of the IR and PI3K in detergent-resistant membrane rafts of rod photoreceptor outer segments (191). Currently, we do not know the specific signaling events mediated by IR and PI3K in rafts. Furthermore, heterozygous disruption of p85 improves insulin signaling and glucose homeostasis in normal mice and mice made insulin-resistant by heterozygous deletion of the IR and/or IRS-1 genes (192). We (68) and others (52) have shown that IR activation does not induce the activation of IRS-1 but does induce activation of IRS-2. It is possible that retinal monomeric p85 subunits may sequester IRS-1 in the retina. Further studies are required to verify this hypothesis. However, the existence of free p85 monomeric subunits was recently challenged by Geering et al. (36), and their study found no free p85 or p110 PI3K subunits in murine WEHI-231 B lymphocyte cell line (36).
Deletion of p85 subunits (α and β) from the heart attenuated Akt signaling, reduced heart size, and altered cardiac gene expression (193). The p85 regulatory subunit of PI3K, a key mediator of insulin's metabolic actions, is also required for the activation of c-Jun N-terminal kinase (JNK) in states of insulin resistance including high-fat-diet-induced obesity and JNK1 overexpression (194). Skeletal muscle lacking both the p85α/p55α/p50α and the p85β regulatory subunits of PI3K had reduced muscle weight and fiber size (144). We previously examined the retinal phenotype in p85α subunit knockout mice (195) and these mice retain the ability to express p50α and p55α subunits (179). These mice do not exhibit any gross phenotype changes in the retinas, although a decreased IR-associated PI3K activity is found in p85α subunit knockout mouse strains (195). The absence of lethal phenotype could be due to the complementation of p50α and p55α for the p85α disruption. Disruption of the mouse gene encoding PI3K adaptor subunit p85α and its splice variants p50α and p55α resulted in a lethal phenotype of impaired B cell development and proliferation (181). Tissue-specific deletion of p85 would give a precise functional role of PI3K in retinal functions. We recently observed that cone photoreceptor-specific deletion of p85α subunit of PI3K resulted in age-related cone photoreceptor degeneration (unpublished observations).
Mice lacking either p110α or p110β are embryonic lethal (196); however, heterozygous mice lacking PI3Kα or β show normal responses to insulin and p110α/p110β compound heterozygotes display decreased insulin sensitivity (196). These results suggest that both proteins may be involved in insulin signaling. Young heterozygous mice expressing a catalytically inactive PI3Kα are normoglycemic; however, mice older than 6 months display glucose intolerance, hyperlipidemia, adiposity, as well as hyperglycemia, and deregulate hepatic gluconeogenesis, thus, assigning a fundamental role of PI3Kα in insulin-dependent signaling (197). PI3Kα is selectively recruited and activated by IRS proteins (197). PI3K inhibitors with significant isoform selectivity show that PI3Kα is the major PI3K effector downstream of the IR and that PI3Kβ maintains a marginal role (198). Inhibitor studies indicate that PI3Kβ influences the PI3Kα function, providing a basal threshold of PI-3,4,5-P3 production that potentiates PI3Kα activity, thus, suggesting that PI3Kβ function may still be necessary for achieving full-scale signaling response. Further studies with mice expressing catalytically inactive mutants of PI3Kβ may help to address this issue. There are no direct studies available on the disruption of the p110 subunit of PI3K in retinal cells. However, our previous work demonstrated that PI3K activity in photoreceptors is regulated through the IR (148). Rod-specific IR knockout mice have reduced PI3K and Akt association with the membranes, suggesting that the IR regulates the downstream PI3K and Akt survival signal in rod photoreceptors. Reduced IR expression in rod photoreceptors significantly impairs the retinal function and loss of photoreceptors in mice exposed to bright light stress (148).
PHOSPHOINOSITIDES IN CELLULAR REGULATION
The lipid products of PI3K serve as second messengers to recruit specific phospholipid-binding proteins to the plasma membrane and control the activity and subcellular localization of a diverse array of signal transduction molecules (199). The phospholipid-binding proteins bind to phosphoinositides through their PH domain. The PH domain, which was initially identified in the platelet PKC substrate pleckstrin, is a 120 amino acid colinear region identified by sequence comparison in more than 100 proteins (16). PH domains are present in cytoskeletal components (spectrin, α actinin), guanine nucleotide exchange proteins, and GTPase-regulating proteins (RAS-GRF, Dbl, VAV, cdc25, SOS, RAS-GAP, Tiam-1, ARNO, GRP1/cytohesin-1), GTPases (dynamin), PI-regulated protein kinases (Akt/protein kinase B, PDK1), other protein kinases (BTK, β ARK), and PLC. All of these proteins bind to phosphoinositides with different affinities and specificities (16). These PH domains do not always bind phosphoinositides; sometimes, PH domains serve as protein-protein interaction domains (200, 201).
Several PH domain-containing proteins, which can be directly or indirectly regulated by phosphoinositides, have been identified in the retina. The best examples are Akt isoforms (52, 135), IRS-1, IRS-2 (52, 68, 69), Grb14 (157), Evictin-1 (202), PHR1 ( p leckstrin h omology domain r etinal protein) (203, 204), and recently identified Akt dephosphorylating enzymes PHLPP (PH domain and leucine rich repeat protein phosphatases) and PHLPPL (PH domain and leucine rich repeat protein phosphatase-like) (205). Deletion of two PI-binding proteins, Akt2 (135) and IRS-2 (69), in the retina results in photoreceptor degeneration.
PHOSPHOINOSITIDE METABOLISM IN THE RETINA
We and others have established that PI metabolism is active in the vertebrate retina and ROS (13–15, 206–228). In ROS, light stimulates the enzymatic activities of PLC (219, 220, 224–226, 229), PKC (230, 231), PI synthetase (12), DG kinase (13), and PI3K (14, 49, 50). ROS PKC can phosphorylate a number of ROS proteins including rhodopsin (232–237) and phosphodiesterase (238, 239). The lipid second messengers generated from phosphatidylcholine, phosphatidic acid and diacylglycerol, are modulated by the different illumination states of the retina. Further, the light-dependent translocation of phototransduction proteins influences the enzymatic activities of phospholipase D, lipid phosphate phosphatase, diacylglyceride lipase, and diacylglyceride kinase, all of which are responsible for the generation of the second messenger molecules. Detailed studies are described by Giusto and her coworkers (to come in this series) in the thematic review on lipid second messengers and related enzymes in ROS.
PHOSPHOINOSITIDES IN RETINA STRUCTURE AND FUNCTION
Emerging evidence from various laboratories shows the importance of phosphoinositides in photoreceptor functions. PI-4,5-P2 can activate phosphodiesterase, which results in the inhibition of current flow through cyclic nucleotide-gated channels in ROSs (240). Modulation of phototransduction gain by changes in PI levels has been reported (241). PI3K-generated phosphoinositides are involved in the membrane targeting of rhodopsin as well as disc morphogenesis in mammalian photoreceptors (242). In the retina, deletion of the PI-binding proteins Akt2 (135) and IRS-2 (69) results in photoreceptor degeneration. Drosophila arrestin has a PI-binding domain that binds PI-3,4,5-P3 and controls the movement of arrestin (243). Two other proteins with PH domains play potentially important roles in the retina. Evictin-1 can localize to rhodopsin-laden, postGolgi membranes in photoreceptor cells, suggesting that the protein may be involved in postGolgi trafficking of membranes (202). PHR1 is present in outer segments and binds to transducin βγ subunits but does not bind to inositol phosphates; however, PI lipids were not tested (204). The PH domain of PHR1 is most similar to that of Akt, which we found in the ROS (135). We demonstrated that PH domain of Ak1 can bind to PI3K-generated phosphoinositides in ROS (61). Myosin II binds to the PH domain of Akt (244), which raises interesting questions, because myosin VIIA gene is mutated in Usher syndrome 1B patients (245). Usher syndrome is the most common condition that affects both hearing and vision. The major symptoms of Usher syndrome are hearing loss and an eye disorder called retinitis pigmentosa. Retinitis pigmentosa causes night-blindness and a loss of peripheral vision (side vision) through the progressive degeneration of the retina. However, the myosin-Akt interaction has not been demonstrated in the visual system. All of these studies clearly underline the importance of phosphoinositides in photoreceptor structure and function.
NEUROPROTECTIVE ROLE OF PI3K IN THE VERTEBRATE RETINA
IR-activated PI3K activity can protect transformed retinal neurons in culture and can inhibit caspase-mediated retinal cell death (246). The neuroprotective activity of 17-β estradiol in the retina is exhibited through PI3K activation (247). Deletion of PTEN, a negative regulator of mTOR pathway in adult retinal ganglion cells (RGCs), promotes robust axon regeneration after optic nerve injury (248). The PDGF-induced Akt activation has shown to protect from the death of retinal pericytes by advanced glycation end-products (249). This activation perhaps could be mediated through PI3K pathway in response to PDGF (249). The PI3K-Akt signaling cascade plays a major role in mouse 661W cone photoreceptors and this pathway can be compromised in response to the activation of pro-apoptotic BCL-2 family members (250). PI3K-generated PI-3,4,5-P3 accumulation is essential for apical membrane morphogenesis in Drosophila photoreceptor epithelial cells (251). During photoreceptor apoptosis in the retinal degeneration mouse model, PTEN, which counteracts the effects of PI3K by decreasing levels of 3′-phosphorylated phosphoinositides, is activated and the downstream effectors of PI3K, Akt, and forkhead in rhabdomyosarcoma are inactivated (252). Interestingly, inactivation of PTEN in RPE cells results in retinal degeneration (253). This phenotype is due to the loss of interaction of signaling proteins to the PDZ-binding domain of PTEN but not due to the enhanced Akt activation (253). These studies suggest that PTEN is essential for normal RPE cell function (253).
Impairment of RPE structural integrity is known to cause age-related macular degeneration (AMD). AMD is a leading cause of vision loss in Americans 60 years of age or older. Activation of PI3K-Akt pathway in RPE protects the RPE cells against the deleterious effects of oxidative stress. One of the major causes of AMD is oxidative stress, which is generated both endogenously during the course of phototransduction and also by a variety of exogenous insults (254–256). AMD-inducing oxidative stresses impact the PI3K-Akt neuron survival pathway (257–260). It has been proposed that this pathway protects RPE cells against the deleterious effects of oxidative stress (261–263).
The goldfish retina has been used extensively for the study of nerve regeneration and the PI3K-mediated process is critical for nerve regrowth (264). PI3K has a central role in mediating the survival of differentiated neurons in vivo and also plays an important role in retinal development (265). BDNF has a potential neuroprotective effect on axotomized RGCs and this protective effect is mediated through PI3K and MAPK signaling pathways (65). Furthermore, PI3K/Akt signaling pathway is activated intrinsically and has a neuroprotective effect on injured RGCs (266). bFGF may mediate activation of Muller glial cells in the ischemic-hypoxic retina and some of the protective effects of bFGF are mediated through PI3K pathway (64). CNTF can also protect the retinal cells in vitro and in vivo via PI3K activation (67). The cytokine EPO promotes regeneration of adult central nervous system neurons via Jak2/Stat3 and PI3K/Akt pathway (66).
Intraocular pressure elevation has often been used as an experimental model to study mechanisms underlying RGC death associated with ocular ischemic injury and glaucoma. PI3K/Akt pathway has differential roles in RGC survival in rats with or without acute ocular hypertension (267). Furthermore, PI3K/Akt pathway inhibition-dependent activation of macrophages is detrimental to RGCs (268). The PI3K pathway can protect the 4-hydroxy-2-nonenal-induced oxidative injury in RPE (269). Heme oxygenase 1 (HO-1) is a representative mediator of antioxidants and cytoprotectants against various stress stimuli including oxidants in vascular cells (270). Insulin treatment can prevent H2O2-induced NF-kappaB and caspase-8 activation and apoptosis via IRS1/PI3K/Akt2/heme oxygenase 1 pathway in retinal pericytes, suggesting a potential explanation for how insulin is retarding the progression of microvascular complications induced by diabetes (270). PI3K-atypical protein kinase C signaling is also required for Wnt attraction and anterior-posterior axon guidance (271). PI3K-Akt mediated atypical protein kinase C signaling is also essential for apical membrane morphogenesis in Drosophila photoreceptor epithelial cells (251). PI3K/Akt signaling pathway serves as the circadian output in the retina (272). PDGF and insulin/IGF-1 can exhibit specific, distinct modes of class IA PI3K activation in normal rat retinas and RGC-5 retinal ganglion cells (62). These studies identify two distinct modes of retinal class IA PI3K activation that occur in response to PDGF receptor and insulin/IGF-1 receptor stimulation (62). Biswas et al. (62) propose the hypothesis that PDGF-induced PI3K/Akt pathway may provide novel therapeutic targets to ameliorate cell death in diabetic retinopathy and other retinal neurodegenerations. We recently reported that light stress activates tyrosine phosphorylation of ROS proteins, including IGF-1R, which promotes the binding of PI3K to ROS membranes (48). The activation of the IGF1-R/PI3K/Akt survival pathway may serve a protective role in the retina. In stress, insulin and p85 activates JNK via cdc42 and mitogen-activated protein kinase kinase 4; this cdc42/JNK pathway requires both an intact N-terminus and functional SH2 domains within the C terminus of the p85α regulatory subunit (194). These studies suggest that p85α plays dual roles in regulating insulin sensitivity and in mediating cross-talk between the PI3K and stress kinase pathways. No data on the cross-talk between PI3K and JNK stress kinase pathways in retina are available.
The IR and IGF-1R are structurally similar. Both are found at the cell surface as α2β2 heterotetramers with transmembrane ligand-binding α-subunits and intracellular, tyrosine kinase-containing β-subunits. Furthermore, once activated, both receptors can phosphorylate and/or interact with the same intracellular protein substrates, including members of the IRS family and Src homologous and collagen domain protein. IR and IGF-1R also activate many of the same downstream signaling molecules such as PI3K and MAPK (273, 274). Insulin and IGF-1 in retinal endothelial cells respond differentially to oxygen (275). Insulin and IGF-1 signaling in endothelium plays a role in retinal neovascularization through the expression of vascular mediators, with the effect of insulin being more important in this process than IGF-1, as demonstrated with the advent of endothelial cell-specific insulin and IGF-1R knockout mice (275). Both IR (148) and IGF-1R (48) in photoreceptors activate neuroprotective PI3K and Akt. These studies clearly suggest redundant and nonredundant tissue-specific roles of IR and IGF-1R. Further studies are required to understand the specific receptor-activated PI3K in retina and photoreceptor functions.
PHOSPHOINOSITIDES IN CYTOSKELETAL ORGANIZATION
Phosphoinositides are the key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane (147). Our studies suggest that light-dependent-generated phosphoinositides regulate the actin cytoskeleton (61). We also observed lower levels of ROS-associated actin (presumably F-actin) in IR knockout mouse retinas than in wild-type mouse retinas (unpublished observations). These observations, along with our current findings, suggest that light-induced activation of IR/PI3K may regulate the actin cytoskeletal organization. Dysfunction of the actin cytoskeleton is a key event in the pathogenesis of diabetic nephropathy (149), diabetic neuropathy (150, 151), and diabetic cardiomyopathy (152, 153). The light-induced activation of IR/PI3K-generated phosphoinositides in the retina may play an important role in the control of diabetic retinopathy. PI3K and Akt activations are downregulated in diabetic retinopathy, supporting this hypothesis (132).
PHOSPHOINOSITIDE SIGNALING IN CONE PHOTORECEPTORS
Cone photoreceptors make up a very small percent (3–5%) of total retinal photoreceptors. In humans, cones are essential for an optimal acute, bright, and colored visual perception. Therefore, the quality of our daily lives relies highly on how healthy and functional our cones are. Specific mechanisms of cone cell death are very different depending on genetic predispositions as well as environmental factors. Many questions regarding these mechanisms are yet to be answered. A canonical Akt survival pathway has been shown to be constitutively active in cone photoreceptors (276). However, rod photoreceptors express the active form of Akt only transiently during the exposure to conditions such as physiological light (61), oxidative (269), hyperosmotic (277), or light stress (135). In mouse models of retinitis pigmentosa having genetic mutations in rods, the active form of Akt stays expressed until the photoreceptors die (109). A possible interpretation is that Akt survival pathway desperately attempts to rescue rods from a genetically predisposed death phenotype. Furthermore, recent findings from the model of retinitis pigmentosa showed that as rods die, cones are starved, primarily due to the downregulation of insulin/Akt/mTOR signaling pathway (109). mTOR plays a crucial role in a nutrient-sensitive signaling pathway that regulates cell growth, proliferation, and metabolic maintenance. Stimulation of the insulin/mTOR signaling pathway delays cone death (109). The classical link between the extracellular signals such as insulin/IR and intracellular survival pathways like Akt/mTOR is PI3K. In diabetic retinopathy, the leading cause of blindness in developed countries, cones rather than rods selectively die, primarily due to downregulation or inactivation of IR/PI3K/Akt survival pathway (132). Evidence showed that PI metabolism at the synapse is important in synaptic ribbon formation and glutamate release, specifically, in cone photoreceptors (278). This PI metabolism is Ca+2-dependent. Furthermore, in dark, when intracellular Ca+2 levels are high, PI metabolism increases at the synaptic termini compared with outer segments (278). Cones then release glutamate at the synapse, which in turn halts PI metabolism in adjacent horizontal cells. The opposite events take place in light, where the highest PI metabolism is in outer segments, perhaps to aid phototransduction processes (278). A drop in intracellular Ca+2 levels and a subsequent decrease in PI metabolism at the synapse causes cones to stop releasing glutamate, which in turn liberates PI metabolism in horizontal cells (278). At present, the biological significance of this cone phenomenon is not well studied. A study has suggested that in rod photoreceptors, proper synaptic ribbon formation and rod synapse pairing with the bipolar and horizontal cells into triads might be important in inner retinal phototransduction signal propagation (278).
Synaptic ribbons are specialized cytoskeletal components of the presynaptic exocytotic machinery in photoreceptors. In cone photoreceptors, these structures are highly dynamic, disappearing during darkness and reforming in the light phase. Synaptic ribbons in cone photoreceptors are very sensitive to both Li+ and IP3, suggesting that inositol polyphosphates may play a physiological role in the disassembly of synaptic ribbons (278). The PI pathway can exist in isolated photoreceptor synapses, and PI metabolism in photoreceptor synapses is activated in the dark and correlated with the disappearance of synaptic ribbons during dark adaptation (278). Furthermore, Li+ and IP3 can influence synaptic ribbons in cones but not in rods; thus, the dark-activated PI metabolism is largely ascribed to cone synapses (278). We recently observed that cone photoreceptor-specific deletion of p85 subunit of PI3K resulted in age-related cone photoreceptor degeneration (unpublished observations). Whether the observed degeneration could be due to lack of PI3K generated phosphoinositides in the regulation of synaptic ribbons needs to be studied.
POSSIBLE PHYSIOLOGICAL ROLES OF THE PI CYCLE IN PHOTORECEPTOR CELLS
Rod and cone photoreceptor cells are involved daily in several activities other than visual transduction. These include the rhythmic shedding of outer segments (279), the synthesis and transport of large amounts of membrane material destined for the outer segments (280), the movement of proteins between the inner and the outer segments in response to light/dark stimuli (281, 282), and the maintenance of a circadian clock (283). In addition, we showed that the photoreceptor cell is highly plastic and is capable of responding to stress by upregulating the synthesis of proteins that provide protection from damaging effects of constant illumination (284, 285). Each of these events requires the transfer of a signal from the outer segment to other regions of the photoreceptor cell. A mutation in several enzymes of the PI cycle can cause a retinal degeneration in invertebrates (286–288). In humans, Lowe's oculocerebrorenal syndrome is caused by a mutation in inositol polyphosphate-5-phosphatase (289, 290). Mutations in the PYK2-binding domain of phosphatidylinositol transfer membrane-associated protein (PIPNM3) caused autosomal dominant cone dystrophy in two Swedish families (291). PIPNM3, known as a human homolog of the Drosophila rdgB, lacks the N-terminal PIT domain needed for the transport of phospholipids, the renewal of photoreceptor membranes, and the electroretinogram response to light (291). A disruption in the orderly flow of metabolic events in the retina can lead to a retinal degeneration, as has been demonstrated repeatedly.
CONCLUSIONS AND FUTURE DIRECTIONS
Studies from various laboratories, including ours, suggest that the PI3K pathway is neuroprotective. Our laboratory was the first to demonstrate the light-dependent regulation of PI3K pathway in rod photoreceptors. PI3K activation has been shown to be downregulated in diabetic retinopathy. Cell type specific activation of PI3K by various growth factors has also been reported in the retina, and the activated PI3K is shown to be neuroprotective. Neurodegeneration is an important component of diabetic retinopathy as demonstrated by an increased apoptosis in the neural retina during experimental and human diabetes. PI3K-Akt survival signals have been shown to be downregulated in diabetic retinopathy. Understanding the regulation of this pathway and identifying new regulator(s) would help to design therapeutic strategies to protect the dying retinal cells. One of the limitations in retina research is the involvement of different PI3K isoforms and their precise functional role in retinal structure and function. Currently, most of the studies are done with the use of chemical inhibitors, which tend to inhibit all isoforms, leading to difficulty in interpreting the isoform involvement. Availability of cell-specific conditional knockout mouse lines would address the individual role of specific isoforms in retina. Research on targeted therapies to activate the PI3K pathway in retina would be a promising avenue to pursue to protect the dying retinal cells in neurodegeneration.
Acknowledgments
The author thanks Dr. Robert E. Anderson and Mr. Steve Brush for reading and commenting on the manuscript.
Footnotes
Abbreviations:
- AMD
- age-related macular degeneration
- BDNF
- brain derived neurotrophic factor
- bFGF
- basic fibroblast growth factor
- CNTF
- ciliary neurotrophic factor
- DG
- diacylglycerol
- DHA
- docosahexaenoic acid
- 4E-BP
- eukaryotic translation initiation factor 4E binding protein
- eIF
- eukaryotic initiation factor
- EPO
- erythropoietin
- GPCR
- G-protein coupled receptor
- Grb14
- growth factor receptor-bound protein 14
- GSK3
- glycogen synthase kinase 3
- IGF-1
- insulin like growth factor 1
- IP3
- inositol 1,4,5-trisphosphate
- IR
- insulin receptor
- IRS
- insulin receptor substrate
- JNK
- c-Jun N-terminal kinase
- MAPK
- mitogen-activated protein kinase
- mTOR
- mammalian target of rapamycin
- PDGF
- platelet-derived growth factor
- PDK1
- phosphoinositide-dependent protein kinase-1
- PH
- pleckstrin homology
- PHR1
- p leckstrin h omology domain r etinal protein
- PI
- phosphatidylinositol
- PI-3,4 P2
- phosphatidylinositol 3,4-bisphosphate
- PI-3,4,5-P3
- phosphatidylinositol 3,4,5-trisphosphate
- PI3K
- phosphoinositide 3-kinase
- PI-3-P
- phosphatidylinositol 3-phosphate
- PI-4,5-P2
- phosphatidylinositol 4,5-bisphosphate
- PKC
- protein kinase C
- PLC
- phospholipase C
- p70S6K
- ribosomal protein S6 kinase
- PTEN
- phosphatase and tensin homolog
- PTP
- protein tyrosine phosphatase
- RGC
- retinal ganglion cell
- ROS
- rod outer segment
- RPE
- retinal pigmented epithelium
- SH
- Src homology
- VSP34
- vacuole sorting protein 34
- 4E-BP
- eukaryotic initiation factor 4E binding protein
This work was supported by grants from the National Institutes of Health (EY016507; EY00871), NEI Core grants (EY12190), NCRR COBRE (P20-RR17703), and Research to Prevent Blindness, Inc. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hokin M. R., Hokin L. E. 1953. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J. Biol. Chem. 203: 967–977. [PubMed] [Google Scholar]
- 2.Hokin L. E., Hokin M. R. 1955. Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim. Biophys. Acta. 18: 102–110. [DOI] [PubMed] [Google Scholar]
- 3.Streb H., Irvine R. F., Berridge M. J., Schulz I. 1983. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 306: 67–69. [DOI] [PubMed] [Google Scholar]
- 4.Nishizuka Y. 1986. Studies and perspectives of protein kinase C. Science. 233: 305–312. [DOI] [PubMed] [Google Scholar]
- 5.Rhee S. G. 1991. Inositol phospholipids-specific phospholipase C: interaction of the gamma 1 isoform with tyrosine kinase. Trends Biochem. Sci. 16: 297–301. [DOI] [PubMed] [Google Scholar]
- 6.Rhee S. G., Choi K. D. 1992. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem. 267: 12393–12396. [PubMed] [Google Scholar]
- 7.Berridge M. J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361: 315–325. [DOI] [PubMed] [Google Scholar]
- 8.Auger K. R., Carpenter C. L., Cantley L. C., Varticovski L. 1989. Phosphatidylinositol 3-kinase and its novel product, phosphatidylinositol 3-phosphate, are present in Saccharomyces cerevisiae. J. Biol. Chem. 264: 20181–20184. [PubMed] [Google Scholar]
- 9.Vanhaesebroeck B., Waterfield M. D. 1999. Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res. 253: 239–254. [DOI] [PubMed] [Google Scholar]
- 10.Datta S. R., Brunet A., Greenberg M. E. 1999. Cellular survival: a play in three Akts. Genes Dev. 13: 2905–2927. [DOI] [PubMed] [Google Scholar]
- 11.Rameh L. E., Cantley L. C. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274: 8347–8350. [DOI] [PubMed] [Google Scholar]
- 12.Ghalayini A. J., Anderson R. E. 1995. Light adaptation of bovine retinas in situ stimulates phosphatidylinositol synthesis in rod outer segments in vitro. Curr. Eye Res. 14: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z., Ghalayini A., Guo X. X., Alvarez K. M., Anderson R. E. 2000. Light-mediated activation of diacylglycerol kinase in rat and bovine rod outer segments. J. Neurochem. 75: 355–362. [DOI] [PubMed] [Google Scholar]
- 14.Guo X., Ghalayini A. J., Chen H., Anderson R. E. 1997. Phosphatidylinositol 3-kinase in bovine photoreceptor rod outer segments. Invest. Ophthalmol. Vis. Sci. 38: 1873–1882. [PubMed] [Google Scholar]
- 15.Guo X. X., Huang Z., Bell M. W., Chen H., Anderson R. E. 2000. Tyrosine phosphorylation is involved in phosphatidylinositol 3-kinase activation in bovine rod outer segments. Mol. Vis. 6: 216–221. [PubMed] [Google Scholar]
- 16.Martin T. F. 1998. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu. Rev. Cell Dev. Biol. 14: 231–264. [DOI] [PubMed] [Google Scholar]
- 17.Grado C., Ballou C. E. 1960. Myo-inositol phosphates from beef brain phosphoinostide. J. Biol. Chem. 235: C23–C24. [PubMed] [Google Scholar]
- 18.Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. 1988. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 332: 644–646. [DOI] [PubMed] [Google Scholar]
- 19.Vanhaesebroeck B., Leevers S. J., Panayotou G., Waterfield M. D. 1997. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 22: 267–272. [DOI] [PubMed] [Google Scholar]
- 20.Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11: 213–221. [DOI] [PubMed] [Google Scholar]
- 21.Goberdhan D. C., Paricio N., Goodman E. C., Mlodzik M., Wilson C. 1999. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 13: 3244–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdu J., Buratovich M. A., Wilder E. L., Birnbaum M. J. 1999. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1: 500–506. [DOI] [PubMed] [Google Scholar]
- 23.Lin K., Dorman J. B., Rodan A., Kenyon C. 1997. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 278: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 24.Ogg S., Ruvkun G. 1998. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol. Cell. 2: 887–893. [DOI] [PubMed] [Google Scholar]
- 25.Kuruvilla F. G., Schreiber S. L. 1999. The PIK-related kinases intercept conventional signaling pathways. Chem. Biol. 6: R129–R136. [DOI] [PubMed] [Google Scholar]
- 26.Engelman J. A., Luo J., Cantley L. C. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7: 606–619. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins P. T., Anderson K. E., Davidson K., Stephens L. R. 2006. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 34: 647–662. [DOI] [PubMed] [Google Scholar]
- 28.Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. 1994. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 77: 83–93. [DOI] [PubMed] [Google Scholar]
- 29.Fruman D. A., Meyers R. E., Cantley L. C. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67: 481–507. [DOI] [PubMed] [Google Scholar]
- 30.Maffucci T., Brancaccio A., Piccolo E., Stein R. C., Falasca M. 2003. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J. 22: 4178–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindmo K., Stenmark H. 2006. Regulation of membrane traffic by phosphoinositide 3-kinases. J. Cell Sci. 119: 605–614. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. 1990. Purification and characterization of phosphoinositide 3-kinase from rat liver. J. Biol. Chem. 265: 19704–19711. [PubMed] [Google Scholar]
- 33.Zhao L., Vogt P. K. 2008. Class I PI3K in oncogenic cellular transformation. Oncogene. 27: 5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch E., Lembo G., Montrucchio G., Rommel C., Costa C., Barberis L. 2006. Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb. Haemost. 95: 29–35. [PubMed] [Google Scholar]
- 35.Hirsch E., Costa C., Ciraolo E. 2007. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J. Endocrinol. 194: 243–256. [DOI] [PubMed] [Google Scholar]
- 36.Geering B., Cutillas P. R., Nock G., Gharbi S. I., Vanhaesebroeck B. 2007. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc. Natl. Acad. Sci. USA. 104: 7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonetti D. A., Algenstaedt P., Kahn C. R. 1996. Insulin receptor substrate 1 binds two novel splice variants of the regulatory subunit of phosphatidylinositol 3-kinase in muscle and brain. Mol. Cell. Biol. 16: 2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inukai K., Anai M., Van Breda E., Hosaka T., Katagiri H., Funaki M., Fukushima Y., Ogihara T., Yazaki Kikuchi Y., Oka Y., Asano T. 1996. A novel 55-kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to p55PIK Is generated by alternative splicing of the p85alpha gene. J. Biol. Chem. 271: 5317–5320. [DOI] [PubMed] [Google Scholar]
- 39.Pons S., Asano T., Glasheen E., Miralpeix M., Zhang Y., Fisher T. L., Myers M. G., Jr., Sun X. J., White M. F. 1995. The structure and function of p55PIK reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol. Cell. Biol. 15: 4453–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. 1992. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 70: 419–429. [DOI] [PubMed] [Google Scholar]
- 41.Murga C., Fukuhara S., Gutkind J. S. 2000. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 275: 12069–12073. [DOI] [PubMed] [Google Scholar]
- 42.Stephens L. R., Eguinoa A., Erdjument-Bromage H., Lui M., Cooke F., Coadwell J., Smrcka A. S., Thelen M., Cadwallader K., Tempst P., et al. 1997. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 89: 105–114. [DOI] [PubMed] [Google Scholar]
- 43.Suire S., Coadwell J., Ferguson G. J., Davidson K., Hawkins P., Stephens L. 2005. p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr. Biol. 15: 566–570. [DOI] [PubMed] [Google Scholar]
- 44.Voigt P., Brock C., Nurnberg B., Schaefer M. 2005. Assigning functional domains within the p101 regulatory subunit of phosphoinositide 3-kinase gamma. J. Biol. Chem. 280: 5121–5127. [DOI] [PubMed] [Google Scholar]
- 45.Clement S., Krause U., Desmedt F., Tanti J. F., Behrends J., Pesesse X., Sasaki T., Penninger J., Doherty M., Malaisse W., et al. 2001. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 409: 92–97. [DOI] [PubMed] [Google Scholar]
- 46.Maehama T., Dixon J. E. 1999. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 9: 125–128. [DOI] [PubMed] [Google Scholar]
- 47.Maehama T., Dixon J. E. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273: 13375–13378. [DOI] [PubMed] [Google Scholar]
- 48.Dilly A. K., Rajala R. V. 2008. Insulin growth factor 1 receptor/PI3K/AKT survival pathway in outer segment membranes of rod photoreceptors. Invest. Ophthalmol. Vis. Sci. 49: 4765–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajala A., Anderson R. E., Ma J. X., Lem J., Al Ubaidi M. R., Rajala R. V. 2007. G-protein-coupled receptor rhodopsin regulates the phosphorylation of retinal insulin receptor. J. Biol. Chem. 282: 9865–9873. [DOI] [PubMed] [Google Scholar]
- 50.Rajala R. V., McClellan M. E., Ash J. D., Anderson R. E. 2002. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J. Biol. Chem. 277: 43319–43326. [DOI] [PubMed] [Google Scholar]
- 51.Rajala R. V., Anderson R. E. 2001. Interaction of the insulin receptor beta-subunit with phosphatidylinositol 3-kinase in bovine ROS. Invest. Ophthalmol. Vis. Sci. 42: 3110–3117. [PubMed] [Google Scholar]
- 52.Reiter C. E., Sandirasegarane L., Wolpert E. B., Klinger M., Simpson I. A., Barber A. J., Antonetti D. A., Kester M., Gardner T. W. 2003. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am. J. Physiol. Endocrinol. Metab. 285: E763–E774. [DOI] [PubMed] [Google Scholar]
- 53.Parish C. A., Smrcka A. V., Rando R. R. 1995. Functional significance of beta gamma-subunit carboxymethylation for the activation of phospholipase C and phosphoinositide 3-kinase. Biochemistry. 34: 7722–7727. [DOI] [PubMed] [Google Scholar]
- 54.Chuang J. Z., Zhao Y., Sung C. H. 2007. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 130: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu P., Mondino A., Skolnik E. Y., Schlessinger J. 1993. Cloning of a novel, ubiquitously expressed human phosphatidylinositol 3-kinase and identification of its binding site on p85. Mol. Cell. Biol. 13: 7677–7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klippel A., Escobedo J. A., Fantl W. J., Williams L. T. 1992. The C-terminal SH2 domain of p85 accounts for the high affinity and specificity of the binding of phosphatidylinositol 3-kinase to phosphorylated platelet-derived growth factor beta receptor. Mol. Cell. Biol. 12: 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White M. F., Kahn C. R. 1994. The insulin signaling system. J. Biol. Chem. 269: 1–4. [PubMed] [Google Scholar]
- 58.DeFronzo R. A., Bonadonna R. C., Ferrannini E. 1992. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 15: 318–368. [DOI] [PubMed] [Google Scholar]
- 59.Van Horn D. J., Myers M. G., Jr., Backer J. M. 1994. Direct activation of the phosphatidylinositol 3′-kinase by the insulin receptor. J. Biol. Chem. 269: 29–32. [PubMed] [Google Scholar]
- 60.Backer J. M., Myers M. G., Jr., Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. 1992. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 11: 3469–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li G., Rajala A., Wiechmann A. F., Anderson R. E., Rajala R. V. 2008. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light- dependent generation of phosphoinositides. J. Neurochem. 107: 1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biswas S. K., Zhao Y., Nagalingam A., Gardner T. W., Sandirasegarane L. 2008. PDGF- and insulin/IGF-1-specific distinct modes of class IA PI 3-kinase activation in normal rat retinas and RGC-5 retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 49: 3687–3698. [DOI] [PubMed] [Google Scholar]
- 63.Hollborn M., Bringmann A., Faude F., Wiedemann P., Kohen L. 2006. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem. Biophys. Res. Commun. 344: 912–919. [DOI] [PubMed] [Google Scholar]
- 64.Hollborn M., Jahn K., Limb G. A., Kohen L., Wiedemann P., Bringmann A. 2004. Characterization of the basic fibroblast growth factor-evoked proliferation of the human Muller cell line, MIO-M1. Graefes Arch. Clin. Exp. Ophthalmol. 242: 414–422. [DOI] [PubMed] [Google Scholar]
- 65.Nakazawa T., Tamai M., Mori N. 2002. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest. Ophthalmol. Vis. Sci. 43: 3319–3326. [PubMed] [Google Scholar]
- 66.Kretz A., Happold C. J., Marticke J. K., Isenmann S. 2005. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol. Cell. Neurosci. 29: 569–579. [DOI] [PubMed] [Google Scholar]
- 67.Ikeda K., Tatsuno T., Noguchi H., Nakayama C. 2004. Ciliary neurotrophic factor protects rat retina cells in vitro and in vivo via PI3 kinase. Curr. Eye Res. 29: 349–355. [DOI] [PubMed] [Google Scholar]
- 68.Rajala R. V., McClellan M. E., Chan M. D., Tsiokas L., Anderson R. E. 2004. Interaction of the retinal insulin receptor beta-subunit with the P85 subunit of phosphoinositide 3-kinase. Biochemistry. 43: 5637–5650. [DOI] [PubMed] [Google Scholar]
- 69.Yi X., Schubert M., Peachey N. S., Suzuma K., Burks D. J., Kushner J. A., Suzuma I., Cahill C., Flint C. L., Dow M. A., et al. 2005. Insulin receptor substrate 2 is essential for maturation and survival of photoreceptor cells. J. Neurosci. 25: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andjelkovic M., Alessi D. R., Meier R., Fernandez A., Lamb N. J., Frech M., Cron P., Cohen P., Lucocq J. M., Hemmings B. A. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272: 31515–31524. [DOI] [PubMed] [Google Scholar]
- 71.Meier R., Alessi D. R., Cron P., Andjelkovic M., Hemmings B. A. 1997. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J. Biol. Chem. 272: 30491–30497. [DOI] [PubMed] [Google Scholar]
- 72.Wijkander J., Holst L. S., Rahn T., Resjo S., Castan I., Manganiello V., Belfrage P., Degerman E. 1997. Regulation of protein kinase B in rat adipocytes by insulin, vanadate, and peroxovanadate. Membrane translocation in response to peroxovanadate. J. Biol. Chem. 272: 21520–21526. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X., Vik T. A. 1997. Growth factor stimulation of hematopoietic cells leads to membrane translocation of AKT1 protein kinase. Leuk. Res. 21: 849–856. [DOI] [PubMed] [Google Scholar]
- 74.Sable C. L., Filippa N., Filloux C., Hemmings B. A., Van Obberghen E. 1998. Involvement of the pleckstrin homology domain in the insulin-stimulated activation of protein kinase B. J. Biol. Chem. 273: 29600–29606. [DOI] [PubMed] [Google Scholar]
- 75.Currie R. A., Walker K. S., Gray A., Deak M., Casamayor A., Downes C. P., Cohen P., Alessi D. R., Lucocq J. 1999. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 337: 575–583. [PMC free article] [PubMed] [Google Scholar]
- 76.Manning B. D., Cantley L. C. 2007. AKT/PKB signaling: navigating downstream. Cell. 129: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fayard E., Tintignac L. A., Baudry A., Hemmings B. A. 2005. Protein kinase B/Akt at a glance. J. Cell Sci. 118: 5675–5678. [DOI] [PubMed] [Google Scholar]
- 78.Alessi D. R., Kozlowski M. T., Weng Q. P., Morrice N., Avruch J. 1998. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol. 8: 69–81. [DOI] [PubMed] [Google Scholar]
- 79.Hresko R. C., Mueckler M. 2005. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3–L1 adipocytes. J. Biol. Chem. 280: 40406–40416. [DOI] [PubMed] [Google Scholar]
- 80.Carvalho E., Eliasson B., Wesslau C., Smith U. 2000. Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/Akt in adipocytes from Type II diabetic subjects. Diabetologia. 43: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 81.Bijur G. N., Jope R. S. 2003. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 87: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Testa J. R., Bellacosa A. 1997. Membrane translocation and activation of the Akt kinase in growth factor-stimulated hematopoietic cells. Leuk. Res. 21: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 83.Borgatti P., Martelli A. M., Bellacosa A., Casto R., Massari L., Capitani S., Neri L. M. 2000. Translocation of Akt/PKB to the nucleus of osteoblast-like MC3T3–E1 cells exposed to proliferative growth factors. FEBS Lett. 477: 27–32. [DOI] [PubMed] [Google Scholar]
- 84.New D. C., Wu K., Kwok A. W., Wong Y. H. 2007. G protein-coupled receptor-induced Akt activity in cellular proliferation and apoptosis. FEBS J. 274: 6025–6036. [DOI] [PubMed] [Google Scholar]
- 85.Parcellier A., Tintignac L. A., Zhuravleva E., Hemmings B. A. 2008. PKB and the mitochondria: AKTing on apoptosis. Cell. Signal. 20: 21–30. [DOI] [PubMed] [Google Scholar]
- 86.Franke T. F., Kaplan D. R., Cantley L. C. 1997. PI3K: downstream AKTion blocks apoptosis. Cell. 88: 435–437. [DOI] [PubMed] [Google Scholar]
- 87.Proud C. G. 2006. Regulation of protein synthesis by insulin. Biochem. Soc. Trans. 34: 213–216. [DOI] [PubMed] [Google Scholar]
- 88.Banerjee P., Ahmad M. F., Grove J. R., Kozlosky C., Price D. J., Avruch J. 1990. Molecular structure of a major insulin/mitogen-activated 70-kDa S6 protein kinase. Proc. Natl. Acad. Sci. USA. 87: 8550–8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kozma S. C., Ferrari S., Bassand P., Siegmann M., Totty N., Thomas G. 1990. Cloning of the mitogen-activated S6 kinase from rat liver reveals an enzyme of the second messenger subfamily. Proc. Natl. Acad. Sci. USA. 87: 7365–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grove J. R., Banerjee P., Balasubramanyam A., Coffer P. J., Price D. J., Avruch J., Woodgett J. R. 1991. Cloning and expression of two human p70 S6 kinase polypeptides differing only at their amino termini. Mol. Cell. Biol. 11: 5541–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reinhard C., Thomas G., Kozma S. C. 1992. A single gene encodes two isoforms of the p70 S6 kinase: activation upon mitogenic stimulation. Proc. Natl. Acad. Sci. USA. 89: 4052–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reinhard C., Fernandez A., Lamb N. J., Thomas G. 1994. Nuclear localization of p85s6k: functional requirement for entry into S phase. EMBO J. 13: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pullen N., Dennis P. B., Andjelkovic M., Dufner A., Kozma S. C., Hemmings B. A., Thomas G. 1998. Phosphorylation and activation of p70s6k by PDK1. Science. 279: 707–710. [DOI] [PubMed] [Google Scholar]
- 94.Dennis P. B., Pullen N., Kozma S. C., Thomas G. 1996. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol. Cell. Biol. 16: 6242–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dufner A., Andjelkovic M., Burgering B. M., Hemmings B. A., Thomas G. 1999. Protein kinase B localization and activation differentially affect S6 kinase 1 activity and eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation. Mol. Cell. Biol. 19: 4525–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15: 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 97.Alessi D. R., Downes C. P. 1998. The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta. 1436: 151–164. [DOI] [PubMed] [Google Scholar]
- 98.Aoki M., Batista O., Bellacosa A., Tsichlis P., Vogt P. K. 1998. The akt kinase: molecular determinants of oncogenicity. Proc. Natl. Acad. Sci. USA. 95: 14950–14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akimoto K., Nakaya M., Yamanaka T., Tanaka J., Matsuda S., Weng Q. P., Avruch J., Ohno S. 1998. Atypical protein kinase Clambda binds and regulates p70 S6 kinase. Biochem. J. 335: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romanelli A., Martin K. A., Toker A., Blenis J. 1999. p70 S6 kinase is regulated by protein kinase Czeta and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol. Cell. Biol. 19: 2921–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sigal N. H., Lin C. S., Siekierka J. J. 1991. Inhibition of human T-cell activation by FK 506, rapamycin, and cyclosporine A. Transplant. Proc. 23: 1–5. [PubMed] [Google Scholar]
- 102.Chung J., Kuo C. J., Crabtree G. R., Blenis J. 1992. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 69: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 103.Kuo C. J., Chung J., Fiorentino D. F., Flanagan W. M., Blenis J., Crabtree G. R. 1992. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 358: 70–73. [DOI] [PubMed] [Google Scholar]
- 104.Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. 1992. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 257: 973–977. [DOI] [PubMed] [Google Scholar]
- 105.Brown E. J., Beal P. A., Keith C. T., Chen J., Shin T. B., Schreiber S. L. 1995. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 377: 441–446. [DOI] [PubMed] [Google Scholar]
- 106.Burnett P. E., Barrow R. K., Cohen N. A., Snyder S. H., Sabatini D. M. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E–BP1. Proc. Natl. Acad. Sci. USA. 95: 1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holcik M., Sonenberg N., Korneluk R. G. 2000. Internal ribosome initiation of translation and the control of cell death. Trends Genet. 16: 469–473. [DOI] [PubMed] [Google Scholar]
- 108.Rehen S. K., Neves D. D., Fragel-Madeira L., Britto L. R., Linden R. 1999. Selective sensitivity of early postmitotic retinal cells to apoptosis induced by inhibition of protein synthesis. Eur. J. Neurosci. 11: 4349–4356. [DOI] [PubMed] [Google Scholar]
- 109.Punzo C., Kornacker K., Cepko C. L. 2009. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat. Neurosci. 12: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahmad F., Azevedo J. L., Cortright R., Dohm G. L., Goldstein B. J. 1997. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J. Clin. Invest. 100: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmad F., Considine R. V., Bauer T. L., Ohannesian J. P., Marco C. C., Goldstein B. J. 1997. Improved sensitivity to insulin in obese subjects following weight loss is accompanied by reduced protein-tyrosine phosphatases in adipose tissue. Metabolism. 46: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 112.Ahmad F., Considine R. V., Goldstein B. J. 1995. Increased abundance of the receptor-type protein-tyrosine phosphatase LAR accounts for the elevated insulin receptor dephosphorylating activity in adipose tissue of obese human subjects. J. Clin. Invest. 95: 2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahmad F., Goldstein B. J. 1995. Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metabolism. 44: 1175–1184. [DOI] [PubMed] [Google Scholar]
- 114.Bleyle L. A., Peng Y., Ellis C., Mooney R. A. 1999. Dissociation of PTPase levels from their modulation of insulin receptor signal transduction. Cell. Signal. 11: 719–725. [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto N., Feener E. P., Zhang W. R., Goldstein B. J. 1992. Insulin receptor protein-tyrosine phosphatases. Leukocyte common antigen-related phosphatase rapidly deactivates the insulin receptor kinase by preferential dephosphorylation of the receptor regulatory domain. J. Biol. Chem. 267: 13811–13814. [PubMed] [Google Scholar]
- 116.Kenner K. A., Hill D. E., Olefsky J. M., Kusari J. 1993. Regulation of protein tyrosine phosphatases by insulin and insulin-like growth factor I. J. Biol. Chem. 268: 25455–25462. [PubMed] [Google Scholar]
- 117.Kenner K. A., Anyanwu E., Olefsky J. M., Kusari J. 1996. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J. Biol. Chem. 271: 19810–19816. [DOI] [PubMed] [Google Scholar]
- 118.Kuhne M. R., Pawson T., Lienhard G. E., Feng G. S. 1993. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J. Biol. Chem. 268: 11479–11481. [PubMed] [Google Scholar]
- 119.Maegawa H., Hasegawa M., Sugai S., Obata T., Ugi S., Morino K., Egawa K., Fujita T., Sakamoto T., Nishio Y., et al. 1999. Expression of a dominant negative SHP-2 in transgenic mice induces insulin resistance. J. Biol. Chem. 274: 30236–30243. [DOI] [PubMed] [Google Scholar]
- 120.Myers M. G., Jr., Mendez R., Shi P., Pierce J. H., Rhoads R., White M. F. 1998. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 273: 26908–26914. [DOI] [PubMed] [Google Scholar]
- 121.Seely B. L., Staubs P. A., Reichart D. R., Berhanu P., Milarski K. L., Saltiel A. R., Kusari J., Olefsky J. M. 1996. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 45: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 122.Byon J. C., Kusari A. B., Kusari J. 1998. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol. Cell. Biochem. 182: 101–108. [PubMed] [Google Scholar]
- 123.Goldstein B. J., Ahmad F., Ding W., Li P. M., Zhang W. R. 1998. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol. Cell. Biochem. 182: 91–99. [PubMed] [Google Scholar]
- 124.Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. 1992. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 68: 545–560. [DOI] [PubMed] [Google Scholar]
- 125.Woodford-Thomas T. A., Rhodes J. D., Dixon J. E. 1992. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J. Cell Biol. 117: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simoncic P. D., McGlade C. J., Tremblay M. L. 2006. PTP1B and TC-PTP: novel roles in immune-cell signaling. Can. J. Physiol. Pharmacol. 84: 667–675. [DOI] [PubMed] [Google Scholar]
- 127.Tonks N. K. 2003. PTP1B: from the sidelines to the front lines! FEBS Lett. 546: 140–148. [DOI] [PubMed] [Google Scholar]
- 128.Byon J. C., Kenner K. A., Kusari A. B., Kusari J. 1997. Regulation of growth factor-induced signaling by protein-tyrosine-phosphatases. Proc. Soc. Exp. Biol. Med. 216: 1–20. [DOI] [PubMed] [Google Scholar]
- 129.Ahmad F., Li P. M., Meyerovitch J., Goldstein B. J. 1995. Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B in negative regulation of the insulin action pathway. J. Biol. Chem. 270: 20503–20508. [DOI] [PubMed] [Google Scholar]
- 130.Klaman L. D., Boss O., Peroni O. D., Kim J. K., Martino J. L., Zabolotny J. M., Moghal N., Lubkin M., Kim Y. B., Sharpe A. H., et al. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20: 5479–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., Normandin D., Cheng A., Himms-Hagen J., Chan C. C., et al. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 283: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 132.Reiter C. E., Wu X., Sandirasegarane L., Nakamura M., Gilbert K. A., Singh R. S., Fort P. E., Antonetti D. A., Gardner T. W. 2006. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 55: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 133.Rajala R. V., Wiskur B., Tanito M., Callegan M., Rajala A. 2009. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Invest. Ophthalmol. Vis. Sci. 50: 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravichandran L. V., Chen H., Li Y., Quon M. J. 2001. Phosphorylation of PTP1B at Ser(50) by Akt impairs its ability to dephosphorylate the insulin receptor. Mol. Endocrinol. 15: 1768–1780. [DOI] [PubMed] [Google Scholar]
- 135.Li G., Anderson R. E., Tomita H., Adler R., Liu X., Zack D. J., Rajala R. V. 2007. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 27: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galetic I., Andjelkovic M., Meier R., Brodbeck D., Park J., Hemmings B. A. 1999. Mechanism of protein kinase B activation by insulin/insulin-like growth factor-1 revealed by specific inhibitors of phosphoinositide 3-kinase–significance for diabetes and cancer. Pharmacol. Ther. 82: 409–425. [DOI] [PubMed] [Google Scholar]
- 137.Tremblay F., Lavigne C., Jacques H., Marette A. 2001. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activities. Diabetes. 50: 1901–1910. [DOI] [PubMed] [Google Scholar]
- 138.Hernandez R., Teruel T., Lorenzo M. 2001. Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett. 494: 225–231. [DOI] [PubMed] [Google Scholar]
- 139.Vosseller K., Wells L., Lane M. D., Hart G. W. 2002. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3–L1 adipocytes. Proc. Natl. Acad. Sci. USA. 99: 5313–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang B. H., Zheng J. Z., Aoki M., Vogt P. K. 2000. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA. 97: 1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gousseva N., Kugathasan K., Chesterman C. N., Khachigian L. M. 2001. Early growth response factor-1 mediates insulin-inducible vascular endothelial cell proliferation and regrowth after injury. J. Cell. Biochem. 81: 523–534. [DOI] [PubMed] [Google Scholar]
- 142.Lal B. K., Varma S., Pappas P. J., Hobson R. W., Duran W. N. 2001. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc. Res. 62: 252–262. [DOI] [PubMed] [Google Scholar]
- 143.Shioi T., McMullen J. R., Kang P. M., Douglas P. S., Obata T., Franke T. F., Cantley L. C., Izumo S. 2002. Akt/protein kinase B promotes organ growth in transgenic mice. Mol. Cell. Biol. 22: 2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luo J., Sobkiw C. L., Hirshman M. F., Logsdon M. N., Li T. Q., Goodyear L. J., Cantley L. C. 2006. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell Metab. 3: 355–366. [DOI] [PubMed] [Google Scholar]
- 145.Reiter C. E., Gardner T. W. 2003. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog. Retin. Eye Res. 22: 545–562. [DOI] [PubMed] [Google Scholar]
- 146.Rajala R. V., Rajala A., Brush R. S., Rotstein N. P., Politi L. E. 2009. Insulin receptor signaling regulates actin cytoskeletal organization in developing photoreceptors. J. Neurochem. 110: 1648–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takenawa T., Itoh T. 2001. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta. 1533: 190–206. [DOI] [PubMed] [Google Scholar]
- 148.Rajala A., Tanito M., Le Y. Z., Kahn C. R., Rajala R. V. 2008. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 283: 19781–19792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Conway B. R., Maxwell A. P., Savage D. A., Patterson C. C., Doran P. P., Murphy M., Brady H. R., Fogarty D. G. 2004. Association between variation in the actin-binding gene caldesmon and diabetic nephropathy in type 1 diabetes. Diabetes. 53: 1162–1165. [DOI] [PubMed] [Google Scholar]
- 150.McLean W. G. 1997. The role of axonal cytoskeleton in diabetic neuropathy. Neurochem. Res. 22: 951–956. [DOI] [PubMed] [Google Scholar]
- 151.McLean W. G., Roberts R. E., Mullins F. H. 1995. Post-translational modifications of microtubule- and growth-associated proteins in nerve regeneration and neuropathy. Biochem. Soc. Trans. 23: 76–80. [DOI] [PubMed] [Google Scholar]
- 152.Olson T. M., Michels V. V., Thibodeau S. N., Tai Y. S., Keating M. T. 1998. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 280: 750–752. [DOI] [PubMed] [Google Scholar]
- 153.Fein F. S., Malhotra A., Miller-Green B., Scheuer J., Sonnenblick E. H. 1984. Diabetic cardiomyopathy in rats: mechanical and biochemical response to different insulin doses. Am. J. Physiol. 247: H817–H823. [DOI] [PubMed] [Google Scholar]
- 154.Zherebitskaya E., Akude E., Smith D. R., Fernyhough P. 2009. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 58: 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tosaki T., Kamiya H., Yasuda Y., Naruse K., Kato K., Kozakae M., Nakamura N., Shibata T., Hamada Y., Nakashima E., et al. 2008. Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp. Neurol. 213: 381–387. [DOI] [PubMed] [Google Scholar]
- 156.Rajala R. V., Chan M. D. 2005. Identification of a NPXY motif in growth factor receptor-bound protein 14 (Grb14) and its interaction with the phosphotyrosine-binding (PTB) domain of IRS-1. Biochemistry. 44: 7929–7935. [DOI] [PubMed] [Google Scholar]
- 157.Rajala R. V., Chan M. D., Rajala A. 2005. Lipid-protein interactions of growth factor receptor-bound protein 14 in insulin receptor signaling. Biochemistry. 44: 15461–15471. [DOI] [PubMed] [Google Scholar]
- 158.Jones N., Master Z., Jones J., Bouchard D., Gunji Y., Sasaki H., Daly R., Alitalo K., Dumont D. J. 1999. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J. Biol. Chem. 274: 30896–30905. [DOI] [PubMed] [Google Scholar]
- 159.Daly R. J., Sanderson G. M., Janes P. W., Sutherland R. L. 1996. Cloning and characterization of GRB14, a novel member of the GRB7 gene family. J. Biol. Chem. 271: 12502–12510. [DOI] [PubMed] [Google Scholar]
- 160.Reilly J. F., Mickey G., Maher P. A. 2000. Association of fibroblast growth factor receptor 1 with the adaptor protein Grb14. Characterization of a new receptor binding partner. J. Biol. Chem. 275: 7771–7778. [DOI] [PubMed] [Google Scholar]
- 161.Kasus-Jacobi A., Perdereau D., Auzan C., Clauser E., Van Obberghen E., Mauvais-Jarvis F., Girard J., Burnol A. F. 1998. Identification of the rat adapter Grb14 as an inhibitor of insulin actions. J. Biol. Chem. 273: 26026–26035. [DOI] [PubMed] [Google Scholar]
- 162.Rajala A., Daly R. J., Tanito M., Allen D. T., Holt L. J., Lobanova E., Arshavsky V. Y., Rajala R. V. 2009. Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role on retinal insulin receptor activation. Biochemistry. 48: 5563–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Luttrell L. M., van Biesen T., Hawes B. E., Koch W. J., Krueger K. M., Touhara K., Lefkowitz R. J. 1997. G-protein-coupled receptors and their regulation: activation of the MAP kinase signaling pathway by G-protein-coupled receptors. Adv. Second Messenger Phosphoprotein Res. 31: 263–277. [PubMed] [Google Scholar]
- 164.Luttrell L. M., Daaka Y., Lefkowitz R. J. 1999. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 11: 177–183. [DOI] [PubMed] [Google Scholar]
- 165.Lev S., Hernandez J., Martinez R., Chen A., Plowman G., Schlessinger J. 1999. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell. Biol. 19: 2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Halet G. 2005. Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol. Cell. 97: 501–518. [DOI] [PubMed] [Google Scholar]
- 167.Lorenzo O., Urbe S., Clague M. J. 2005. Analysis of phosphoinositide binding domain properties within the myotubularin-related protein MTMR3. J. Cell Sci. 118: 2005–2012. [DOI] [PubMed] [Google Scholar]
- 168.Dormann D., Weijer G., Dowler S., Weijer C. J. 2004. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J. Cell Sci. 117: 6497–6509. [DOI] [PubMed] [Google Scholar]
- 169.Pattni K., Jepson M., Stenmark H., Banting G. 2001. A PtdIns(3)P-specific probe cycles on and off host cell membranes during Salmonella invasion of mammalian cells. Curr. Biol. 11: 1636–1642. [DOI] [PubMed] [Google Scholar]
- 170.Rusten T. E., Stenmark H. 2006. Analyzing phosphoinositides and their interacting proteins. Nat. Methods. 3: 251–258. [DOI] [PubMed] [Google Scholar]
- 171.Basinger S., Hoffman R., Matthes M. 1976. Photoreceptor shedding is initiated by light in the frog retina. Science. 194: 1074–1076. [DOI] [PubMed] [Google Scholar]
- 172.Hollyfield J. G., Rayborn M. E. 1979. Photoreceptor outer segment development: light and dark regulate the rate of membrane addition and loss. Invest. Ophthalmol. Vis. Sci. 18: 117–132. [PubMed] [Google Scholar]
- 173.Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y. 2001. Adaptation in vertebrate photoreceptors. Physiol. Rev. 81: 117–151. [DOI] [PubMed] [Google Scholar]
- 174.D'Mello S. R., Borodezt K., Soltoff S. P. 1997. Insulin-like growth factor and potassium depolarization maintain neuronal survival by distinct pathways: possible involvement of PI 3-kinase in IGF-1 signaling. J. Neurosci. 17: 1548–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spear N., Estevez A. G., Barbeito L., Beckman J. S., Johnson G. V. 1997. Nerve growth factor protects PC12 cells against peroxynitrite-induced apoptosis via a mechanism dependent on phosphatidylinositol 3-kinase. J. Neurochem. 69: 53–59. [DOI] [PubMed] [Google Scholar]
- 176.Noell W. K., Albrecht R. 1971. Irreversible effects on visible light on the retina: role of vitamin A. Science. 172: 76–79. [DOI] [PubMed] [Google Scholar]
- 177.Noell W. K. 1980. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 20: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 178.Wang W., Fridman A., Blackledge W., Connelly S., Wilson I. A., Pilz R. B., Boss G. R. 2009. The phosphatidylinositol 3-kinase/akt cassette regulates purine nucleotide synthesis. J. Biol. Chem. 284: 3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Suzuki H., Terauchi Y., Fujiwara M., Aizawa S., Yazaki Y., Kadowaki T., Koyasu S. 1999. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 283: 390–392. [DOI] [PubMed] [Google Scholar]
- 180.Terauchi Y., Tsuji Y., Satoh S., Minoura H., Murakami K., Okuno A., Inukai K., Asano T., Kaburagi Y., Ueki K., et al. 1999. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 21: 230–235. [DOI] [PubMed] [Google Scholar]
- 181.Fruman D. A., Snapper S. B., Yballe C. M., Davidson L., Yu J. Y., Alt F. W., Cantley L. C. 1999. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 283: 393–397. [DOI] [PubMed] [Google Scholar]
- 182.Chen D., Mauvais-Jarvis F., Bluher M., Fisher S. J., Jozsi A., Goodyear L. J., Ueki K., Kahn C. R. 2004. p50alpha/p55alpha phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol. Cell. Biol. 24: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ueki K., Yballe C. M., Brachmann S. M., Vicent D., Watt J. M., Kahn C. R., Cantley L. C. 2002. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA. 99: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ueki K., Fruman D. A., Yballe C. M., Fasshauer M., Klein J., Asano T., Cantley L. C., Kahn C. R. 2003. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 278: 48453–48466. [DOI] [PubMed] [Google Scholar]
- 185.Ueki K., Fruman D. A., Brachmann S. M., Tseng Y. H., Cantley L. C., Kahn C. R. 2002. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol. 22: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Inoue G., Cheatham B., Emkey R., Kahn C. R. 1998. Dynamics of insulin signaling in 3T3–L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J. Biol. Chem. 273: 11548–11555. [DOI] [PubMed] [Google Scholar]
- 187.Luo J., Field S. J., Lee J. Y., Engelman J. A., Cantley L. C. 2005. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J. Cell Biol. 170: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cinar B., Mukhopadhyay N. K., Meng G., Freeman M. R. 2007. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J. Biol. Chem. 282: 29584–29593. [DOI] [PubMed] [Google Scholar]
- 189.Arcaro A., Aubert M., Espinosa del Hierro M. E., Khanzada U. K., Angelidou S., Tetley T. D., Bittermann A. G., Frame M. C., Seckl M. J. 2007. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell. Signal. 19: 1081–1092. [DOI] [PubMed] [Google Scholar]
- 190.Peres C., Yart A., Perret B., Salles J. P., Raynal P. 2003. Modulation of phosphoinositide 3-kinase activation by cholesterol level suggests a novel positive role for lipid rafts in lysophosphatidic acid signalling. FEBS Lett. 534: 164–168. [DOI] [PubMed] [Google Scholar]
- 191.Rajala R. V., Elliott M. H., McClellan M. E., Anderson R. E. 2006. Localization of the insulin receptor and phosphoinositide 3-kinase in detergent-resistant membrane rafts of rod photoreceptor outer segments. Adv. Exp. Med. Biol. 572: 491–497. [DOI] [PubMed] [Google Scholar]
- 192.Mauvais-Jarvis F., Ueki K., Fruman D. A., Hirshman M. F., Sakamoto K., Goodyear L. J., Iannacone M., Accili D., Cantley L. C., Kahn C. R. 2002. Reduced expression of the murine p85alpha subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J. Clin. Invest. 109: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Luo J., McMullen J. R., Sobkiw C. L., Zhang L., Dorfman A. L., Sherwood M. C., Logsdon M. N., Horner J. W., DePinho R. A., Izumo S., et al. 2005. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol. Cell. Biol. 25: 9491–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Taniguchi C. M., Aleman J. O., Ueki K., Luo J., Asano T., Kaneto H., Stephanopoulos G., Cantley L. C., Kahn C. R. 2007. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol. Cell. Biol. 27: 2830–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rajala R. V., McClellan M. E., Ash J. D., Anderson R. E. 2003. Regulation of retinal phosphoinositide 3-kinase activity in p85alpha-subunit knockout mice. Adv. Exp. Med. Biol. 533: 369–376. [DOI] [PubMed] [Google Scholar]
- 196.Brachmann S. M., Ueki K., Engelman J. A., Kahn R. C., Cantley L. C. 2005. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol. Cell. Biol. 25: 1596–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Foukas L. C., Claret M., Pearce W., Okkenhaug K., Meek S., Peskett E., Sancho S., Smith A. J., Withers D. J., Vanhaesebroeck B. 2006. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 441: 366–370. [DOI] [PubMed] [Google Scholar]
- 198.Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., et al. 2006. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 125: 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases. Cell. 103: 211–225. [DOI] [PubMed] [Google Scholar]
- 200.Touhara K., Inglese J., Pitcher J. A., Shaw G., Lefkowitz R. J. 1994. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J. Biol. Chem. 269: 10217–10220. [PubMed] [Google Scholar]
- 201.Rodriguez M. M., Ron D., Touhara K., Chen C. H., Mochly-Rosen D. 1999. RACK1, a protein kinase C anchoring protein, coordinates the binding of activated protein kinase C and select pleckstrin homology domains in vitro. Biochemistry. 38: 13787–13794. [DOI] [PubMed] [Google Scholar]
- 202.Krappa R., Nguyen A., Burrola P., Deretic D., Lemke G. 1999. Evectins: vesicular proteins that carry a pleckstrin homology domain and localize to post-Golgi membranes. Proc. Natl. Acad. Sci. USA. 96: 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu S., Wang Y., Zhao H., Zhang L., Xiong W., Yau K. W., Hiel H., Glowatzki E., Ryugo D. K., Valle D. 2004. PHR1, a PH domain-containing protein expressed in primary sensory neurons. Mol. Cell. Biol. 24: 9137–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xu S., Ladak R., Swanson D. A., Soltyk A., Sun H., Ploder L., Vidgen D., Duncan A. M., Garami E., Valle D., et al. 1999. PHR1 encodes an abundant, pleckstrin homology domain-containing integral membrane protein in the photoreceptor outer segments. J. Biol. Chem. 274: 35676–35685. [DOI] [PubMed] [Google Scholar]
- 205.Brognard J., Sierecki E., Gao T., Newton A. C. 2007. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol. Cell. 25: 917–931. [DOI] [PubMed] [Google Scholar]
- 206.Anderson R. E., Maude M. B., Kelleher P. A., Maida T. M., Basinger S. F. 1980. Metabolism of phosphatidylcholine in the frog retina. Biochim. Biophys. Acta. 620: 212–226. [DOI] [PubMed] [Google Scholar]
- 207.Anderson R. E., Kelleher P. A., Maude M. B. 1980. Metabolism of phosphatidylethanolamine in the frog retina. Biochim. Biophys. Acta. 620: 227–235. [DOI] [PubMed] [Google Scholar]
- 208.Anderson R. E., Maude M. B., Kelleher P. A., Rayborn M. E., Hollyfield J. G. 1983. Phosphoinositide metabolism in the retina: localization to horizontal cells and regulation by light and divalent cations. J. Neurochem. 41: 764–771. [DOI] [PubMed] [Google Scholar]
- 209.Anderson R. E., Hollyfield J. G. 1984. Inositol incorporation into phosphoinositides in retinal horizontal cells of Xenopus laevis: enhancement by acetylcholine, inhibition by glycine. J. Cell Biol. 99: 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Anderson R. E., Maude M. B., Pu G. A., Hollyfield J. G. 1985. Effect of light on the metabolism of lipids in the rat retina. J. Neurochem. 44: 773–778. [DOI] [PubMed] [Google Scholar]
- 211.Jung H. H., Reme C. E., Pfeilschifter J. 1993. Light evoked inositol trisphosphate release in the rat retina in vitro. Curr. Eye Res. 12: 727–732. [DOI] [PubMed] [Google Scholar]
- 212.Schmidt S. Y. 1983. Phosphatidylinositol synthesis and phosphorylation are enhanced by light in rat retinas. J. Biol. Chem. 258: 6863–6868. [PubMed] [Google Scholar]
- 213.Schmidt S. Y. 1983. Light enhances the turnover of phosphatidylinositol in rat retinas. J. Neurochem. 40: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 214.Schmidt S. Y. 1983. Light- and cytidine-dependent phosphatidylinositol synthesis in photoreceptor cells of the rat. J. Cell Biol. 97: 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Choe H. G., Ghalayini A. J., Anderson R. E. 1990. Phosphoinositide metabolism in frog rod outer segments. Exp. Eye Res. 51: 167–176. [DOI] [PubMed] [Google Scholar]
- 216.Ghalayini A. J., Guo X. X., Koutz C. A., Anderson R. E. 1998. Light stimulates tyrosine phosphorylation of rat rod outer segments in vivo. Exp. Eye Res. 66: 817–821. [DOI] [PubMed] [Google Scholar]
- 217.Brown J. E., Blazynski C., Cohen A. I. 1987. Light induces a rapid and transient increase in inositol-trisphosphate in toad rod outer segments. Biochem. Biophys. Res. Commun. 146: 1392–1396. [DOI] [PubMed] [Google Scholar]
- 218.Brown J. E., Rubin L. J., Ghalayini A. J., Tarver A. P., Irvine R. F., Berridge M. J., Anderson R. E. 1984. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 311: 160–163. [DOI] [PubMed] [Google Scholar]
- 219.Das N. D., Yoshioka T., Samuelson D., Shichi H. 1986. Immunocytochemical localization of phosphatidylinositol-4,5-bisphosphate in dark- and light-adapted rat retinas. Cell Struct. Funct. 11: 53–63. [DOI] [PubMed] [Google Scholar]
- 220.Das N. D., Yoshioka T., Samuelson D., Cohen R. J., Shichi H. 1987. Immunochemical evidence for the light-regulated modulation of phosphatidylinositol-4,5-bisphosphate in rat photoreceptor cells. Cell Struct. Funct. 12: 471–481. [DOI] [PubMed] [Google Scholar]
- 221.Ferreira P. A., Shortridge R. D., Pak W. L. 1993. Distinctive subtypes of bovine phospholipase C that have preferential expression in the retina and high homology to the norpA gene product of Drosophila. Proc. Natl. Acad. Sci. USA. 90: 6042–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gehm B. D., Mc Connell D. G. 1990. Phosphoinositide synthesis in bovine rod outer segments. Biochemistry. 29: 5442–5446. [DOI] [PubMed] [Google Scholar]
- 223.Gehm B. D., Pinke R. M., Laquerre S., Chafouleas J. G., Schultz D. A., Pepperl D. J., McConnell D. G. 1991. Activation of bovine rod outer segment phosphatidylinositol-4,5-bisphosphate phospholipase C by calmodulin antagonists does not depend on calmodulin. Biochemistry. 30: 11302–11306. [DOI] [PubMed] [Google Scholar]
- 224.Hayashi F., Amakawa T. 1985. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem. Biophys. Res. Commun. 128: 954–959. [DOI] [PubMed] [Google Scholar]
- 225.Jelsema C. L. 1989. Regulation of phospholipase A2 and phospholipase C in rod outer segments of bovine retina involves a common GTP-binding protein but different mechanisms of action. Ann. N. Y. Acad. Sci. 559: 158–177. [DOI] [PubMed] [Google Scholar]
- 226.Millar F. A., Fisher S. C., Muir C. A., Edwards E., Hawthorne J. N. 1988. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. Biochim. Biophys. Acta. 970: 205–211. [DOI] [PubMed] [Google Scholar]
- 227.Peng Y. W., Rhee S. G., Yu W. P., Ho Y. K., Schoen T., Chader G. J., Yau K. W. 1997. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc. Natl. Acad. Sci. USA. 94: 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huang Z., Guo X. X., Chen S. X., Alvarez K. M., Bell M. W., Anderson R. E. 2001. Regulation of type II phosphatidylinositol phosphate kinase by tyrosine phosphorylation in bovine rod outer segments. Biochemistry. 40: 4550–4559. [DOI] [PubMed] [Google Scholar]
- 229.Ghalayini A., Anderson R. E. 1984. Phosphatidylinositol 4,5-bisphosphate: light-mediated breakdown in the vertebrate retina. Biochem. Biophys. Res. Commun. 124: 503–506. [DOI] [PubMed] [Google Scholar]
- 230.Kapoor C. L., O'Brien P. J., Chader G. J. 1987. Phorbol ester- and light-induced endogenous phosphorylation of rat rod outer-segment proteins. Exp. Eye Res. 45: 545–556. [DOI] [PubMed] [Google Scholar]
- 231.Kapoor C. L., Chader G. J. 1984. Endogenous phosphorylation of retinal photoreceptor outer segment proteins by calcium phospholipid-dependent protein kinase. Biochem. Biophys. Res. Commun. 122: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 232.Kelleher D. J., Johnson G. L. 1985. Purification of protein kinase C from bovine rod outer segments. J. Cyclic Nucleotide Protein Phosphor. Res. 10: 579–591. [PubMed] [Google Scholar]
- 233.Kelleher D. J., Johnson G. L. 1986. Phosphorylation of rhodopsin by protein kinase C in vitro. J. Biol. Chem. 261: 4749–4757. [PubMed] [Google Scholar]
- 234.Newton A. C., Williams D. S. 1993. Rhodopsin is the major in situ substrate of protein kinase C in rod outer segments of photoreceptors. J. Biol. Chem. 268: 18181–18186. [PubMed] [Google Scholar]
- 235.Greene N. M., Williams D. S., Newton A. C. 1997. Identification of protein kinase C phosphorylation sites on bovine rhodopsin. J. Biol. Chem. 272: 10341–10344. [DOI] [PubMed] [Google Scholar]
- 236.Greene N. M., Williams D. S., Newton A. C. 1995. Kinetics and localization of the phosphorylation of rhodopsin by protein kinase C. J. Biol. Chem. 270: 6710–6717. [DOI] [PubMed] [Google Scholar]
- 237.Williams D. S., Liu X., Schlamp C. L., Ondek B., Jaken S., Newton A. C. 1997. Characterization of protein kinase C in photoreceptor outer segments. J. Neurochem. 69: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 238.Udovichenko I. P., Cunnick J., Gonzalez K., Yakhnin A., Takemoto D. J. 1996. Protein kinase C in rod outer segments: effects of phosphorylation of the phosphodiesterase inhibitory subunit. Biochem. J. 317: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Udovichenko I. P., Cunnick J., Gonzalez K., Takemoto D. J. 1994. Functional effect of phosphorylation of the photoreceptor phosphodiesterase inhibitory subunit by protein kinase C. J. Biol. Chem. 269: 9850–9856. [PubMed] [Google Scholar]
- 240.Womack K. B., Gordon S. E., He F., Wensel T. G., Lu C. C., Hilgemann D. W. 2000. Do phosphatidylinositides modulate vertebrate phototransduction? J. Neurosci. 20: 2792–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.He F., Mao M., Wensel T. G. 2004. Enhancement of phototransduction g protein-effector interactions by phosphoinositides. J. Biol. Chem. 279: 8986–8990. [DOI] [PubMed] [Google Scholar]
- 242.Chuang J. Z., Zhao Y., Sung C. H. 2007. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 130: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lee S. J., Xu H., Kang L. W., Amzel L. M., Montell C. 2003. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 39: 121–132. [DOI] [PubMed] [Google Scholar]
- 244.Tanaka M., Konishi H., Touhara K., Sakane F., Hirata M., Ono Y., Kikkawa U. 1999. Identification of myosin II as a binding protein to the PH domain of protein kinase B. Biochem. Biophys. Res. Commun. 255: 169–174. [DOI] [PubMed] [Google Scholar]
- 245.Weil D., Blanchard S., Kaplan J., Guilford P., Gibson F., Walsh J., Mburu P., Varela A., Levilliers J., Weston M. D. 1995. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 374: 60–61. [DOI] [PubMed] [Google Scholar]
- 246.Barber A. J., Nakamura M., Wolpert E. B., Reiter C. E., Seigel G. M., Antonetti D. A., Gardner T. W. 2001. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 276: 32814–32821. [DOI] [PubMed] [Google Scholar]
- 247.Yu X., Rajala R. V., McGinnis J. F., Li F., Anderson R. E., Yan X., Li S., Elias R. V., Knapp R. R., Zhou X., et al. 2004. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J. Biol. Chem. 279: 13086–13094. [DOI] [PubMed] [Google Scholar]
- 248.Park K. K., Liu K., Hu Y., Smith P. D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., et al. 2008. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 322: 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stitt A. W., Hughes S. J., Canning P., Lynch O., Cox O., Frizzell N., Thorpe S. R., Cotter T. G., Curtis T. M., Gardiner T. A. 2004. Substrates modified by advanced glycation end-products cause dysfunction and death in retinal pericytes by reducing survival signals mediated by platelet-derived growth factor. Diabetologia. 47: 1735–1746. [DOI] [PubMed] [Google Scholar]
- 250.Gomez-Vicente V., Doonan F., Donovan M., Cotter T. G. 2006. Induction of BIM(EL) following growth factor withdrawal is a key event in caspase-dependent apoptosis of 661W photoreceptor cells. Eur. J. Neurosci. 24: 981–990. [DOI] [PubMed] [Google Scholar]
- 251.Pinal N., Goberdhan D. C., Collinson L., Fujita Y., Cox I. M., Wilson C., Pichaud F. 2006. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr. Biol. 16: 140–149. [DOI] [PubMed] [Google Scholar]
- 252.Jomary C., Cullen J., Jones S. E. 2006. Inactivation of the Akt survival pathway during photoreceptor apoptosis in the retinal degeneration mouse. Invest. Ophthalmol. Vis. Sci. 47: 1620–1629. [DOI] [PubMed] [Google Scholar]
- 253.Kim J. W., Kang K. H., Burrola P., Mak T. W., Lemke G. 2008. Retinal degeneration triggered by inactivation of PTEN in the retinal pigment epithelium. Genes Dev. 22: 3147–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cai J., Nelson K. C., Wu M., Sternberg P., Jr., Jones D. P. 2000. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 19: 205–221. [DOI] [PubMed] [Google Scholar]
- 255.Bailey T. A., Kanuga N., Romero I. A., Greenwood J., Luthert P. J., Cheetham M. E. 2004. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 45: 675–684. [DOI] [PubMed] [Google Scholar]
- 256.Wenzel A., Grimm C., Samardzija M., Reme C. E. 2005. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 24: 275–306. [DOI] [PubMed] [Google Scholar]
- 257.Lee S. R., Yang K. S., Kwon J., Lee C., Jeong W., Rhee S. G. 2002. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277: 20336–20342. [DOI] [PubMed] [Google Scholar]
- 258.Leslie N. R., Bennett D., Lindsay Y. E., Stewart H., Gray A., Downes C. P. 2003. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 22: 5501–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yang P., Peairs J. J., Tano R., Jaffe G. J. 2006. Oxidant-mediated Akt activation in human RPE cells. Invest. Ophthalmol. Vis. Sci. 47: 4598–4606. [DOI] [PubMed] [Google Scholar]
- 260.Barbieri S. S., Ruggiero L., Tremoli E., Weksler B. B. 2008. Suppressing PTEN activity by tobacco smoke plus interleukin-1beta modulates dissociation of VE-cadherin/beta-catenin complexes in endothelium. Arterioscler. Thromb. Vasc. Biol. 28: 732–738. [DOI] [PubMed] [Google Scholar]
- 261.Wang K., Spector A. 2000. alpha-crystallin prevents irreversible protein denaturation and acts cooperatively with other heat-shock proteins to renature the stabilized partially denatured protein in an ATP-dependent manner. Eur. J. Biochem. 267: 4705–4712. [DOI] [PubMed] [Google Scholar]
- 262.Defoe D. M., Grindstaff R. D. 2004. Epidermal growth factor stimulation of RPE cell survival: contribution of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. Exp. Eye Res. 79: 51–59. [DOI] [PubMed] [Google Scholar]
- 263.Cai J., Nelson K. C., Wu M., Sternberg P., Jr., Jones D. P. 2000. Oxidative damage and protection of the RPE. Prog.Retin.Eye Res. 19: 205–221. [DOI] [PubMed] [Google Scholar]
- 264.Lavie Y., Dybowski J., Agranoff B. W. 1997. Wortmannin blocks goldfish retinal phosphatidylinositol 3-kinase and neurite outgrowth. Neurochem. Res. 22: 373–378. [DOI] [PubMed] [Google Scholar]
- 265.Pimentel B., Rodriguez-Borlado L., Hernandez C., Carrera A. C. 2002. A role for phosphoinositide 3-kinase in the control of cell division and survival during retinal development. Dev. Biol. 247: 295–306. [DOI] [PubMed] [Google Scholar]
- 266.Nakazawa T., Shimura M., Tomita H., Akiyama H., Yoshioka Y., Kudou H., Tamai M. 2003. Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr. Eye Res. 26: 55–63. [DOI] [PubMed] [Google Scholar]
- 267.Huang Y., Cen L. P., Luo J. M., Wang N., Zhang M. Z., van Rooijen N., Pang C. P., Cui Q. 2008. Differential roles of phosphatidylinositol 3-kinase/akt pathway in retinal ganglion cell survival in rats with or without acute ocular hypertension. Neuroscience. 153: 214–225. [DOI] [PubMed] [Google Scholar]
- 268.Huang Y., Li Z., Wang N., van Rooijen N., Cui Q. 2008. Roles of PI3K and JAK pathways in viability of retinal ganglion cells after acute elevation of intraocular pressure in rats with different autoimmune backgrounds. BMC Neurosci. 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chen J., Wang L., Chen Y., Sternberg P., Cai J. 2009. Phosphatidylinositol 3 kinase pathway and 4-hydroxy-2-nonenal-induced oxidative injury in the RPE. Invest. Ophthalmol. Vis. Sci. 50: 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Geraldes P., Yagi K., Ohshiro Y., He Z., Maeno Y., Yamamoto-Hiraoka J., Rask-Madsen C., Chung S. W., Perrella M. A., King G. L. 2008. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J. Biol. Chem. 283: 34327–34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wolf A. M., Lyuksyutova A. I., Fenstermaker A. G., Shafer B., Lo C. G., Zou Y. 2008. Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J. Neurosci. 28: 3456–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ko M. L., Jian K., Shi L., Ko G. Y. 2009. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J. Neurochem. 108: 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Blakesley V. A., Scrimgeour A., Esposito D., Le Roith D. 1996. Signaling via the insulin-like growth factor-I receptor: does it differ from insulin receptor signaling? Cytokine Growth Factor Rev. 7: 153–159. [DOI] [PubMed] [Google Scholar]
- 274.Saltiel A. R., Kahn C. R. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 414: 799–806. [DOI] [PubMed] [Google Scholar]
- 275.Kondo T., Vicent D., Suzuma K., Yanagisawa M., King G. L., Holzenberger M., Kahn C. R. 2003. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J. Clin. Invest. 111: 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Johnson L. E., van Veen T., Ekstrom P. A. 2005. Differential Akt activation in the photoreceptors of normal and rd1 mice. Cell Tissue Res. 320: 213–222. [DOI] [PubMed] [Google Scholar]
- 277.Rajala R. V., Ivanovic I., Dilly A. K. 2009. Retinal insulin receptor signaling in hyperosmotic stress. Vitam. Horm. 80: 583–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schmitz F., Drenckhahn D. 1993. Li(+)-induced structural changes of synaptic ribbons are related to the phosphoinositide metabolism in photoreceptor synapses. Brain Res. 604: 142–148. [DOI] [PubMed] [Google Scholar]
- 279.LaVail M. M. 1976. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 194: 1071–1074. [DOI] [PubMed] [Google Scholar]
- 280.Papermaster D. S., Converse C. A., Siuss J. 1975. Membrane biosynthesis in the frog retina: opsin transport in the photoreceptor cell. Biochemistry. 14: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 281.McGinnis J. F., Whelan J. P., Donoso L. A. 1992. Transient, cyclic changes in mouse visual cell gene products during the light-dark cycle. J. Neurosci. Res. 31: 584–590. [DOI] [PubMed] [Google Scholar]
- 282.Calvert P. D., Strissel K. J., Schiesser W. E., Pugh E. N., Jr., Arshavsky V. Y. 2006. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 16: 560–568. [DOI] [PubMed] [Google Scholar]
- 283.Steenhard B. M., Besharse J. C. 2000. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J. Neurosci. 20: 8572–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li F., Cao W., Anderson R. E. 2001. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp. Eye Res. 73: 569–577. [DOI] [PubMed] [Google Scholar]
- 285.Penn J. S., Anderson R. E. 1987. Effect of light history on rod outer-segment membrane composition in the rat. Exp. Eye Res. 44: 767–778. [DOI] [PubMed] [Google Scholar]
- 286.Inoue H., Yoshioka T., Hotta Y. 1989. Diacylglycerol kinase defect in a Drosophila retinal degeneration mutant rdgA. J. Biol. Chem. 264: 5996–6000. [PubMed] [Google Scholar]
- 287.Kim S., McKay R. R., Miller K., Shortridge R. D. 1995. Multiple subtypes of phospholipase C are encoded by the norpA gene of Drosophila melanogaster. J. Biol. Chem. 270: 14376–14382. [DOI] [PubMed] [Google Scholar]
- 288.McKay R. R., Chen D. M., Miller K., Kim S., Stark W. S., Shortridge R. D. 1995. Phospholipase C rescues visual defect in norpA mutant of Drosophila melanogaster. J. Biol. Chem. 270: 13271–13276. [DOI] [PubMed] [Google Scholar]
- 289.Attree O., Olivos I. M., Okabe I., Bailey L. C., Nelson D. L., Lewis R. A., McInnes R. R., Nussbaum R. L. 1992. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 358: 239–242. [DOI] [PubMed] [Google Scholar]
- 290.Majerus P. W., Kisseleva M. V., Norris F. A. 1999. The role of phosphatases in inositol signaling reactions. J. Biol. Chem. 274: 10669–10672. [DOI] [PubMed] [Google Scholar]
- 291.Kohn L., Kadzhaev K., Burstedt M. S., Haraldsson S., Hallberg B., Sandgren O., Golovleva I. 2007. Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. Eur. J. Hum. Genet. 15: 664–671. [DOI] [PubMed] [Google Scholar]