Abstract
The event-related potential (ERP) P3b, a cognitive electrophysiological measure that has been linked to working memory processing in many experimental paradigms, was measured in Inuit children from Nunavik (Arctic Québec, Canada) to assess lead (Pb) neurotoxicity. Visual and auditory oddball paradigms were administered at 5 (N=27) and 11 (N=110) years of age, respectively, to elicit this ERP component. Pearson correlations and multiple regression analyses were performed to examine the associations between Pb levels and P3b parameters (peak latency and amplitude). Greater prenatal Pb exposure was related to a decrease in P3b amplitude at 5 years of age, and early childhood Pb exposure was associated with delayed P3b latency at 5 years. No significant association was observed at 11 years. These results, in line with those from previous neurobehavioral studies, suggest that Pb exposure affects cognitive processing in children even though the Pb levels measured in a large majority of our sample were below the threshold value for public health intervention used by federal agencies. This study strengthens the arguments for reducing sources of Pb exposure in Nunavik and for lowering the blood Pb concentrations considered “acceptable” in governmental policies.
Keywords: Children, Event-related potentials, Environmental contaminants, Lead, Neurotoxicity, P300
Introduction
Inuit communities from Nunavik (Arctic Québec, Canada) are exposed to substantial levels of environmental contaminants through consumption of traditional food, such as marine mammals, fish and game birds (Muckle et al., 2001). Among these environmental contaminants, lead (Pb) has been the focus of much attention since its neurotoxicity is well recognized (World Health Organization, 1995). Children are at increased risk for exhibiting neurotoxic effects of Pb exposure compared to adults, notably because of greater absorption from the gastrointestinal tract, weaker efficacy of the blood-brain barrier, and greater vulnerability of the developing nervous system (Lidsky and Schneider, 2003). Documented effects of environmental Pb exposure in children include impaired neuropsychological (e.g. visuo-spatial memory, executive functioning and attention) and motor functions as well as decreased IQ (Banks et al., 1997; Canfield et al., 2004; Després et al., 2005; Lidsky and Schneider, 2006; Walkowiak et al., 1998; Wasserman et al., 2000). More persistent effects of greater magnitude have been related to postnatal exposure compared to prenatal exposure. Although most Nunavik children have blood Pb levels below the values considered at risk by federal agencies (Lévesque et al., 2003), recent studies suggest there might be no lower bound threshold for observing effects of postnatal Pb exposure on neuropsychological function (Chiodo et al., 2004; Jusko et al., 2008; Lanphear et al., 2000).
Until now, the vast majority of studies assessing the neurotoxicity of Pb in children have used neurobehavioral test batteries. These measures use overt behaviours (e.g. verbal responses, button press, drawings, etc.) to reflect the brain processes involved in a task’s demands. However, behavioural measures typically solicit several interacting functions (at the sensory, cognitive and motor levels). It is thus difficult to identify which one of the solicited functions is affected when observing alterations in performance on a given test. Furthermore, the nature of the brain processes assessed by these measures are often inferred from studies with adults; whereas children might use different processes and strategies than adults to achieve the same task due to brain immaturity (Segalowitz and Schmidt, 2008). Direct measures of brain functioning could be more sensitive for detecting effects of environmental contaminants and might provide information about the specific processes affected by exposure. Twenty years ago, Otto (1987) recommended the inclusion of electrophysiological measures, such as evoked potentials, to assess the effects of neurotoxicants in children, to avoid some of the aforementioned limitations of neurobehavioral testing. To date, only a handful of studies have used evoked potentials to detect Pb-induced alterations of sensory functions in children, which were found sensitive to Pb-associated sensory deficits in some (Altmann et al., 1997; Rothenberg et al., 2000) but not all (Counter, 2003; Winneke et al., 1994) studies. Cognitive event-related potentials could also appear as a sensitive tool to detect the neurotoxic effects of Pb on neuropsychological functioning.
Event-related potentials (ERPs) are small deflections in electroencephalogram (EEG) recordings elicited by an external or internal event. They are typically obtained by averaging the EEG activity recorded coinciding with the repeated occurrence of a specific stimulus to which the participant has been given specific instructions regarding how to respond. This neurophysiologic technique has the potential of measuring, with high time resolution, brain processes that are fundamental to cognitive functioning but not directly observable through overt behaviour.
The P3b wave is one of the most extensively studied ERP components. It is elicited when an attended but unpredictable stimulus is detected (Hansenne, 2000). It is typically assessed during auditory or visual oddball detection paradigms, in which the subject must respond to rare targets randomly distributed among a series of standard stimuli. It is functionally and anatomically distinct from other late positive ERP components such as the P3a/novelty component (Courchesne et al., 1975; Squires et al., 1975; Sutton and Rushkin, 1984). The functional role of the P3b wave in the oddball paradigm has been most frequently associated with working memory. According to the leading “context-updating” theory, the P3b is a manifestation of activity occurring when the subject’s model of the environment must be revised; that is, it represents the adaptation of working memory to the changing data in the environment (Donchin, 1981; Donchin and Coles, 1988). Its latency is used as an index of categorization speed independent from response-associated processes (Kutas et al., 1977; McCarthy and Donchin 1981) and is correlated with performance on speeded attention tasks and neuropsychological measures of short-term memory (Gurrera et al., 2005; Polich, et al., 1983; Portin et al., 2000; Walhovd and Fjell, 2003). P3b amplitude is believed to reflect the allocation of attentional resources for information processing (Kok, 1997) and correlates with behavioural performance on attentional (Gurrera et al., 2005; Portin et al., 2000) and memory (Karis et al., 1984) tasks. P3b is thought to be relatively independent from task modality. Although slight differences in scalp distribution have been reported between the P3b wave obtained from visual and auditory stimuli (Johnson, 1989), most studies report more similarities than differences (Ji et al., 1999; Linden, 2005; Naumann et al., 1992). P3b waves from both auditory and visual stimuli also appear to be similarly affected by experimental conditions, such as stimulus intensity and number of trial blocks employed (Covington and Polich, 1996; Romero and Polich, 1996).
Although to date, no study has used the P3b wave to study the effects of Pb exposure in children, a few studies have documented this component in adults occupationally exposed to Pb. Using either auditory or visual oddball paradigms to measure the ERP, these studies all found that Pb-exposed workers had delayed P3b latency in comparison to controls (Araki et al., 1992; Hirata et al., 2004; Solliway et al., 1994). Decreased P3b amplitude was also reported in one of these studies (Solliway et al., 1994). These results suggest that occupational Pb exposure during adulthood slows information processing and may decrease the capacity to allocate attentional resources in a cognitive task.
This study was designed to assess the neurotoxicity of Pb in Nunavik children using the P3b wave elicited during an oddball task. Based on the neurobehavioral effects reported in Pb-exposed children and the ERP results obtained with adults, it was hypothesized that Pb exposure would be related to delayed latency and decreased amplitude of the P3b component in our sample of Inuit children.
Materials and Methods
Participants and study design
The study sample included 104 children assessed at 5 years of age from whom umbilical cord blood samples had been obtained under the auspices of the Cord Blood Monitoring Program, conducted between 1993 and 1998. Two hundred one children with archived cord blood samples were assessed at 11 years of age, 80 of whom had been seen at 5 years. An informed consent was obtained from a parent of the participant at each phase of the study. The research was approved by Laval University and Wayne State University ethics committees and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
To be included in this ERP study, the following inclusion criteria were used: children aged between 4.5 and 6.5 years (5-year assessment) and between 10.0 and 13.0 years (11-year assessment), birth weight ≥ 2500 g, gestation duration ≥ 36 weeks, no known neurological or developmental disorder. Participants with a medical condition that could affect ERP recording (e.g. history of epilepsy), and those on medication, such as methylphenidate, were also excluded. For the 5-year assessment, visual acuity was assessed (Snellen E chart); vision was considered abnormal when visual acuity ≥ 20/40. Two participants were excluded for this reason. For the 11-year assessment, pure-tone audiometry (1000 and 2000 Hz) was performed to document hearing impairment. One child with a hearing threshold ≥35 dB in his best ear was excluded. Characteristics of study sample participants are presented in Table 1.
Table 1.
Sample characteristics
| 5-year assessment |
11-year assessment |
|||
|---|---|---|---|---|
| N | Mean (Range) | N | Mean (Range) | |
| Child characteristics | ||||
| Gender (% female) | 104 | 59% | 201 | 55% |
| Age at testing (years) | 104 | 5.4 (4.8 – 6.2) | 201 | 11.3 (10.2 – 12.9) |
| Cord blood Pb levels (μg/dL) | 104 | 4.9 (0.8 – 27.1) | 198 | 4.8 (0.8 – 20.9) |
| Child blood Pb levels at testing (μg/dL) | 104 | 5.2 (1.0 – 37.1) | 198 | 2.66 (0.4 – 12.8) |
| Cord blood Hg (nmol/L) | 104 | 111 (9 – 520) | 198 | 105 (5 – 495) |
| Child blood Hg levels (nmol/L) | 104 | 48 (1 – 191) | 198 | 23 (1 – 170) |
| Cord PCB-153 (ng/g fat) | 104 | 124.1 (21.6 – 653.6) | 196 | 127.0 (9.7 – 653.6) |
| Child blood PCB-153 levels (ng/g fat) | 103 | 157.6 (7.5 – 1467.2) | 197 | 77.8 (4.1 – 809.5) |
| Haemoglobin level at testing (g/L) | 104 | 123 (88 – 172) | 198 | 130 (98 – 149) |
| Maternal characteristics | ||||
| Age (years) | 104 | 30.4 (21.4 – 45.1) | 200 | 39.1 (22.3 – 71.6) |
| Breastfeeding (% Yes) | 103 | 82% | 195 | 66% |
| Breastfeeding duration (months)a | 82 | 17.2 (0.1 – 60.0) | 128 | 18.2 (0.1 – 108.0) |
| Socioeconomic status (SES)b | 102 | 28.5 (8 – 57) | 201 | 28.8 (8 – 66) |
| Nonverbal intelligencec | 98 | 35.5 (13 – 54) | 195 | 35 (4 – 56) |
Mean duration of breastfeeding for mothers who breastfed;
Hollingshead index for the mother and her partner or, if she was not self-supporting, for her primary source of support (Hollingshead, 1975);
Raven Progressive Matrices (Raven et al., 1992).
ERP protocol and recording
At the 5-year assessment, a visual oddball paradigm was used to elicit the P3b wave. Although we initially planned to use an auditory protocol because our prior experience suggested that this is the best modality to elicit clear and well-defined P3b components, a visual paradigm was used because ear infections were frequent and we found in a pilot study that auditory attention was limited in young children. The child was asked to sit still in front of a computer screen and to press a button as quickly as possible to the deviant stimulus (blue circle) but not to the standard stimulus (red square). The button box was connected to the computer and placed on the arm of the chair, so that the child only had to move his/her thumb to respond. All stimuli were displayed in the centre of the screen for 50 ms duration. Onset to onset inter-stimulus interval was set at 1.2 s. Standard stimuli occurred with an 80% probability; deviant stimuli, with a 20% probability. The total protocol included three trials of 125 stimuli, for a total of 7.5 min of stimulus presentation. Data acquisition was performed with InstEP® v4.2 (InstEP Systems, Montréal, Canada). The electro-oculogram (EOG) was recorded using four tin electrodes (Beckman) placed at the outer canthus of each eye (horizontal EOG) and above and below the right eye (vertical EOG). ERPs were recorded with Ag-AgCl electrodes from mid-line central (Fz, Cz, Pz, Oz) and from the mastoid (M2) derivations according to the international 10–20 system. Reference and ground electrodes were located on nose and forehead, respectively. The impedance was kept below 5 kΩ. While the EOG gain was set at 7500, the EEG signal was amplified with a gain of 15,000. The digitization rate was 256 Hz.
At the 11-year-old assessment, an auditory oddball paradigm was used to elicit the P3b wave. The children were asked to sit still in front of a computer screen and to press the space key on the computer keyboard as quickly as possible to the deviant stimulus (2000 Hz tone), but not to the standard stimulus (1000 Hz tone). They were also instructed to be as accurate as possible. All stimuli were 70 dB (hearing level) tones, presented for 50 ms and had a rise/fall time of 5 ms. Onset to onset inter-stimulus interval was set at 2 s. Standard stimuli had an 80% probability; target stimuli, a 20% probability. Auditory tones were presented binaurally through ear inserts attached to auditory apparel « E-A-RTONE » from Auditory Systems, and their generation was controlled by the Presentation Software (Neurobehavioral Sciences). Stimuli were presented in a pseudo-randomized sequence: targets were always separated by a minimum of 2 standard tones. The total protocol included 4 trials of 100 stimuli, for a total of 13 min of stimulus presentation. Data acquisition was performed with InstEP® v5.17 (InstEP Systems, Montréal, Canada). The electro-oculogram (EOG) was recorded for both eyes with tin electrodes placed at the supraorbital ridge of one eye and the infra-orbital ridge of the other. ERPs were recorded with 29 Ag-AgCl electrodes (Fz, F3, F4, F7, F8, AF3, AF4, AF7, AF8, FCz, FC1, FC2, Cz, C3, C4, T3, T4, T5, T6, Pz, P3, P4, Oz, O1, O2, M1, M2, A1, and A2 according to the international 10–20 system) referred to linked ear lobes, with forehead ground. The impedance was kept below 10 kΩ. EOG and EEG gain were amplified with a gain of 7500 and 15,000, respectively, with Grass Model 15A54 amplifiers. The digitization rate was 512 Hz.
ERP analyses
ERP analyses were performed using Analyzer 1.05 (Brain Vision©) software. EOG correction was performed from the vertical EOG electrode. High and low pass filters were set at 0.16 and 30 Hz, respectively. Artefact rejection was performed with a ± 100 μV criterion. ERPs were time locked to stimulus onset. Baseline correction was applied using a 100 ms pre-stimulus baseline. Trials with omission (false negative response) and commission (false positive response) errors as well as impulsive responses (<200 ms post-stimuli) were excluded from averaging. The P3b was identified for target condition at Pz position and defined as the maximum positive deflection occurring between 400 and 600 ms post-stimulus for the 5-year assessment, and between 250 and 500 ms at age 11 years. These different latency range values at the two ages were based on previous research reporting a strong decrease in P3b latency across childhood (Johnson, 1989; Johnstone et al., 1996; Picton, 1992; Polich et al., 1990). Latency for each component was determined from stimulus onset to maximal peak, and amplitude was calculated from baseline. Semi-automatic peak detection was used, with co-validation from two different scorers. When discrepancies were found between scores, a decision was made by an expert neurophysiologist (CB). Interrater reliability was high; Pearson correlations between both initial peak scorings were 0.86 for P3b latency and 0.97 for P3b amplitude.
Exclusion criteria
After ERP analysis, participants were excluded from further statistical analyses if they did not have a minimum of 20 recorded deviant stimuli left for averaging (Cohen and Polich, 1997) or if they made commission errors on 15% or more of the standard stimuli or omission errors on 50% or more of the target stimuli.
Biological measures
Umbilical cord blood was used to document prenatal Pb exposure, and child’s blood samples were collected to quantify Pb exposure at time of neurophysiological assessment. The analyses were performed at the Laboratoire de Toxicologie INSPQ, which is accredited by the Canadian Association for Environmental Analytical Laboratories. Pb concentrations were analysed in cord and child blood by graphite furnace atomic absorption with Zeeman background correction (Perkin Elmer model ZL4100). Quality control and detailed laboratory procedures are presented elsewhere (Rhainds et al., 1999). Pb detection limit in cord blood and blood samples at 5 years was 0.2 μg/dL, and was 0.002 μg/dL at 11 years. Pb was detected in all blood samples. Blood samples were also analysed for mercury (Hg) and polychlorinated biphenyls (PCBs).
Statistical analyses
The normality of each variable’s distribution was subjected to visual inspection and checked for skewness/kurtosis values (normality range: −2.0 to 2.0). Log transformations were conducted on the Pb concentration variables since they followed log-normal distributions. Extreme values (>3 standard deviations from the mean) for normally distributed variables were recoded to one point greater than the highest observed non-outlying value following the procedure recommended by Winer (1971). One outlier was recoded for each of the following variables: P3b latency at both 5 and 11 years of age, socio-economic status and haemoglobin level at 11 years.
The associations between Pb exposure (cord-blood and blood levels at 5 and 11 years) and P3b parameters (latency and amplitude at ages 5 and 11 years) were first tested by performing Pearson correlation analyses. Hierarchical multiple regression analyses were then conducted in order to assess the relation between Pb exposure and P3b parameters when controlling for significant confounders. Selection of confounders was determined using a hybrid strategy combining significance test and change-in-estimate procedures. First, among a set of potential confounders (see Table 1), each variable related even weakly to the P3b dependent variable (at p < 0.20) was selected. Thereafter, each of the selected variables was tested to see whether its inclusion in the regression model altered the relation of Pb to the outcome in question. Pb was entered in the first step of the regression analysis. Each potential confounder was then entered hierarchically and retained if its inclusion changed the association (β coefficient) between Pb exposure and the outcome by at least 10%. The 0.20 alpha level and 10% change value criteria were based on the work of Greenland and associates (Greenland and Rothman, 1998; Maldonado and Greenland, 1993). Associations between Pb and ERP parameters were considered significant when p ≤ 0.05 after control for the potential confounders retained in the model. Missing values from significant confounding variables were replaced by the sample median value. This was done for maternal non-verbal intelligence at 5 years (n = 1), and haemoglobin (n = 3) and PCB-153 levels at 11 years (n = 4).
Results
Descriptive data
In the 5-year assessment sample, cord blood Pb levels averaged 4.90 μg/dL and blood Pb levels at testing averaged 5.24 μg/dL. Although this is lower than the threshold value used in action guidelines of the U.S. and Canadian public health agencies (10 μg/dL) (Centers for Disease Control and Prevention, 1991; Health Canada, 1994), it is similar to levels found in other cohorts where low Pb exposure was related to subtle cognitive impairments (e.g. Chiodo et al., 2004; Jusko et al., 2008) and it is more than twice as high as the mean blood level from a representative sample of U.S. preschool children taking part to the National Health and Nutrition Examination Survey III, 3rd phase (Jones et al., 2009). Twelve children (11.5%) had blood levels above the recommended value of 10 μg/dL. In the 11-year assessment sample, average blood Pb levels were 4.80 μg/dL and 2.66 μg/dL at birth and at 11 years, respectively. Pb levels were significantly lower at 11 years compared to 5 years (t (77) = 5.16, p < 0.01). At 11 years, six children (3.0%) had blood levels that were above the recommended maximum value. Pearson correlations revealed low-to-moderate associations between cord and blood Pb levels at 5 (r = 0.26, p < 0.01; n = 104) and 11 years (r = 0.20, p < 0.01; n = 195), and between Pb levels at 5 and 11 years (r = 0.28, p < 0.01; n = 80).
ERP results
At the 5-year assessment, ERP data from 34 children could not be used due to data loss following a major computer problem that occurred after their assessments. Of the 70 children remaining, only 27 (39%) satisfied the criteria for the analyses of the ERPs. Reasons for exclusion were: too much noise in the waveform to identify the P3b component (n = 18), lack of collaboration (n = 11), technical problems during the task (e.g. problem with software, power failure; n = 6), < 20 deviant trials left for averaging after artefact rejection (n = 5) and too many errors while completing task (n = 3). Among the retained participants, three had blood levels ≥ 10 μg/dL. Univariate analysis of variance (ANOVA) revealed that the retained and excluded participants did not differ in terms of cord blood (F (1, 69) = 0.18, p > 0.20) or blood Pb levels at time of testing (F (1, 69) = 0.83, p > 0.20).
Of the 201 children participating in the 11-year ERP protocol, 110 (55%) met the criteria for inclusion in the statistical analysis. Reasons for exclusion were: no evidence of P3b wave following averaging (n = 59), lack of cooperation (n = 15), technical problems (n = 9), too many errors during the task (n = 7) and not enough deviant stimuli in the averaged waveform (n = 1). Among the retained participants, two had blood Pb levels above 10 μg/dL. Blood Pb levels were slightly higher among the excluded participants (3.01 μg/dL) compared to those who were included (2.37 μg/dL) (F (1,196) = 4.04; p = 0.05).
The weighted grand averages at Pz electrode location for the 5-year and 11-year assessments are shown on Figure 1, while data for the P3b measures are summarized in Table 2. P3b appears much later and was larger at 5 years compared to 11 years. T-tests performed for participants included at both assessments (n = 9) revealed that the differences in P3b parameters of the P3b wave (Pz) elicited in the deviant condition are statistically significant for both latency (mean = 476.6 ms at 5 years vs 343.3 ms at 11 years; t (8) = 7.80, p < 0.001) and amplitude (mean = 23.2 μV at 5 years vs 9.10 μV at 11 years; t (8) = 5.51, p = 0.001). At each assessment, Pearson correlations revealed no association between P3b latencies and amplitudes (all p > 0.20).
Figure 1.
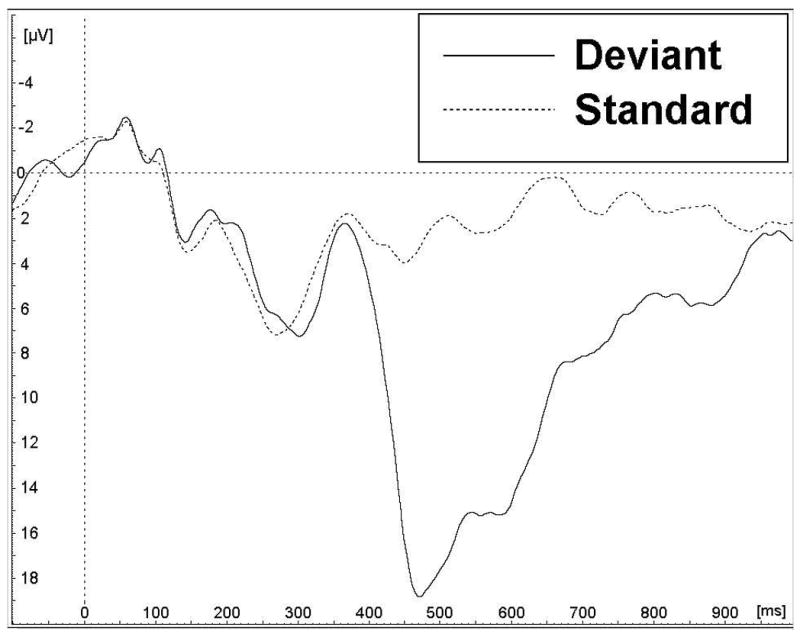
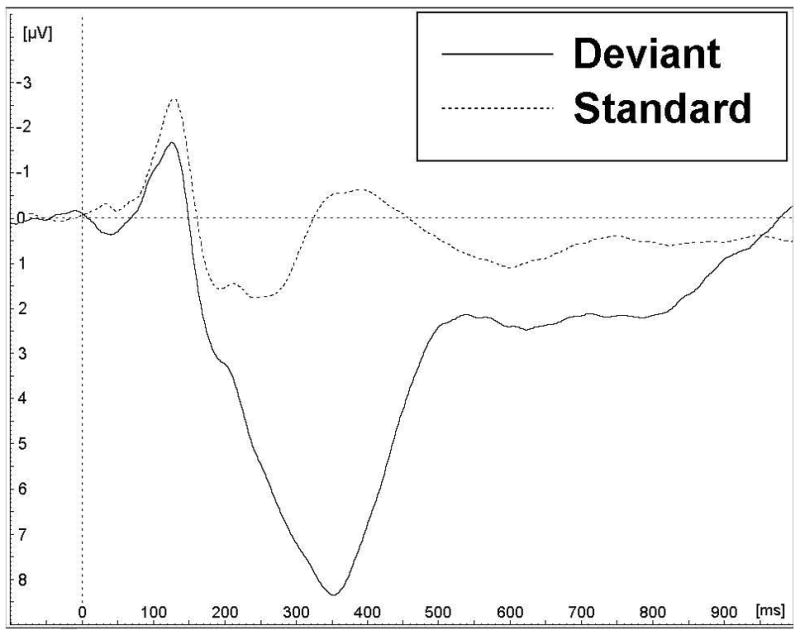
Grand averages for the retained participants, for both assessment phases (5 years and 11 years old) for parietal (Pz) lead. Y-axis scales were adjusted in order to maximize the waveform’s size in each image. 1a) At 5 years (N = 27), a clear positive wave peaks about 450–500 ms post-stimulus in the deviant condition and was considered the P3b; 1b) At 11 years (N = 110), the late positive wave is still present, but peaks earlier, about 350 ms post-stimulus.
Table 2.
Descriptive characteristics for P3b in target condition at Pz (5 and 11 years)
| Latency (ms) |
Amplitude (μV) |
||||||
|---|---|---|---|---|---|---|---|
| N | Mean | Range | Std Dev | Mean | Range | Std Dev | |
| 5 years | 27 | 481.3 | 445.3 – 585.9 | 31.4 | 22.8 | 8.9 – 34.5 | 8.0 |
| 11 years | 110 | 347.9 | 267.6 – 484.4 | 39.4 | 10.8 | 4.3 – 21.1 | 3.8 |
Note. 5-year data were obtained from a visual oddball paradigm whereas 11-year data were obtained from an auditory oddball paradigm. ERP data prior to winzorizing are presented.
Relation of Pb exposure to P3b (deviant condition) at 5 years
Results from correlation and regression analyses involving ERP parameters are reported in Table 3. Regression analyses revealed that cord blood Pb concentrations were not related to 5-year P3b latency (β = −0.08, p = 0.65) but were associated with a decrease in 5-year P3b amplitude (β = −0.38, p = 0.05). Blood Pb levels at age 5 years were related to an increased P3b latency (β = 0.37, p = 0.04) but were not associated with P3b amplitude (β = −0.04, p = 0.83). As illustrated in Figure 2a) and b), these associations did not appear only above a certain threshold of exposure. -Insert Figure 2 about here--Insert Table 3 about here-
Table 3.
Relation of Pb exposure to P3b parameters (Pz)
| Pb levels in cord blood |
Pb levels at 5 years |
Pb levels at 11 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Pearson r | Standardized β | N | Pearson r | Standardized β | N | Pearson r | Standardized β | |
| 5 years | |||||||||
| P3b latency | 27 | −0.25 | −0.08 | 27 | 0.55** | 0.37* | - | - | - |
| P3b amplitude | 27 | −0.38* | −0.38* | 27 | −0.04 | −0.04 | - | - | - |
| 11 years | |||||||||
| P3b latency | 108 | 0.07 | 0.00 | 40 | −0.03 | −0.02 | 107 | 0.04 | −0.01 |
| P3b amplitude | 108 | −0.06 | −0.04 | 40 | −0.30† | −0.27† | 107 | −0.05 | −0.03 |
Note. 5-year data were obtained from a visual oddball paradigm whereas 11-year data were obtained from an auditory oddball paradigm. Analyses were performed with log-transformed blood Pb concentrations. Covariables included in the models were: maternal non-verbal intelligence, breastfeeding status and Hg levels at testing time (P3b latency at 5 years), haemoglobin, PCB-153 levels and age at testing (P3b latency at 11 years), and socioeconomic status (P3b amplitude at 11 years).
p ≤ 0.01
p ≤ 0.05
p ≤ 0.10
Figure 2.
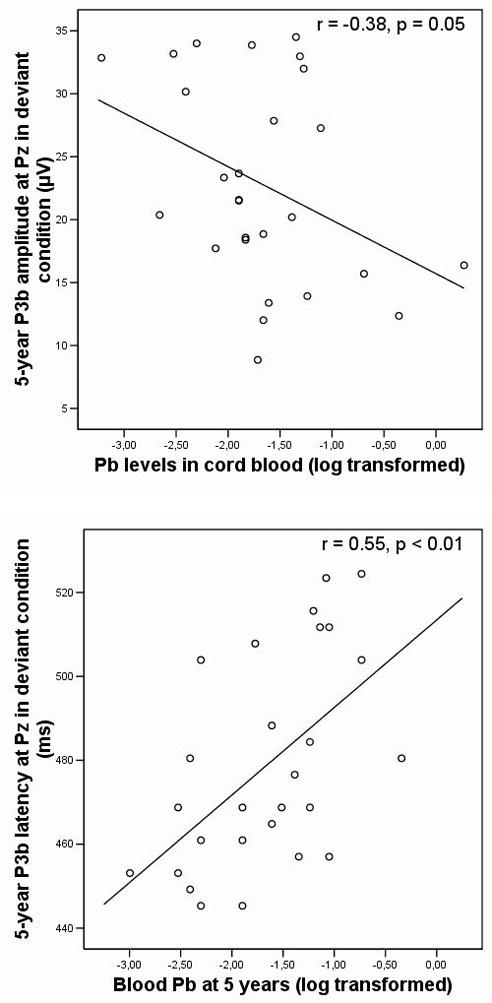
Scatterplots showing the relationships between Pb levels and P3b parameters at 5 years of age (N = 27). Dots represent individual values. Both graphs suggest the Pb-P3b relationship is linear.
Relation of Pb exposure to P3b (deviant condition) at 11 years
As reported in Table 3, cord blood Pb concentrations were not related to ERP parameters at 11 years of age. Blood Pb concentrations during early childhood were not associated with P3b latency but showed a negative association with P3b amplitude falling short of statistical significance in both Pearson correlation (r = −0.30, p = 0.06) and regression (β = −0.27, p = 0.10) analyses. Finally, blood Pb concentrations at 11 years were not related to either P3b parameter.
Discussion
This study was designed to assess Pb neurotoxicity in children aged 5 and 11 years in relation to the ERP P3b wave in oddball paradigms. Prenatal Pb exposure was related to decreased P3b amplitude at 5 years of age. Exposure occurring before school age, as assessed by 5-year Pb levels, was related to delayed P3b latency at 5 years and tended to be associated with decreased P3b amplitude at 11 years. Results from this electrophysiological study corroborate those from neurobehavioural studies involving children (e.g. Chiodo et al., 2004; Lanphear et al., 2000; Surkan et al., 2007), in that we found that early exposure to background levels of Pb affects a specific ERP component believed to be involved in working memory, an important domain of human cognition required for learning and optimal school performance.
In this study, the nature of the associations differed according to the timing of Pb exposure. Results from the 5-year assessment suggested that prenatal Pb exposure is related to decreased allocation of resources for working memory, whereas delayed information processing speed is related to Pb exposure during early childhood. This supports the hypothesis that Pb, like other neurotoxicants, has different mechanisms of action depending on the degree of brain maturation. Early processes of brain development, such as neuronal migration, might be indirectly altered by prenatal Pb exposure (Hu et al., 2008). In contrast, Pb exposure during early childhood is likely to affect developmental processes occurring mainly after birth, such as myelinisation. Myelinating cells are known to be especially sensitive to Pb toxicity (Tiffany-Castiglioni, 1993).
The associations also differed with age of children at testing. At both the 5- and 11-year assessments, the strongest associations were found for blood Pb levels assessed at 5 years of age. This may be attributable to the fact that bioaccumulation of Pb is usually greatest during the toddler and preschool periods, which are presumably, therefore, the periods of greatest impact of this exposure on brain development (Lidsky and Schneider, 2003). Because no auditory protocol was used at 5 years and because the visual protocol was not repeated at 11 years, we cannot assume that modality differences in the oddball tasks account for the different findings at both ages.
The P3b is thought to reflect working memory processing (Donchin, 1981; Donchin and Coles, 1988). Associations between Pb exposure and this ERP component are consistent with the adverse effects of Pb documented with neurobehavioral measures in previous studies (Lanphear et al., 2000; Surkan et al., 2007) and suggest that working memory is a cognitive domain especially vulnerable to the effects of Pb exposure. Another domain that has been consistently shown to be affected by Pb exposure is executive function (Canfield et al., 2004; Chiodo et al., 2004; Surkan et al., 2007). Recent developments in electrophysiological techniques have led to the elaboration of new ERP protocols (e.g. with Go/No-go, Stop-signal and Flanker paradigms) for specific assessment of executive processes, such as conflict monitoring and response inhibition (Dimoska et al., 2007; Jonkman, 2006; Rueda et al., 2004). Such measures could provide other sensitive indexes of Pb toxicity in future studies.
Despite their importance from a public health perspective, the results of this study must be cautiously interpreted. Valid data were available only for a small number of the children who were assessed at 5 years, and the non-significant tendency towards a decreased P3b amplitude at 11 years was observed in relation to 5-year blood Pb levels, which were not documented for all children participating in the 11-year-old assessment. Also, because of the relatively short half-life of Pb in blood (Rabinowitz et al., 1976), exposure assessment would have benefited from multiple blood samplings during childhood. This approach could have provided key information on the developmental trajectory of exposure and helped identify the age period at increased risk for developing Pb-related cognitive dysfunction.
Very few studies have assessed the P3b wave of the ERPs in children at or before 5 years of age. In our study with 5-year-old children, a clear positive component peaking between 450 and 550 ms at Pz was elicited by target stimuli in a visual oddball protocol, which we assumed to be the P3b component. The obtained P3b latency values were higher than what is usually observed in older subjects with similar protocols (e.g. Veiga et al., 2004) but were comparable to those found in other studies of young children (Johnson, 1989; Polich et al., 1990). Although clearly present in some children, however, this component was not always identifiable, resulting in a high rejection rate. Many children failed to complete the entire protocol, which may explain why so little information on the P3b is available for children this age. In addition, noise in the EEG recordings was frequent.
Nonetheless, the present study supports and extends the results from previous neurobehavioral studies of Pb neurotoxicity in children and adults by demonstrating the sensitivity of the P3b component of ERPs, an electrophysiological measure attributed to working memory processing, to early childhood Pb exposure. Associations between Pb exposure and P3b alterations were observed in children with blood levels which, for the most part, were lower than the minimum level necessary to initiate public health interventions. These results strengthen arguments for reducing the blood Pb level considered as acceptable by governmental agencies and for continuing local and international actions to reduce human exposure to this contaminant.
Acknowledgments
We are grateful to the Nunavik population for their participation in this study and to the medical and health care professionals from the health centers and nursing stations involved for their assistance. We acknowledge the long time support of the Nunavik Nutrition and Health Committee, the public health director of the Nunavik Regional Board of Health and Social Services, the Municipal Councils of Puvirnituq, Inukjuak, Kuujjuaq and Kuujjuarapik, Inuit Tapiriit Kanatami, Pauktuutit Inuit Women's Association, and Nunalituqait Ikaluqatigiitut Association. We are thankful to Jocelyne Gagnon, Christine Després, Karine Poitras, Carole Vézina, Renee Sun, Brenda Tuttle, Line Roy, Renée Dallaire, Suzanne Bruneau, Mary Nuluki and Germain Lebel for their involvement in many phases of this research.
This research was funded by annual grants from Indian and Northern Affairs Canada (Northern Contaminants Program) and grants from the National Institute of Environmental Health and Sciences/U.S. National Institutes of Health (R01 ES007902), Health Canada (Toxic Substances Research Initiative #239), March of Dimes Birth Defect Foundation (#12-FY99-49), FRSQ-Hydro-Québec (Environmental Child Health Initiative), the Joseph Young, Sr., Fund from the State of Michigan, and Nunavik Regional Board of Health and Social Services. O. Boucher was supported by doctoral grants from the Canadian Institutes of Health Research and from the Nasivvik Centre for Inuit Health and Changing Environments.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann L, Sveinsson K, Krämer U, Weishoff-Houben M, Turfeld M, Winneke G, et al. Visual functions in 6-year-old children in relation to lead and mercury levels. Neurotoxicol Teratol. 1998;20:9–17. doi: 10.1016/s0892-0362(97)00070-6. [DOI] [PubMed] [Google Scholar]
- Araki S, Murata K, Yokoyama K, Uchida E. Auditory event-related potential (P300) in relation to peripheral nerve conduction in workers exposed to lead, zinc, and copper: effects of lead on cognitive function and central nervous system. Am J Ind Med. 1992;21:539–47. doi: 10.1002/ajim.4700210409. [DOI] [PubMed] [Google Scholar]
- Banks EC, Ferretti LE, Shucard DW. Effects of low level lead exposure on cognitive function in children: a review of behavioural, neuropsychological and biological evidence. Neurotoxicology. 1997;18:237–81. [PubMed] [Google Scholar]
- Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–40. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Preventing lead poisoning in young children: a statement by the Centers for Disease Control and Prevention. Atlanta: Centers for Disease Control and Prevention; 2001. [accessed 15 August 2008]. 4th Revision. Available: http://www.cdc.gov/nceh/lead/publications/books/plpyc/contents.htm. [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26:359–71. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Cohen J, Polich J. On the number of trials needed for P300. Int J Psychophysiol. 1997;25:249–55. doi: 10.1016/s0167-8760(96)00743-x. [DOI] [PubMed] [Google Scholar]
- Counter SA. Brainstem neural conduction biomarkers in lead-exposed children of Andean lead-glaze workers. J Occup Environ Med. 2003;44:855–64. doi: 10.1097/00043764-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–43. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Covington JW, Polich J. P300, stimulus intensity, and modality. Electroencephalogr Clin Neurophysiol. 1996;100:579–84. doi: 10.1016/s0168-5597(96)96013-x. [DOI] [PubMed] [Google Scholar]
- Després C, Beuter A, Richer F, Poitras K, Veilleux A, Ayotte P, et al. Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol Teratol. 2005;27:245–57. doi: 10.1016/j.ntt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!... Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–74. [Google Scholar]
- Greenland S, Rothman KJ. Introduction to stratified analysis. In: Rothman KJ, Greenland S, editors. Modern epidemiology. Philadephia: Lippincott, Williams, & Wilkins; 1998. pp. 253–79. [Google Scholar]
- Gurrera RJ, Salisbury DF, O'Donnell BF, Nestor PG, McCarley RW. Auditory P3 indexes personality traits and cognitive function in healthy men and women. Psychiatry Res. 2005;133:215–28. doi: 10.1016/j.psychres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hansenne M. Le potential évoqué cognitif P300 (I): aspects théorique et psychobiologique [in French] Neurophysiol Clin. 2000;30:191–210. doi: 10.1016/s0987-7053(00)00223-9. [DOI] [PubMed] [Google Scholar]
- Health Canada EHD. Update of evidence for low-level effects of lead and blood lead intervention levels and strategies. Ottawa: Health Canada; 1995. Federal-Provincial Committee on Environmantal and Occupational Health. [Google Scholar]
- Hirata M, Kosaka H, Yoshida T. A study on the effect of lead on event-related potentials among lead-exposed workers. Ind Health. 2004;42:431–34. doi: 10.2486/indhealth.42.431. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Heaven: 1975. Unpublished Manuscript. [Google Scholar]
- Hu Q, Fu H, Ren T, Wang S, Zhou W, Song H, et al. Maternal low-level lead exposure reduces the expression of PSA-NCAM and the activity of sialyltransferase in the hippocampi of neonatal rat pups. Neurotoxicology. 2008;29:675–681. doi: 10.1016/j.neuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Ji J, Porjesz B, Begleiter H, Chorlian D. P300: The similarities and differences in the scalp distribution of visual and auditory modality. Brain Topogr. 1999;11:315–27. doi: 10.1023/a:1022262721343. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr Developmental evidence for modality-dependent P300 generators: a normative study. Psychophysiology. 1989;26:651–67. doi: 10.1111/j.1469-8986.1989.tb03167.x. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Anderson JW, Coyle SF. Age-related changes in child and adolescent event-related potential component morphology, amplitude and latency to standard and target stimuli in an auditory oddball task. Int J Psychophysiol. 1996;24:223–38. doi: 10.1016/s0167-8760(96)00065-7. [DOI] [PubMed] [Google Scholar]
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123:e376–e386. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Jonkman LM. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood: a Go/Nogo ERP study. Brain Res. 2006;1097:181–93. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Jr, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield R. Blood lead concentrations < 10 μg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–48. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis D, Fabiani M, Donchin E. “P300” and memory: individual differences in the von Restorff effect. Cogn Psychol. 1984;16:177–216. [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 μg/dL in US children and adolescents. Public Health Rep. 2000;115:521–29. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque B, Duchesne JF, Gariépy C, Rhainds M, Dumas P, Scheuhammer AM, et al. Monitoring of umbilical cord blood lead levels and sources assessment among the Inuit. Occup Environ Med. 2003;60:693–95. doi: 10.1136/oem.60.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environ Res. 2006;100:284–93. doi: 10.1016/j.envres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: A comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109:1291–99. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann E, Huber C, Maier S, Plihal W, Wustmans A, Diedrich O, et al. The scalp topography of P300 in the visual and auditory modalities: a comparison of three normalization methods and the control of statistical type II error. Electroencephalogr Clin Neurophysiol. 1992;83:254–264. doi: 10.1016/0013-4694(92)90119-3. [DOI] [PubMed] [Google Scholar]
- Otto DA. The assessment of neurotoxicity in children. Electrophysiological methods. Monogr Am Assoc Ment Defic. 1987;8:139–58. [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–79. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J, Howard L, Starr A. P300 latency correlates with Digit Span. Psychophysiology. 1983;20:665–69. doi: 10.1111/j.1469-8986.1983.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, Burns T. Normal variation of P300 in children: age, memory span, and head size. Int J Psychophysiol. 1990;9:237–48. doi: 10.1016/0167-8760(90)90056-j. [DOI] [PubMed] [Google Scholar]
- Portin R, Kovala T, Polo-Kantola P, Revonsuo A, Müller K, Matikainen E. Does P3 reflect attentional or memory performances, or cognition more generally? Scand J Psychol. 2000;41:31–40. doi: 10.1111/1467-9450.00168. [DOI] [PubMed] [Google Scholar]
- Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58:260–270. doi: 10.1172/JCI108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary scales: standard progressive matrices. Oxford: Psychologists; 1992. [Google Scholar]
- Rhainds M, Levallois P, Dewailly E, Ayotte P. Lead, mercury, and organochlorine compound levels in cord blood in Québec, Canada. Arch Environ Health. 1999;54:40–7. doi: 10.1080/00039899909602235. [DOI] [PubMed] [Google Scholar]
- Romero R, Polich J. P3(00) habituation from auditory and visual stimuli. Physiol Behav. 1996;59:517–22. doi: 10.1016/0031-9384(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Rothenberg SJ, Poblano A, Schnaas L. Brainstem auditory evoked response at five years and prenatal and postnatal blood lead. Neurotoxicol Teratol. 2000;22:503–10. doi: 10.1016/s0892-0362(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK, Davis-Stober CP. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci. 2004;5:39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz SJ, Schmidt LA. Capturing the dynamic endophenotype: a developmental psychophysiological manifesto. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology: Theory, Systems, and Methods. Cambridge: Cambridge University Press; 2008. pp. 1–12. [Google Scholar]
- Solliway BM, Schaffer A, Pratt H, Yannai S. A multidisciplanary study of lead-exposed subjects. I. Delayed target detection P-300 latency, an electrophysiological parameter, correlates with urinary δ-ALA. Environ Res. 1994;67:168–82. doi: 10.1006/enrs.1994.1072. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 μg/dL. Neurotoxicology. 2007;28:1170–77. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S, Ruchkin DS. The late positive complex. Advances and new problems. Ann NY Acad Sci. 1984;425:1–23. doi: 10.1111/j.1749-6632.1984.tb23520.x. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E. Cell culture models for lead toxicity in neuronal and glial cells. Neurotoxicology. 1993;14:513–536. [PubMed] [Google Scholar]
- Veiga H, Deslandes A, Cagy M, McDowell K, Pompeu F, Piedade R, et al. Visual event-related potentials (P300): a normative study. Arq Neuropsiquiatr. 2004;62:575–81. doi: 10.1590/s0004-282x2004000400002. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. The relationship between P3 and neuropsychological function in an adult life span sample. Biol Psychol. 2003;62:65–87. doi: 10.1016/s0301-0511(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Walkowiak J, Altmann L, Krämer U, Sveinsson K, Turfeld M, Weishoff-Houben M, et al. Cognitive and sensorimotor functions in 6-year-old children in relation to lead and mercury levels: Adjustment for intelligence and contrast sensitivity in computerized testing. Neurotoxicol Teratol. 1998;20:511–21. doi: 10.1016/s0892-0362(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Popovac D, Factor-Litvak P, Kline J, Waternaux C, et al. The Yugoslavia Prospective Lead Study: contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol Teratol. 2000;22:811–18. doi: 10.1016/s0892-0362(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2. NewYork: McGraw-Hill; 1971. [Google Scholar]
- Winneke G, Altmann L, Krämer U, Turfeld M, Behler R, Gutsmuths FJ, et al. Neurobehavioral and neurophysiological observations in six year old children with low lead levels in East and West Germany. Neurotoxicology. 1994;15:705–13. [PubMed] [Google Scholar]
- Wold Health Organization. Inorganic lead. International Program on Chemical Safety; Geneva: 1995. Environmental health criteria 165. [Google Scholar]


