Abstract
Any insult that affects survival of ovarian antral follicles can cause abnormal estradiol production and fertility problems. Phthalate esters (PEs) are plasticizers used in wide range of consumer and industrial products. Exposure to these chemicals has been linked to reduced fertility in humans and animal models. Di-(2-ethylhexyl) phthalate (DEHP) and Mono-(2-ethylhexyl) phthalate (MEHP) decreases serum estradiol levels and aromatase (Arom) expression, prolongs estrous cycles, and causes anovulation in animal and culture models. These observations suggest PEs directly target antral follicles. We therefore tested the hypothesis that DEHP (1–100 µg/ml) and MEHP (0.1–10 µg/ml) directly inhibit antral follicular growth and estradiol production. Antral follicles from adult mice were cultured with DEHP or MEHP, and/or estradiol for 96 hrs. During culture, follicle size was measured every 24 hrs as a measurement of follicle growth. After culture, media was collected for measurement of estradiol levels and follicles were subjected to measurement of cylin-D-2 (Ccnd2), cyclin-dependant-kinase-4 (Cdk4), and Arom. We found that DEHP and MEHP inhibited growth of follicles and decreased estradiol production compared to controls at highest doses. DEHP and MEHP also decreased mRNA expression of Ccnd2, Cdk4, and Arom with highest dose. Addition of estradiol to the culture medium prevented the follicles from DEHP- and MEHP-induced inhibition of growth, reduction in estradiol levels, and decreased Ccnd2 and Cdk4 expression. Collectively, our results indicate that DEHP and MEHP may directly inhibit antral follicle growth via a mechanism that partially includes reduction in levels of estradiol production and decreased expression of cell cycle regulators.
Keywords: phthalates, DEHP, MEHP, ovary, antral follicles, cell cycle
INTRODUCTION
Endocrine disrupting chemicals (EDCs) pose threats to human development and reproduction by interfering with normal physiological processes. Some EDCs mimic or block endocrine action directly or indirectly by competing with endogenous hormones. Sex steroids, particularly estrogens, are essential for normal development and function of the reproductive system (Britt et al., 2004; Hirshfield 1991). In females, the majority of sex steroids are produced by antral follicles in the ovary. Estradiol (E2) produced by antral follicles is essential for maintaining fertility (Britt et al., 2004; Hirshfield 1991). Exposure to EDCs that affect the production and/or action of estrogens could lead to infertility.
Phthalates, or phthalate esters (PEs), are one class of chemicals that have been shown to reduce E2 synthesis in ovarian cells (Davis et al., 1994a; Davis et al., 1994b; Lovekamp and Davis, 2001). Phthalates are plasticizers which are used in a wide range of consumer and industrial products including toys, nail polish, household vinyl, automobiles, and medical devices. Dozens of PEs have been developed over the years, including di-n-butyl phthalate (DBP), di-(2-ethylhexyl) phthalate (DEHP), and butyl benzyl phthalate (BBP). More than 18 billion pounds of phthalates are used worldwide each year and humans are exposed through inhalation, ingestion and dermal absorption on a daily basis (Muncke 2009; Heudorf et al., 2007). The levels of phthalates in household dust can reach as high as 400–700 mg/kg (Becker et al., 2004). DEHP is one of the most commonly used plasticizer (Tanaka et al., 1975). Aristech Chemical Company reported in 1999 that the production volume of DEHP is about 2 million tons (cited in CERHR Evaluation, 2000). The Agency for Toxic Substances and Disease Registry reported that the maximum daily exposure to DEHP for the general population is about 2 mg/day, but certain populations may be exposed to much higher levels (ATSDR, 1993). For example, exposure to phthalates can occur via plastic medical devices, with patients undergoing hemodialysis receiving as much as 150 mg of DEHP in 5 h (Gibson et al., 1976).
Many of these PEs are considered to be EDCs based on their effects on reproductive organs. In humans, exposure of females to PE, such as DEHP and DBP during pregnancy resulted in defects in the genitalia of the male offspring (Marsee et al., 2006). In laboratory male rats, PE exposure is associated with various degrees of cryptorchidism, testicular injury, and malformations of the epididymis, vas deferens, seminal vesicles, prostate, and external genitalia (Foster, 2006).
In humans, a positive correlation between high urine phthalate levels and pregnancy complications such as anemia, toxemia, and preeclampsia was found in pregnant women living near a plastics manufacturing company (Tabacova et al., 1999). Chronic occupational exposure to high phthalate levels also has been associated with decreased pregnancy rates, increased rates of miscarriage, and decreased estrogen levels in a group of women factory workers in Russia (Aldyreva et al., 1975). In female laboratory rats, DEHP has been shown to increase the number of atretic tertiary follicles in the ovary (Grande et al., 2007). In another study, DEHP-treated rats had prolonged estrous cycles, reduced serum E2 levels, and defects in ovulation (Davis et al., 1994a). Mono-(2-ethylhexyl) phthalate (MEHP), a metabolite of DEHP, has been shown to inhibit follicle-stimulating hormone (FSH)- stimulated cAMP accumulation and progesterone production in rat granulosa cells (Treinen et al., 1990). MEHP also reduces E2 production by decreasing aromatase (Arom), the rate-limiting enzyme that converts testosterone to E2, in rat granulosa cell cultures (Lovekamp and Davis, 2001).
Despite the potential adverse reproductive outcomes associated with PE exposure, very little is known about the mechanisms by which PEs cause toxicity in the ovary. This work was designed to investigate whether PEs (specifically DEHP and MEHP) directly inhibit antral follicle growth by reducing E2 levels. Growth of cells in antral follicles is, in part, controlled by cell cycle related factors. PEs could affect follicle growth by altering the expression of selected cell cycle regulators in antral follicles. We chose to focus on cyclin-D2 (Ccnd2) and cyclin dependant kinase-4 (Cdk4) because these factors are required for the first steps in the cell cycle progression, the progression of cell cycle from G1 to S phase. E2 is known to be an important factor regulating growth of follicles. We supplemented our cultures with E2 to test whether E2 replacement would rescue follicles from toxic effects of DEHP and MEHP on antral follicles.
MATERIALS AND METHODS
Chemicals
DEHP and MEHP were purchased from Sigma (St. Louis, MO). Stock solutions of DEHP and MEHP were prepared using dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO) as the solvent in various concentrations (133, 13.3, 1.33, and 0.133 mg/ml) that allowed an equal volume to be added to culture wells for each treatment group to control for solvent concentration. Final concentrations in culture were 1, 10, and 100 µg/ml of DEHP, which is equivalent to approximately 2.77, 27.7, and 277 µM. Final concentrations of MEHP in culture were 0.1, 1, and 10 µg/ml, which are equivalent to 0.34, 3.4 and 34 µM. We chose these doses based on previous studies on the effects of DEHP and MEHP on cultured cells or follicles and were shown to be clinically relevant (Gillum et al., 2009; Lenie and Smith, 2009; Lovekamp and Davis, 2001; Mlynarcíková et al., 2009). We also chose these doses based on our preliminary dose-response experiment. The final concentrations of E2 (Sigma, St. Louis, MO) in culture were 1 and 10 nM. Our choice for E2 concentration was based on previous publications (Miller et al., 2006 and Symonds et al., 2005). These studies used E2 at the concentration of 1–50nM in follicle and ovarian surface epithelium cell cultures without any overt or supra physiologic responses. Further, the E2 levels observed in our follicle cultures and other studies (Lenie and Smitz, 2009) very closely match the levels we used to supplement the cultures. In our experiments, from the figures 2A and 2B, we observed that the level of E2 in vitro follicle culture ranges from 1200 to 4500 pg/ml of media. The amount used as co-treatment in our cultures is 1 nM and 10 nM, which is equivalent to 300 and 2000 pg/ml. Thus, the doses used for our follicle culture fall below and within the range of the E2 levels observed in our follicle cultures. For experiments involving E2 treatment, we used follicles treated with the highest dose of DEHP (100 µg/ml) and MEHP (10 µg/ml) because PEs were observed to inhibit follicle growth at these doses.
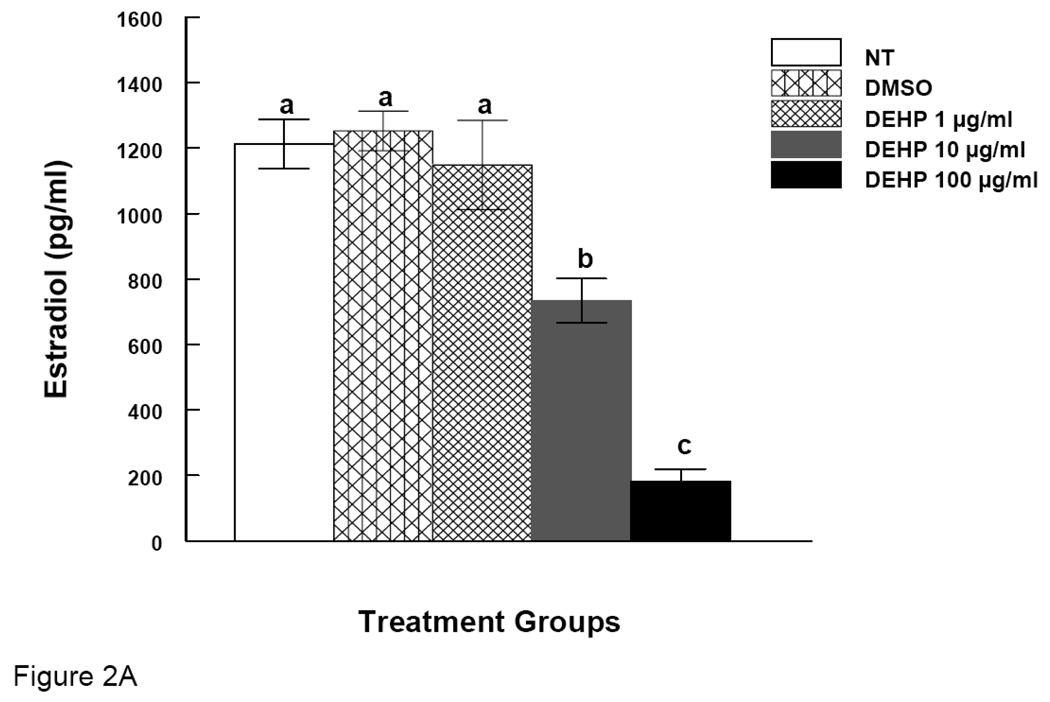
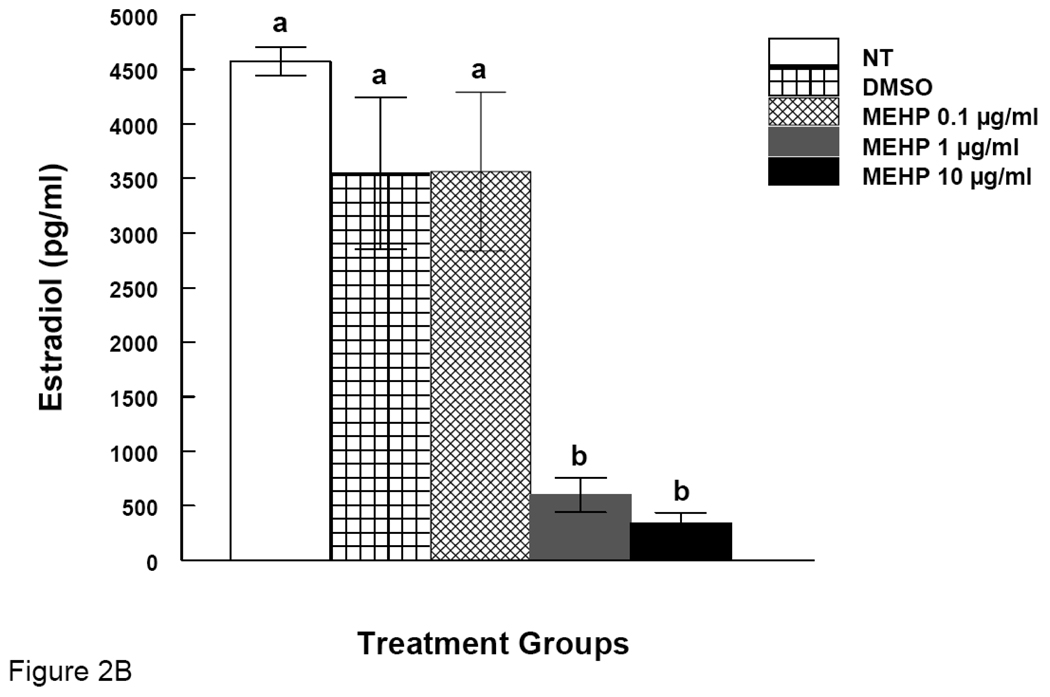
Figure 2. Effect of DEHP or MEHP Treatment on Estradiol Levels.
Antral follicles were cultured in the presence of either DEHP (1, 10, or 100 µg/ml in A) or MEHP (0.1, 1, or 10 µg/ml in B) for 96 h. After culture, media were collected for measurements of estradiol levels by ELISA. NT = nontreated, DMSO = dimethylsulphoxide. Graph represents means ± SEM from three separate experiments. Bars with different letters are significantly different from controls (p ≤ 0.05).
Animals
Cycling female CD-1 (Charles River Laboratories, Charles River, CA) mice (32–35 days old) were housed 4 animals per cage at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility and provided food and water ad libitum. Temperature was maintained at 22 ±1 °C and animals were subjected to 12-hour light-dark cycles. Cycling mice (39 days old) were euthanized for ovary collection and follicle isolation as described below. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
In Vitro Follicle Culture
Antral follicles were isolated mechanically from the ovary based on size (more than 200 µm) and cleaned of interstitial tissue using fine watchmaker forceps by teasing it off from the follicle (Gupta et al., 2006; Miller et al., 2005). Sufficient numbers (10–15 follicles per treatment) of antral follicles for experimental significance were isolated from unprimed (not been stimulated by any treatment) mouse ovaries; follicles from 2–4 mice were isolated providing approximately 20–40 antral follicles from each mouse. Upon isolation, follicles were placed individually in a 96-well culture plate with unsupplemented α-minimal essential media (α-MEM) prior to treatment. Each experiment contained a minimum of 10–16 follicles per treatment.
Treatment groups included DEHP (1, 10, or 100 µg/ml), MEHP (0.1, 1, or 10 µg/ ml) and DMSO which were prepared in α-MEM. In addition, DEHP (100 µg/ml), MEHP (10 µg/ml), DEHP (100 µg/ml) + E2 (1 or 10 nM), MEHP (10 µg/ml) + E2 (1 or 10 nM), and DMSO controls were also prepared in supplemented α-MEM for experiments involving E2. An equal volume of chemical was added for each dose to control for the amount of vehicle in each preparation. Supplemented α-MEM was prepared with: 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant FSH (Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). Follicles culture was performed for 96 hrs as described in previous publications (Gupta et al., 2006; Miller et al., 2005). Follicles were exposed to the treatments for the whole 96 hrs.
Analysis of Follicle Growth
Follicle growth was examined at 24 hr intervals by measuring follicle diameter on two perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Antral follicles were follicles with diameters of 200 µm or greater (Cortvrindt and Smitz, 2002; Miller et al., 2005). Follicle diameter measurements were averaged among treatment groups and plotted to compare the effects of chemical treatments on growth over time. Data were presented as percent change over time.
Measurement of Estradiol Levels
Media collected from antral follicle culture was subjected to measurement of E2 using enzyme linked immunoassay (ELISA). The ELISA kits were purchased from DRG International Inc. (Mountainside, NJ) and the experiments were performed following manufacturer’s protocol. α-MEM media was used as background control. The samples were diluted 1:5 ratio and adjusted later while calculating the concentrations. The laboratory personnel that performed this assay were kept blind with respect to any information concerning study groups. All samples were run in duplicate and mean values for each sample were used in the analysis. For quality control purposes, positive controls containing known amounts of estradiol were included in each batch. This also made sure that the assay values did not dramatically shift over time. Intra-assay co-efficient of variation and inter-assay coefficient of variation for all assays were <5%. The minimum detection limit for the estradiol assay was 25 pg/ml. No samples were below the limit of detection.
Quantitative Real Time Polymerase Chain Reaction (qPCR)
Immediately after the 96 hrs follicle culture, follicles were snap-frozen at −80°C until qPCR analysis. Total RNA was extracted from follicles using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. Reverse-transcriptase generation of cDNA was performed with 0.5 µg of total RNA using an Omniscript RT kit (Qiagen, Inc., Valencia, CA) with random primers provided by the manufacturer. qPCR was conducted using an MJ Research Chromo4 Real Time PCR machine (MJ Research, Inc., Waltham, MA) and accompanying software. Primer sequences used were as follows: Arom: (forward) 5′- TCC-CAT-GGC-AGA-TTC-TTG-TGG-ATG -3′, (reverse) 5′- TCG-GGA-GAT-GTA-GTG-ACT-GTG-CTT -3′; Ccnd2 (forward) 5′- AGC-TGT-CCC-TGA-TCC-GCA-AG -3′, (reverse) 5′- GTC-AAC-ATC-CCG-CAC-GTC-TG -3′ (Piatelli et al., 2002); Cdk4 (forward) 5′- TGG-CTG-CCA-CTC-GAT-ATG-AAC -3′, (reverse) 5′- CCT-CAG-GTC-CTG-GTC-TAT-ATG -3′ (de Castro et al., 1999) and, β-actin (forward) 5′- GGG-CAC-AGT-GTG-GGT-GAC-3′, (reverse) 5′- CTG-GCA-CCA-CAC-CTT-CTAC -3′ (Zhang et al., 2000). β-Actin was used as an internal standard for each sample. qPCR analysis was performed using 3 µl cDNA, forward and reverse primers (5 pmol) for Arom, Ccnd2, Cdk4 or β-actin in conjunction with a DyNAmo SYBR Green qPCR kit (Finnzymes, c/o Bio-Rad Laboratories, Hercules, CA). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 s, annealing at 59.5°C (Arom), 57°C (Ccnd2) or 55°C (Cdk4 and β-actin) for 10 s, and extension at 72°C for 10 s, for 50 cycles, followed by final extension at 72°C for 10 min. A melting curve was generated at 55–90°C to monitor the generation of a single product. A standard curve was generated from five serial dilutions of one of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. The software generated a standard curve from the given known amount of sample. Readings for the genes were based off this generated standard curve. β-Actin was used as an internal standard for each sample. All experiments were performed in triplicate. Final values were calculated and expressed as the ratio Arom:β-actin Ccnd2:β-actin and Cdk4:β-actin.
Statistical Analysis
Data were expressed as means ± SEM and comparisons between experimental groups were made using analysis of variance (ANOVA) followed by Tukey’s post hoc comparison. Statistical significance was assigned at p < 0.05.
RESULTS
Effects of DEHP or MEHP Treatment on Antral Follicles Growth
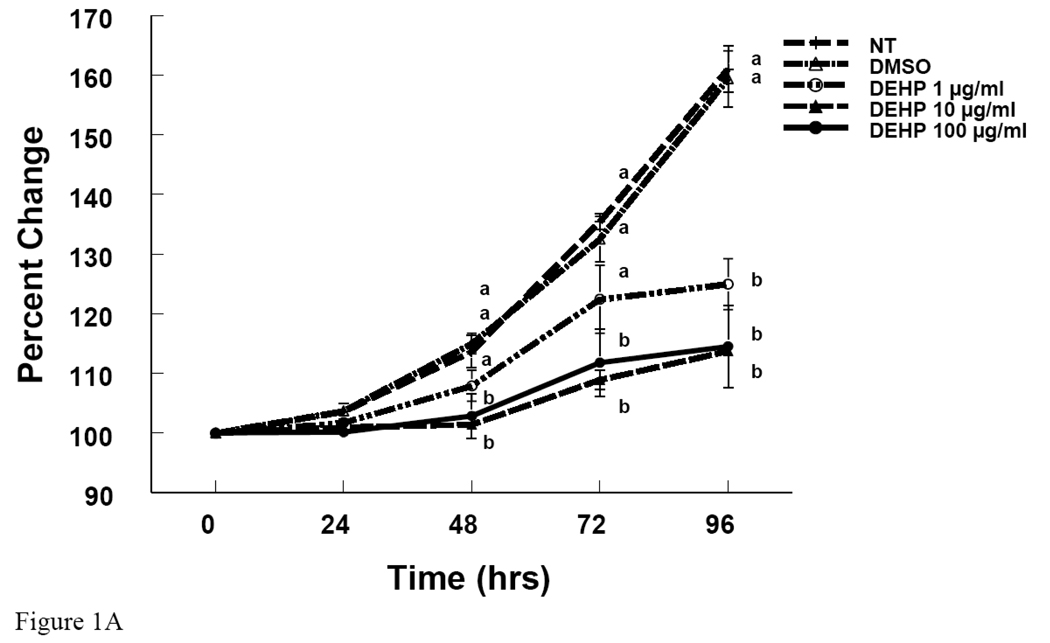
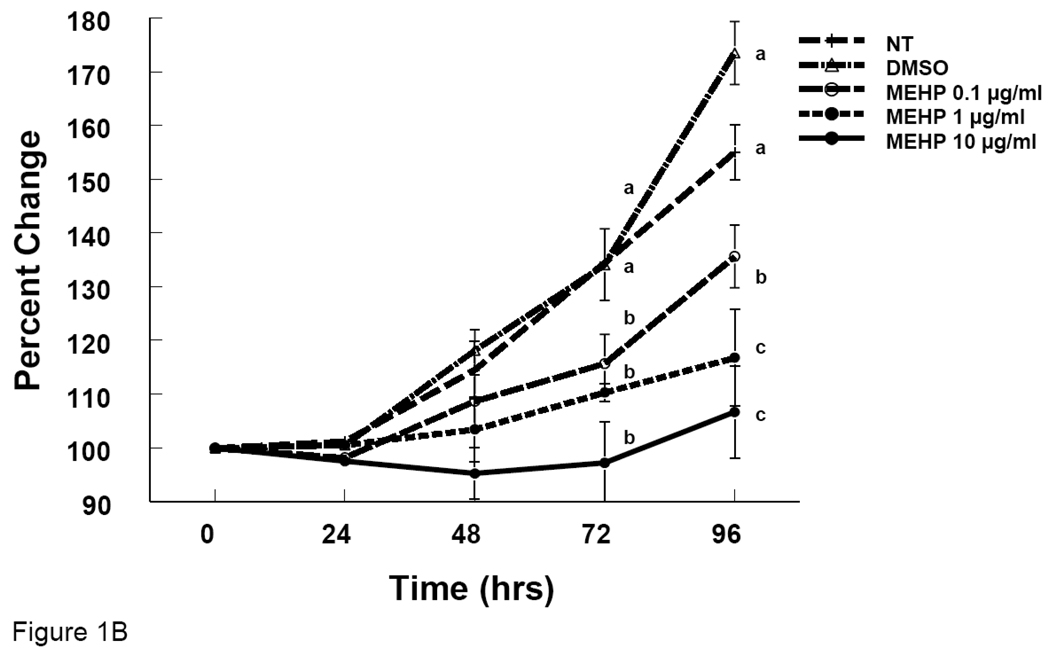
To determine whether DEHP and MEHP affect antral follicle growth, antral follicles were treated with either DEHP or MEHP in culture. DEHP (1–100 µg/ml) significantly inhibited antral follicle growth at 72 and 96 hrs compared to non-treated and DMSO-treated controls (Fig 1A). MEHP (0.1–10 µg/ml) also significantly inhibited antral follicle growth at 72 and 96 hrs compared to the controls (Fig 1B).
Figure 1. Effect of DEHP or MEHP Treatment on Antral Follicle Growth.
Antral follicles were cultured in the presence of either DEHP (1, 10, or 100 µg/ml in A) or MEHP (0.1, 1, or 10 µg/ml in B) for 96 h. Growth of follicles was monitored every 24 h and reported as percent change compared to time zero at the beginning of the culture. NT = nontreated, DMSO = dimethylsulphoxide. Graph represents means ± SEM from three separate experiments. Lines with different letters are significantly different from controls at 48h, 72 h and 96 h timepoint and not between the timepoints (p ≤ 0.05).
Effects of DEHP or MEHP Treatment on Estradiol Production
It is known that E2 is required for follicle growth. Since we observed that DEHP and MEHP inhibit antral follicle growth, we measured E2 levels in the media of cultured follicles. E2 was measured after 96 hrs of culture because at this time point we observed definitive inhibition of follicle growth. Both DEHP and MEHP reduced the levels of E2 in media. DEHP (10 and 100 µg/ml) significantly reduced E2 levels compared to controls (Fig 2A). There was significant dose response relationship between DEHP 10 and 100 µg/ml groups (Fig 2A). MEHP (0.1 and 10 µg/ml) also significantly reduced E2 levels compared to controls (Fig 2B).
Effects of Estradiol Supplement on DEHP- or MEHP-Induced Follicle Growth Inhibition
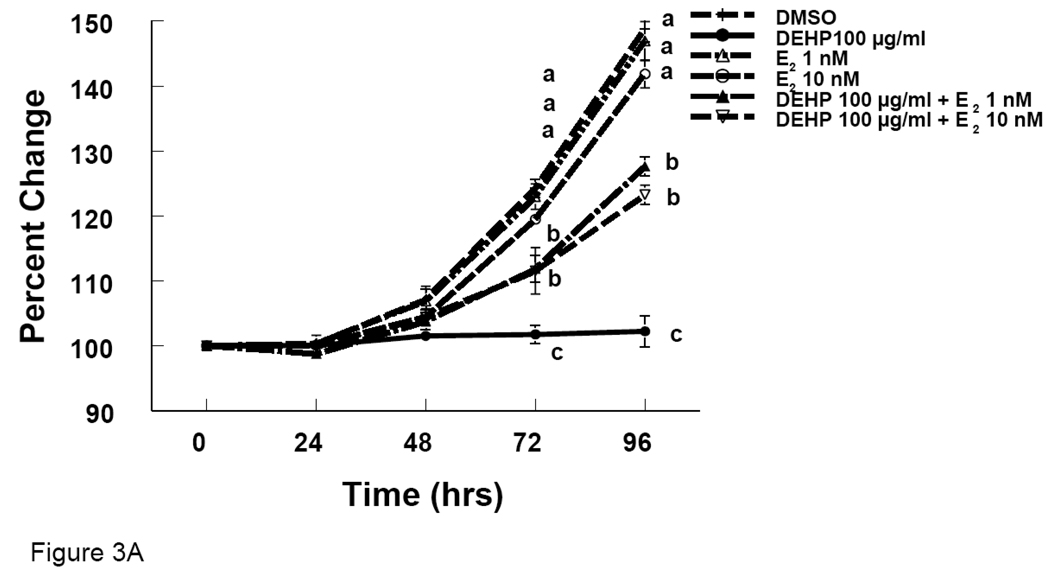
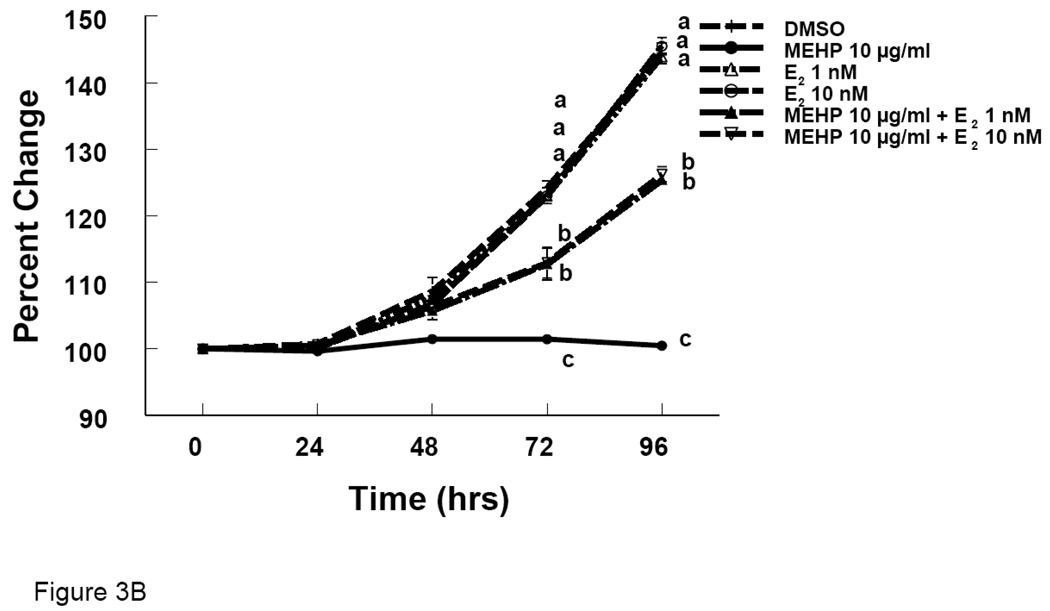
We observed reduced growth of antral follicles and reduction in E2 levels with DEHP and MEHP treatments. To determine whether E2 replacement would prevent the effects of DEHP and MEHP on follicle growth, we supplemented the media with E2. Follicles co-treated with E2 and DEHP (100 µg/ml) grew significantly more than follicles treated with DEHP (100 µg/ml) alone, but grew significantly less than follicles treated with DMSO (Fig 3A). Similarly, follicles co-treated with E2 and MEHP (10 µg/ml) grew significantly more than follicles treated with MEHP (10 µg/ml) alone, but grew significantly less than follicles treated with DMSO (Fig 3B).
Figure 3. Effect of DEHP or MEHP and Estradiol Co-Treatment on Antral Follicle Growth.
Antral follicles were cultured in the presence of either DEHP (100 µg/ml in A) or MEHP (10 µg/ml in B) supplemented with estradiol (1 or 10 nM) for 96 hrs. Growth of follicles was monitored during culture and reported as percent change compared to time zero at the beginning of the culture. DMSO = dimethylsulphoxide, E2 = estradiol. Graph represents means ± SEM from three separate experiments. Lines with different letters are significantly different from controls at 72 h and 96 h timepoint and not between the timepoints (p ≤ 0.05).
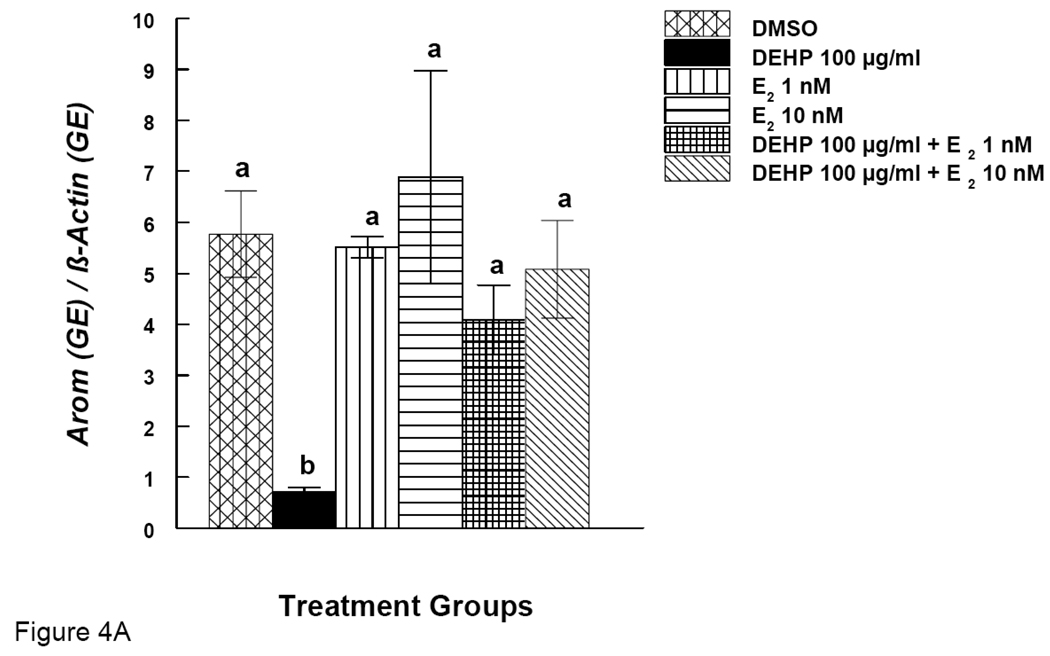
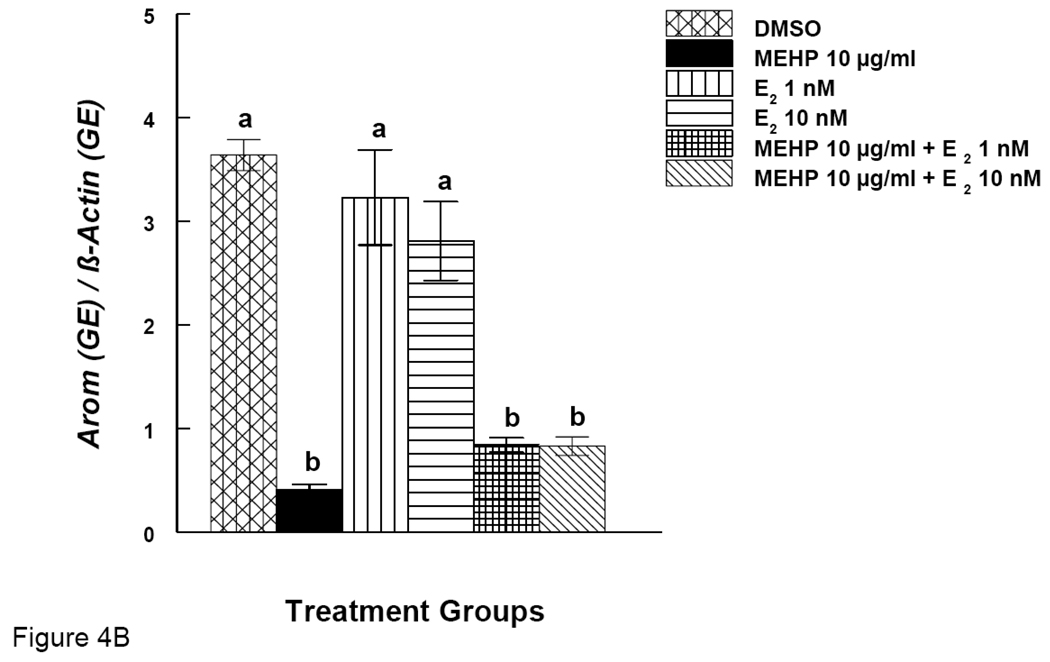
Effects of DEHP or MEHP and Estradiol Supplement on mRNA expression of Arom in Antral Follicles
Arom is an enzyme known for converting testosterone to E2. We therefore investigated the expression of Arom gene. Simultaneously, we also examined Arom gene expression after E2 supplementation. DEHP (100 µg/ml) significantly reduced the expression of Arom (Fig. 4A). E2 supplement (1 and 10 nM), however, restored Arom expression to control levels (Fig 4A). MEHP (10 µg/ml) significantly reduced the expression of Arom, but E2 supplement (1 and 10 nM) did not restore Arom levels to control levels (Fig 4B).
Figure 4. Effect of DEHP or MEHP and Estradiol Co-Treatment on mRNA expression of Arom in Antral Follicles.
Antral follicles were cultured in the presence of either DEHP (100 µg/ml in A) or MEHP (10 µg/ml in B) supplemented with estradiol (1 or 10 nM) for 96 hrs. Total mRNAs were isolated from the follicle for qPCR analysis of Arom mRNA expression. All values were normalized with β-actin as a loading control. DMSO = dimethylsulphoxide, E2 = estradiol. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other within the time point (p ≤ 0.05).
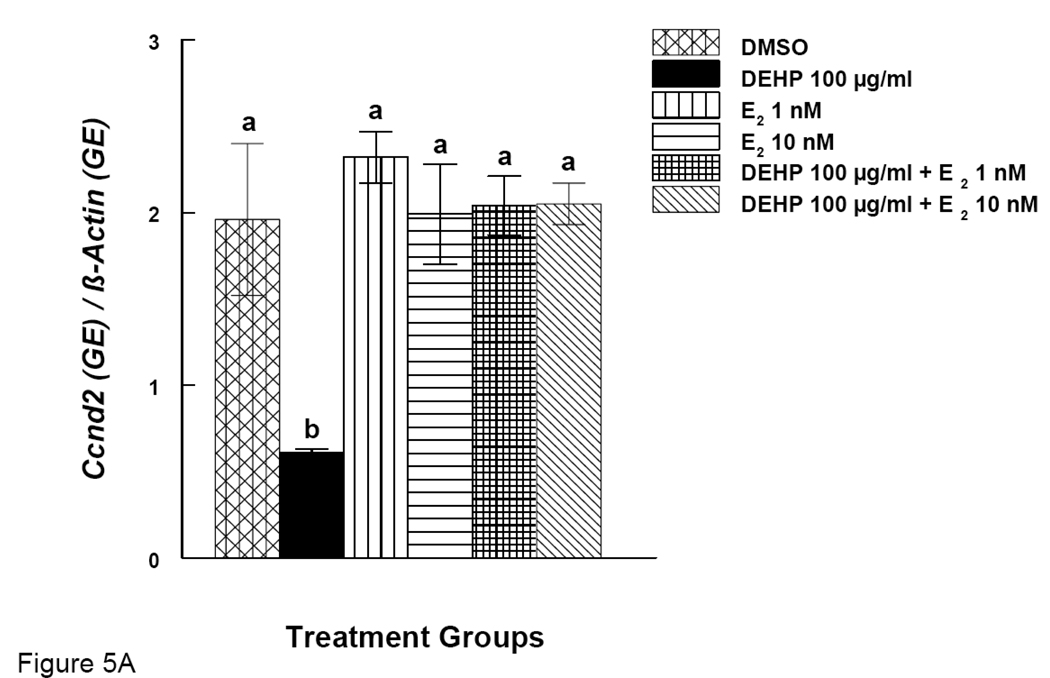
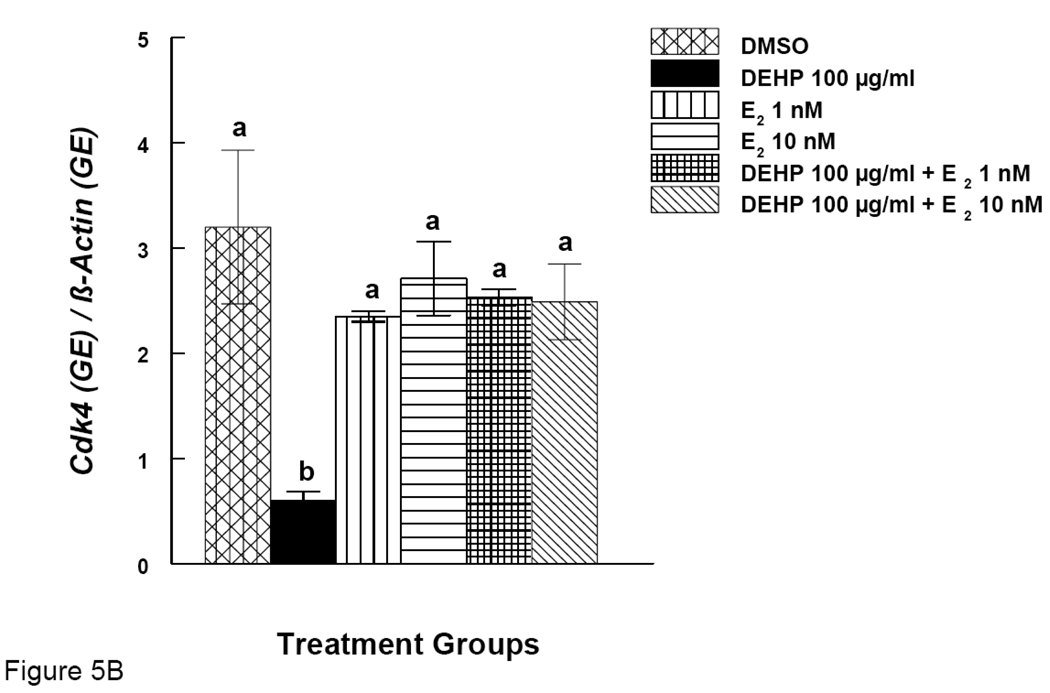
Effects of DEHP and Estradiol Supplement on mRNA expression of Ccnd2 and Cdk4 in Antral Follicles
Our results show that DEHP inhibits antral follicle growth and co-treatment with E2 offers protection from DEHP-induced growth inhibition. We therefore looked at expression of certain cell cycle regulators (Ccnd2 and Cdk4) after DEHP exposure. Expression of the same genes was also examined after DEHP exposure and co-treatment with E2. DEHP (100 µg/ml) significantly reduced the expression of Ccnd2 (Fig. 5A). E2 supplement (1 and 10 nM), however, restored Ccnd2 levels to control levels (Fig 5A). Similarly, DEHP (100 µg/ml) significantly reduced the expression of Cdk4 and E2 supplement (1 and 10 nM) restored Cdk4 levels to control levels (Fig 5B).
Figure 5. Effect of DEHP and Estradiol Co-Treatment on mRNA expression of Ccnd2 and Cdk4 in Antral Follicles.
Antral follicles were cultured in the presence of either DEHP (100 µg/ml) supplemented with estradiol (1 or 10 nM) for 96 hrs. Total mRNAs were isolated from the follicle for qPCR analysis of Ccnd2 (A) and Cdk4 (B) mRNA expression. All values were normalized with β-actin as a loading control. DMSO = dimethylsulphoxide, E2 = estradiol. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other within the time point (p ≤ 0.05).
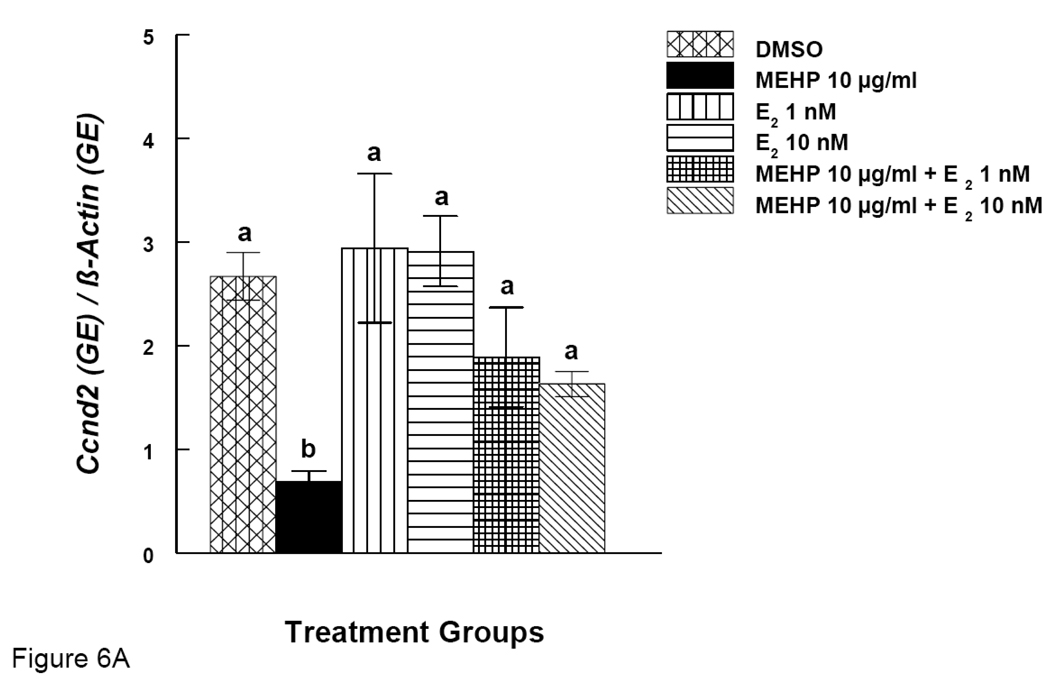
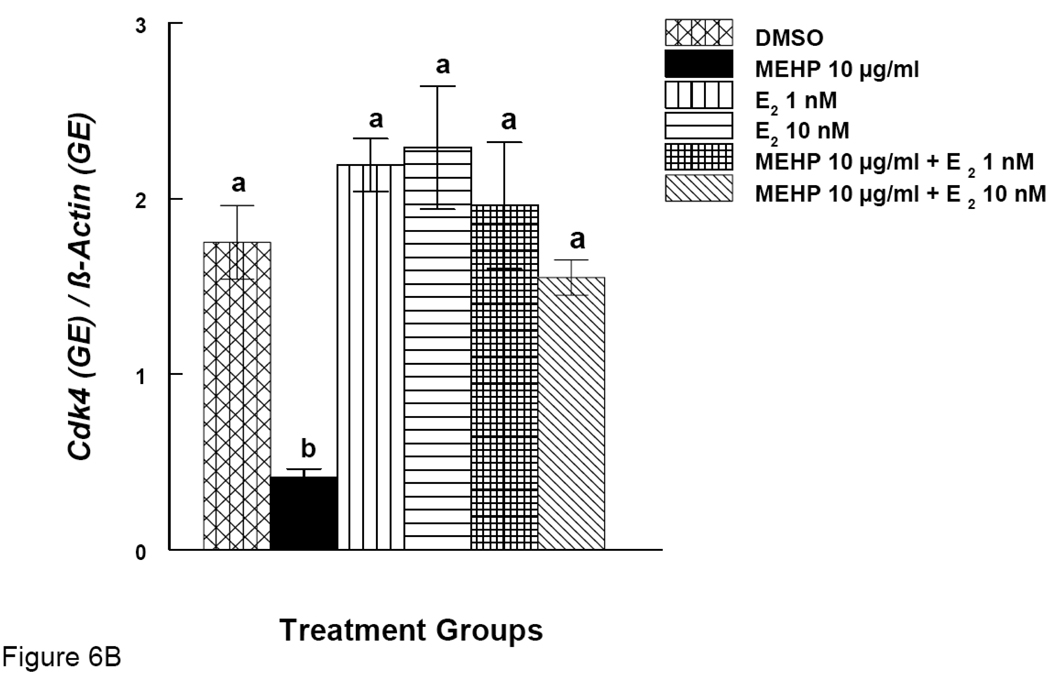
Effects of MEHP and Estradiol Supplement on mRNA expression of Ccnd2 and Cdk4 in Antral Follicles
Our results show that MEHP inhibits antral follicle growth and co-treatment with E2 offers protection from MEHP-induced growth inhibition. We therefore examined expression of certain cell cycle regulators (Ccnd2 and Cdk4) after MEHP exposure. Expression of same the genes was also examined after MEHP exposure and co-treatment with E2. MEHP (10 µg/ml) significantly reduced the expression of Ccnd2 (Fig. 6A). Co-treatment of MEHP (10 µg/ml) with E2 (1 and 10 nM), however, restored Ccnd2 levels to control levels (Fig 6A). Similarly, MEHP (10 µg/ml) significantly reduced the expression of Cdk4 and co-treatment with E2 (1 and 10 nM) restored Cdk4 levels to control levels (Fig 6B).
Figure 6. Effect of MEHP and Estradiol Co-Treatment on mRNA expression of Ccnd2 and Cdk4 in Antral Follicles.
Antral follicles were cultured in the presence of MEHP (10 µg/ml) supplemented with estradiol (1 or 10 nM) for 96 hrs. Total mRNAs were isolated from the follicle for qPCR analysis of Ccnd2 (A) and Cdk4 (B) mRNA expression. All values were normalized with β-actin as a loading control. DMSO = dimethylsulphoxide, E2 = estradiol. Graph represents means ± SE from three separate experiments. Bars with different letters are significantly different from each other within the time point (p ≤ 0.05).
DISCUSSION
We have shown that DEHP and MEHP inhibit growth of antral follicles, decrease E2 levels, and decrease mRNA expression of Arom, Ccnd2, and Cdk4 in vitro. In addition, we found that co-treatment with E2, a known factor required for growth and maturation of follicles, partially rescues antral follicles from DEHP- and MEHP-induced growth inhibition and restores expression of Ccnd2 and Cdk4 mRNA in DEHP-and MEHP treated follicles to control levels. Co-treatment with E2 also restores expression of Arom in DEHP-treated follicles to control levels, but does not restore expression Arom in MEHP-treated follicles to control levels.
To our knowledge, this is first study which examined the effects of DEHP and MEHP on mouse antral follicles. Previous studies have either treated rats or utilized rat granulosa cell culture systems to examine the effects of DEHP and MEHP. These previous studies have shown that rats exposed to DEHP were hypo-estrogenic, anovulatory, and had polycystic ovaries (Davis et al., 1994a). Specific cellular targets of PEs are difficult to pinpoint using these whole animal in vivo studies because of the concerns of systemic effects of PEs. Our in vitro follicle culture study demonstrates that DEHP and its metabolite MEHP directly affect antral follicles. Follicle culture techniques could be more advantageous than other in vitro techniques. In vitro granulosa cell culture models are often criticized due to the fact that isolated granulosa cells do not function similarly to those in the native follicles. Follicles are a unique tissue containing different cell types, such as theca and granulosa cells. It is the interaction between these two major cell types in the follicle that are responsible for its growth. Follicles cultured in vitro have been shown to develop similar to those in vivo in terms of growth, hormone production, and hormone responsiveness (Cortvrindt and Smitz, 2002). Also, antral follicles are the major follicle type that have the capability of steroidogenesis, making it biologically relevant to study xenobiotics that affect steroid synthesis (Cortvrindt and Smitz, 2002; Gupta R et al., 2006; Lenie and Smitz, 2009; Miller K et al., 2005).
MEHP was shown to suppress Arom transcript levels and decrease E2 production in rat granulosa cells (Lovekamp and Davis, 2001) and in human granulosa cell culture systems (Reinsberg et al., 2009). In a study by Lenie and Smitz (2009), it was shown that MEHP exposure up to 200µM during follicular growth and maturation provoked only subtle alterations in steroidogenesis. MEHP resulted in an increased metabolism of E2 but not a decrease in production of E2 and a premature progesterone production by the follicle, implying that MEHP could disrupt the critical timing of follicle differentiation causing premature luteinization of preantral follicles in this case. Since MEHP did not substantially alter estrogen production, the authors in this study hypothesize that potentially adverse effects of MEHP on aromatase gene expression are less pronounced in follicle culture than in granulosa cell culture, due to a difference in differentiation stage of granulosa cells. We on the other hand found that both DEHP and MEHP reduce E2 production and reduce Arom transcript levels in whole antral follicles from mice. Therefore, our results with the mouse follicle culture are consistent with the findings seen in whole animal studies and also with the studies using granulosa cell from rats and human (Davis et al., 1994a; Lovekamp and Davis, 2001; Reinsberg et al., 2009). Our study does not compare to the study done by Lenie and Smitz (2009) because, preantral follicles were used in Lenie and Smitz (2009) study versus our current study. Further, Lenie and Smitz (2009) observe premature luteinization, so it is not possible predict the effects on mature antral follicles from this study.
The mechanism by which DEHP and MEHP affect growth of antral follicles is not known. Growth of follicles requires an increase in number of granulosa and thecal cells. Granulosa and theca cells must proliferate by undergoing cell cycle progression so that follicles can reach the penultimate stage for ovulation. The progression of the cell cycle is controlled by activities of protein complexes known as cyclins and cyclin-dependent kinases (cdk). Different cyclin/cdk complexes are assembled and activated at different points in the cell cycle (Iliakis, 1995; Morgan, 1995; Nigg, 1995). In our study, we have shown that DEHP and MEHP exposure reduces the levels of Ccnd2 and Cdk4 mRNA. Thus, it is likely that DEHP and MEHP inhibit follicle growth by reducing expression of cell cycle regulators.
While no studies have reported the effect of DEHP and MEHP on cell cycle regulators in ovarian follicles, there are studies showing that E2 affects cell cycle regulators (Fujita et al., 2002; Kanda and Watanabe, 2004). E2 is required for C cnd2 expression in human keratinocytes (Kanda and Watanabe, 2004). In osteoclasts, E2 activates Cdk4 through Ccnd2 to promote growth (Fujita et al., 2002). In our study, we found that DEHP and MEHP reduce Ccnd2 and Cdk4 expression as well as E2 production. Thus, it is possible that the reduction in Ccnd2 and Cdk4 expression in DEHP- and MEHP-treated follicles results indirectly from reduced E2 production. This is supported by the fact that in our study, E2 supplement restores Ccnd2 and Cdk4 expression to control levels in DEHP- and MEHP-treated follicles.
Several studies indicate that E2 is one factor required for follicle growth and therefore, a reduction in E2 levels would lead to growth reduction (Britt et al., 2004; Hirshfield 1991). Rat granulosa cells treated with MEHP had significantly reduced E2 levels, which lead to decrease in growth of these cells (Lovekamp and Davis, 2001). We observed that DEHP and MEHP-treated follicles have reduced E2 levels. Therefore, reduction in E2 could be responsible for DEHP- and MEHP-induced growth inhibition. It is also possible that inhibition of follicle growth by DEHP and MEHP could lead to the reduction in E2 levels. Future experiments need to be done to test this possibility.
Studies have shown that rat granulosa cells treated with MEHP had significantly reduced E2 levels, which was attributed to a decrease in expression of Arom (Lovekamp and Davis, 2001), the enzyme that convert testosterone to estradiol. We also observed that DEHP and MEHP-treated follicles have reduced E2 levels and a decrease in expression of Arom gene in follicles. We further tested whether Arom gene was a direct target of DEHP and/or MEHP. For this, we co-treated follicles with E2 to see if E2 offers any protection. E2 co-treatment partially protected follicles from DEHP- and MEHP-induced growth inhibition. To our surprise, E2 co-treatment helped bring the expression of Arom back to control levels in DEHP-treated follicles but not in MEHP-treated follicles. This could be due to different sensitivity of follicles to the parent (DEHP) compound versus the active metabolite (MEHP). Since we observe that replacing E2 in media allowed better follicle growth and recovery of Arom expression, it could be unlikely that Arom expression is a direct target of DEHP. But, it could also be that E2 regulates the transcription of Arom resulting in up regulation observed in our experiments. Although no studies directly show this in antral follicles there are studies showing positive relationship between E2 and Arom gene expression (Kumar et al., 2009; Bourguiba et al., 2003, and Nakamura et al., 1999). Specifically, Kumar et al. (2009) show that during trophoblast differentiation in human placenta, E2/ERalpha exerts a positive feedback role, which promotes permissive histone modifications that are associated with induction of Arom gene transcription. Bourguiba et al. (2003) suggest a presence of androgen and estrogen response element in Arom gene in germ cells of adult rats. Further, Nakamura et al. (1999) suggest an estrogen regulate transcription of both Arom in the mammary gland, suggesting a regulatory loop. Having said that, our MEHP groups co-treated with E2 do not show such rescue of Arom gene. This could be due to the fact that MEHP is more toxic, overwhelming to the system, and/or acting through different pathways other than DEHP. This hypothesis is supported by studies showing different sites of action of MEHP. For example, in one study, MEHP inhibited FSH-stimulated cAMP and progesterone production in rat ovarian granulosa cell cultures (Treinen et al., 1990). The decrease in progesterone was prevented by stimulators of cAMP or by pregnenolone, the precursor of progesterone. In another study, however, MEHP decreased estradiol production when rat granulosa cells were stimulated either with FSH or a non degradable analogue of cAMP, 8br-cAMP (Davis et al., 1994b). Therefore, the effect on estradiol was separate from the effect on FSH-stimulated cAMP and progesterone production in the granulosa cells. Further, it has also been proposed that MEHP may act as a ligand for the fatty acid binding protein and/or as activator of the peroxisome proliferator-activated receptor γ (PPARγ) or PPAR α pathway (Lovekamp et al., 2003). Thus, it is possible that MEHP is acting through different pathways than DEHP.
Although it is known that cell cycle regulators and E2 play important roles in follicle growth, the connection between cell cycle regulators and E2 on follicle growth remains to be determined. E2 and its receptors are known to be responsible for cAMP production, which is required for growth of granulosa cells (Conti et al., 1984; Deroo et al., 2009). Growth of any particular cell requires cell cycle regulators such as Ccnd2 and Cdk4. Therefore, it is possible that decrease in E2 production induced by DEHP and MEHP leads to a reduction in cAMP, and this reduction in cAMP leads to lowered Ccnd2 and Cdk4 expression. In turn, this could cause the inhibition of follicle growth observed in our study. We also observed partial rescue of follicular growth with E2 co-treatment. Thus, it is possible that replacement with E2 replenishes cAMP, therefore restoring the Ccnd2 and Cdk4 levels to control levels and protecting follicles from DEHP-and MEHP-induced growth inhibition. The reason why we observe partial protection in follicle growth after co-treatment with E2 could stem from the fact that numerous other growth factors such as epidermal growth factor, insulin like growth factors, along with E2 may be altered in response to PEs. Further, PE treatment could also affect E2 receptors. Future studies need to be done to ascertain the relationship between cAMP and cell cycle regulators and explore other modes of action.
In conclusion, our study shows that DEHP and MEHP inhibit growth of antral follicles and that inhibition of growth of antral follicles is mediated in part by reduction in E2 levels and cell cycle regulators. Further, the reduction in E2 levels in response to DEHP and MEHP is due to reduction in Arom expression. This study expands our knowledge on how PEs can affect ovarian function. Better understanding the mechanism how PEs elicit ovarian toxicity may help in the development of preventive measures and treatments against the toxic effects of PEs.
Acknowledgments
Supported by NIH R01 HD 46861, NIH R01 ES012893-01A2, NIEHS T32 ES07326
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rupesh K. Gupta, Email: drrupesh@illinois.edu.
Jeffery M. Singh, Email: jsingh20@illinois.edu.
Tracie C. Leslie, Email: tleslie2@illinois.edu.
Sharon Meachum, Email: smeachum@illinois.edu.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Atlanta, GA: Toxicologica Profile for Di-(2-ethylhexyl) Phthalate, Update. NTIS Publication Number PB/93/182400. 1993
- Aldyreva MV, Klimova TS, Iziumova AS, Timofe vskaia LA. The effect of phthalate plasticizers on the generative function. Gig Tr Prof Zabol. 1975;19:25–29. [PubMed] [Google Scholar]
- Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schlüter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71(5):1712–1723. doi: 10.1095/biolreprod.104.028175. [DOI] [PubMed] [Google Scholar]
- Center for the Evaluation of Risks to Human Reproduction (CERHR) NTP-CERHR Export Panel Report on Di(2-ethylhexyl)Phthalate. Alexandria, VA: Science International, Inc.; 2000. [Google Scholar]
- Conti M, Kasson BG, Hsueh AJ. Hormonal regulation of 3',5'-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology. 1984;114(6):2361–2368. doi: 10.1210/endo-114-6-2361. [DOI] [PubMed] [Google Scholar]
- Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod, Update. 2002;8(3):243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994a;128(2):216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of fsh-camp stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994b;128(2):224–228. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Rodriguez KF, Couse JF, Hamilton KJ, Collins JB, Grissom SF, Korach KS. Estrogen receptor {beta} is required for optimal camp production in mouse granulosa cells. Mol Endocrinol. 2009;23(7):955–965. doi: 10.1210/me.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29(1):140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Fujita M, Urano T, Horie K, Ikeda K, Tsukui T, Fukuoka H, Tsutsumi O, Ouchi Y, Inoue S. Estrogen activates cyclin-dependent kinases 4 and 6 through induction of cyclin D in rat primary osteoblasts. Biochem Biophys Res Commun. 2002;299(2):222–228. doi: 10.1016/s0006-291x(02)02640-2. [DOI] [PubMed] [Google Scholar]
- Gibson TP, Briggs WA, Boone BJ. Delivery of di-2-ethylhexyl phthalate to patients during hemodialysis. J Lab Clin Med. 1976;87:519–524. [PubMed] [Google Scholar]
- Gillum N, Karabekian Z, Swift LM, Brown RP, Kay MW, Sarvazyan N. Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicol Appl Pharmacol. 2009;236(1):25–38. doi: 10.1016/j.taap.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande SW, Andrade JM, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Reproductive effects on adult female offspring rats. Toxicology. 2007;229(1–2):114–122. doi: 10.1016/j.tox.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93(2):382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Iliakis G. Cell cycle regulation in irradiated and nonirradiated cell. Semin Oncol. 1995;24:602–615. [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17[beta]-estradiol stimulates the growth of human keratinocytes by inducing cyclin d2 expression. J Investig Dermatol. 2004;123(2):319–328. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- Lenie S, Smitz JE. Steroidogenesis-disrupting compounds can be effectively studied for major fertility-related endpoints using in vitro cultured mouse follicles. Toxicol Let. 2009;185(3):143–152. doi: 10.1016/j.toxlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172(3):217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Lovekamp TN, Jetten AM, Davis BJ. Dual activation of PPAR[alpha] and PPAR[gamma] by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201(1–2):133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114(6):805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93(1):180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88(1):213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Mlynarcíková A, Nagyová E, Ficková M, Scsuková S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol In Vitro. 2009;23(3):371–377. doi: 10.1016/j.tiv.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Muncke J. Exposure to endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci Total Environ. 2009;407:4549–4559. doi: 10.1016/j.scitotenv.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17(6):471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- de Castro IP, Malumbres M, Santos J, Pellicer A, Fernández-Piqueras J. Cooperative alterations of Rb pathway regulators in mouse primary T cell lymphomas. Carcinogenesis. 1999;20(9):1675–1682. doi: 10.1093/carcin/20.9.1675. [DOI] [PubMed] [Google Scholar]
- Piatelli MJ, Doughty C, Chiles TC. Requirement for a hsp90 chaperone-dependent MEK1/2-ERK pathway for B cell antigen receptor-induced cyclin D2 expression in mature B lymphocytes. J Biol Chem. 2002;277(14):12144–12150. doi: 10.1074/jbc.M200102200. [DOI] [PubMed] [Google Scholar]
- Reinsberg J, Wegener-Toper P, van der Ven K, van der Ven H, Klingmueller D. Effect of mono-(2-ethylhexyl) phthalate on steroid production of human granulosa cells. Toxicol Appl Pharmacol. 2009;239(1):116–123. doi: 10.1016/j.taap.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Tabacova S, Little R, Balabaeva L. Maternal exposure to phthalates and complications of pregnancy. Epidemiology. 1999;10 suppl:S127. [Google Scholar]
- Tanaka A, Adachi T, Takahashi T, Yamaha T. Biochemical studies on phthalic esters I. Elimination, distribution and metabolism of di-(2-ethylhexyl) phthalate in rats. Toxicology. 1975;4:253–264. doi: 10.1016/0300-483x(75)90105-5. [DOI] [PubMed] [Google Scholar]
- Treinen KA, Dodson WC, Heindel JJ. Inhibition of FSH-stimulated cAMP accumulation and progesterone production by mono(2-ethylhexyl) phthalate in rat granulosa cell cultures. Toxicol Appl Pharmacol. 1990;106(2):334–340. doi: 10.1016/0041-008x(90)90252-p. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shigehira S, Sirpa M, Guojun C, Elwood VJ, Margaret W, Jan-Åke G. Estrogen receptor (ER) β, a modulator of ERα in the uterus. Proc Natl Acad Sci, USA. 2000;97(11):5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]