Abstract
Guanylyl cyclase C a receptor for bacterial diarrheagenic enterotoxins is expressed selectively by intestinal epithelium and is an endogenous downstream target of CDX2. The expression of Guanylyl cyclase C is preserved throughout the adenoma/carcinoma sequence in the colorectum. Detection of Guanylyl cyclase C expression by RT-PCR is currently being validated as a technique to identify occult lymph node metastases in patients with colorectal cancer and for circulating cells in the blood for postoperative surveillance. Although Guanylyl cyclase C is widely expressed by well differentiated colorectal cancer, its expression in poorly differentiated colorectal cancer has not been evaluated. A tissue microarray was created from 69 archival specimens including 44 poorly differentiated, 15 undifferentiated or medullary and 10 signet ring cell colorectal carcinomas. Matched normal colonic mucosa was used as a positive control. Immunohistochemical staining for Guanylyl cyclase C and CDX2 was evaluated as positive or negative based on at least a 10% extent of staining. Out of the 69 tumor samples 75%, 47%, and 90% of the poorly differentiated, medullary and signet ring cell tumors were positive for Guanylyl cyclase C and 75%, 40% and 90% of these subsets were positive for CDX2 respectively. There was excellent correlation between Guanylyl cyclase C and CDX2 expression on a case per case basis (p<0.0001). There was also a statistically significant difference in the GCC staining pattern between MC and PDC (p=0.05). Immunopositivity for Guanylyl cyclase C was greater than 95% in a separately stained microarray series of well/moderately differentiated colorectal carcinomas. In conclusion, Guanylyl cyclase C expression is lost in a quarter of poorly differentiated and half of undifferentiated colorectal carcinomas. Therefore the utility of Guanylyl cyclase C expression as a diagnostic marker for colorectal carcinoma may be questionable in poorly differentiated colorectal neoplasms.
Keywords: Guanylyl cyclase C, Poorly differentiated, Colon, Carcinoma, CDX2
Introduction
Colorectal carcinoma (CRC) is the third most common cause of cancer death in the United States [1]. CRC can be divided into well, moderately and poorly differentiated adenocarcinomas based on the histologic presence of glandular differentiation. By definition CRC is classified as being poorly differentiated (or high grade) if more than 50% of the tumor is formed by non-gland forming neoplastic cells [2]. Subtypes of poorly differentiated CRCs include undifferentiated or medullary carcinomas (MC), signet ring cell carcinomas (SRC), and poorly differentiated not otherwise specified (PDC) [2]. Approximately 20% of all CRC fall into the poorly differentiated category [3].
A number of immunohistochemical and PCR markers specific for intestinal epithelium have been characterized and their retention in many CRCs has been utilized for the identification of metastatic tumors, detection of occult lymph node metastases and for detecting circulating tumor cells in the peripheral blood [4–6]. Guanylyl cyclase C (GCC) is an N-linked glycoprotein receptor for heat stable enterotoxin that is expressed by intestinal epithelium [7,8]. The intestine specific expression of the GCC promoter requires CDX2 [9]. GCC has been shown to be a sensitive and specific marker for CRCs, and its expression is retained in the adenoma to adenocarcinoma sequence [10,11]. Molecular studies to detect the presence of GCC have been proposed as a method of detecting occult micrometastases to regional lymph nodes in CRCs [12–15]. To date, the expression of GCC in poorly differentiated CRCs has not been thoroughly investigated.
CDX2 is a transcription factor expressed in intestinal epithelial cells and is thought to play a role in the differentiation and proliferation of intestinal epithelium [16]. Several studies have shown CDX2 to be both a sensitive and specific marker of intestinal differentiation; furthermore, it appears to be overexpressed in CRC tumor cells compared with normal intestinal epithelium [17–19]. While the majority of well and moderately differentiated CRCs stain strongly for CDX2, certain studies have shown that a lower proportion of poorly differentiated tumors express CDX2 [20–21].
The focus of this study was to examine the pattern of staining of GCC expression in a large cohort of poorly differentiated CRCs and to correlate these findings with the expression of CDX2.
Material and Methods
Patients and samples
Formalin-fixed paraffin embedded tissues from 69 specimens including 44 poorly differentiated (PDC), 15 undifferentiated or medullary (MC) and 10 signet ring cell (SRC) CRCs were collected from the archives of the Department of Pathology at the Rhode Island and Miriam Hospitals in accordance with Institutional Review Board approvals from both hospitals. The written reports of all colon cancer patients diagnosed between the years 1984–2008 were reviewed and all cases classified as PDC, MC and SRC were selected. Histological sections were reviewed by MR and BW and were reclassified based on the current WHO criteria [2].
Tissue microarray construction
Areas of pure invasive carcinoma were identified on hematoxylin-eosin-stained slides from each case. In cases of PDC that showed focal well-differentiated areas, only the poorly differentiated components were selected. The corresponding areas on the paraffin embedded source blocks were identified and marked. The source blocks were then cored and a 1-mm core was transferred to the “master block” using the Beecher Tissue Microarrayer (Beecher Instruments, Silver Spring, MD). Approximately three to five tissue cores of tumor were obtained for each case. Additionally, two to three cores of histologically normal adjacent colonic mucosal tissue were obtained and arrayed in the same manner. Whole sections of five cases of PDC which had focal well differentiated areas were also assessed. In addition a tissue microarray (TMA) constructed previously containing 111 cases of primarily moderately to well differentiated CRC was assessed [22].
Immunohistochemistry
Immunohistochemical staining for each antigen was performed on 6 micron paraffin sections of each of the TMA masterblocks. The TMA sections were stained for CDX2 (mouse antibody, clone CDX2–88, 1:50, Biogenex, San Ramon, CA) and GCC (rabbit polyclonal, Gift from SA Waldman, first described by Birbe et al [11]) were performed using the Dako Autostainer with the Dako Envision Plus kit or manually with the Dako Envision Plus kit. The slides were counter stained with hematoxylin and dehydrated in graded alcohols through to xylene and coverslipped. Normal colonic mucosa served as a positive control for both CDX2 and GCC.
Immunohistochemical assessment
The immunohistochemical stains were scored using a two-tiered scoring system: positive or negative. A positive score required moderate to strong staining of more than 10% of the tumor cell population, whereas a negative score included tumors with focal (less than 10%) or weak staining. This cutoff was reached based on personal experience and allowed for minimal interobserver variability in the scoring. BW and MR independently scored each of the sections without knowledge of the histologic diagnosis or staining pattern with the other marker. There was a high correlation between the two scores and in the few discrepant cases, a consensus was reached after joint review.
Statistical analysis
Staining for GCC and CDX2 was compared between well differentiated CRC and PDC, MC and SRC using Fischer’s exact test. Correlation between GCC staining and CDX2 staining and the clinicopathologic comparison of MC and PDC was also analyzed using Fischer’s exact test. Two-tailed P values of 0.05 or less were considered to be statistically significant.
Results
The clinicopathologic characteristics of the 69 cases of CRC are summarized in Table 1. The MC group had the highest mean age at the time of surgery (76yrs) as compared to the SRC and PDC groups which were 71yrs and 68yrs respectively. The majority of patients with MC (87%) and SRC (80%) were female whereas only 45% of patients with PDC were female. The majority of tumors occurred on the right side in the MC (73%) and SRC (80%) groups whereas PDC was right sided in only 45% of cases.
Table 1.
Clinicopathologic Characteristics of 69 Cases of Colonic Carcinoma.
| Variable | MC (n = 15) | PDC (n = 44) | SRC (n = 10) |
|---|---|---|---|
| Age at surgery (y) | |||
| Mean | 75.8 | 67.7 | 71.2 |
| Range | 55–89 | 27–89 | 35–87 |
| Sex | |||
| Male | *2 (13%) | 24 (55%) | 2 (20%) |
| Female | 13 (87%) | 20 (45%) | 8 (80%) |
| Locationa | |||
| Right colon | 11 (73%) | 20 (45%) | 8 (80%) |
| Left colon | 4 (27%) | 24 (55%) | 2 (20%) |
Cutoff for right vs left is hepatic flexure
Statistically significant (P<.05).
MC: Medullary carcinoma, PDC: Poorly differentiated carcinoma, SRC: Signet ring cell carcinoma.
An example of each of the tumor types studied, PDC, MC and SRC is shown in Figure 1. MC lacked glandular differentiation and exhibited solid sheets of neoplastic cells with a syncytial growth pattern and a moderate amount of eosinophilic cytoplasm. An intra-tumoral and peri-tumoral lymphocytic infiltrate was present. The PDC tumors were composed of at least 50% solid sheets or single neoplastic cells. Typically the tumor cells were more pleomorphic than the MC group. SRC were composed of at least 50% of cells with prominent cytoplasmic mucin and characteristic displacement of the nucleus.
Fig. 1.
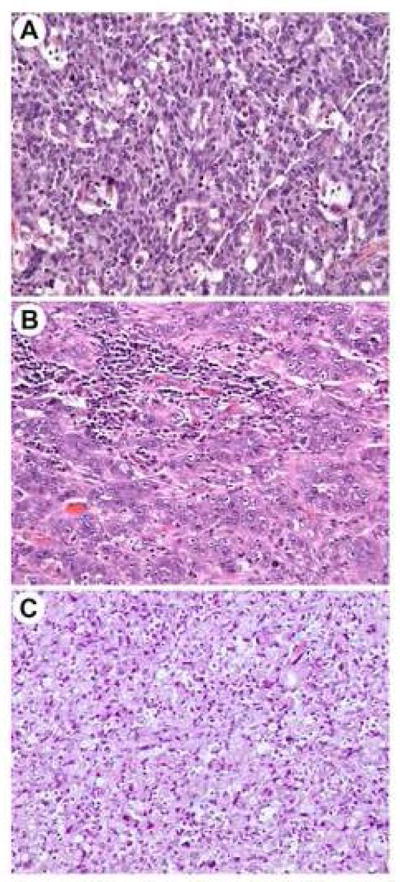
Hematoxylin and eosin stains of poorly differentiated adenocarcinoma (A), medullary carcinoma (B) and signet ring cell carcinoma (C) (original magnification ×200).
The normal colonic epithelium served as a positive control for GCC and showed typical apical membranous staining in all cases examined (Fig. 2A). GCC stained the apical luminal membrane of the tumor cells in the well to moderately differentiated tumors (Fig. 2B), whereas a predominantly cytoplasmic staining pattern was seen in the more poorly differentiated areas, which was most pronounced in the MC group (Fig. 2C, 2D). The SRC tumors exhibited a cytoplasmic with focal membranous staining (Fig. 2E). The pattern of CDX2 staining was nuclear in both the tumor cells and the normal colonic epithelium.
Fig. 2.
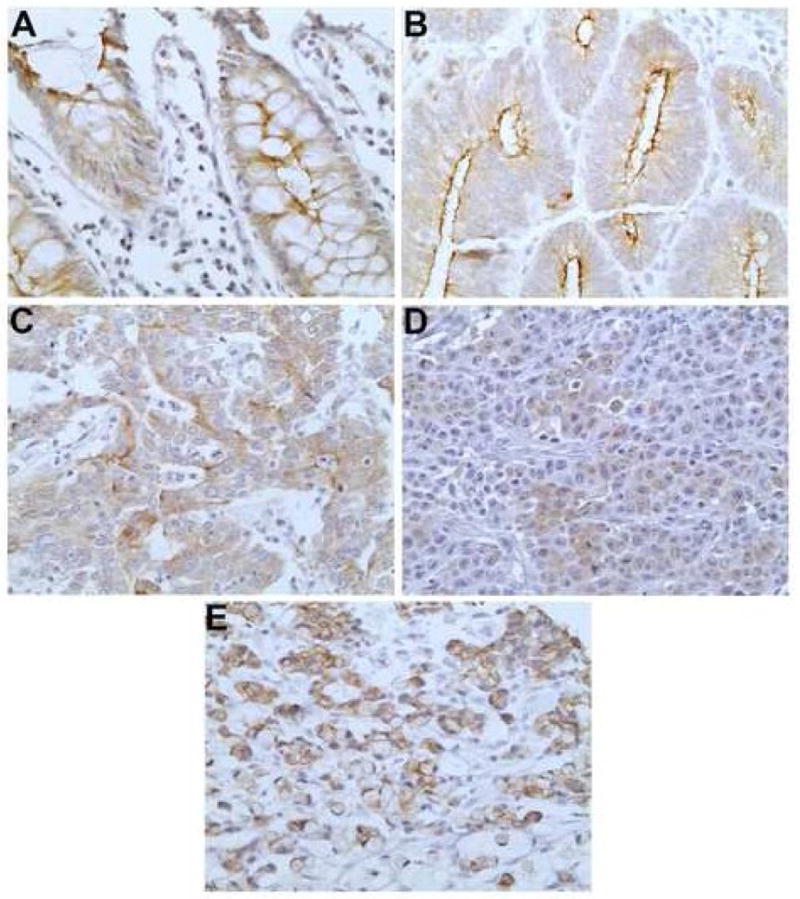
GCC staining pattern in normal colonic mucosa (A), well differentiated adenocarcinoma (B), poorly differentiated adenocarcinoma (C), medullary carcinoma (D) and signet ring cell carcinoma (E) (original magnification ×400).
Out of the 69 tumor samples 75%, 47% and 90% of the PDC, MC and SRC tumors were positive for GCC and 75%, 40% and 90% of these subsets were positive for CDX2 (Table 2). In contrast all but two of the 111 moderately to well differentiated cases examined stained strongly for both GCC and CDX2. There was excellent correlation between GCC and CDX2 expression on a case per case basis (p<0.0001). GCC staining was more commonly lost in MC as compared to PDC (p=0.05). GCC staining in SRC as opposed to PDC shows a positive trend towards staining of the signet ring cell tumors (p=0.2). Several separately stained whole sections from five tumors showing areas of both well and poorly differentiated components were also examined. Positive GCC staining in the well-differentiated component and negative staining in the poorly differentiated areas was seen in these cases as depicted in Figure 3.
Table 2.
Positive staining with CDX2 and GCC in subtypes of colorectal carcinomas.
| PDC (n = 44) | MC (n = 15) | SRC (n = 10) | WDC (n = 111) | |
|---|---|---|---|---|
| CDX2 | 33 (75%) | 6 (40%) | 9 (90%) | 109 (98%) |
| GCC | 33 (75%) | 7 (47%) | 9 (90%) | 109 (98%) |
MC and WDC showed statistically significant different extent of staining as compared with PDC (p≤0.05).
MC: Medullary carcinoma, PDC: Poorly differentiated carcinoma, SRC: Signet ring cell carcinoma.
Fig. 3.
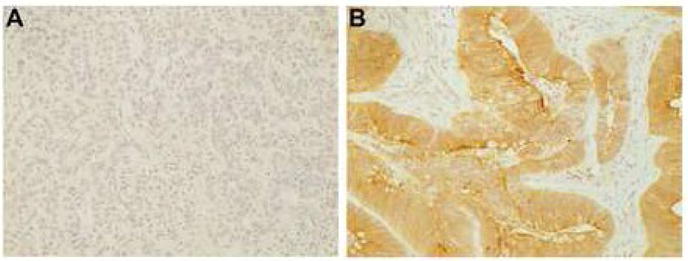
GCC staining in the same whole section of tumor displaying both poorly differentiated (A) and well differentiated areas (B) (original magnification ×200).
Discussion
GCC has been shown to be both a specific and sensitive marker of intestinal differentiation and is being investigated as a biomarker for occult lymph node metastases and circulating peripheral tumor cells [10–15]. While essentially all well or moderately differentiated CRCs have been shown to stain positively with GCC there is only limited data on expression of GCC in PDC [10,11,14]. Birbe et al. showed positive GCC staining in 10 out of 10 PDCs and 5 of 5 SRCs and Buc et al. showed 5 out of 5 PDCs stained with GCC [10,11]. In contrast to the studies described above our study included a larger cohort of PDCs and also included the specific poorly differentiated subtypes. Our results indicate that a significant percentage of PDCs (25%) and MCs (53%) are negative for GCC expression as compared to well differentiated CRCs. There was a statistically significant difference in the GCC staining pattern between MC and PDC. Interestingly, 90% of the SRC cases stained for both markers which shows a positive trend when compared with the extent of GCC staining in PDC. This further strengthens the fact that medullary carcinomas should be classified separately from other less distinct poorly differentiated tumors and that signet ring cell carcinomas as a whole are probably “better differentiated”. There was also a strong positive correlation between CDX2 and GCC expression. This was not surprising as intestinal GCC expression requires a CDX2 binding element in the proximal promoter of CDX2 [9].
One of the most important factors in guiding treatment in patients with colorectal carcinoma is regional lymph node metastases [23]. Detection of micrometastases has import clinical implications and may lead to more aggressive chemotherapeutic treatment; however traditional techniques may fail to detect a significant proportion of lymph node metastases in CRC and lead to under-staging [24,25]. Several studies have demonstrated the effectiveness of molecular studies using reverse transcriptase PCR for GCC in detecting occult micrometastases to lymph nodes in CRCs [12–15]. Schulz et al. showed quantitative RT-PCR could be employed as a highly sensitive and specific method of detecting lymph node metastases [14]. Carrithers et al also showed that by reverse transcriptase PCR GCC was present in peripheral blood samples of all patients with metastatic colorectal cancer and none of the control group and that the presence of GCC in peripheral blood samples may correlate with increased risk of recurrence or poorer prognosis [15]. Bustin et al found that only 1/21 healthy patients had reverse transcriptase PCR positivity for GCC in the peripheral blood while 20/27 peri-operative patients with CRC were positive. In the same study CK19 and CK20, two markers typically positive in CRC, were found to be unreliable as indicators of circulating CRC tumors cells [6]. Buc et al. also proposed GCC as a potential target for delivering chemotherapeutic agents directly to tumor cells by binding heat stable enterotoxin to an chemotherapeutic protein with subsequent GCC dependant tumor cell uptake [10].
Microsatellite instability (MSI) as a result of defective DNA mismatch repair is a well recognized factor in the development of some colorectal carcinomas, is more frequently present in PDC and has clinicopathologic significance [26]. Relying for the most part on this same cohort, we recently studied the expression pattern of a number of markers, including MLH-which is considered a good indicator of MSI [20] in PDC and MC. We found that only 20% of the MC and about half of the PDC stained with MLH-1. Relying on data generated in the previous study on a case by case basis, there was no correlation between loss of MLH-1 and staining for GCC (p=0.39). In this same study we also found that poorly differentiated CRCs often have loss of expression of markers normally expressed in colonic epithelium, such as CK20 [20]. There was a strong correlation between CK20 staining and GCC staining (p=0.0016), showing that poorly differentiated tumors with loss of CK20 expression are also likely to lack GCC expression.
Our findings suggest that if the primary CRC is poorly differentiated, there is a significant chance that the lesion would not express GCC and thus metastases to lymph nodes or circulating tumor cells in the peripheral blood may not be detected by molecular methods. This would also be of significance if future modalities utilize GCC as a target for delivery of proteins directly to tumor cells for imaging or chemotherapeutic purposes, as PDC or poorly differentiated components of well differentiated CRC might not express GCC. Finally, the observation that a significant portion of these PDCs and poorly differentiated components of well or moderately differentiated CRCs may lack staining with GCC is an important consideration when using this immunohistochemical marker to aide in determining the primary site in a metastatic carcinoma of unknown origin.
Footnotes
Disclosure/Conflict of Interest
S.A. Waldman is a paid consultant for Merck Research Laboratories and is the Chair (uncompensated) of the Scientific Advisory Board for Targeted Diagnostics & Therapeutics, Inc, which sponsors research and has a licence to commercialize diagnostic and therapeutic products related to guanylyl cyclase C.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton SR, Vogelstein B, Kudo S, Riboli E, Nakamura S, Hainaut P, et al. Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen L, editors. World Health Organization Classification of Tumors: Pathology and Genetics. Tumors of the Digestive System. IARC Press; Lyon, France: 2000. pp. 103–142. [Google Scholar]
- 3.Lewin KJ, Ridell RH, Weinstein WM. Gastrointestinal pathology and its clinical implications. New York: Igaku-Shoin; 1992. pp. 1272–1273. [Google Scholar]
- 4.Lagendijk JH, Mullink H, Van Diest PJ, Meijer GA, Meijer CJ. Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol. 1998;29:491–497. doi: 10.1016/s0046-8177(98)90065-x. [DOI] [PubMed] [Google Scholar]
- 5.Gervasoni A, Monasterio Munoz RM, Wengler GS, Rizzi A, Zaniboni A, Parolini O. Molecular signature detection of circulating tumor cells using a panel of selected genes. Cancer Lett. 2008;263:267–279. doi: 10.1016/j.canlet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Bustin SA, Gyselman VG, Williams NS, Dorudi S. Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer. 1999;79:1813–1820. doi: 10.1038/sj.bjc.6990289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat stable enterotoxin receptor. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 8.Vaandrager AB, Schulz S, De Jonge HR, Garbers DL. Guanylyl cyclase C is an N-linked glycoprotein receptor that accounts for multiple heat stable enterotoxin-binding proteins in the intestine. J Biol Chem. 1993;268:2174–2179. [PubMed] [Google Scholar]
- 9.Park J, Schulz S, Waldman SA. Intestine-specific activity of the human guanylyl cyclase C promoter is regulated by CDX2. Gastroenterology. 2000;119:89–96. doi: 10.1053/gast.2000.8520. [DOI] [PubMed] [Google Scholar]
- 10.Buc E, Vartanian MD, Darcha C, Dechelotte, Pezet D. Guanylyl cyclase C as a reliable immunohistochemical marker and its ligand Escherichia coli heat-stable enterotoxin as a potential protein-delivering vehicle for colorectal cancer cells. Eur J Cancer. 2005;41:1618–1627. doi: 10.1016/j.ejca.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36:170–179. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Frick GS, Pitari GM, Weinberg DS, Hyslop T, Schulz S, Waldman SA. Guanylyl cyclase C: a marker for staging and postoperative surveillance of patients with colorectal cancer. Expert Rev Mol Diagn. 2005;5:701–713. doi: 10.1586/14737159.5.5.701. [DOI] [PubMed] [Google Scholar]
- 13.Waldman SA, Cagir B, Rakinic J, Fry RD, Goldstein SD, Isenberg G, Barber M, Biswas S, Minimo C, Palazzo J, Park PK, Weinberg D. Use of Guanylyl cylase C for detecting micrometastases in lymph nodes of patients with colon cancer. Dis Colon Rectum. 1998;41:310–315. doi: 10.1007/BF02237484. [DOI] [PubMed] [Google Scholar]
- 14.Schulz S, Hyslop T, Haaf J, Bonacorso C, Nielsen K, Witek ME, Birbe R, Palazzo J, Weinberg D, Waldman SA. A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase C in patients with colorectal cancer. Clin Cancer Res. 2006;12:4545–4552. doi: 10.1158/1078-0432.CCR-06-0865. [DOI] [PubMed] [Google Scholar]
- 15.Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci USA. 1996;93:14827–14832. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond F, Putt W, Fox M, et al. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet. 1997;61:393–400. doi: 10.1046/j.1469-1809.1997.6150393.x. [DOI] [PubMed] [Google Scholar]
- 17.De Lott LB, Morrison C, Suster S, Cohn DE, Frankel WL. CDX2 is a useful marker of intestinal type differentiation: A tissue microarray-based study of 629 tumors from various sites. Arch Pathol Lab Med. 2005;129:1100–1105. doi: 10.5858/2005-129-1100-CIAUMO. [DOI] [PubMed] [Google Scholar]
- 18.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Witek ME, Nielson K, Walters R, Hyslop T, Palazzo J, Schulz S, Waldman SA. The putative tumor suppressor CDX2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11:8549–8556. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 20.Winn B, Tavares R, Fanion J, Noble L, Gao J, Sabo E, Resnick MB. Differentiating the undifferentiated: immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum Pathol. 2009;40:398–404. doi: 10.1016/j.humpath.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakaris S, Cetinkaya A, Ezberci F, Ekerbicer H. Expression of homeodomain protein CDX2 in colorectal adenoma and adenocarcinoma. Histol Histopathol. 2008;23:1043–1047. doi: 10.14670/HH-23.1043. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, de la Monte SM, Sabo E, Kethu S, Tavares R, Branda M, Simao L, Wands JR, Resnick MB. Prognostic value of humbug gene overexpression in stage II colon cancer. Hum Pathol. 2007;38:17–25. doi: 10.1016/j.humpath.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Greene FL, Balch CM, Fleming ID, et al. AJCC cancer staging handbook. 6. New York: Springer; 2002. [Google Scholar]
- 24.Lugo TG, Braun S, Cote RJ, Pantel K, Rusch V. Detection and measurement of occult disease for the prognosis of solid tumors. J Clin Oncol. 2003;21:2609–2615. doi: 10.1200/JCO.2003.01.153. [DOI] [PubMed] [Google Scholar]
- 25.Ratto C, Sofo L, Ippoliti M, et al. Accurate lymph node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum. 1999;42:143–154. doi: 10.1007/BF02237119. [DOI] [PubMed] [Google Scholar]
- 26.Kazama Y, Watanabe T, Kanazawa T, Tanaka J, Tanaka T, Nagawa H. Microsatellite instability in poorly differentiated adenocarcinomas of the colon and rectum: relationship to clinicopathological features. J Clin Pathol. 2007;60:701–4. doi: 10.1136/jcp.2006.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]


