Abstract
Release of neurotransmitters from synaptic vesicles is a central event in synaptic transmission. Recent evidence suggests that synaptic vesicles fuse with the plasma membrane by multiple routes during exocytosis, but the regulation and physiological implications of this choice are unclear. At hippocampal synapses, two modes of synaptic vesicle exocytosis can be distinguished by virtue of the rate and extent of loss of a fluorescent lipid marker (FM1-43). Here these two modes of exocytosis were investigated with a combination of electrophysiological recording and fluorescence imaging. It is shown that these exocytic modes result in distinct postsynaptic consequences, such that so-called ‘kiss-and-run’ exocytosis results in negligible activation of AMPA receptors, compared to the robust postsynaptic responses elicited by apparent full fusion. In contrast NMDA receptors are robustly activated by this form of glutamate delivery. Addition of cyclothiazide, which blocks AMPA receptor desensitization, reveals that the relatively slow rate of release of glutamate during kiss-and-run exocytosis shifts the population of AMPA receptors into a desensitized state, rather than simply being insufficient for receptor activation. These findings provide further support for the existence of a fusion pore mediated mode of exocytosis, and demonstrate that these two exocytic modes directly affect the throughput of synaptic transmission.
Introduction
Synaptic transmission is dependent on the fusion of neurotransmitter-containing synaptic vesicles (SVs) with the plasma membrane, which opens an aqueous connection to the extracellular space, allowing transmitter to escape into the synaptic cleft. This process of vesicular cycling between exocytosis and endocysis is an intricate one, with multiple steps occurring between initiation of exocytosis and final availability of a recycled vesicle for a second round of exocytosis (Edwards, 2007). Exocytosis and endocytosis are highly inter-dependent processes in neuronal cells. Endocytosis swiftly follows exocytosis, and in many systems operates via multiple mechanisms (Koenig & Ikeda, 1996; Richards et al. 2000; Verstreken et al. 2002; Voglmaier et al. 2006); in some cases these are organized in a vesicle pool-dependent fashion (Kuromi & Kidokoro, 1998; Richards et al. 2003; Evans & Cousin, 2007; Xu et al. 2008). Perhaps the most extreme case of interdependence of exocytic and endocytic cyling is that of so-called ‘kiss-and-run’ exocytosis, where a transient fusion pore connects the lumen of the vesicle to the extracellular space, and vesicular contents are released without the vesicle fully collapsing into the plasma membrane. In this scenario, exocytosis and endocytosis are inextricably linked in the process of opening and closing the fusion pore. Strong evidence has been provided to show that both classical full fusion involving the collapse of the vesicle or granule membrane into the plasmalemma, and ‘kiss-and-run’ exocytosis occur in neuroendocrine cells (Chow et al. 1992; Klyachko & Jackson, 2002; Wang et al. 2003; Doreian et al. 2008), while the evidence for this at central synapses is less direct (Klingauf et al. 1998; Gandhi & Stevens, 2003; Aravanis et al. 2003; Richards et al. 2005; Zhang et al. 2009).
FM dyes, which bind reversibly to membranes, and thus provide a means to study vesicle cycling (Betz & Bewick, 1992), have been used to demonstrate that in some cases exocytosis terminates prior to the complete escape of the dye from labelled vesicles, an observation most compatible with kiss-and-run exocytosis (Aravanis et al. 2003; Richards et al. 2005). I have examined vesicle cycling in hippocampal nerve terminals, in response to action potentials, and used the postsynaptic response at identified synapses as a complementary means to characterize release of glutamate. Evidence is provided that fusion pore mediated release (kiss and run) and full fusion give rise to different concentration profiles of glutamate in the synaptic cleft. The result of this is very weak activation of AMPA receptors which gives rise to a predominant receptor desensitization, while (under conditions where the magnesium blockade is lifted) the NMDA receptors are activated at levels more equivalent to those elicited by full fusion.
Methods
Cell culture
Hippocampi were removed from 0–2 day postnatal rat pups, and were then coarsely minced before being incubated for 20–30 min at 37°C in Dulbecco's modified Eagle's medium (DMEM) containing 1 mg ml−1 trypsin. The tissue suspension was then washed in DMEM containing 1% fetal bovine serum (FBS) before the suspension being triturated through a series of Pasteur pipettes of decreasing tip diameter. The resulting cell suspension was centrifuged at 1000 g to pellet the cells before resuspension in culture medium. The cells were initially plated in a small drop of medium at a density of approximately 40 000 per 16 mm coverslip, which had previously been coated with poly-l-lysine, then left for 2 h to settle, at which point DMEM containing 10% FBS is added to fill the culture dish. After 48 h this medium was replaced by the following medium: DMEM 40 ml, minimal essential medium (MEM) 45 ml, Ham's F12 medium 10 ml, FBS 1 ml, bovine serum albumin 0.25 g, glutamine 50 μm, 0.5 ml insulin–transferrin–selenium and 1 ml of Di Porzio mixture (comprising progesterone, 1.25 mg ml−1, hydrocortisone 2 mg ml−1, triiodothyronine 1 mg ml−1, putrescine 20 mg ml−1, superoxide dismutase 20 000 units ml−1, and transferrin 20 mg ml−1). The l-arginine content of the tissue culture medium is 0.66 mm. To minimize glial growth 0.7 mg 5-fluoro-2-deoxyuridine and 1.6 mg uridine are also added. Cultures were maintained for ∼14 days prior to use to allow full maturation. Pyramidal neurons were identified by their morphology and appearance under phase contrast microscopy (phase bright cell bodies possessing long spiny neurites with few recurrent branches). All animal procedures were carried out according to guidelines laid out by the institutional animal care and use committee of the University of Cincinnati.
Imaging
Imaging was carried out on a Zeiss Axiovert 200M inverted microscope with harmonic focus drive, integrated into the Marianas workstation from Intelligent Imaging Innovations (Boulder, CO, USA). The camera was a Roper Cascade 512B, viewing the cover slip through a 100× 1.4 NA objective (Zeiss), illumination was provided by a Sutter Instrument Co. (Novato, CA, USA) lambda DG4 through a scrambled light pipe to provide even illumination of the specimen. Imaging frequency was 5 Hz, with 170 ms exposure time. Subsequent analysis was carried out using ImageJ. The bathing saline had the following composition: 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 5.5 mm glucose, 20 mm Hepes buffered to pH 7.3 using NaOH. Release was evoked by action potentials initiated via a presynaptic patch pipette. The amplitude of fluorescence drops was assessed by comparing fluorescence intensity prior to action potential stimulation with the intensity once the fluorescence had reached a new steady state. In the case of full fusion events this was ∼0.6 s, and 1.2–1.8 s in the case of kiss and run events. No photobleaching correction was required, as under these imaging conditions bleaching was estimated at a maximum of ∼1% of signal.
Electrophysiology
To record the currents resulting from synaptic release of transmitter, synaptic currents were recorded from pyramidal cells in primary culture in whole cell voltage clamp mode. To allow patching under visual guidance, and simultaneous imaging, the cells on their coverslips were mounted in a glass-bottomed incubation chamber on an inverted microscope (Zeiss Axiovert 200M), and the final approach viewed with Nomarski optics using a 100× objective. The extracellular bathing solution was (in mm): NaCl 145, KCl 3, CaCl2 2, MgCl2 2, glucose 11, Hepes 10, buffered to pH 7.4, and 1 μm tetrodotoxin (TTX). Pipettes (5–7 MΩ) were pulled on a Sutter Instrument microelectrode puller. Pipette tips were coated with wax containing 1:1 (by weight) paraffin:mineral oil to reduce noise due to tip capacitance. The internal solution was (in mm): CsMeSO3 130, NaCl 8, Na2ATP 3, MgSO3 3, EGTA 0.5, Hepes 10; pH 7.3. Patch seal resistances were between 2 and 10 GΩ, and zero current potentials (resting membrane potential) were more negative than −65 mV. The signal was amplified by an Axopatch 200 amplifier (Axon Instruments, Union City, CA, USA), low-pass filtered at 2–5 kHz and digitized at 20 kHz in real time to a computer hard drive using Axon Instruments pCLAMP 9 software. Activation was spatially restricted by a push–pull local perfusion via two small glass pipettes driven by syringe pump. In these experiments bathing CaCl2 was reduced to zero, and MgCl2 increased to 4 mm; CaCl2 was introduced only in the region of microperfusion.
Focal FM labelling and analysis of electrophysiological and fluorescence correlation
FM1-43 dye was added to the bathing medium in Ca2+ free medium. Loading was induced by stimulation with localized high-K+ (15 mm, for 2 min, with 2 mm Ca2+), after which the perfusion was stopped, and the bathing saline was exchanged 5 times with low Ca2+ saline in order to wash away any external FM1-43. Patch recordings were then established. Synaptic events were subsequently restricted by focal perfusion in 10 s bursts, with 1 min recovery time, using a solution containing 2 mm Ca2+; the surrounding medium was calcium free. Events were considered simultaneous if the onset of the FM1-43 fluorescence loss occurred within the fluorescence time point (200 ms) that contained the postsynaptic current. If another postsynaptic current was present within 200 ms (+/−) the fluorescence event was considered unattributable, and discarded from analysis. NMDA and AMPA antagonists ((±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX)) were bath applied. Control experiments (not shown) demonstrated that it took 40–60 s for the antagonists to wash from the focal region.
Results
Single action potential evoked destaining events
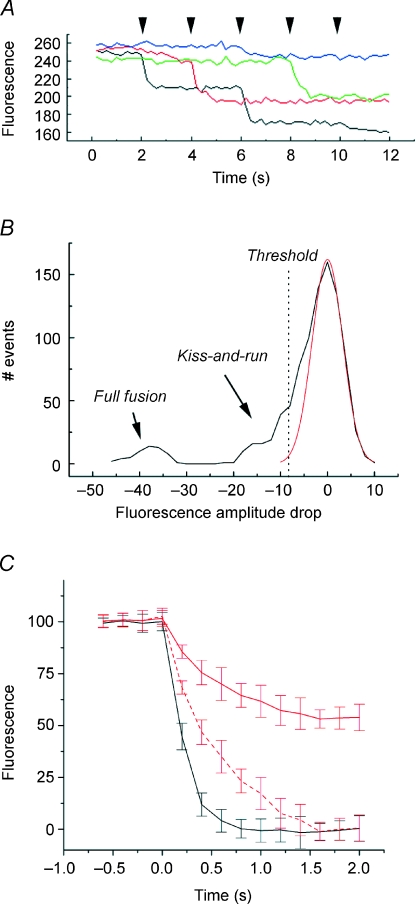
In order to confirm the interpretation of our previous study, which relied on hyper-kalemic stimulation (Richards et al. 2005), I wished to examine the loss of FM1-43 from synaptic vesicles using action potentials elicited at the cell soma with brief depolarisations applied via presynaptic patch pipette. An estimated ∼5 synaptic vesicles per bouton were loaded by eliciting 15 action potentials at 10 Hz. The excess dye was then washed, and after 10 min, imaging began. Five action potentials were applied at 0.5 Hz while imaging at 5 Hz for 12 s. Four representative traces are shown in Fig. 1A, illustrating both large amplitude drops in fluorescence, which Richards et al. (2005) have proposed to correspond to full fusion of synaptic vesicles with the plasma membrane, together with much smaller amplitude events, which are interpreted as fusion pore mediated release, also known as kiss-and-run. Figure 1B is a plot of the fluorescence change following action potentials, which includes both success (loss of fluorescence by either route) and failure (little change in fluorescence). As described previously, this consists of noise, which merges with negative, small amplitude fluorescence changes (kiss-and-run). An additional peak, consisting of full fusion events, is significantly more negative and well resolved from the other events.
Figure 1. Action potentials elicit both kiss-and-run and full fusion FM1-43 destaining events.
A, representative traces from 4 boutons, illustrating both kiss-and-run and full fusion release modes. Action potentials were elicited presynaptically at the times indicated by the arrowheads. B, fluorescence changes following presynaptic action potentials; this includes both failures, where no change in FM1-43 was observed, kiss-and-run events, and full fusion events. Boutons showing no change in fluorescence throughout the experiment (i.e. showing no stimulus-evoked destaining) were excluded. The red curve is a theoretical Gaussian based on the noise. Threshold line indicates where events were accepted for further analysis. C, comparison of rate of loss of dye during full fusion events and kiss-and-run events. Curves are the means of 25 individual events. Normalization consisted of finding the largest amplitude event in a time series, corresponding to loss of a single vesicle's worth of FM1-43, and setting this as 100%. Consequently, kiss-and-run events in this analysis only include those that occurred in a bouton that also exhibited full fusion. Black corresponds to full fusion events, and red to kiss-and-run. The upper kiss and run trace (red) is directly comparable to the full fusion trace (black). The second kiss and run trace has been stretched between 0 and 100% to provide a relative comparison of rate of fluorescence loss.
Previously, Richards et al. (2005) reported that individual boutons can show both modes of release, and this is directly demonstrated here (e.g. in the red trace there is a small ‘kiss-and-run’ response followed by a larger ‘full fusion’ response to action potential stimulation (arrowheads). The significance of this is that it rules out mechanisms for these disparate results based upon synapse heterogeneity. Importantly, in the light of the electrophysiological results which follow, it rules out differences in the complement of postsynaptic receptors as a causal factor in the establishment of the two groups.
By establishing a threshold, events can be classified into three groups: failures, kiss-and-run events, and full fusion events. In Fig. 1C, the decay profiles of each group are shown, based on the average of 25 events each. Large amplitude events (single vesicles releasing the entirety of their contents) lose fluorescence faster than those which only lose a portion of their fluorescence. This is true both in absolute terms, and when fluorescence loss is scaled.
FM1-43 does not alter release properties at central synapses
One possible explanation for the detection of kiss-and-run type release modes by FM1-43 fluorescence measurements (Aravanis et al. 2003; Richards et al. 2005), but not by pH-sensor measurements (Granseth et al. 2006; Balaji & Ryan, 2007) although Zhu et al. (2009) propose an argument based on signal to noise ratio) is that FM1-43 somehow perturbs the release machinery to make this release mode more favoured (although this cannot explain the successful detection of kiss-and-run like release by capacitance at the calyx of Held; He et al. 2006). It is important to exclude this from consideration, however, as it would clearly complicate the analyses described herein. To this end, a series of control experiments was carried out to investigate the effects of 0, 1, 4 and 10 μm FM1-43 on EPSCs in these cultures. To make it as comparable to the experimental system as possible, I loaded and evoked release using elevated K+ (30 min in 20 mm K+, which provides ∼80% labelling of the releasable pool), with a 15 min wash in normal artificial cerebrospinal fluid (ACSF) prior to recording and stimulated release (this time with 15 mm K+). EPSC frequency, 20–80% rise time, amplitude and decay were then measured, and the results are summarized in Table 1. No evidence was found that the presence of FM1-43 caused any change in release parameters, indicating that it is unlikely that the presence of the dye perturbs the release apparatus.
Table 1.
FM1-43 has no measurable effect on release properties of hippocampal synapses, as measured by miniEPSC analysis
| [FM1-43] (μm) | n | Frequency (Hz) | Rise time (20–80%) (ms) | Amplitude (pA) | τ decay (ms) |
|---|---|---|---|---|---|
| Control (no stim) | 953 | 0.87 ± 0.13 | 0.34 ± 0.05 | 22.82 ± 4.81 | 3.18 ± 0.11 |
| 0 | 897 | 6.58 ± 0.22 | 0.32 ± 0.04 | 23.12 ± 4.73 | 3.23 ± 0.12 |
| 1 | 921 | 6.87 ± 0.27 | 0.35 ± 0.05 | 20.87 ± 4.02 | 3.05 ± 0.10 |
| 4 | 980 | 6.43 ± 0.26 | 0.33 ± 0.04 | 24.74 ± 5.11 | 3.09 ± 0.14 |
| 10 | 864 | 6.61 ± 0.24 | 0.34 ± 0.03 | 23.54 ± 4.36 | 3.13 ± 0.13 |
Stimulation groups were bathed in 20 mm K+-containing ACSF.
Kiss-and-run and full fusion have different postsynaptic consequences
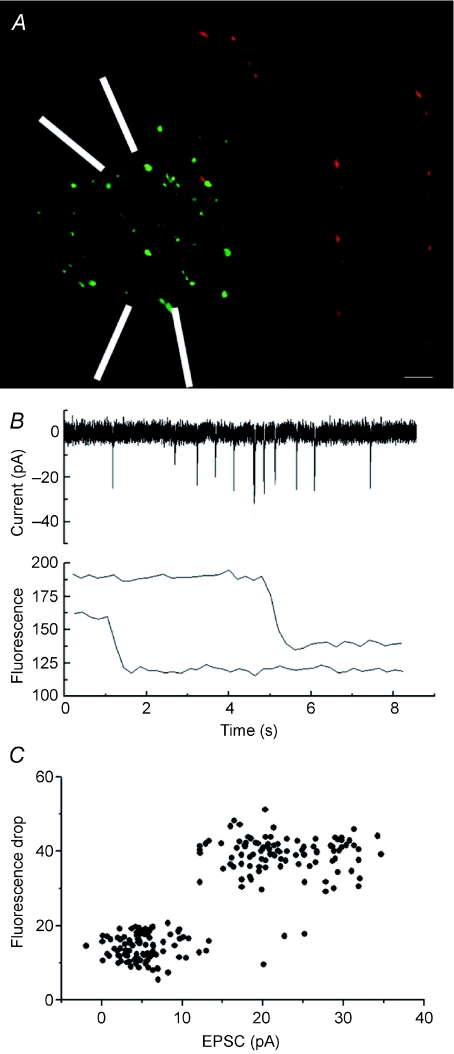
With two very different exocytic release modes co-existing at the same bouton, it becomes important to ask what is the postsynaptic result of each mode? This was addressed by patching neurons, and filling them with an inactive red dye (Alexa 594). FM labelling was restricted to a limited region, to reduce the impact of dendritic filtering, by focal application of K+ and Ca2+ (Fig. 2A). In other experiments (not shown) I attempted to resolve the postsynaptic responses due to each release mode by imaging the entire cell and all attached boutons. This failed due to the large number of events (and the impossibility of distinguishing which were linked) and the high degree of variability in the mEPSCs. Resolving individual events depended on restricting excitation to a very small area. This was achieved using two pipettes, the first to perfuse, the second to provide local extraction of the medium (white arrowheads in Fig. 2A; phase image also superimposed on the fluorescence). The bathing medium lacked extracellular Ca2+. This restricted excitation to a very small region, and consequently both triggering of mEPSCs and FM1-43 labelling were confined to a region of ∼25 μm. After 10 min to allow diffusion of the red label through the postsynaptic cell, the culture was again locally stimulated with elevated potassium (15 mm), and EPSCs were recorded while imaging at 5 Hz. Synchronization was obtained by sending a logic pulse from the imaging software at the initiation of recording. During offline analysis, the fluorescence and electrical traces were time-aligned, and scanned for postsynaptic currents synchronous with a fluorescence drop at a bouton in apparent contact with the postsynaptic cell (i.e. overlapping by at least 1 pixel with the red-labelled neural process; Fig. 2B). The resulting data were then plotted with postsynaptic current against the amplitude of the fluorescence drop (Fig. 2C). As summarized in Fig. 2C, the data indicate that large amplitude fluorescence signals (putative full fusion events) corresponded with well-resolved postsynaptic EPSCs, while small amplitude (putative kiss-and-run type) events did not elicit robust EPSCs.
Figure 2. Postsynaptic consequences of vesicular release mode.
A, fluorescence image of a neuron filled via patch pipette with Alexa 594, together with FM1-43 puncta (green). Synaptic release was confined to a limited area by local perfusion of K+ and Ca2+ from one pipette, with retrieval by another (pipettes indicated by white lines). Release in surrounding areas was restricted by the absence of extracellular Ca2+. Scale bar 1 μm. B, synchronous current and fluorescence recordings. Upper panel shows a stretch of postsynaptic current recording, with numerous EPSCs, all derived from the focal region (since both depolarization and extracellular calcium are restricted to the micro perfused region). In the lower panel, fluorescence from 2 colocalized boutons shows drops which coincide with large EPSCs. C, postsynaptic consequences of release mode. The amplitude of fluorescence drops in co-localized boutons is plotted against the peak current simultaneously recorded by patch pipette. While large full fusion events clearly elicit robust postsynaptic responses, kiss-and-run type release events do not.
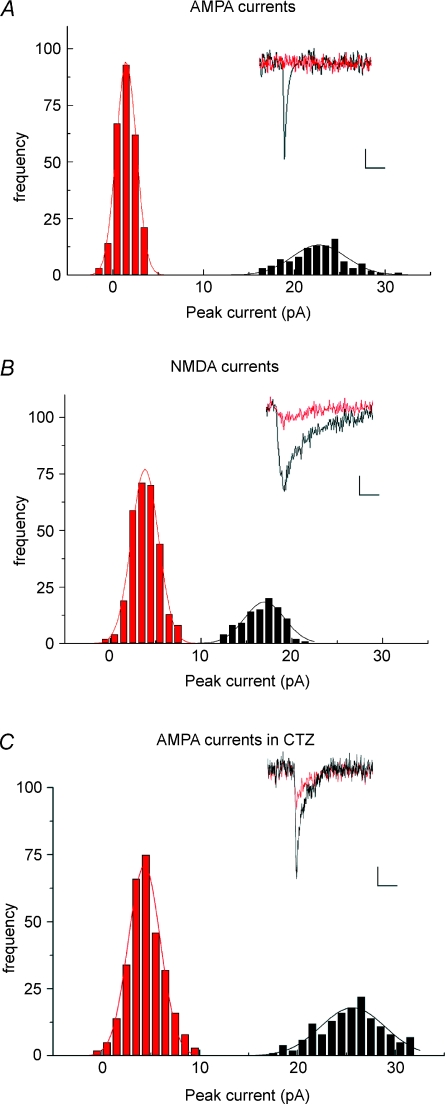
A potential explanation for the inefficient postsynaptic activation following kiss-and-run mediated release is that the slowed efflux of glutamate is both activating and desensitizing AMPA receptors, resulting in very modest postsynaptic currents. To test this the extent of activation of AMPA receptors and NMDA receptors following individual release events was investigated. Two groups of experiments were carried out; the first monitored EPSCs in the presence of CPP and 2 mM Mg2+, while the second was carried out in the presence of NBQX and the absence of extracellular Mg2+. In each case, the peak current amplitude coincident with the fluorescence drop was measured, and is plotted as an amplitude frequency histogram. For descriptive purposes, a threshold of 25 fluorescence units was used to separate kiss-and-run from full fusion events. Although in both groups synchronous EPSCs and large amplitude fluorescence drops were observed (consistent with Fig. 2), very little AMPA mediated current was observed synchronous with the small amplitude fluorescence drops (Fig. 3A). In contrast, reduced NMDA currents synchronous with small amplitude fluorescence drops were seen in the NMDA traces (Fig. 3B). No currents or fluorescence drops were seen in either group following addition of tetanus toxin to the preparation (not shown).
Figure 3. Kiss-and-run release elicits detectable NMDA but not AMPA currents.
A, using the focal approach demonstrated in Fig. 2, the activation of AMPA receptors specifically was investigated by including CPP (10 μm) in the bathing medium to block NMDA receptors. Kiss-and-run and full fusion events were defined as being either larger than 25 fluorescence units (full fusion, black) or smaller than this (kiss-and-run, red). While full fusion events evoked robust AMPA currents, the peak currents coincident with kiss-and-run exocytosis were not different from noise. Each current trace represents the average of 5 responses from the same cell. Scale bars 5 pA, 10 ms. B, NBQX (15 μm) was included in the bathing medium to block AMPA receptors, and magnesium was removed, to probe the extent of activation of NMDA receptors. Full fusion events evoked robust NMDA currents; however, unlike AMPA, the peak NMDA currents coincident with kiss-and-run could be resolved, indicating that kiss-and-run events do result in significant levels of glutamate release. C, inclusion of cyclothiazide (CTZ) in the bathing medium unmasked a small AMPA current in response to putative kiss-and-run mediated release.
Apparent failure to activate AMPA-receptors, while successfully stimulating NMDA-receptors, could be interpreted to indicate a glutamate concentration below the level required for activation of low affinity AMPA receptors, while still being high enough to activate the higher affinity NMDA-receptors. An alternative interpretation is that the lowered glutamate concentration, coupled with slowed entry of glutamate into the cleft, leads to a significant degree of receptor desensitization. This hypothesis was tested using cyclothiazide, an agent which blocks AMPA-receptor desensitization. Consistent with previous reports (Gasparini et al. 2001), an increase was seen in overall release rate of 181.5% (data not shown), while the ratio between kiss-and-run and full fusion release modes changed only slightly (67.6 ± 6.8% kiss-and-run in control solution compared to 61.9 ± 7.3% in CTZ, not significant; data are mean ± s.e.m. from 5 experiments. Significance was assessed using Student's t test.). In the presence of cyclothiazide, both kiss-and-run type and full fusion type fusion events produced clear postsynaptic currents (Fig. 3C), indicating that receptor desensitization was the principal cause for the failure of kiss-and-run fusion events to elicit AMPA responses under normal conditions.
Discussion
In the present work I have investigated the properties of two modes of synaptic vesicle release at hippocampal boutons, which can be separated on the basis of the rate at which they lose FM1-43 (Aravanis et al. 2003; Richards et al. 2005). I find that those events which result in reduced, slowed FM1-43 efflux give rise to small or undetectable postsynaptic currents, while those events where a ‘vesicle equivalent’ of FM1-43 is lost result in robust EPSCs comparable to typical mEPSCs. The loss of postsynaptic currents seen in response to the putative ‘kiss-and-run’ events can be ameliorated by cyclothiazide treatment, indicating that desensitization of AMPA receptors plays a role in masking the true extent of glutamate release during this mode, a conclusion reinforced by the observation that NMDA-specific EPSCs show less differentiation between the two modes. These results support previous studies indicating that synaptic vesicle exocytosis at this synapse can progress through (at least) two routes: one an apparently fusion-pore based route that restricts neurotransmitter and FM1-43 flux, and another that exhibits no apparent restrictions. The results also demonstrate that both mechanisms result in significant glutamate release, while the relative ‘set-point’ for synapses (between the extremes of kiss-and-run-only and full-fusion-only) will have a significant impact on neural circuits.
These data confirm that the small amplitude events are consistent with a fusion pore mediated model for ‘kiss-and-run’ exocytosis, and indicate that the rate of release of glutamate, together with the reduced peak concentration, is insufficient to synchronously activate large numbers of AMPA receptors in the postsynaptic membrane. This finding is consistent with the estimated pore size (∼1–2 nm) previously described for kiss-and-run exocytosis (Klyachko & Jackson, 2002; Richards et al. 2005; Kasai et al. 2005). It is important to note, however, that the electrophysiology is likely to reflect only the first few milliseconds of exocytosis (due to the rapid efflux of glutamate even in a restricted fusion pore – see the extensive discussion of this issue in Choi et al. 2003).
The debate over the contribution of kiss-and-run and full fusion exocytotic mechanisms at synapses arose in the context of presynaptic vesicle pools recovering from stimulation (Heuser & Reese, 1973; Ceccarelli et al. 1973). Kiss-and-run exocytosis was seen as potentially significant mainly in terms of speeding re-use of vesicles, an argument that was strengthened by findings that recovery from synaptic depression depended on vesicle pool regeneration (Kuromi & Kidokoro, 1998; Richards et al. 2003). The findings presented here complicate this picture, indicating that modes of exocytosis can be complementary, as they may serve different postsynaptic ‘purposes’.
The data in this study indicate that hippocampal boutons are making continual decisions as to which exocytic mode to use. This process has important implications for information flow through the CNS, since one mode (full fusion) provides robust postsynaptic activation, which the other does not. Nevertheless, the postsynaptic consequences of kiss-and-run mediated release are potentially highly significant – by only weakly activating AMPA receptors, kiss-and-run mediated release will tend to shift this population of receptors into a somewhat desensitized state. Additionally, this release mode will tend to sharpen coincidence detection; by occupying ligand binding sites on the NMDA receptors, they will increase the magnitude of the NMDA current when coincident pre- and postsynaptic activation occurs. Finally, it is possible that a portion of the silent synapses, the unsilencing of which has been reported to play a role in synaptic plasticity in this region, are in fact presynaptically silent (Gasparini et al. 2001; Choi et al. 2000, 2003).
There has been extensive debate over the last few years concerning not only the relative contribution of so-called ‘kiss-and-run’ exocytosis to neurotransmitter release at various synapses, but also the very existence of this phenomenon. While kiss-and-run has become a well established mechanism in the fusion of large dense-core vesicles, potentially providing an additional locus for regulation of secretion, at synapses using small clear synaptic vesicles its role has become the subject of heated debate. In general this falls between reports indicating no clear evidence for kiss-and-run-like exocytosis (Granseth et al. 2006; Balaji & Ryan, 2007), and those which do report it (Klingauf et al. 1998; Gandhi & Stevens, 2003; Aravanis et al. 2003; Richards et al. 2005; Zhang et al. 2009). It is possible that this may reflect the different methodologies used. Studies indicating no detectable kiss-and-run exocytosis have used pH-sensitive green fluorescent protein (GFP) variants tagged to synaptobrevin or synaptophysin (Granseth et al. 2006; Balaji & Ryan, 2007), while those reporting the presence of kiss-and-run have used FM1-43 efflux rates (Aravanis et al. 2003; Richards et al. 2005), low molecular weight quenchers (Harata et al. 2006), capacitance (He et al. 2006), and pH sensitive quantum dots (Zhang et al. 2009). A recent report appears to have addressed this discrepancy (Zhu et al. 2009), indicating that a lack of signal to noise in pHlourin (pH sensitive GFP) based reporters is to blame – the authors inserted four of these reporter moieties into a luminal domain of synaptophysin, and with this they were able to resolve both fast and slow modes of endocytosis at hippocampal synapses, while their pooled data were broadly consistent with what had been reported before (Granseth et al. 2006; Balaji & Ryan, 2007). The ability to distinguish between release modes electrophysiologically, as described in the present work, fills what is perhaps the greatest remaining hole in the kiss-and-run debate as it presently stands.
The next challenge at the level of the cell biology of the synaptic terminal will be to distinguish the detailed mechanism of fast endocytosis. It appears to involve clathrin (Granseth et al. 2006; Zhu et al. 2009) and may not involve complete retention of vesicle identity (Zhu et al. 2009) at the protein level. These findings are not necessarily at odds with a fusion-pore mediated release mode (termed kiss-and-run in this paper). While multiple models can be conjectured to fit this, perhaps the simplest is a modification of the process thought to occur in neuroendocrine cells. In dense core granule secretion, formation of an initial fusion pore is often followed by dilatation and complete fusion (the pre-spike foot and spike detected by amperometric recording). Perhaps synaptic vesicle fusion either proceeds by very rapid dilatation or by halted dilatation involving accessory proteins (dynamin, clathrin) followed by rapid retrieval.
In the field of long-term plasticity, in particular LTP and LTD, which have dominated the hippocampal field over recent decades, the demonstration of electrophysiologically distinct release modes has important implications. LTP has been well documented to involve postsynaptic mechanisms such as insertion of AMPA receptors, and this has become the de facto explanation for the disappearance of ‘silent synapse’ (NMDA only synapses) following LTP induction. Our finding that kiss-and-run type exocytosis effectively gives rise to NMDA only responses in the absence of cyclothiazide provides an additional mechanism for un-silencing of synapses, as well as for the variability of cleft glutamate concentration (Liu et al. 1999; Renger et al. 2001).
Acknowledgments
This research was supported by National Institutes of Health R01 grant NS054750, an American Heart Association Scientist Development Grant, and Millennium Scholar funds from the University of Cincinnati. There are no competing interests.
Author's present address
D. A. Richards: Department of Anesthesia, Cincinnati Children's Hospital Medical Center, MLC2001, 3333 Burnet Avenue, Cincinnati, OH 45229, USA.
References
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;54:30–38. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at ‘silent synapses’. Nat Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Fusion pore modulation as a presynaptic mechanism contributing to expression of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:695–705. doi: 10.1098/rstb.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Doreian BW, Fulop TG, Smith CB. Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J Neurosci. 2008;28:4470–4478. doi: 10.1523/JNEUROSCI.0008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:385–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27:401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Savinae C, Voronin LL, Cherubini E. Proc Natl Acad Sci U S A. 2001;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Hatakeyama H, Kishimoto T, Liu TT, Nemoto T, Takahashi N. A new quantitative (two-photon extracellular polar-tracer imaging-based quantification (TEPIQ)) analysis for diameters of exocytic vesicles and its application to mouse pancreatic islets. J Physiol. 2005;568:891–903. doi: 10.1113/jphysiol.2005.093047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Kavalali ET, Tsien RW. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Klyachko VA, Jackson MB. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;20:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Egles C, Liu G. A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Richards DA, Bai J, Chapman ER. Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles. J Cell Biol. 2005;168:929–939. doi: 10.1083/jcb.200407148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO, Betz WJ. Synaptic vesicle pools at the frog neuromuscular junction. Neuron. 2003;39:529–541. doi: 10.1016/s0896-6273(03)00405-7. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Wang CT, Lu JC, Bai J, Chang PY, Martin TF, Chapman ER, Jackson MB. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Heinemann SF. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron. 2009;61:397–411. doi: 10.1016/j.neuron.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]