Abstract
The etiology of motor neuron degeneration in amyotrophic lateral sclerosis (ALS) remains to be better understood. Based on the studies from ALS patients and transgenic animal models, it is believed that ALS is likely to be a multifactorial and multisystem disease. Many mechanisms have been postulated to be involved in the pathology of ALS, such as oxidative stress, glutamate excitotoxicity, mitochondrial damage, defective axonal transport, glia cell pathology and aberrant RNA metabolism. Mitochondria, which play crucial roles in excitotoxicity, apoptosis and cell survival, have shown to be an early target in ALS pathogenesis and contribute to the disease progression. Morphological and functional defects in mitochondria were found in both human patients and ALS mice overexpressing mutant SOD1. Mutant SOD1 was found to be preferentially associated with mitochondria and subsequently impair mitochondrial function. Recent studies suggest that axonal transport of mitochondria along microtubules and mitochondrial dynamics may also be disrupted in ALS. These results also illustrate the critical importance of maintaining proper mitochondrial function in axons and neuromuscular junctions, supporting the emerging “dying-back” axonopathy model of ALS. In this review, we will discuss how mitochondrial dysfunction has been linked to the ALS variants of SOD1 and the mechanisms by which mitochondrial damage contributes to the disease etiology.
Keywords: amyotrophic lateral sclerosis, mutant SOD1, mitochondrial function, axonal transport, mitochondrial dynamics
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by preferential motor neuron death. Approximately 10-20% of the cases are familial whereas the majority of them are sporadic. Among the familial cases, the most common disease-causing mutations are found in the copper-zinc superoxide dismutase (SOD1) gene [1, 2]. Mutations in other genes have also been found to cause various subsets of familial ALS, including the recently discovered RNA processing proteins TDP-43 [3, 4] and FUS/TLS [5, 6]. While the new gene mutations in TDP-43 and FUS/TLS will help understanding the etiology of ALS, particularly the potential mechanisms underlying both sporadic and familial ALS, the large body of knowledge on the disease pathogenesis has been based on the studies of mutant SOD1 mediated familial ALS in the past decade. This review is also focused on the mitochondrial dysfunction induced by mutant SOD1.
The mitochondrion is a critical organelle within cells executing multiple functions. Mitochondria are the primary site of ATP production, maintain calcium homeostasis and participate in calcium signaling, and regulate intrinsic apoptosis. Mitochondrial malfunction confers pleiotropic effects to the cells, especially neurons with an elevated susceptibility to aging and stress. Mitochondrial pathology is a key player among multiple working hypotheses in the ALS studies [2, 7]. Mutant SOD1 has been reported to be associated with mitochondria, and the morphology and bioenergetic function of mitochondria can consequently be impacted. More recent studies also support that axonal transport of mitochondria is disrupted by mutant SOD1 in ALS. Furthermore, mitochondrial dynamics and function can be altered when the axonal transport in motor neurons is compromised. The above mechanisms are not mutually exclusive, but rather consist a vicious cycle that deteriorates mitochondria in motor neurons especially the distal nerve terminals. These observations suggest that mitochondrial dysfunction is likely an important causal or contributing factor to ALS pathogenesis and progression. This review will discuss the findings and the mechanistic insights garnered from the studies.
Subcellular localization of SOD1
SOD1 is a ubiquitous protein that functions as a primary antioxidant by catalyzing the disproportionation of superoxide radicals to hydrogen peroxide and molecular oxygen. The homodimeric protein is localized predominantly in the cytoplasm, but it is also found in other cellular compartments including the nucleus [8], endoplasmic reticulum [9] and mitochondria [10, 11]. Wild type (WT) SOD1 as well as copper chaperone for SOD1 (CCS) has been found in the intermembrane space (IMS) of mitochondria [10, 11]. It has been proposed that the nascent SOD1 polypeptide with no metal ion bound can efficiently enter the mitochondria and that the maturation of SOD1 (including metal ion binding and intramolecular disulfide bond formation) inside mitochondria and the subsequent retention in IMS involve the SOD1-CCS interaction. Moreover, high levels of CCS in the mitochondrial intermembrane space could result in enhanced mitochondrial accumulation of SOD1 [12].
The ALS-related mutant SOD1 proteins have also been found in the IMS, matrix and outer membrane of mitochondria [13-16]. It is likely that mutant SOD1 fails to fold properly and perturbs the physiological regulation of mitochondrial import and retention [16]. However, it remains unclear how mutant SOD1 becomes aggregated on the outer membrane or in the matrix of mitochondria. It is also unclear how mutant SOD1 is selectively recruited to spinal, but not liver, mitochondria [17].
Once associated with mitochondria, the mutant SOD1 is believed to cause multiple damages to mitochondria. An early event could be damage to the mitochondrial membrane leading to loss of mitochondrial membrane potential and swelling of the important organelle [18, 19]. Potential consequences include impaired respiratory complex [20-22], disrupted redox homeostasis and decreased ATP production [23]. Furthermore, calcium homeostasis can be disrupted [22, 24-26] and apoptosis may be activated [27-29]. Details of the above inter-related mechanisms underlying mutant SOD1 induced mitochondrial dysfunction will be discussed in the sections in the first half of this review.
Abnormal mitochondrial morphology and bioenergetics in ALS
Early studies have shown degenerating mitochondrial vacuoles in axons and dendrites of motor neurons in presymptomatic mice expressing mutant SOD1 [18, 19, 30]. In addition, dense conglomerates of mitochondria in the anterior horn of lumbar spinal cord and proximal axons have been found in sporadic ALS patients [31, 32]. Abnormal clustering of mitochondria was recently reported in motor axons in mutant SOD1 transgenic mice [33]. Extensive fragmentation of mitochondria was also reported in cultured NSC34 cells overexpressing mutant SOD1 [34, 35]. A further study found that vacuolation of mitochondria in the spinal motor neurons of G93A transgenic mice was caused by expansion of the IMS and the outer mitochondrial membrane. The same study found that the degenerative vacuoles were bounded by mutant SOD1 that colocalized with mitochondrial outer membrane markers [36]. Besides alteration of mitochondrial morphology, damage to the mitochondrial membrane caused by mutant SOD1 can yield loss of mitochondrial membrane potential, disruption of mitochondrial respiratory chain activity [20, 21], and reduction in mitochondrial Ca2+ buffering capability [26]. The dysregulation in electron transfer chain complexes was observed in both G93A SOD1 transgenic mice and human ALS patients [20, 22].
Disruption of calcium homeostasis in ALS
Another important function of mitochondria is to buffer intracellular surges of Ca2+ in excited cells. In excitable cells like motor neurons, mitochondria play an important role in short-term handling of rapid cytosolic Ca2+ transients. Ca2+ is a ubiquitous second messenger and participates in many signaling pathways that are crucial for cell survival. Increased Ca2+ concentration and mitochondrial damage were found in ALS patients as well as animal and cellular ALS models [25, 26, 37-39]. A significant decrease in mitochondrial Ca2+ loading capacity in brain and spinal cord, but not in liver, was observed in presymptomatic G93A mutant transgenic mice [38]. Elevated Ca2+ can induce reactive oxygen species and oxidative stress in primary motor neurons isolated from G93A transgenic mice [25]. Alternatively, Ca2+ mediated glutamate excitotoxicity might contribute to the mutant SOD1 toxicity in motor neurons. Altogether, the mitochondrial dysfunction induced by mutant SOD1, disrupted calcium homeostasis and subsequent excitotoxicity are likely to be interrelated mechanisms that collectively contribute to motor neuron degeneration in ALS.
Involvement of mitochondria-mediated apoptosis in ALS
Mitochondria-mediated apoptosis was found to be involved in motor neuron degeneration in early studies of ALS. In G93A SOD1 transgenic mice, cytosolic release of cytochrome c was observed [27-29], and levels of pro-apoptotic proteins Bad and Bax were increased while those of anti-apoptotic proteins Bcl2, Bcl-xL and XIAP were decreased [40-42]. It has been proposed that mutant SOD1 can sequester anti-apoptotic protein Bcl-2 [27], reduce mitochondrial membrane potential, and trigger cytochrome c release from mitochondria [27-29]. Caspase-1 and caspase-3 were also found to be sequentially activated in motor neurons and astrocytes in G93A SOD1 mice as well as in G37R SOD1 and G85R SOD1 mice [43-45]. Strategies intervening in mitochondria-mediated apoptosis were demonstrated to be effective in G93A SOD1 mice: (i) intraventricular administration of minocycline, which inhibits cytochrome c release from mitochondria, was shown to delay disease onset and extend survival [46]; (ii) over-expression of anti-apoptotic protein Bcl-2 could delay activation of the caspases, attenuate neuron degeneration and delay disease onset and mortality [41, 47]; and (iii) intraventricular administration of a broad spectrum caspase inhibitor zVAD-fmk could delay disease onset and mortality [43]. However, the clinical trials based on the above apoptosis-inhibiting approaches (e.g. minocycline) failed in human ALS patients [48].
Interestingly, Gould et al. crossed the mutant SOD1 transgenic mice with Bax knockout mice and showed that neuromuscular denervation and mitochondrial vacuolization persisted in the absence of apoptotic death of motor neuron cell bodies in the double mutant mice [49]. Neuromuscular denervation was observed to begin long before the activation of apoptotic proteins, and Bax deficiency delayed the onset of neuromuscular denervation. Motor neurons exhibited mitochondrial abnormalities at the innervated neuromuscular junction at the onset of neuromuscular denervation. In addition, presynaptic terminals of motor neurons accumulated high levels of mutant SOD1 before the axons were withdrawn from the neuromuscular junction. A separate study showed denervation of 40% neuromuscular junctions in pre-symptomatic G93A SOD1 transgenic mice (47 days), 60% loss of ventral root axon in 80 days old G93A mice (immediately prior to onset), and significant motor neuron death until 100 days old (post-symptomatic) [50]. The results from these studies support the “dying back” hypothesis that clinical symptoms in the G93A SOD1 mice result from damages to the distal motor axon rather than the activation of the cell death pathway in cell bodies. Furthermore, the results suggest that local mitochondrial changes in distal axons may represent a triggering mechanism for axonal degeneration and denervation. The findings inspired more careful studies of mitochondrial abonormalities in ALS with respect to the subcellular localization of such changes as well as the transport and dynamics of mitochondria. The significance and findings of mitochondrial transport and dynamics in the context of ALS are discussed below.
Disruption of axonal transport in ALS
Neurons have extensive dendritic arbors and axonal processes that can extend far from the cell body. Transport of materials (proteins and organelles) and signals between the cell body and neuronal processes is crucial to neuronal function and survival. Disruption of slow axonal transport of the cytoskeleton is one of the earliest pathological events in mutant SOD1 mice [51, 52]. Fast axonal transport mediated by kinesin and dynein motor complexes is responsible for transporting membrane-bound organelles (e.g. mitochondria) for axonal and synaptic function. Mutations in the anterograde transport motor protein kinesin (KIF5A) can cause spastic paraplegia [53] and mutations in the retrograde motor complex dynein-dynactin cause motor neuron degeneration in humans and mice [54, 55]. Mouse strains carrying various dynein or dynactin mutations also showed retrograde transport impairment and motor neuron degeneration [54, 56-59]. Decreased kinesin-mediated (anterograde) and dynein-mediated (retrograde) axonal transport have been observed both in ALS patients and in transgenic animal models [51, 60-65]. More intriguingly, crossing of G93A SOD1 ALS mice with different mouse strains carrying dynein or dynactin mutations resulted in various degrees of protection in the double mutant mice [56-58, 66, 67]. Nevertheless, the results strongly support that axonal transport is likely a critical component in ALS disease etiology.
The detailed mechanisms by which axonal transport is affected in ALS have yet to be established. In the last few years, ALS-related mutant SOD1 proteins have been shown to interact with both the anterograde motor protein kinesin-2 complex via kinesin-associated protein 3 (KAP3) and the retrograde motor protein complex dynein-dynactin [68-70], providing potential molecular mechanisms how mutant SOD1 may interfere with axonal transport. Most recently, a genome-wide single nucleotide polymorphism analysis in a set of 1,821 sporadic ALS cases and 2,258 controls from the U.S. and Europe revealed that a variant within the KAP3 gene was associated with decreased KAP3 expression and increased survival in sporadic ALS [71]. Examination of the literature revealed a report showing increased expression of KAP3 in mutant SOD1 transgenic mice [72]. These findings provide an emerging concept that alterations of axonal transport might be involved in both familial and sporadic ALS.
Axonal transport of mitochondria and its regulation
Different from other transported organelles, mitochondria are unique since they do not have a defined destination. Their dynamics are influenced by moment-to-moment changes in the energy demands of the cell [73]. Appropriate distribution of mitochondria is critical for meeting cellular energy demands or regulation of Ca2+ levels, especially in neurons [74]. Mitochondria are frequently found in axon terminals due to the high demand of ATP and Ca2+ handling at synapses [75, 76]. In response to synaptic excitation, mitochondria redistribute to the dendrites and facilitate spine morphogenesis and synaptogenesis [77]. In neurons, mitochondria migrate in both anterograde and retrograde directions through association with kinesin and dynein motor complexes. Disruption of kinesin heavy chain KIF5B causes perinuclear clustering of mitochondria in mouse neurons, indicating that KIF5B is essential for mitochondrial dispersion [78]. In addition, other kinesin superfamily members KIF1B and KLC3 are also implicated in anterograde transport of mitochondria [79, 80]. Dynein is important not only for axonal retrograde transport of mitochondria but also for mitochondrial fission [81].
The precise mechanisms that regulate the kinesin and dynein motor complexes and their attachment to the organelles are yet to be fully understood [74]. One mechanism by which mitochondria are attached to kinesin has been described. Milton is an adaptor protein interacting with both kinesin heavy chain (KHC) and the mitochondrial protein Miro and recruits mitochondria to the kinesin motor in microtubule-dependent transport [82-86]. The interaction between Milton and KHC is independent of kinesin light chain (KLC). Miro mutants in Drosophila cause enrichment of mitochondria in neuronal somata and reduction in neuropil [84]. GTPase defective mutants of Miro resulted in the aggregation of mitochondria [85]. The C-terminal transmembrane domain within Miro is required for mitochondrial outer membrane targeting. The N-terminus within Miro is responsible for binding with the kinesin-interacting protein GABA-A receptor-interacting factor 1 (GRIF-1) and O-linked N-acetylglucosamine transferase interacting protein 106 (OIP106), linking mitochondria to kinesin-mediated axonal transport [87-89].
Miro contains two Ca2+-binding EF-hand motifs, providing a calcium-responsive mechanism to regulate mitochondrial transport. Ca2+ binding to the EF-hand motif of Miro can promote direct binding of Miro to KHC rather than via Milton, and prevent the attachment of KHC to microtubules [86]. In response to excitation, an influx of Ca2+ in both pre- and post-synaptic cytosol will consequently inhibit mitochondrial transport. The locally arrested mitochondria are then able to provide more ATP and to buffer high Ca2+ to avoid overexcitement. Mitochondria subsequently move away when the local Ca2+ concentration returns to normal and ATP is supplied [90, 91]. This provides a mechanism for responding to local calcium homestasis and energy demand.
Neurotrophic factors, which are critical to neuronal differentiation and survival and axonal growth and maintenance, can also regulate axonal transport of mitochondria. For instance, nerve growth factor (NGF) has been shown to regulate the motility and distribution of axonal mitochondria and cause the local accumulation of mitochondria in cultured primary neurons [92, 93].
Disruption of axonal transport of mitochondria in ALS
Disruption of mitochondrial transport has been implicated in neurodegenerative diseases including ALS [74, 94, 95]. Mitochondria, which display saltatory movement along microtubules, are transported by kinesin and dynein motors at the speed of approximately 1 μm/s (i.e. 86.4 mm/day). In one study using both primary neurons isolated from G93A SOD1 transgenic mice and cortical neurons transfected with G93A SOD1, mitochondrial transport was observed to be selectively reduced in the anterograde direction [94]. In another study using differentiated NSC34 cells overexpressing mutant SOD1, mitochondrial transport was found to be altered in both anterograde and retrograde directions [95].
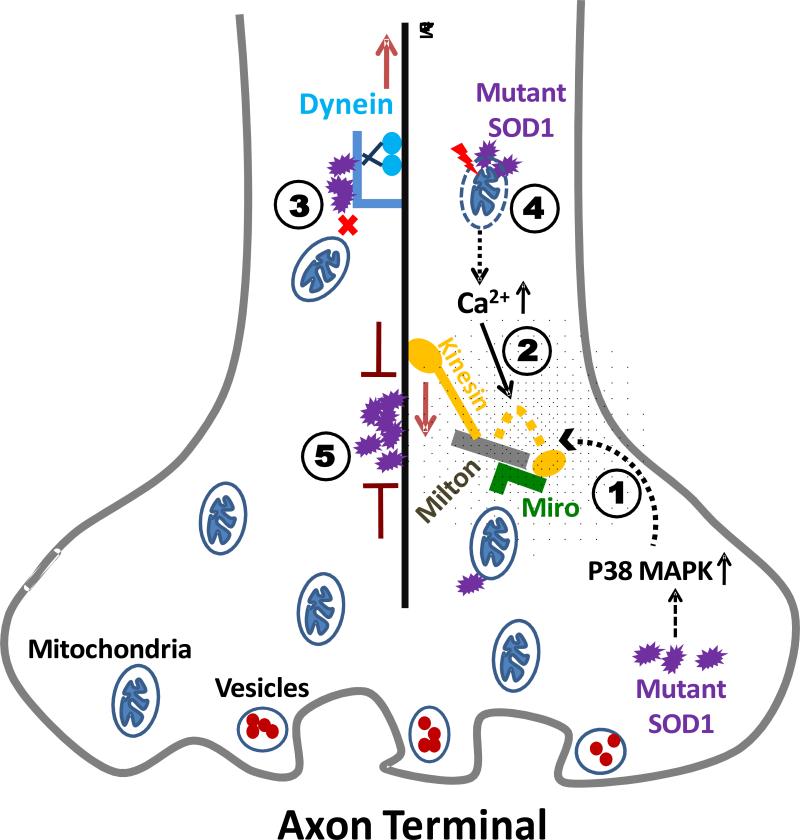
The mechanism by which mutant SOD1 interferes with mitochondrial transport is yet to be elucidated. Several scenarios can be envisioned and illustrated as in Figure 1. First, various kinases have been reported to be activated by pathogenic proteins implicated in different neurodegenerative diseases to phosphorylate kinesin heavy chain (KHC) or kinesin light chain (KLC), and to affect anterograde transport [96-99]. The p38 MAP kinase stress pathway was reported to be activated in mutant SOD1 transgenic mice [100, 101], which potentially provides a connection between mutant SOD1 and kinesin-mediated axonal transport. Second, elevated Ca2+ levels caused by disrupted calcium homeostasis can promote direct binding of KHC to Miro and detachment of KHC from microtubules as discussed earlier [86], which would cause decreased anterograde axonal transport. Third, our previous studies showed that mutant SOD1 could interact with the dynein-dynactin complex [68, 69], linking mutant SOD1 to retrograde transport. Fourth, mitochondrial transport and membrane potential are correlated [102]. Thus, loss of mitochondrial membrane potential induced by mutant SOD1 as discussed earlier can be another underlying mechanism. Fifth, mutant SOD1 aggregation along microtubules may interfere with both anterograde and retrograde transport like blockades along highways. Sixth, anterograde and retrograde transport are inter-related and highly regulated, thus it is not surprising that disruption of the transport in one direction would affect the transport in the other direction. Lastly, it is also unclear whether all transported cargos would be affected similarly or differentially by mutant SOD1. For instance, a recent study showed that mutant SOD1 interacted with the kinesin-2 complex via kinesin-associated protein 3 (KAP3) and impaired anterograde transport of choline acetyltransferase [70]. Will the axonal transport of mitochondria and of choline acetyltransferase be affected in the same fashion in the presence of mutant SOD1? Or will transport of pro-survival and pro-death signals be affected similarly or differentially? It is conceivable that transport of different organelles, cargos or signals can be differentially altered in the presence of the ALS-linked SOD1 mutants. Future studies are needed to clarify how axonal transport of mitochondria is altered in ALS and to understand the mechanisms.
Figure 1. Hypotheses of abnormal axonal transport of mitochondria in ALS.
Several mechanisms by which mutant SOD1 can interfere with axonal transport of mitochondria have been discussed. (1) Kinesin heavy chain and kinesin light chain can be phosphorylated by various kinases and anterograde transport would be affected. (2) Elevated Ca2+ levels can prevent kinesin from binding to microtubules and lead to decreased anterograde axonal transport. (3) Mutant SOD1 can interact with the dynein-dynactin complex and may result in impairment of retrograde transport. (4) Disturbed mitochondrial membrane potential can impact mitochondrial transport. (5) Aggregation of mutant SOD1 along microtubules may interfere with both anterograde and retrograde transport like a blockade.
Despite the unclear mechanism, impaired transport of mitochondria can result in abnormal mitochondrial morphology, redistribution of mitochondria with fewer mitochondria in axons and nerve terminals, reduced ATP production from mitochondrial metabolism, and decreased calcium handling capacity. Moreover, altered mitochondrial transport can lead to abnormal mitochondrial dynamics as discussed in the next section.
Potential role of mitochondrial dynamics in ALS
Mitochondria are highly dynamic organelles. Mitochondria are abundant in areas of active neurons with intense ATP demands such as the axon hillock, the nodes of Ranvier, and the synaptic regions [103]. They are actively transported and they can have defined subcellular distributions that can change rapidly according to physiological needs. Mitochondria maintain their overall morphology, distribution and activity through two essential mechanisms, fusion and fission. An imbalance of these two opposing processes results in mitochondrial fragmentation or elongation [104]. It is logical to speculate that mitochondrial morphology, metabolic function, membrane potential, axonal transport, and fission and fussion are highly inter-dependent.
In mammalian cells, the fusion machinery includes Mfn1, Mfn2 and OPA1. Mfn1 and Mfn2 belong to the large GTPase family and localize to the mitochondrial outer membrane. OPA1 is a dynamin family GTPase and localizes within the mitochondrial intermembrane space. Mfn1 and Mfn2 deletion leads to loss of mitochondria fusion, high fragmentation, and correspondingly no mitochondrial tubules. In addition, Mfn1 and Mfn2 deletion results in severe cellular defects, including widespread heterogeneity of mitochondrial membrane potential and decreased cellular respiration [105]. In humans, mutations in Mfn2 cause Charcot-Marie-Tooth neuropathy type 2A and mutations in OPA1 cause the most common form of hereditary optic atrophy [106, 107]. On the other hand, the components of mitochondrial fission machinery in mammals include Drp1 and Fis1. Dominant-negative mutants of Drp1 inhibit mitochondrial division and result in highly interconnected mitochondrial tubules [108]. Overexpression of Fis1 leads to mitochondrial fragmentation, release of cytochrome c, and ultimately apoptosis [109].
Although no studies have been reported specifically regarding mitochondrial fission and fusion in ALS, abnormal mitochondrial clustering and fragmentation [31-35] and altered mitochondrial transport [94, 95] are highly suggestive that mitochondrial dynamics may be influenced in the presence of mutant SOD1. Changes in mitochondrial dynamics have been found in other neurodegenerative diseases such as Alzheimer's Disease (AD). Wang et al. found that mitochondria were much shorter and round in sporadic AD patients’ fibroblasts, indicating imbalance of fission and fusion [110]. The same study also found that the levels of dynamin-like protein 1 (Dlp1), a regulator of mitochondrial fission and distribution, were decreased significantly in sporadic AD fibroblasts. Wang et al. also reported that levels of Dlp1 and OPA1 were significantly decreased whereas levels of Fis1 were significantly increased in M17 cells overexpressing the AD-causing APP mutant [111]. Moreover, Dlp1 and OPA1 overexpression could ameliorate the abnormal mitochondrial morphology and distribution and synaptic loss induced by oligomeric amyloid-β derived diffusible ligands [112]. The results provide the best evidence thus far that APP mutant can cause an imbalance of mitochondrial fission/fusion and consequently result in mitochondrial fragmentation and abnormal distribution. It remains to be determined whether ALS mutant SOD1 may also impair mitochondrial dynamics.
Mitochondria in the “dying back” axonopathy hypothesis of ALS
Recent studies provide convincing evidence that devervation of motor neuron from muscles occurs in early stage of the disease pathogenesis prior to clinical symptoms in mutant SOD1 transgenic mice [49, 50, 65, 113, 114]. A zebrafish model overexpressing mutant human SOD1 also caused motor neuron axonopathy in a dose-dependent manner [115]. A “dying-back” axonopathy hypothesis of ALS thus is emerging in the field. Interestingly, transgenic mice with muscular overexpression of uncoupling protein 1 (UCP1), which only caused mitochondrial defects in muscles, displayed age-dependent deterioration of neuromuscular junctions and subsequent motor neuron pathology [116]. The results are supportive that the distal pathology at neuromuscular junction can contribute to motor neuron degeneration in ALS. The results also illustrate the critical importance of proper function of mitochondria at both the presynaptic and postsynaptic compartments of the neuromuscular junction. As discussed earlier, mutant SOD1 may interfere with the mitochondrial function via multiple mechanisms, particularly via impairment of mitochondrial transport and dynamics. Thus, maintaining appropriate population of properly functioning mitochondria in distal axons could provide a critical therapeutic avenue for potential ALS treatment.
Conclusion
It is believed that alteration in multiple pathways in multiple cell types can contribute to ALS pathogenesis and progression. Mitochondrial dysfunction plays a critical role in mutant SOD1 mediated familial ALS. Various aspects of the underlying mechanisms as well as functional consequences of mitochondrial dysfunction are discussed in this review. They include association of mutant SOD1 aggregates with mitochondria, abnormal mitochondrial morphology, impaired mitochondrial bioenergetics, loss of mitochondrial membrane potential, reduced mitochondrial calcium buffering capacity and disrupted calcium homestasis, impaired axonal transport of mitochondria, and potential imbalance of mitochondrial fission and fusion. In fact, many of the events can be cause for and consequence of each other and they create a vicious cycle that results in motor axon denervation and ultimately motor neuron degeneration in ALS.
It is evidently critical to determine the very first event induced by mutant SOD1 and devise a strategy to stop or delay it before the cycle becomes unstoppable. Misfolding of mutant SOD1 due to the intrinsic structural properties of the mutation is probably the first event. The impairment of axonal transport by misfolded mutant SOD1 is likely the immediate next event. The mitochondrial abnormalities can be secondary effects caused by compromised axonal transport as discussed in the review. This hypothesis would explain the accumulation of dysfunctional mitochondria in distal axon terminals and the axon degeneration that were observed in very early stage of presymptomatic ALS mice. This speculation is supportive of the “dying back” axonopathy model that is emerging in the ALS field. It is also essential to study the mechanisms in the context of each other to understand the crosstalk among the events so that potential therapeutic strategies may be designed to tackle multiple pathways simultaneously.
Acknowledgements
This study was in part supported by the NIH grants R01NS049126 and R21AG032567 to HZ. The support from NIH/NCRR Center of Biomedical Research Excellence in the Molecular Basis of Human Disease (COBRE, P20RR020171) is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 3.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 4.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 7.Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi H, Almer G, Yamashita S, Guegan C, Nagai M, Xu Z, Sosunov AA, McKhann GM, 2nd, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci U S A. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 11.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 12.Field LS, Furukawa Y, O'Halloran TV, Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem. 2003;278:28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 13.Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci. 2002;22:RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc Natl Acad Sci U S A. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamata H, Manfredi G. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum Mol Genet. 2008;17:3303–3317. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brannstrom T, Gredal O, Wong PC, Williams DS, Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 19.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann Neurol. 1999;46:787–790. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Jung C, Higgins CM, Xu Z. A quantitative histochemical assay for activities of mitochondrial electron transport chain complexes in mouse spinal cord sections. J Neurosci Methods. 2002;114:165–172. doi: 10.1016/s0165-0270(01)00524-6. [DOI] [PubMed] [Google Scholar]
- 22.Bowling AC, Schulz JB, Brown RH, Jr., Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61:2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferri A, Cozzolino M, Crosio C, Nencini M, Casciati A, Gralla EB, Rotilio G, Valentine JS, Carri MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci U S A. 2006;103:13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger C, Lanius RA, Pelech SL, Shaw CA. Amyotrophic lateral sclerosis: the involvement of intracellular Ca2+ and protein kinase C. Trends Pharmacol Sci. 1996;17:114–120. doi: 10.1016/0165-6147(96)10004-3. [DOI] [PubMed] [Google Scholar]
- 25.Kruman WA, II, Pedersen JE, Springer MP. Mattson, ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- 26.Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- 27.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi H, Kobayashi Y, Ishigaki S, Doyu M, Sobue G. Mitochondrial localization of mutant superoxide dismutase 1 triggers caspase-dependent cell death in a cellular model of familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:50966–50972. doi: 10.1074/jbc.M209356200. [DOI] [PubMed] [Google Scholar]
- 30.Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki S, Iwata M. Ultrastructural study of synapses in the anterior horn neurons of patients with amyotrophic lateral sclerosis. Neurosci Lett. 1996;204:53–56. doi: 10.1016/0304-3940(96)12314-4. [DOI] [PubMed] [Google Scholar]
- 32.Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43:471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Sotelo-Silveira JR, Lepanto P, Elizondo MV, Horjales S, Palacios F, Martinez Palma L, Marin M, Beckman JS, Barbeito L. Axonal mitochondrial clusters containing mutant SOD1 in transgenic models of ALS. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raimondi A, Mangolini A, Rizzardini M, Tartari S, Massari S, Bendotti C, Francolini M, Borgese N, Cantoni L, Pietrini G. Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1. Eur J Neurosci. 2006;24:387–399. doi: 10.1111/j.1460-9568.2006.04922.x. [DOI] [PubMed] [Google Scholar]
- 35.Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZM, Dong L, Figlewicz DA, Shaw PJ. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 2002;125:1522–1533. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- 36.Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiner A, Medina L, Figueredo-Cardenas G, Anfinson S. Brainstem motoneuron pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS. Exp Neurol. 1995;131:239–250. doi: 10.1016/0014-4886(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Flint Beal M, Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal MK, Zech WD, Goos M, Leutbecher C, Ferri A, Zippelius A, Carri MT, Nau R, Keller BU. Impairment of mitochondrial calcium handling in a mtSOD1 cell culture model of motoneuron disease. BMC Neurosci. 2009;10:64. doi: 10.1186/1471-2202-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guegan C, Vila M, Rosoklija G, Hays AP, Przedborski S. Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. J Neurosci. 2001;21:6569–6576. doi: 10.1523/JNEUROSCI.21-17-06569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vukosavic S, Dubois-Dauphin M, Romero N, Przedborski S. Bax and Bcl-2 interaction in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999;73:2460–2468. doi: 10.1046/j.1471-4159.1999.0732460.x. [DOI] [PubMed] [Google Scholar]
- 42.Ishigaki S, Liang Y, Yamamoto M, Niwa J, Ando Y, Yoshihara T, Takeuchi H, Doyu M, Sobue G. X-Linked inhibitor of apoptosis protein is involved in mutant SOD1-mediated neuronal degeneration. J Neurochem. 2002;82:576–584. doi: 10.1046/j.1471-4159.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 44.Pasinelli P, Borchelt DR, Houseweart MK, Cleveland DW, Brown RH., Jr. Caspase-1 is activated in neural cells and tissue with amyotrophic lateral sclerosis-associated mutations in copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A. 1998;95:15763–15768. doi: 10.1073/pnas.95.26.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasinelli P, Houseweart MK, Brown RH, Jr., Cleveland DW. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu,Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2000;97:13901–13906. doi: 10.1073/pnas.240305897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 47.Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- 48.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 49.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, Rubinsztein DC, Marchuk DA. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe de Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 55.Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, Finnegan K, Holzbaur EL, Fischbeck KH, Ludlow CL. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai C, Lin X, Chandran J, Shim H, Yang WJ, Cai H. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27:13982–13990. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teuling E, van Dis V, Wulf PS, Haasdijk ED, Akhmanova A, Hoogenraad CC, Jaarsma D. A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Hum Mol Genet. 2008;17:2849–2862. doi: 10.1093/hmg/ddn182. [DOI] [PubMed] [Google Scholar]
- 59.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Price DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28:1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breuer AC, Lynn MP, Atkinson MB, Chou SM, Wilbourn AJ, Marks KE, Culver JE, Fleegler EJ. Fast axonal transport in amyotrophic lateral sclerosis: an intra-axonal organelle traffic analysis. Neurology. 1987;37:738–748. doi: 10.1212/wnl.37.5.738. [DOI] [PubMed] [Google Scholar]
- 61.Breuer AC, Atkinson MB. Fast axonal transport alterations in amyotrophic lateral sclerosis (ALS) and in parathyroid hormone (PTH)-treated axons. Cell Motil Cytoskeleton. 1988;10:321–330. doi: 10.1002/cm.970100136. [DOI] [PubMed] [Google Scholar]
- 62.Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 63.Sasaki S, Iwata M. Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology. 1996;47:535–540. doi: 10.1212/wnl.47.2.535. [DOI] [PubMed] [Google Scholar]
- 64.Ligon LA, LaMonte BH, Wallace KE, Weber N, Kalb RG, Holzbaur EL. Mutant superoxide dismutase disrupts cytoplasmic dynein in motor neurons. Neuroreport. 2005;16:533–536. doi: 10.1097/00001756-200504250-00002. [DOI] [PubMed] [Google Scholar]
- 65.Parkhouse WS, Cunningham L, McFee I, Miller JM, Whitney D, Pelech SL, Krieger C. Neuromuscular dysfunction in the mutant superoxide dismutase mouse model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:24–34. doi: 10.1080/17482960701725646. [DOI] [PubMed] [Google Scholar]
- 66.Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, Fisher EM, Greensmith L. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169:561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teuchert M, Fischer D, Schwalenstoecker B, Habisch HJ, Bockers TM, Ludolph AC. A dynein mutation attenuates motor neuron degeneration in SOD1G93A mice. Experimental Neurology. 2006;198:271–274. doi: 10.1016/j.expneurol.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Zhang F, Strom AL, Fukada K, Lee S, Hayward LJ, Zhu H. Interaction between familial amyotrophic lateral sclerosis (ALS)-linked SOD1 mutants and the dynein complex. J Biol Chem. 2007;282:16691–16699. doi: 10.1074/jbc.M609743200. [DOI] [PubMed] [Google Scholar]
- 69.Strom AL, Shi P, Zhang F, Gal J, Kilty R, Hayward LJ, Zhu H. Interaction of ALS-related mutant copper-zinc superoxide dismutase with the dynein-dynactin complex contributes to inclusion formation. J Biol Chem. 2008;283:22795–22805. doi: 10.1074/jbc.M800276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tateno M, Kato S, Sakurai T, Nukina N, Takahashi R, Araki T. Mutant SOD1 impairs axonal transport of choline acetyltransferase and acetylcholine release by sequestering KAP3. Hum Mol Genet. 2009;18:942–955. doi: 10.1093/hmg/ddn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Landers JE, Melki J, Meininger V, Glass JD, van den Berg LH, van Es MA, Sapp PC, van Vught PW, McKenna-Yasek DM, Blauw HM, Cho TJ, Polak M, Shi L, Wills AM, Broom WJ, Ticozzi N, Silani V, Ozoguz A, Rodriguez-Leyva I, Veldink JH, Ivinson AJ, Saris CG, Hosler BA, Barnes-Nessa A, Couture N, Wokke JH, Kwiatkowski TJ, Jr., Ophoff RA, Cronin S, Hardiman O, Diekstra FP, Leigh PN, Shaw CE, Simpson CL, Hansen VK, Powell JF, Corcia P, Salachas F, Heath S, Galan P, Georges F, Horvitz HR, Lathrop M, Purcell S, Al-Chalabi A, Brown RH., Jr. Reduced expression of the Kinesin-Associated Protein 3 (KIFAP3) gene increases survival in sporadic amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812937106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dupuis L, de Tapia M, Rene F, Lutz-Bucher B, Gordon JW, Mercken L, Pradier L, Loeffler JP. Differential screening of mutated SOD1 transgenic mice reveals early up-regulation of a fast axonal transport component in spinal cord motor neurons. Neurobiol Dis. 2000;7:274–285. doi: 10.1006/nbdi.2000.0292. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein AY, Wang X, Schwarz TL. Axonal transport and the delivery of pre-synaptic components. Curr Opin Neurobiol. 2008;18:495–503. doi: 10.1016/j.conb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 79.Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Oko R, van der Hoorn FA. Rat kinesin light chain 3 associates with spermatid mitochondria. Dev Biol. 2004;275:23–33. doi: 10.1016/j.ydbio.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 82.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 84.Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 85.Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- 88.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278:5399–5409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- 89.Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- 90.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 93.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 94.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, Brownlees J, Ackerley S, Shaw PJ, McLoughlin DM, Shaw CE, Leigh PN, Miller CC, Grierson AJ. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magrane J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2604. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morfini G, Pigino G, Opalach K, Serulle Y, Moreira JE, Sugimori M, Llinas RR, Brady ST. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci U S A. 2007;104:2442–2447. doi: 10.1073/pnas.0611231104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shibata N, Kawaguchi-Niida M, Yamamoto T, Toi S, Hirano A, Kobayashi M. Effects of the PPARgamma activator pioglitazone on p38 MAP kinase and IkappaBalpha in the spinal cord of a transgenic mouse model of amyotrophic lateral sclerosis. Neuropathology. 2008;28:387–398. doi: 10.1111/j.1440-1789.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 98.Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, Huang CF, Banker G, Pigino G, Brady ST. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci U S A. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tortarolo M, Veglianese P, Calvaresi N, Botturi A, Rossi C, Giorgini A, Migheli A, Bendotti C. Persistent activation of p38 mitogen-activated protein kinase in a mouse model of familial amyotrophic lateral sclerosis correlates with disease progression. Mol Cell Neurosci. 2003;23:180–192. doi: 10.1016/s1044-7431(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 101.Dewil M, dela Cruz VF, Van Den Bosch L, Robberecht W. Inhibition of p38 mitogen activated protein kinase activation and mutant SOD1(G93A)-induced motor neuron death. Neurobiol Dis. 2007;26:332–341. doi: 10.1016/j.nbd.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 103.Strom AL, Gal J, Shi P, Kasarskis EJ, Hayward LJ, Zhu H. Retrograde axonal transport and motor neuron disease. J Neurochem. 2008;106:495–505. doi: 10.1111/j.1471-4159.2008.05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 105.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 106.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 107.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 108.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park KH, Vincent I. Presymptomatic biochemical changes in hindlimb muscle of G93A human Cu/Zn superoxide dismutase 1 transgenic mouse model of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2008;1782:462–468. doi: 10.1016/j.bbadis.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gordon T, Ly V, Hegedus J, Tyreman N. Early detection of denervated muscle fibers in hindlimb muscles after sciatic nerve transection in wild type mice and in the G93A mouse model of amyotrophic lateral sclerosis. Neurol Res. 2009;31:28–42. doi: 10.1179/174313208X332977. [DOI] [PubMed] [Google Scholar]
- 115.Lemmens R, Van Hoecke A, Hersmus N, Geelen V, D'Hollander I, Thijs V, Van Den Bosch L, Carmeliet P, Robberecht W. Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish. Hum. Mol. Genet. 2007;16:2359–2365. doi: 10.1093/hmg/ddm193. [DOI] [PubMed] [Google Scholar]
- 116.Dupuis L, Gonzalez de Aguilar J-L, Echaniz-Laguna A, Eschbach J, Rene F.d.r., Oudart H, Halter B, Huze C, Schaeffer L, Bouillaud F.d.r., Loeffler J-P. Muscle Mitochondrial Uncoupling Dismantles Neuromuscular Junction and Triggers Distal Degeneration of Motor Neurons. PLoS ONE. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]