Abstract
The myogenic transcription factor Pax3 plays an essential role in early skeletal muscle development and is a key component in Alveolar rhabdomyosarcoma (ARMS), a childhood solid muscle tumor. ARMS is characterized by a t(2;13) chromosomal translocation resulting in the fusion of the 5′ Pax3 sequences to the 3′ FOXO1 sequences to encode the oncogenic fusion protein, Pax3-FOXO1. Posttranslational modifications such as phosphorylation are common mechanisms by which transcription factors are regulated. Consistent with this fact, we demonstrated in a previous report that Pax3 is phosphorylated on Ser205 in proliferating, but not differentiated, primary myoblasts. However, the kinase that mediates this phosphorylation event has yet to be identified. In addition, it is not known whether Pax3-FOXO1 is phosphorylated at this site or how the phosphorylation of the fusion protein changes during early myogenic differentiation. In this report we identify CK2 (formerly termed “casein kinase II”) as the kinase responsible for phosphorylating Pax3 and Pax3-FOXO1 at Ser205 in proliferating mouse primary myoblasts. Furthermore, we demonstrate that in contrast to wild-type Pax3, phosphorylation at Ser205 persists on Pax3-FOXO1 throughout early myogenic differentiation. Finally, we show that Pax3-FOXO1 is phosphorylated at Ser205 in a variety of translocation-containing ARMS cell lines. The results presented in this report not only suggest a possible mechanism by which the disregulation of Pax3-FOXO1 may contribute to tumorigenesis, but also identifies a novel target for the development of therapies for the treatment of ARMS.
Pax3 is a member of the paired class homeodomain family of transcription factors and plays an essential role in early skeletal muscle development. As such, it is required for the formation of muscles of the trunk and for the delamination and migration of myogenic progenitor cells to the limb buds (1). In particular, Pax3 controls critical biological aspects of myogenic progenitor cells including cell survival, proliferation, and entry of the progenitor cells into the myogenic program (2). Highlighting the importance of Pax3 throughout embryogenesis, Pax3 null mice lack limbs due to defects in the skeletal musculature and die midgestation due to defective neural crest cell migration and consequent cardiac defects (3).
In addition to its role in early muscle development, Pax3 is also involved in the formation of the solid childhood muscle tumor alveolar rhabdomyosarcoma (ARMS). The most prevalent genetic mutation in ARMS, a t(2;13)(q35-37;q14) chromosomal translocation, results in the fusion of the 5′ Pax3 sequences to the 3′ sequences of a member of the forkhead family of transcription factors, FOXO1, to encode an 836 amino acid oncogenic fusion protein (3). The Pax3-FOXO1 fusion protein retains the DNA-binding and protein-protein interaction domains of Pax3. However, the Pax3 transcriptional activation domain is replaced by the bisected DNA binding domain and potent transcriptional activation domain of FOXO1 (Figure 1A) (4, 5). As a result of the fusion, several biological properties of Pax3-FOXO1 are altered when compared to wildtype Pax3, which include increased mRNA levels (6), more potent transcriptional activity despite having a reduced DNA binding capability (7), unresponsiveness to the Pax3 co-repressor hDaxx (8), greater post-translational stability in early myogenesis (9), and the ability to regulate genes not normally regulated by wild type Pax3 (10, 11). As a consequence of these altered activities, Pax3-FOXO1 is thought to be a key contributor to the development of ARMS highlighting the importance of the identification of new molecular targets for potential drug development.
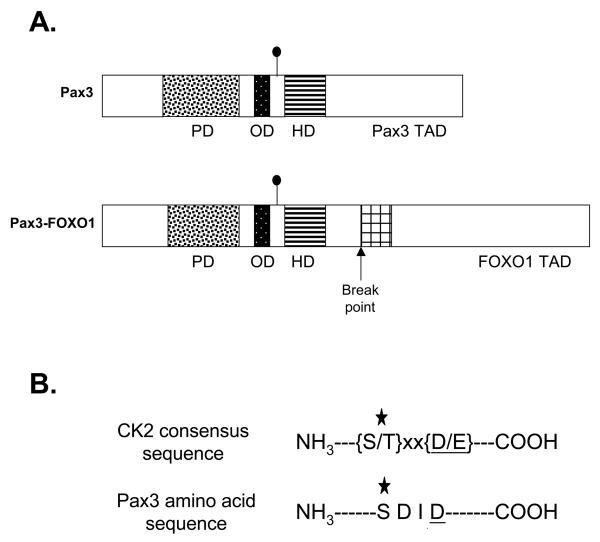
Figure 1.
Ser205 is present in a CK2 consensus amino acid sequence. A) Schematic of Pax3 and Pax3-FOXO1. The domains are as follows: the paired DNA binding domain (PD) is indicated by the speckled box, the octapeptide domain (OD) by the black box, the homeodomain (HD) by the striped box, and the bisected FOXO1 DNA binding domain by the cross-hatched box. The Pax3 and FOXO1 transcriptional activation domains (TAD) are indicated. The filled circle indicates the site of Ser205 phosphorylation. B) Amino acid sequence of the CK2 consensus sequence and the region surrounding Ser205. The star indicates the phosphorylated amino acid and the underlined residue at position (n + 3) indicates the most critical amino acid of the CK2 consensus sequence.
Posttranslational modifications such as phosphorylation are common mechanisms for the regulation of myogenic transcription factors. Consistent with this fact, we previously demonstrated that Pax3 is phosphorylated at Ser205 in proliferating primary myoblasts and that this phosphorylation event is rapidly lost upon the induction of differentiation (12). More recently it was demonstrated that Pax3-FOXO1 is also phosphorylated at unidentified sites in non-physiologically relevant cells (13). Despite this information, the kinase responsible for phosphorylating Pax3 at Ser205 has yet to be identified. In addition, it is not known whether Pax3-FOXO1 is also phosphorylated at Ser205 or how the pattern of phosphorylation at this site differs between wild-type Pax3 and the fusion protein during myogenesis.
Ser205 is present in the context of a protein kinase CK2 (formerly termed “casein kinase II”) consensus sequence, suggesting that CK2 may be responsible for phosphorylating Pax3, and potentially Pax3-FOXO1, at Ser205. CK2 is a ubiquitous, highly pleiotropic, and highly conserved Serine/Threonine protein kinase with a heterotetrameric structure comprised of two catalytic α or α′ subunits and two regulatory β subunits (14). It has been postulated that the constitutive activity of CK2 creates a constitutive post-translational modification of its target proteins, whereby the “regulatory” actions of CK2 result from the active dephosphorylation of the target protein (15). In further support of CK2 as the responsible kinase, CK2 is expressed in both proliferating myoblasts and throughout myogenic differentiation (16) whereby it controls the transcriptional activity of muscle regulatory factors such as MRF4, myf5, and MyoD (17-19). In addition to its role in muscle development, CK2 activity is enhanced in ARMS cells and as a result contributes to the development of ARMS by inhibiting apoptosis (20). Therefore, it is conceivable that Pax3 and Pax3-FOXO1, two proteins key in myogenesis and the development of ARMS, may represent additional targets of CK2.
In this report, we show that both Pax3 and Pax3-FOXO1 are phosphorylated at Ser205 by CK2 in proliferating mouse primary myoblasts, consistent with a well-conserved CK2 consensus sequence at this site (21). Furthermore, we demonstrate that phosphorylation of Ser205 persists on Pax3-FOXO1, but not Pax3, throughout early myogenic differentiation. Finally, consistent with the prominent role of both CK2 and Pax3-FOXO1 in the development of ARMS, we show that Pax3-FOXO1 is phosphorylated at Ser205 in a variety of t(2;13) translocation-containing ARMS cell lines. The results presented in this report not only suggest a possible mechanism by which the disregulation of Pax3-FOXO1 may contribute to tumorigenesis, but also identifies potential novel targets for the development of therapies to be used for the treatment of ARMS.
EXPERIMENTAL PROCEDURES (MATERIALS & METHODS)
Cells and cell culture conditions
Mouse primary myoblasts were isolated from 2 – 4 day old C57/Bl6 mice as previously described (12). Proliferation medium for the mouse primary myoblasts consisted of Ham's F-10 nutrient medium (Mediatech Cellgro, Herndon, VA) supplemented with 20% FBS (HyClone Laboratories, Inc., Logan, UT), 2.5ng/ml bFGF (Promega Corp., Madison, WI), and 15mM HEPES (HyClone). Differentiation medium consisted of Dulbecco's Modification of Eagle's Medium (DMEM, Gibco BRL) supplemented with 2% horse serum (HyClone). All media contained penicillin G (200U/ml) and streptomycin (200μg/ml). DMEM was additionally supplemented with 2mM L-glutamine (GIBCO BRL) and when prepared in this manner referred to as DMEM-complete. Cells were grown in a humidified incubator at 37°C in 5% CO2. All cells were grown on collagen-coated dishes (Becton Dickinson Labware, Bedford, MA), were passage-matched to prevent possible differences due to different passage conditions, were not used past passage 9 to prevent the cells from entering crisis, and were not allowed to grow past approximately 80% confluency to maintain the cells in an undifferentiated state. To induce differentiation of primary myoblasts, the proliferation media was removed, the cells were washed twice with PBS, the media was replaced with 10ml of differentiation media, and the cells were grown as described above until needed for further analysis.
Mouse primary myoblasts stably expressing FLAG epitope-tagged Pax3 (FLAG-Pax3) or FLAG epitope-tagged Pax3-FOXO1 (FLAG-Pax3-FOXO1) were generated by a modification of retroviral transduction, as previously described (7, 12), using an MSCV-IRES-Puro construct, which contains the gene for Puromycin resistance. The myoblasts were allowed to incubate for three to seven days post-transduction to allow for the expression of the Puromycin resistance gene. The myoblasts containing the desired constructs were selected by incubating the cells in proliferation media containing Puromycin (2.5μg/ml) overnight in a humidified incubator at 37°C in 5% CO2. The following day the media was changed to proliferation media without Puromycin and the cells were allowed to recover for 1 – 3 days. The selection process was repeated exactly as described for a total of three times. Cells selected in this manner were cultured and expanded as previously described (12).
Human rhabdomyosarcoma Rh30 cells were purchased from American Type Culture Collection (Manassas, VA). Human rhabdomyosarcoma Rh3, Rh4, Rh28 and Rh41 cells were a kind gift of Dr. Gerard Grosveld, St. Jude Children's Research Hospital (Memphis, TN). Rhabdomyosarcoma cells were grown in RPMI 1640 medium (Gibco BRL) supplemented with 2mM L-glutamine, 10mM HEPES, 1mM sodium pyruvate, 4.5g/L glucose, 1.5g/L sodium bicarbonate, 10% fetal bovine serum and Penicillin-Streptomycin in a humidified incubator at 37°C in 5% CO2.
Creation of expression vector constructs
The GST fusion constructs pGEX-5X-1-Pax3 and pGEX-5X-1-Pax3-FOXO1 were a kind gift from Dr. Gerard Grosveld, St. Jude Children's Research Hospital (Memphis, TN). The phosphoincompetent pGEX-5X-1-Pax3(S205A) point mutant, in which Ser205 was mutated to a phosphoincompetent alanine, was created as previously described (8). The wild-type and point mutant vectors were individually transformed into Rosetta(DE3)pLysS chemically competent cells (EMD Chemicals, Gibbstown, NJ) and subsequently used for expression and purification using the MagneSphere GST resin (Promega), as previously described (12). Bacterially expressed and purified GST-Pax3, GST-Pax3(S205A) or GST-Pax3-FOXO1 were used without elution from the resin. Protein expression and purity were confirmed by SDS-PAGE analysis with Coomassie blue staining and the relative protein concentrations on the resin were estimated by comparison to proteins of known concentration (data not shown).
[32P]-Orthophosphate Metabolic Labeling
Mouse primary myoblasts containing FLAG-Pax3 or FLAG-Pax3-FOXO1 were grown to 70%-80% confluency and were metabolically labeled with [35S]-Methionine or [32P]-Orthophosphate (MP Biomedicals, Aurora, OH) as previously described (12). FLAG-Pax3 and FLAG-Pax3-FOXO1 proteins were isolated using the anti-FLAG M2 magnetic resin (Clemente Associates Inc., Madison, CT). The resin was then washed 3X with TBS. After removing all traces of TBS, SDS-PAGE loading buffer was added, the samples were boiled for 5 minutes, and the eluted proteins were separated on a 10% SDS-PAGE gel. The gel was dried and visualized by autoradiography.
In vitro kinase assays
GST-Pax3, GST-Pax3(S205A), or GST-Pax3-FOXO1 present on the resin (approximately 1μg of protein), prepared as described above, were used for in vitro kinase assays using either purified CK2 or proliferating mouse primary myoblast total cell extract. For the kinase assay using purified CK2, GST-Pax3, GST-Pax3(S205A), or GST-Pax3-FOXO1 were individually mixed with 5X CK2 reaction buffer (20mM Tris-HCl [pH 7.5], 50mM KCl, and 10mM MgCl2), 30μM ATP, and 50μCi [γ-32P]-ATP [MP Biomedicals]) prior to the addition of 50ng purified CK2 enzyme (Calbiochem, LaJolla, CA). For the kinase assays using proliferating mouse primary myoblast total cell extract, GST-Pax3, GST-Pax3(S205A), or GST-Pax3-FOXO1 was mixed with 26μl of the 2X kinase stock solution [80mM HEPES, 20mM MgCl2, 100mM KCl, 2mM DTT], 2X phosphatase inhibitor cocktails I and II, 84μM ATP, and 50μCi [γ-32P]-ATP [MP Biomedicals]) prior to the addition of 25μl of proliferating mouse primary myoblasts total cell extract (2μg/μl), prepared as previously described (12). Following the addition of either purified CK2 or the total cell extract, the reaction mixture was incubated at 30°C for 1 hour with periodic gentle agitation. After incubation, the beads were washed 3X with 100μl PBS, radiolabeled protein was eluted by boiling in 25μl SDS-PAGE loading buffer, and the proteins were separated by 10% SDS-PAGE. The resulting gels were dried and visualized by autoradiography. Alternatively, the above described in vitro kinase assays were performed using 84 μM GTP and 50μCi [γ-32P]-GTP (MP Biomedicals) as the phosphate donor in place of ATP and [γ-32P]-ATP
For the kinase assays performed in the presence of the CK2-specific inhibitors, mouse primary myoblast total cell extract was incubated at 30°C for 1 hour with 10mM of either DRB (Calbiochem) or heparin (Sigma-Aldrich) prior to the addition of unlabeled ATP and bacterially expressed and purified GST-Pax3 or GST-Pax3-FOXO1. Following the addition of GST-Pax3 or GST-Pax3-FOXO1, the mixture was allowed to incubate at 30°C for 1 hour with periodic gentle agitation after which the phosphorylated protein was separated by SDS-PAGE. The phosphorylated protein was then transferred to Immobilon-P membrane (Millipore, Bedford, MA) to be used for Western blot analysis using either the anti-Pax3 or the anti-Pax3(p205) antibody as described below.
Antibodies and Western blot analysis
The antibody specific for phosphorylation of Pax3 at serine 205, anti-Pax3(p205), was developed, characterized, and used as described previously (12). The mono-specific, affinity purified Pax3-specific antibody was described previously (22).
Total cell extracts from proliferating primary myoblasts or myoblasts that were induced to differentiate for a specific period of time were prepared as described above. A constant amount of total cell extract (50μg) was separated by 10% SDS-PAGE, proteins were transferred to Immobilon-P membrane (Millipore), and the presence of Pax3, Pax3-FOXO1, Pax3 phosphorylated at serine 205, or Pax3-FOXO1 phosphorylated at serine 205 was detected using the previously described affinity purified, monospecific Pax3 antibody (22) or the Pax3(p205) antibody (12) , using previously described conditions (16).
RESULTS
Pax3 is phosphorylated by CK2 at Ser205
Pax3 is a developmentally regulated transcription factor that is phosphorylated on Ser205 in proliferating, but not differentiated, primary myoblasts (12). However, the kinase that mediates this phosphorylation event has yet to be identified. A close visual examination of the Pax3 amino acid sequence surrounding Ser205 demonstrates that this site is present in the context of a perfect CK2 consensus sequence (Figure 1B) (21). This fact, combined with the key role that CK2 plays in myogenesis, strongly suggests that Pax3 might be phosphorylated by CK2. To demonstrate whether CK2 is capable of phosphorylating Pax3, we performed an in vitro kinase assay using purified CK2, radiolabeled ATP, and bacterially expressed and purified GST-tagged Pax3 (GSTPax3). In addition, we took advantage of the fact that CK2 is unique in its ability to utilize GTP as a phosphate donor (11, 23) and used radiolabeled GTP in independent assays. Using both ATP and GTP we observed the efficient incorporation of radiolabel onto Pax3. This incorporation was specific for Pax3 since pure CK2 was unable to phosphorylate GST alone either in the presence of ATP or GTP (Figure 2A). Taken together these results demonstrate that Pax3 can be phosphorylated by purified CK2.
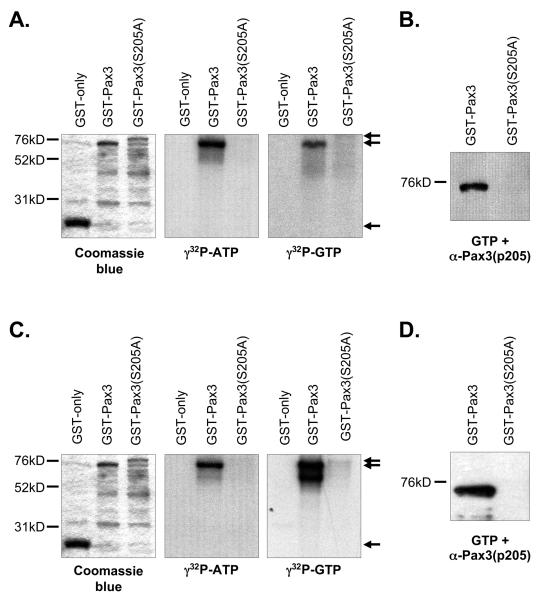
Figure 2.
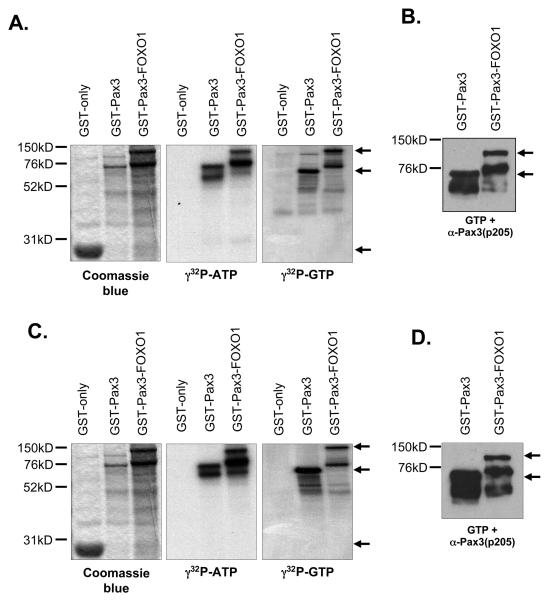
Casein kinase II phosphorylates Pax3 on Ser205. Bacterially expressed and purified GST (27kD), GST-Pax3 (74kD), or GST-Pax3(S205A) (80kD) were phosphorylated in vitro using purified CK2 (A and B) or proliferating primary myoblast total cell extracts (C and D) as described in the Experimental procedures. The phosphorylated proteins were eluted from the resin, separated by 8% SDS-PAGE, and visualized by Coomasie staining (A and C, left panels), autoradiography (A and C, middle and right panels) or Western blot analysis using our phospho-specific anti-Pax3(p205) antibody (12) (B and D). The arrows in panels A and C indicate the major protein species for GST (lower arrow), GST-Pax3 (middle arrow) and GST-Pax3(S205A) (upper arrow). All other observable protein species are degradation products, which are commonly seen in these protein preparations.
In order to demonstrate that purified CK2 can phosphorylate Pax3 on Ser205, we used bacterially expressed and purified GST-Pax3 in which Ser205 has been mutated to the phosphoincompetent alanine, GST-Pax3(S205A), in our in vitro kinase assays with purified CK2 and either radiolabeled ATP or GTP. In contrast to the results observed for wild-type GST-Pax3, we observed no radiolabeling of the phosphoincompetent GST-Pax3(S205A) (Figure 2A), despite the presence of protein sufficient enough to provide a specific signal. In addition, we performed a Western blot analysis on bacterially expressed and purified GST-Pax3 and GSTPax3(S205A) that was phosphorylated by purified CK2 and unlabeled GTP (Figure 2B). The phosphorylated proteins were detected using an antibody that we have demonstrated in previous work to be highly specific for phosphorylation of Pax3 at Ser205 [anti-Pax3(p205)] (12). We observed a strong reactivity of the phospho-specific antibody with wildtype Pax3 but not when Ser205 was mutated to an alanine (Figure 2B). Taken together, these results demonstrate that purified CK2 specifically phosphorylates Pax3 at Ser205.
We next wanted to determine whether CK2 is the kinase present in proliferating primary myoblast total cell extracts that is responsible for phosphorylating Pax3 at Ser205. Therefore, we used proliferating primary myoblast total cell extracts in our previously published in vitro kinase assay (12). We again took advantage of the unique nature of CK2 in its use of GTP and tested the ability of total cell extracts to used both radioactive GTP or ATP to phosphorylate either GST-Pax3 or GST-Pax3(S205A). Consistent with previous reports (12), proliferating primary myoblast total cell extracts are capable of utilizing radiolabeled ATP to phosphorylate GST-Pax3 and this phosphorylation is greatly diminished upon the removal of Ser205 (Figure 2C). We also observed radiolabeling of GST-Pax3, but not GST-Pax3(S205A), in the presence of radiolabeled GTP. Although it was previously reported that total cell extracts from human medulloblastoma cell lines were capable of phosphorylating GST alone (24), we observed no radiolabel incorporation of the GST only negative control in our assay (Figure 2C) demonstrating that the radiolabel present on GST-Pax3 is specific for Pax3 itself. We then performed a Western blot analysis on bacterially expressed and purified GST-Pax3 and GSTPax3(S205A) phosphorylated by proliferating myoblast total cell extracts and unlabeled GTP using our phospho-specific antibody. Consistent with our results using purified CK2, we observed a distinct band corresponding to phosphorylation of Ser205 on wildtype Pax3, but not Pax3(S205A) (Figure 2D). Taken together, the ability of proliferating primary myoblast total cell extracts to utilize GTP to specifically phosphorylate Pax3 at Ser205 supports the hypothesis that CK2 is the kinase responsible for this event.
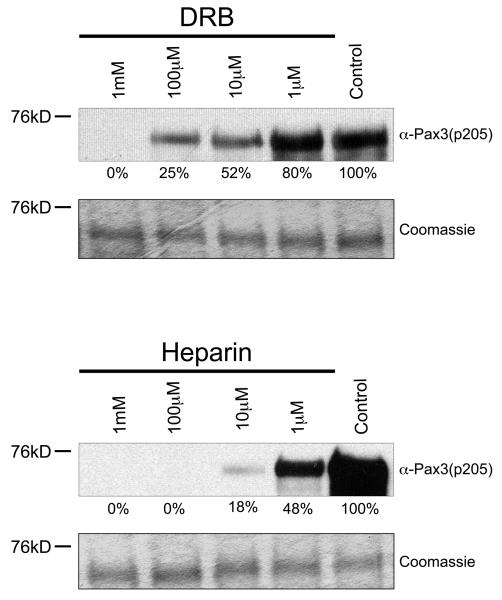
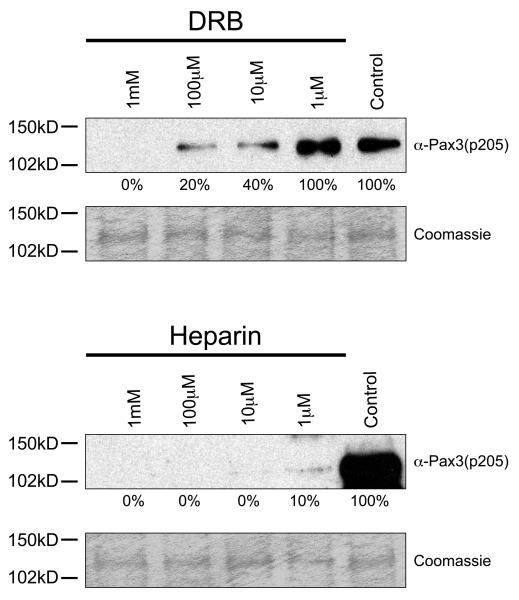
In order to provide additional evidence that CK2 is the kinase responsible for phosphorylating Pax3 at Ser205, we pre-incubated proliferating primary myoblast total cell extracts in the presence or absence of increasing concentrations of two CK2 inhibitors, heparin or 5,6-Dichloro-1-(β-D-ribofuranosyl)benzimidazole (DRB), for 1 hour prior to the addition of unlabeled ATP and bacterially expressed and purified GST-Pax3. Heparin and DRB are inhibitors commonly used in the analysis of CK2 activity and are known to be relatively specific for CK2 in tests against a wide range of kinases (25, 26). The resulting phosphorylated proteins were then analyzed by Western blot analysis using our anti-Pax3(p205) antibody. As shown in Figure 3, preincubation of total cell extracts with increasing amounts of inhibitors resulted in a titratable reduction in the phosphorylation of Pax3, despite the presence of equal amounts of bacterially expressed and purified GST-Pax3, with an approximate IC50 of 10μM and 1μM for DRB and Heparin, respectively, which is in good agreement with published reports (27, 28). Combined with our results described above using GTP, this result provides further evidence to support the conclusion that CK2 is the kinase present in proliferating mouse primary myoblasts that phosphorylates Pax3 at Ser205. Unfortunately, in multiple attempts to determine the involvement of CK2 in intact cells, these same inhibitors used at concentrations commonly reported in the literature for non-primary cells resulted in a large amount of cell death within six hours (data not shown), preventing an in vivo analysis. This result is consistent with multiple reports that show chemical inhibition of CK2 results in apoptosis within the first 24 hours of exposure of primary cells to the inhibitors (29-31). In addition, multiple attempts using a fluorescently labeled control siRNA molecule under a variety of transfection conditions determined that primary myoblasts had a transfection efficiency of <5% (data not shown), thereby preventing the use of this technique to genetically knockdown CK2 in these cells.
Figure 3.
Casein kinase II specific inhibitors prevent phosphorylation of Pax3 at Ser205. Bacterially expressed and purified GST-Pax3 was phosphorylated in vitro using proliferating mouse primary myoblast total cell extract that had been previously incubated in the presence or absence of increasing amounts of the commonly used CK2 inhibitors DRB (top panel) or heparin (bottom panel), as described in the Experimental Procedures. Western blot analysis was performed using our anti-Pax3(p205) antibody (12) to visualize the phosphorylation status of Ser205 and Coomassie staining was performed to confirm the presence of equal amounts of protein. The numbers under the gels indicate the percent maximal phosphorylation, determined relative to the control, providing an approximate IC50 of 10 μM and 1 μM for DRB and Heparin, respectively.
Pax3-FOXO1 is phosphorylated at Ser205 by CK2
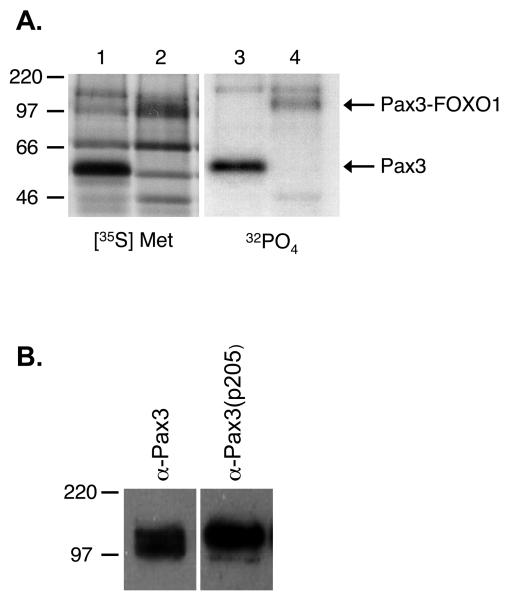
Pax3-FOXO1, the characteristic mutation in ARMS, retains key regulatory domains of Pax3, which include Ser205 within the octapeptide domain region (Figure 1A). Because the fusion protein retains this amino acid in the context of a good CK2 consensus sequence, it is possible that CK2 may also phosphorylate Pax3-FOXO1 at Ser205. Although a previous report demonstrated that Pax3-FOXO1 is phosphorylated on the Pax3 domain, they did not conclusively identify the site(s) of phosphorylation nor did they show that Pax3-FOXO1 was phosphorylated in a physiologically relevant cell type (13). Therefore, it was necessary for us to initially determine if Pax3-FOXO1 exists as a phosphoprotein in the physiologically relevant proliferating primary myoblasts. We stably transduced proliferating mouse primary myoblasts with a retroviral construct containing a FLAG epitope-tagged Pax3 (FLAG-Pax3) or Pax3-FOXO1 (FLAG-Pax3-FOXO1) construct, metabolically labeled the cells with either [35S]-methionine or [32P]-orthophosphate as previously described (12), immunoprecipitated the proteins with a FLAG-specific antibody, and examined incorporated radiolabel by SDS-PAGE analysis and autoradiography. Consistent with our previous results (12), we observed the specific incorporation of both [35S]-Methionine and [32P]-orthophosphate radiolabels into Pax3. In addition, we noted the specific incorporation of both radiolabels into Pax3-FOXO1 (Figure 4A), demonstrating that the fusion protein is both expressed and phosphorylated in the physiologically relevant proliferating primary myoblasts. To determine if the observed phosphorylation occurs on the Pax3 portion of the fusion protein at Ser205, we performed a standard Western blot analysis on total cell extracts isolated from proliferating primary myoblasts transduced with FLAG-Pax3-FOXO1 using either the anti-Pax3 or anti-Pax3(p205) antibodies. As shown in Figure 4B, we observed specific reactivity against Pax3-FOXO1 using both antibodies, directly demonstrating that like Pax3 (12), Pax3-FOXO1 is phosphorylated at Ser205 in proliferating primary myoblasts.
Figure 4.
Pax3-FOXO1 is phosphorylated on Ser205 in proliferating mouse primary myoblasts. A) Proliferating mouse primary myoblasts stably transduced with an amino-terminal FLAG epitope-tagged Pax3 (lanes 1 and 3) or FLAG epitope-tagged Pax3-FOXO1 (lanes 2 and 4) were metabolically labeled with either [35S]-methionine (lanes 1 and 2), in order to label all cellular proteins, or [32P]-orthophosphate (lanes 3 and 4), to label only phosphorylated proteins. The resulting labeled proteins were immunoprecipitated from total cell extracts using an anti-FLAG M2 magnetic resin, the proteins were released from the resin, separated by 12% SDS-PAGE, and the radiolabeled species were detected by autoradiography, as described in the Experimental Procedures. Fewer proteins species are present in the [32P]-orthophosphate sample relative to the [35S]-methionine sample due to not all protein species being present as phosphoproteins. B) Total cell extract was isolated from proliferating mouse primary myoblasts stably transduced with an amino-terminal FLAG epitope-tagged Pax3-FOXO1. The resulting extract was separated by 8% SDS-PAGE and analyzed by Western blot using either the anti-Pax3 or the phospho-specific anti-Pax3(p205).
To determine if CK2 phosphorylates Pax3-FOXO1, we performed an in vitro kinase assay using purified CK2, radiolabeled ATP or GTP, and bacterially expressed and purified GSTPax3-FOXO1. As positive and negative controls for the phosphorylation reaction, we also used GST-Pax3 and GST in parallel experiments, respectively. Staining with Coomassie blue demonstrated similar protein levels of GST-Pax3 and GST-Pax3-FOXO1 (Figure 5A). Using both ATP and GTP, we observed efficient incorporation of both radiolabels onto Pax3 and Pax3-FOXO1, but not GST alone, demonstrating that, like Pax3, Pax3-FOXO1 is phosphorylated by purified CK2 (Figure 5A). We observed a small amount of radiolabel incorporation onto the GST-FOXO1 control (data not shown), which may result from the presence of multiple FOXO1 phosphorylation sites on that portion of the fusion protein. Because of this residual phosphorylation on FOXO1, we were unable to utilize a Pax3-FOXO1(S205A) mutant as we did with wild-type Pax3. Therefore, in order to demonstrate that CK2 phosphorylates Pax3-FOXO1 specifically on Ser205, we phosphorylated Pax3-FOXO1 with purified CK2 and unlabeled GTP and performed a Western blot analysis using the anti-Pax3(p205) antibody. As shown in Figure 5B, we observed reactivity with both the GST-Pax3 and GST-Pax3-FOXO1 demonstrating that like Pax3, Pax3-FOXO1 is phosphorylated at Ser205 in vitro by pure CK2.
Figure 5.
Pax3-FOXO1 is phosphorylated on Ser205 by CK2. Bacterially expressed and purified GST (27kD), GST-Pax3 (74kD), or GST-Pax3-FOXO1 (125kD) were phosphorylated in vitro using purified CK2 (A and B) or proliferating primary myoblast total cell extracts (C and D) as described in the Experimental procedures. The phosphorylated proteins were eluted from the resin, separated by 8% SDS-PAGE, and visualized by Coomasie staining (A and C, left panels), autoradiography (A and C, middle and right panels) or Western blot analysis using our phospho-specific anti-Pax3(p205) antibody (12) (B and D). The arrows in panels A and C indicate the major protein species for GST (lower arrow), GST-Pax3 (middle arrow) and GST-Pax3-FOXO1 (upper arrow). The arrows in panels B and D indicate the major protein species for GST-Pax3 (lower arrow) and GST-Pax3-FOXO1 (upper arrow). All other observable protein species are degradation products, which are commonly seen in these protein preparations.
To determine whether CK2 is the kinase present in proliferating primary myoblasts responsible for phosphorylating Pax3-FOXO1 at Ser205, we examined the ability of proliferating primary myoblast total cell extracts to use either radioactive GTP or ATP to phosphorylate GST-Pax3-FOXO1. GST-Pax3 and GST alone were used in parallel experiments as a positive and negative control, respectively. As observed for Pax3, total cell extracts are capable of utilizing both ATP and GTP to phosphorylate Pax3-FOXO1 (Figure 5C), but not GST alone. Since the observed radiolabel incorporation may be present on FOXO1 sites, we analyzed Pax3-FOXO1 for phosphorylation at Ser205 by performing a Western blot analysis with our phospho-specific antibody on bacterially expressed and purified GST-Pax3 and GST-Pax3-FOXO1 phosphorylated by proliferating myoblast total cell extracts and unlabeled GTP. We observed a distinct band corresponding to phosphorylation of Ser205 on Pax3 and on Pax3-FOXO1 in the presence of GTP (Figure 5D). Therefore, these results suggest that CK2 is the kinase in proliferating mouse primary myoblast total cell extracts that phosphorylates Pax3-FOXO1 at Ser205.
In order to provide additional evidence that CK2 is the kinase responsible for phosphorylating Pax3-FOXO1 at Ser205, we used increasing concentrations of the CK2-specific inhibitors DRB and heparin to inhibit the phosphorylation of Pax3-FOXO1 by CK2 present in total cell extract as described above. We bacterially expressed and purified GST-Pax3-FOXO1 and performed an in vitro kinase assay using proliferating primary myoblast total cell extract that had been pre-incubated either in the presence or absence of commonly used CK2 inhibitors, DRB and heparin. The resulting phosphorylated proteins were then analyzed by Western blot analysis using our anti-Pax3(p205) antibody. We observed reactivity of Pax3-FOXO1 with the phospho-specific antibody with a titratable decrease in the phosphorylation, again despite the presence of equal amounts of bacterially expressed and purified GST-Pax3-FOXO1, with an approximate IC50 of 6μM and 0.5μM for DRB and Heparin, respectively, again in good agreement with published reports. In conjunction with our results using GTP, the inhibitor study provides solid evidence that CK2 is the kinase in proliferating primary myoblast total cell extract that phosphorylates Pax3-FOXO1 at Ser205.
Pax3-FOXO1, but not Pax3, is phosphorylated at Ser205 throughout early myogenic differentiation
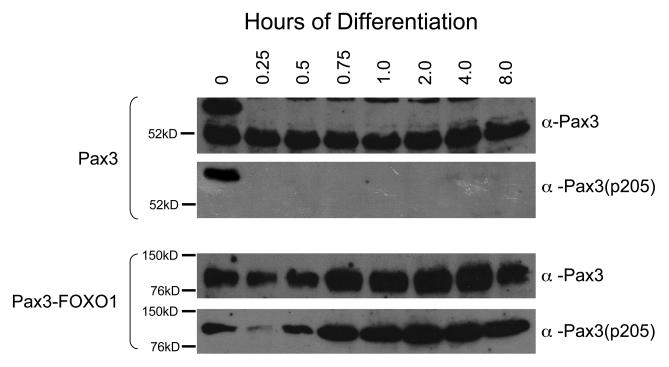
We previously reported that Pax3 is phosphorylated on Ser205 in proliferating primary myoblasts and that this phosphorylation is rapidly lost upon the induction of myogenic differentiation (12). We have also demonstrated that the Pax3 is expressed in the first eight hours of differentiation after which its stability is greatly reduced (8). In this report, we demonstrate that, like Pax3, Pax3-FOXO1 is phosphorylated at Ser205 by CK2 in proliferating primary myoblasts; however, the phosphorylation status of Pax3-FOXO1 throughout early myogenic differentiation remains unknown. Therefore, we were interested in examining the phosphorylation status of Pax3-FOXO1 during the same time of early differentiation that Pax3 is present. Therefore, we used primary myoblasts stably transduced with FLAG-Pax3-FOXO1 and induced differentiation for up to eight hours as previously described (12). Although the presence of Pax3-FOXO1 prevents the terminal fusion of primary myoblasts (8), the morphological and molecular changes in differentiation are indistinguishable between the uninfected primary myoblasts and primary myoblasts stably transduced with Pax3-FOXO1 during the first eight hours of differentiation (data not shown), consistent with previous reports (8). We isolated total cell extracts at various time points during differentiation and performed a Western blot analysis on equal amounts of total cell extract using the anti-Pax3 or the anti-Pax3(p205) antibodies. As a positive control, we performed a parallel experiment on non-transfected mouse primary myoblasts and determined the phosphorylation status of endogenous Pax3. Consistent with previous results, we observed two distinctly migrating species of Pax3 in proliferating myoblasts. Only the apparent 66kD species specifically reacted with the anti-Pax3(p205) antibody (Figure 7, top panel), consistent with previous work demonstrating that phosphorylation of Pax3 at Ser205 contributes in part to a change in electrophoretic mobility (12). By 15 minutes of differentiation, we observed the complete loss of the apparent 66kD species and the corresponding reactivity of the anti-Pax3(p205) antibody confirming that Pax3 is phosphorylated on Ser205 in proliferating, but not differentiated, mouse primary myoblasts. We also observed the presence of an 118kD band corresponding to Pax3-FOXO1 in proliferating primary myoblasts that reacted with both the anti-Pax3 and the phospho-specific antibody. However, in contrast to the results observed with Pax3 and after an initial apparent decrease in phosphorylation, phosphorylation of Pax3-FOXO1 persisted throughout the first eight hours of myogenic differentiation (Figure 7, bottom panel), directly demonstrating that the phosphorylation status of the fusion protein is altered relative to wild-type Pax3 throughout myogenic differentiation.
Figure 7.
Phosphorylation of Ser205 persists on Pax3-FOXO1, but not Pax3, throughout myogenic differentiation. Mouse primary myoblasts or myoblasts stably transduced with FLAG-Pax3-FOXO1 were induced to differentiate as previously described (12). Total cell extract was isolated at various time points post induction of differentiation (hours) and a Western blot analysis was performed on equal amounts of total cell extract using antibodies specific for Pax3 (anti-Pax3), to determine the qualitative presence of Pax3, or phosphorylation at Ser205 [anti-Pax3(p205)] (12).
Pax3-FOXO1 is phosphorylated at Ser205 in ARMS cell lines
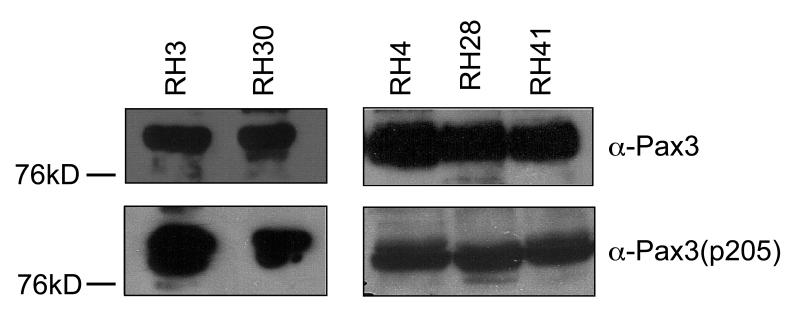
The aberrant and persistent phosphorylation of Pax3-FOXO1 at Ser205 throughout myogenic differentiation suggests a possible model by which Pax3-FOXO1 may contribute to the development of ARMS. Therefore, it was necessary to qualitatively determine if phosphorylation at Ser205 is present on endogenous Pax3-FOXO1 in established ARMS cell lines. We obtained a series of human ARMS cell lines (Rh3, Rh4, Rh28, Rh30 and Rh41) known to contain the characteristic t(2;13)(q35;q14) chromosomal translocation (32-34). In order to determine if Pax3-FOXO1 is phosphorylated at Ser205 in rhabdomyosarcoma cells, we isolated total cell extracts from the ARMS cells and performed a qualitative Western blot analysis using either the anti-Pax3 or the anti-Pax3(p205) antibody. We observed reactivity with the Pax3 antibody, demonstrating the presence of Pax3-FOXO1. After stripping of the blots we also observed reactivity with the phospho-specific antibodies (Figure 8) demonstrating on a qualitative level that Pax3-FOXO1 is both expressed and phosphorylated at Ser205 in a variety of rhabdomyosarcoma cells.
Figure 8.
Pax3-FOXO1 is phosphorylated at Ser205 in a variety of human ARMS cell lines. Total cell extract was isolated from a variety of human ARMS cell lines, as described in the Experimental Procedures. Standard Western blot analysis was performed on equal amounts of total cell extracts in two separate experiments (Experiment #1 – RH3 and RH30; Experiment #2 – RH4, RH28, and RH41) using antibodies specific for Pax3 (anti-Pax3), to determine the qualitative presence of Pax3-FOXO1. After complete stripping, the blot was re-probed using antibodies specific for phosphorylation at Ser205 [anti-Pax3(p205)].
DISCUSSION
CK2 phosphorylates Pax3 and Pax3-FOXO1 at Ser205
Pax3, a member of the Paired-box family of transcription factors, plays an important role in early skeletal muscle development (35). In addition to its role in myogenesis, Pax3 is also a key component in the childhood solid muscle tumor ARMS, which is characterized by a t(2;1) chromosomal translocation resulting in the formation of the oncogenic fusion protein Pax3-FOXO1. Recent evidence indicates that post-translational modifications such as phosphorylation may contribute to the biological activities of both Pax3 and Pax3-FOXO1 (8, 13). Consistent with this fact, we reported that Pax3 is phosphorylated at Ser205 in proliferating primary myoblasts and this phosphorylation event is rapidly lost upon the induction of differentiation (12). Despite this knowledge, the kinase responsible for phosphorylating Pax3 has yet to be identified. In addition, it is not known whether Pax3-FOXO1 is also phosphorylated at Ser205 or how its phosphorylation status at this site changes during myogenesis. In this report we demonstrate that the protein kinase CK2 phosphorylates both Pax3 and Pax3-FOXO1 at Ser205 and in contrast to wild-type Pax3, phosphorylation of the fusion protein at this site persists throughout muscle differentiation.
Normally, it is optimal to use in vivo inhibitor studies or genetic knockdown experiments to demonstrate that CK2 phosphorylates Pax3 and Pax3-FOXO1 in intact cells. However, the presence of commonly used CK2 inhibitors caused extensive cell death in our primary myoblast system (data not shown), a result that is consistent with previous reports (29-31). In addition, primary myoblasts were intractable to transfection of siRNA reagents (data not shown). Despite our inability to utilize in vivo studies, we feel that we have provided compelling evidence in support of our conclusion that CK2 is the kinase responsible for phosphorylating Pax3 and Pax3-FOXO1 at Ser205. First, Ser205 is present within the context of an optimal CK2 consensus sequence (Figure 1B). Second, Pax3 and Pax3-FOXO1 are both directly phosphorylated by pure CK2 (Figures 2A and 4A). Third, pure CK2 and proliferating primary myoblast total cell extracts are capable of utilizing GTP as the phospho-donor to phosphorylate Pax3 and Pax3-FOXO1 (Figures 2 and 5). The use of GTP as a phospho-donor is a characteristic unique to CK2 (11, 23). Fourth, the phosphorylation of Pax3 and Pax3-FOXO1 by pure CK2 or total cell extracts in the presence of GTP occurs on Ser205, as determined by mutational analysis (Figure 2) or through the use of an antibody specific for Pax3 when phosphorylated at Ser205 (Figure 5). Finally, the commonly used CK2 inhibitors heparin (26) and DRB (25) significantly reduce or completely inhibit the phosphorylation of Pax3 (Figure 3) and Pax3-FOXO1 (Figure 6) by proliferating primary myoblast total cell extracts.
Figure 6.
Casein kinase II specific inhibitors prevent phosphorylation of Pax3-FOXO1 at Ser205. Bacterially expressed and purified GST-Pax3-FOXO1 was phosphorylated in vitro using proliferating mouse primary myoblast total cell extract that had been previously incubated in the presence or absence of increasing amounts of the commonly used CK2 inhibitors DRB (top panel) or heparin (bottom panel), as described in the Experimental Procedures. Western blot analysis was performed using our anti-Pax3(p205) antibody (12) to visualize the phosphorylation status of Ser205 and Coomassie staining was performed to confirm the presence of equal amounts of protein. The numbers under the gels indicate the percent maximal phosphorylation, determined relative to the control, providing an approximate IC50 of 6 M and 0.5 M for DRB and Heparin, respectively.
Although our experimental data, in particular our inhibition studies with DRB, strongly supports the conclusion that CK2 is indeed the kinase responsible for phosphorylating Pax3 and Pax3-FOXO1 at Ser205, several other kinases have also been demonstrated to be specifically inhibited by DRB including CK1, cyclin dependent kinase 7 (CDK7), CDK8, and CDK9 (27, 36, 37). However, we demonstrate that ATP and GTP are used by the kinase present in total cell extracts with nearly equal efficiency to phosphorylate Pax3 and Pax3-FOXO1 (Figures 2C and 5C). Of the additional kinases inhibited by DRB, only CDK9 is capable of utilizing GTP as a substrate. Unlike CK2, which is unique in its ability to use ATP and GTP with nearly equal efficiency, CDK9 uses GTP nearly 10-fold less efficiently under physiological conditions (38). Additionally, we demonstrate that Ser205 is present in the context of a nearly perfect CK2 recognition sequence (S*—X–X–D/E, where the asterisk represents the phosphorylated amino acid), a sequence that is significantly and distinctly different from the identified consensus sequence for CDK9 (Y–S–P–T–S*–P–S, where the asterisk represents the phosphorylated amino acid). Therefore, based on our experimental evidence, combined with what is known about the biochemical characteristics of CK2 and other kinases, we conclude that CK2 phosphorylates Pax3 and Pax3-FOXO1 at Ser205.
CK2, Pax3 and myogenesis
CK2 is a ubiquitously expressed, constitutively active Serine/Threonine protein kinase that has been implicated in the regulation of many different fundamental cellular processes. In particular, CK2 is expressed in proliferating and differentiating myoblasts where it is important for the progression of myogenesis by phosphorylating and regulating multiple muscle-specific transcription factors. In proliferating myoblasts, CK2 activates the transcriptional activity of Myf5 through a direct phosphorylation at Ser49 and Ser133 (17). Upon the induction of differentiation, MEF2 is phosphorylated at Ser59 by CK2 resulting in the enhancement of MEF2 DNA binding (39). Finally, CK2 indirectly activates MyoD transcriptional activity by phosphorylating the basic helix-loop-helix E protein E47. Phosphorylation of E47 promotes its heterodimerization with MyoD thereby promoting the DNA binding and transcriptional activity of the E47:MyoD heterodimers (16). Therefore, our results demonstrating the phosphorylation of Pax3 at Ser205 by CK2 is consistent with the role of CK2 in the regulation of the biological activities of muscle-specific transcription factors in early myogenesis.
At present the exact mechanism by which CK2 regulates the biological activities of Pax3 is not completely understood. A recent report demonstrated that phosphorylation of Pax3 at unidentified sites contributes to its DNA binding and transcriptional activities (13). Along these same lines, the DNA binding activity of Pax3 is rapidly down regulated during cellular differentiation (40). This information, combined with the constitutive activity of CK2 and the rapid loss of phosphorylation at Ser205 upon the induction of myogenic differentiation (12), suggest a possible role for the phosphorylation of Pax3 by CK2 in myogenesis. Pax3 transcriptional activity is essential for promoting cellular proliferation (41, 42) while simultaneously inhibiting entry into the differentiation program (41, 43), two processes important for the creation of an enlarged precursor pool necessary to generate an optimal environment for the induction of myogenesis (42). Therefore, it is conceivable that the constitutive phosphorylation of Pax3 by CK2 in proliferating primary myoblasts would promote the transcriptional activity of Pax3 thereby inducing the expression of genes important for the maintenance of the cells in a proliferative, undifferentiated state. Upon the induction of differentiation, the rapid dephosphorylation of Pax3, by an as of yet unknown phosphatase would modulate its activity thereby allowing the cells to initiate the myogenic program.
CK2, Pax3-FOXO1 and ARMS
CK2 has been implicated as an important player in tumor progression and as such has been targeted for the development of potential tumor therapies (29, 31, 44). CK2 activity is enhanced in many human cancers including solid tumors (45-47), where it has a predominantly nuclear localization (48), and the overexpression of CK2 in transgenic mice promotes tumorigenesis in the tissues where it is expressed (47, 49). In particular, CK2 is important in the development of ARMS where it acts as a potent suppressor of apoptosis (20). Consistent with its role in the development of ARMS, we demonstrate in this report that in addition to suppressing apoptosis, CK2 also phosphorylates the oncogenic fusion protein Pax3-FOXO1, the characteristic genetic mutation in ARMS. Furthermore, we show that phosphorylation of Pax3-FOXO1 at Ser205 persists throughout differentiation, which is in direct contrast to the pattern of phosphorylation observed for wild-type Pax3 (Figure 7). Finally, our results demonstrating that endogenous Pax3-FOXO1 is phosphorylated at Ser205 in a variety of ARMS cell lines (Figure 8) provides compelling evidence supporting a potential role of this aberrant phosphorylation in the development of ARMS.
Pax3-FOXO1 has multiple roles in its ability to affect myogenesis. The fusion protein increases the proliferation of myogenic precursor cells and it induces the myogenic program while subsequently inhibiting the ability of muscle cells to fuse to achieve terminal differentiation (8, 50). Like wild-type Pax3, phosphorylation of Pax3-FOXO1 at unidentified sites is believed to contribute to its DNA binding and transcriptional activity (13). This fact, combined with our present results demonstrating the aberrant and persistent phosphorylation of the fusion protein at Ser205 during myogenesis and in ARMS cell lines, suggests a possible model whereby Pax3-FOXO1 commits a precursor cell to the myogenic lineage while ultimately inhibiting terminal differentiation. Like Pax3, the constitutive phosphorylation of Pax3-FOXO1 in proliferating myoblasts would promote the DNA binding and transcriptional activity of the fusion protein, which is capable of binding to Pax3 recognition sequences, thereby inducing the expression of genes necessary to maintain the cells in a proliferative and undifferentiated state. However, unlike Pax3, upon the induction of differentiation, Pax3-FOXO1 is unable to be dephosphorylated, which promotes the persistent phosphorylation of Pax3-FOXO1 at Ser205 thereby maintaining the protein in an active state resulting in the aberrant expression of genes that would inhibit terminal differentiation.
In summary, we present compelling evidence in this report that supports the hypothesis that the protein kinase CK2 phosphorylates both wild-type Pax3 and the oncogenic fusion protein Pax3-FOXO1 at Ser205 in proliferating primary myoblasts. The observation that phosphorylation at Ser205 of the fusion protein persists throughout early myogenic differentiation and is present on Pax3-FOXO1 in a variety of ARMS cell lines suggests that this phosphorylation event may be important for the altering of normal myogenesis and the development of the tumor. Experiments are presently being performed to examine how phosphorylation by CK2 at Ser205 regulates the biological activities of Pax3 and Pax3-FOXO1 and how these events contribute to normal muscle differentiation and the development of ARMS.
ACKNOWLEDGEMENTS
We would like to thank Dr. Gerard Grosveld, Department of Genetics, St. Jude Children's Research Hospital, Memphis, TN, for the generous gift of the ARMS cell lines RH3, RH4, RH28, and RH31 and the GST fusion constructs pGEX-5X-1-Pax3 and pGEX-5X-1-Pax3-FOXO1.
ABBREVIATIONS
- CK2
Casein kinase II
- DRB
5,6-Dichloro-1-(β-D-ribofuranosyl)benzimidazole
- HD
homeodomain
- OD
octapeptide domain
- PD
paired domain
- TAD
transcriptional activation domain
Footnotes
Funding for this work was provided by grant numbers 1P20RR020152 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), 1R01CA138656 from the National Cancer Institute (NCI), and the Louisiana Cancer Research Consortium (LCRC) Immediate Response Fund.
REFERNCES
- 1.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annual review of cell and developmental biology. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 2.Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovascular research. 1997;36:163–173. doi: 10.1016/s0008-6363(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 3.Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;20:5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, Bridge JA, Crist WM, Triche TJ, Barr FG. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children's oncology group. J Clin Oncol. 2002;20:2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 5.Kelly KM, Womer RB, Sorensen PH, Xiong QB, Barr FG. Common and variant gene fusions predict distinct clinical phenotypes in rhabdomyosarcoma. J Clin Oncol. 1997;15:1831–1836. doi: 10.1200/JCO.1997.15.5.1831. [DOI] [PubMed] [Google Scholar]
- 6.Fredericks WJ, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr FG, Rauscher FJ., 3rd. The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol. 1995;15:1522–1535. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollenbach AD, Sublett JE, McPherson CJ, Grosveld G. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. Embo J. 1999;18:3702–3711. doi: 10.1093/emboj/18.13.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller PJ, Hollenbach AD. The oncogenic fusion protein Pax3-FKHR has a greater post-translational stability relative to Pax3 during early myogenesis. Biochim Biophys Acta. 2007;1770:1450–1458. doi: 10.1016/j.bbagen.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein JA, Song B, Lakkis M, Wang C. Tumor-specific PAX3-FKHR transcription factor, but not PAX3, activates the platelet-derived growth factor alpha receptor. Mol Cell Biol. 1998;18:4118–4130. doi: 10.1128/mcb.18.7.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter T. A thousand and one protein kinases. Cell. 1987;50:823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- 11.Edelman AM, Blumenthal DK, Krebs EG. Protein serine/threonine kinases. Annual review of biochemistry. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- 12.Miller PJ, Dietz KN, Hollenbach AD. Identification of serine 205 as a site of phosphorylation on Pax3 in proliferating but not differentiating primary myoblasts. Protein Sci. 2008;17:1979–1986. doi: 10.1110/ps.035956.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amstutz R, Wachtel M, Troxler H, Kleinert P, Ebauer M, Haneke T, Oehler-Janne C, Fabbro D, Niggli FK, Schafer BW. Phosphorylation regulates transcriptional activity of PAX3/FKHR and reveals novel therapeutic possibilities. Cancer Res. 2008;68:3767–3776. doi: 10.1158/0008-5472.CAN-07-2447. [DOI] [PubMed] [Google Scholar]
- 14.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinna LA. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990;1054:267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SE, Wang X, Hardy S, Taparowsky EJ, Konieczny SF. Casein kinase II increases the transcriptional activities of MRF4 and MyoD independently of their direct phosphorylation. Mol Cell Biol. 1996;16:1604–1613. doi: 10.1128/mcb.16.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter B, Kautzner I, Issinger OG, Arnold HH. Two putative protein kinase CK2 phosphorylation sites are important for Myf-5 activity. Biol Chem. 1997;378:1445–1456. doi: 10.1515/bchm.1997.378.12.1445. [DOI] [PubMed] [Google Scholar]
- 18.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell stem cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Molecular and cellular biochemistry. 2005;274:63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- 20.Izeradjene K, Douglas L, Delaney A, Houghton JA. Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res. 2004;10:6650–6660. doi: 10.1158/1078-0432.CCR-04-0576. [DOI] [PubMed] [Google Scholar]
- 21.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 22.Lam PY, Sublett JE, Hollenbach AD, Roussel MF. The oncogenic potential of the Pax3-FKHR fusion protein requires the Pax3 homeodomain recognition helix but not the Pax3 paired-box DNA binding domain. Mol Cell Biol. 1999;19:594–601. doi: 10.1128/mcb.19.1.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allende JE, Allende CC. Protein kinases. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 24.Srivenugopal KS, Mullapudi SR, Ali-Osman F. Phosphorylation of O6-alkylguanine-DNA alkyltransferase: experience with a GST-fusion protein and a new pull-down assay. Cancer Lett. 2002;181:87–93. doi: 10.1016/s0304-3835(01)00823-0. [DOI] [PubMed] [Google Scholar]
- 25.Zandomeni RO. Kinetics of inhibition by 5,6-dichloro-1-beta-Dribofuranosylbenzimidazole on calf thymus casein kinase II. Biochem J. 1989;262:469–473. doi: 10.1042/bj2620469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hathaway GM, Lubben TH, Traugh JA. Inhibition of casein kinase II by heparin. J Biol Chem. 1980;255:8038–8041. [PubMed] [Google Scholar]
- 27.Meggio F, Shugar D, Pinna LA. Ribofuranosyl-benzimidazole derivatives as inhibitors of casein kinase-2 and casein kinase-1. Eur J Biochem. 1990;187:89–94. doi: 10.1111/j.1432-1033.1990.tb15280.x. [DOI] [PubMed] [Google Scholar]
- 28.Hardtke CS, Gohda K, Osterlund MT, Oyama T, Okada K, Deng XW. HY5 stability and activity in arabidopsis is regulated by phosphorylation in its COP1 binding domain. Embo J. 2000;19:4997–5006. doi: 10.1093/emboj/19.18.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Ruzzene M, Penzo D, Pinna LA. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J. 2002;364:41–47. doi: 10.1042/bj3640041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells--a potential approach to cancer therapy. Mol Cell Biochem. 2005;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]
- 32.Khan J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, Smith PD, Jiang Y, Gooden GC, Trent JM, Meltzer PS. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res. 1998;58:5009–5013. [PubMed] [Google Scholar]
- 33.Hazelton BJ, Houghton JA, Parham DM, Douglass EC, Torrance PM, Holt H, Houghton PJ. Characterization of cell lines derived from xenografts of childhood rhabdomyosarcoma. Cancer Res. 1987;47:4501–4507. [PubMed] [Google Scholar]
- 34.Petak I, Douglas L, Tillman DM, Vernes R, Houghton JA. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6:4119–4127. [PubMed] [Google Scholar]
- 35.Bober E, Franz T, Arnold HH, Gruss P, Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development (Cambridge, England) 1994;120:603–612. doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- 36.Rickert P, Corden JL, Lees E. Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene. 1999;18:1093–1102. doi: 10.1038/sj.onc.1202399. [DOI] [PubMed] [Google Scholar]
- 37.Schang LM. Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother. 2002;50:779–792. doi: 10.1093/jac/dkf227. [DOI] [PubMed] [Google Scholar]
- 38.Pei Y, Shuman S. Characterization of the Schizosaccharomyces pombe Cdk9/Pch1 protein kinase: Spt5 phosphorylation, autophosphorylation, and mutational analysis. J Biol Chem. 2003;278:43346–43356. doi: 10.1074/jbc.M307319200. [DOI] [PubMed] [Google Scholar]
- 39.Molkentin JD, Li L, Olson EN. Phosphorylation of the MADS-Box transcription factor MEF2C enhances its DNA binding activity. J Biol Chem. 1996;271:17199–17204. doi: 10.1074/jbc.271.29.17199. [DOI] [PubMed] [Google Scholar]
- 40.Reeves FC, Fredericks WJ, Rauscher FJ, 3rd, Lillycrop KA. The DNA binding activity of the paired box transcription factor Pax-3 is rapidly downregulated during neuronal cell differentiation. FEBS letters. 1998;422:118–122. doi: 10.1016/s0014-5793(97)01598-6. [DOI] [PubMed] [Google Scholar]
- 41.Epstein JA, Lam P, Jepeal L, Maas RL, Shapiro DN. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem. 1995;270:11719–11722. doi: 10.1074/jbc.270.20.11719. [DOI] [PubMed] [Google Scholar]
- 42.Mennerich D, Braun T. Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. Embo J. 2001;20:7174–7183. doi: 10.1093/emboj/20.24.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- 44.Sarno S, Ruzzene M, Frascella P, Pagano MA, Meggio F, Zambon A, Mazzorana M, Di Maira G, Lucchini V, Pinna LA. Development and exploitation of CK2 inhibitors. Mol Cell Biochem. 2005;274:69–76. doi: 10.1007/s11010-005-3079-z. [DOI] [PubMed] [Google Scholar]
- 45.Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: association with malignant transformation. Cancer Lett. 1996;101:31–35. doi: 10.1016/0304-3835(96)04110-9. [DOI] [PubMed] [Google Scholar]
- 46.Munstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG. Casein kinase II is elevated in solid human tumours and rapidly proliferating nonneoplastic tissue. Eur J Biochem. 1990;189:251–257. doi: 10.1111/j.1432-1033.1990.tb15484.x. [DOI] [PubMed] [Google Scholar]
- 47.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 48.Faust RA, Niehans G, Gapany M, Hoistad D, Knapp D, Cherwitz D, Davis A, Adams GL, Ahmed K. Subcellular immunolocalization of protein kinase CK2 in normal and carcinoma cells. Int J Biochem Cell Biol. 1999;31:941–949. doi: 10.1016/s1357-2725(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 49.Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267:894–897. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 50.Graf Finckenstein F, Shahbazian V, Davicioni E, Ren YX, Anderson MJ. PAX-FKHR function as pangenes by simultaneously inducing and inhibiting myogenesis. Oncogene. 2008;27:2004–2014. doi: 10.1038/sj.onc.1210835. [DOI] [PubMed] [Google Scholar]