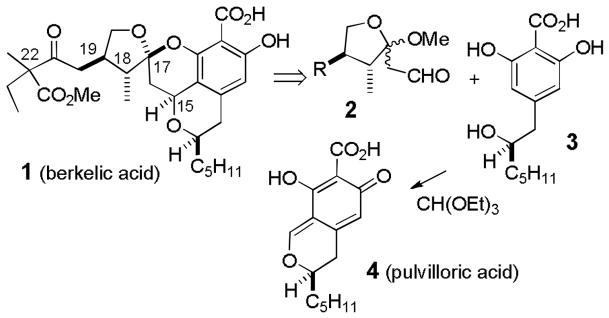
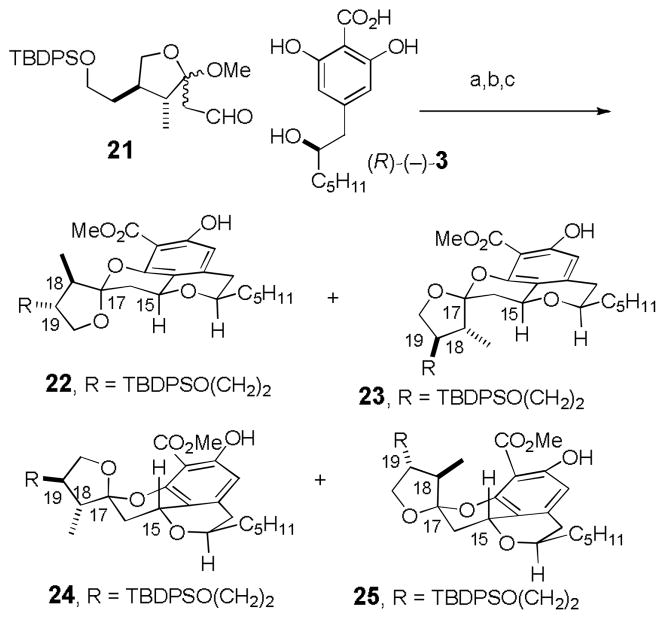
Stierle and coworkers recently isolated berkelic acid (1) from an acid mine waste fungal extremophile (see Scheme 1).[1] The structure was determined to be a novel spiroketal based on the analysis of the NMR and mass spectral data. The relative stereochemistry of the side chain stereocenter (C-22) and the absolute stereochemistry were not assigned. Berkelic acid inhibits MMP-3 and caspase-1 and shows selective activity toward ovarian cancer OVCAR-3 with a GI50 of 91 nM. This potent and selective activity and its limited accessibility from natural sources make it a significant synthetic target. We thought that 1 should be accessible by a highly convergent route starting from ketal aldehyde 2 and 2,6-dihydroxybenzoic acid 3. Acid 3, a synthetic, and possibly biosynthetic, precursor of pulvilloric acid (4), has been prepared in both racemic[2] and optically pure form.[3]
Scheme 1.
Retrosynthesis of berkelic acid (1).
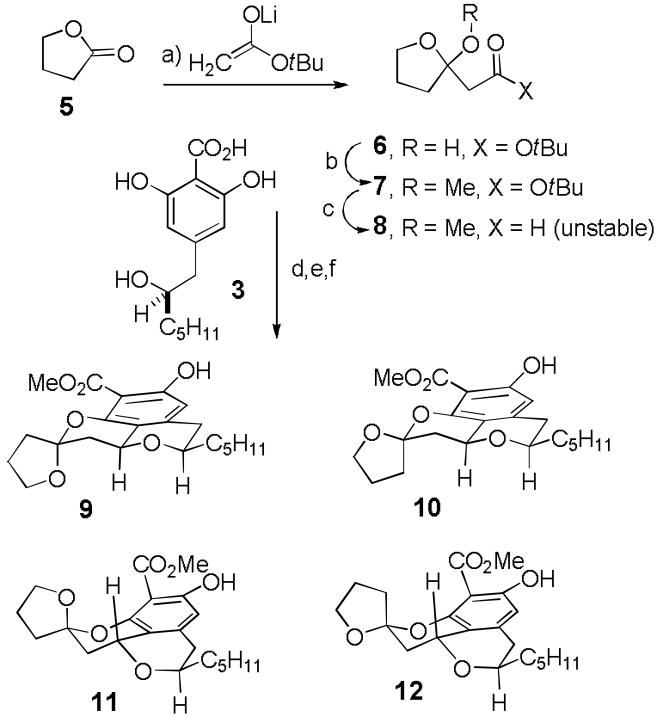
In 2007,[4] we reported an efficient route to the tetracyclic core of berkelic acid by condensing racemic 3[2] with model ketal aldehyde 8 in an oxa-Pictet-Spengler reaction[5] (see Scheme 2). Addition of the enolate of t-butyl acetate to butyrolactone (5) afforded hemiketal ester 6[6] which was converted to ketal ester 7 in 56% overall yield by treatment with Dowex 50WX8-400-H+ in MeOH for 12 h at 25 °C. Reduction with DIBAL-H in ether at −78 °C afforded unstable ketal aldehyde 8, which was treated with (±)-3 and Dowex 50WX8-400-H+ in MeOH for 12 h at 25 °C to provide a mixture of the tetracyclic acids that was treated with diazomethane to give a 4:1:4:1 mixture of tetracycles 9–12, respectively. MMX calculations with conformational searching provided relative strain energies for 9–12 of 28.1, 28.6, 30.5, and 30.8 kcal/mol, respectively,[7] indicating that the desired product 9 is more stable than the other major product 11 by 2.4 kcal/mol. Therefore, we explored the further equilibration of this mixture by treatment with 0.2% TFA in CDCl3 for 12 h at 25 °C, which provided a 20:2:1:0 mixture of 9–12, respectively, from which 9 could be isolated in 50% overall yield from acid (±)-3.[8]
Scheme 2.
Reagents and conditions: a) LDA, tBuOAc, THF, −78 to −30 °C, 2 h; b) Dowex 50WX8-400-H+, MeOH, 25 °C, 12 h (56% from 5); c) DIBAL-H, ether, −78 °C, 1.5 h; d) (±)-3, Dowex 50WX8-400-H+, MeOH, 25 °C, 12 h; e) CH2N2, ether; f) 0.2% TFA in CDCl3, 25 °C, 12 h (50% of 9 from 3).
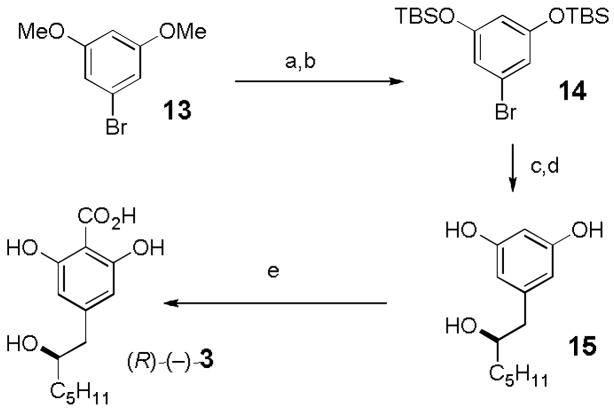
We report here the preparation of fully functionalized ketal aldehydes 21 and ent-21 and their condensation with (R)-(−)-3 leading to the first synthesis of (−)-berkelic acid, the reassignment of the stereochemistry at C-18 and C-19, the assignment of the relative stereochemistry at C-22 and the assignment of the absolute stereochemistry. As this work was being completed, Fürstner reported a synthesis of the enantiomer of the methyl ester of berkelic acid and reassigned the stereochemistry at C-18 and C-19.[9] We had difficulty in scaling up Gerlach’s synthesis[3] of (R)-(−)-3 and modified it as shown in Scheme 3. BBr3 deprotection[10] of 13 afforded the resorcinol in 97% yield, which was protected to give bis TBS ether 14[11] in 94% yield. Halogen-metal exchange with tBuLi at −78 °C and addition of (R)-(+)-2-pentyloxirane[12] followed by deprotection[13] of the TBS ethers with KOH in EtOH at 55 °C for 8 h provided 15. Carboxylation as previously described[2,3] gave (R)-(−)-3.
Scheme 3.
Reagents and conditions: a) BBr3, CH2Cl2, −78 to 25 °C, 12 h (97%); b) TBSCl, imidazole, DMF, 25 °C, 12 h (94%); c) tBuLi (2.2 equiv), THF, −78 °C, 30 min, then (R)-(+)-1,2-epoxyheptane (1.2 equiv), −25 °C, 16 h (74%); d) KOH, EtOH, 55 °C, 8 h (80%); e) CO2, KHCO3, glycerol, 150 °C, 5 h (59%).
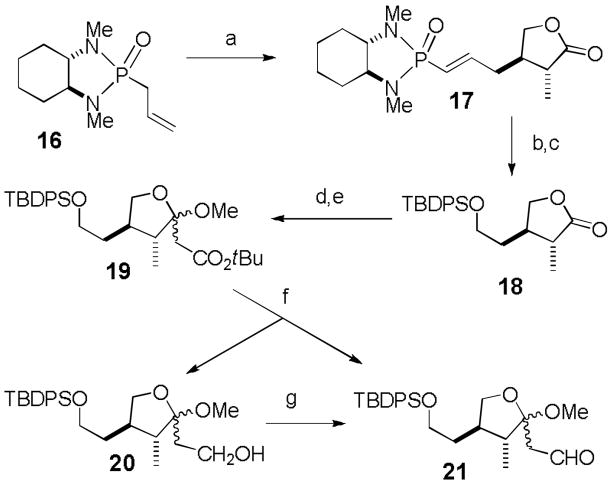
Ketal aldehyde 21 was prepared efficiently using Hanessian’s procedure as shown in Scheme 4.[14] Metalation of 16 with nBuLi at −100 °C, addition of 2-butenolide at −100 °C and trapping with excess MeI at −78 °C afforded 17 in 73% yield with >95% selectivity. Ozonolysis followed by reduction with NaBH4 and protection provided 18 in 52% yield. Lactone 18 was converted to ketal aldehyde 21 using the procedure developed for the conversion of 5 to 8. Addition of the lithium enolate of t-butyl acetate and ketal formation afforded 19 in 78% yield. DIBAL-H reduction gave aldehyde 21 (43%) and alcohol 20 (39%), which was subjected to Swern oxidation to give additional aldehyde 21 (52%).
Scheme 4.
Reagents and conditions: a) nBuLi (1.2 equiv), −100°C, 15 min, −78 °C, 10 min, recool to −100 °C, then 2-butenolide (1.2 equiv), −100 °C, 10 min, −78 °C, 30 min, then MeI (5 equiv), −78 to 25 °C, 4 h (73%); b) O3, 1:1 MeOH/CH2Cl2, −78 °C, 20 min, then NaBH4, −78 to 25 °C, 3 h; c) TBDPSCl, imidazole, DMF, 25 °C, 12 h (52% from 17); d) tBuOAc (6 equiv), LHMDS (6 equiv), −78 °C, 1 h, −78 to 25 °C, 3 h; e) Dowex 50WX8-400-H+, MeOH, 25 °C, 12 h (78% from 18); f) DIBAL-H (4.1 equiv), ether, −78 °C, 1.5 h (39% of 20 and 43% of 21); g) oxalyl chloride (3 equiv), DMSO (5 equiv), Et3N (1 equiv), CH2Cl2, −78 °C, 2 h, −78 to −30 °C, 1 h (52%).
Condensation of 21 with (R)-(−)-3 using Dowex 50WX8-400-H+ in MeOH for 12 h followed by diazomethane esterification gave a 57% yield of an approximately 4:1:3:0 mixture of 22–25, respectively, whose stereochemistry was assigned by analogy to 9–12 (see Scheme 5).[4] Surprisingly, attempted equilibration with 0.2% TFA in CDCl3 had little effect, giving an approximately 2:trace:1:0 mixture of 22–25. This was of considerable concern, because we had hypothesized that the stereochemistry at both C-15 and C-17 in the natural product was thermodynamically controlled. However, the MMX relative strain energies for 22–25 of 30.5, 31.0, 31.3, and 33.6 kcal/mol, respectively,[7,15] indicated that 22 is only 0.8 kcal/mol more stable than 24, whereas model tetracycle 9 was more stable than 11 by 2.4 kcal/mol. Therefore the presence of considerable quantities of both 22 and 24 at equilibrium is not surprising.
Scheme 5.
Reactions and conditions: a) Dowex 50WX8-400-H+, MeOH, 25 °C, 12 h; b) CH2N2, ether; c) 0.2% TFA in CDCl3, 25 °C, 12 h.
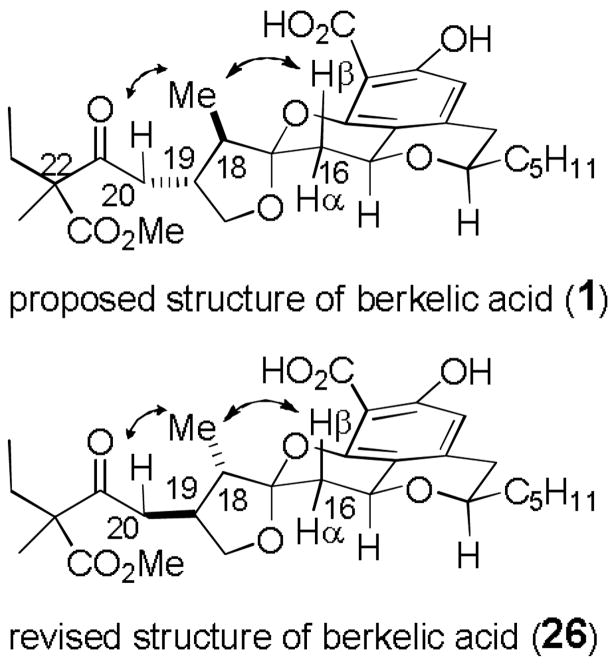
Of greater concern, the 1H NMR spectrum of the desired product 22, which could be isolated in low yield from the mixture, did not fit well with the data for berkelic acid methyl ester (see supporting information) even taking into account the differences in the side chain. This suggested that the stereochemistry of the natural product is not the same as that of 22, which has since been established by the synthesis of berkelic acid methyl ester by Fürstner.[9] The stereochemistry of berkelic acid was assigned based on an NOE from the methyl group (C-25) to H-16β and one H-20. However, MMX calculations indicated that the shortest distance from a methyl hydrogen in 1 is 2.61 Å to H-16β and 2.46 Å to H-16α. Therefore, if 1 were the structure of berkelic acid, there should be an NOE from the methyl group to both H-16β and H-16α. MMX calculations indicated that the observed NOE in berkelic acid to only H-16β fits compound 26, with opposite stereochemistry at both C-18 and C-19. In this isomer, the shortest distance from a methyl hydrogen is 2.49 Å to H-16β and 3.51 Å to H-16α (see Figure 1). Furthermore, the relative strain energy of the four diastereomers of 22–25 with the stereochemistry inverted at both C-18 and C-19 are 28.9, 31.3, 32.8, and 33.3 kcal/mol respectively.[7,15] The desired isomer 27a is calculated to be 3.9 kcal/mol more stable than the diastereomer of 24 with stereochemistry inverted at C-18 and C-19. This analysis suggested that the structure of berkelic acid is 26 rather than 1.
Figure 1.
Proposed and revised structures of berkelic acid showing NOEs to the methyl group.
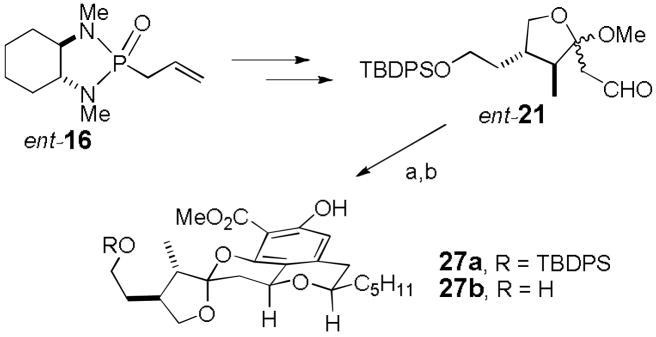
Fortunately this was easy to establish. ent-16 was converted to ent-21 and condensed with (R)-(−)-3 with Dowex 50WX8-400-H+ in MeOH (see Scheme 6). This reaction appears to be slower than that with 21, requiring 60 h for complete reaction, which led to partial cleavage of the TBDPS group. Esterification with diazomethane afforded 30% of 27a and 22% of 27b as the only products. No change occurred on TFA equilibration confirming that 27a is much more stable than the other three diastereomers as calculated. As we had hoped the spectral data of 27a fit well with those of berkelic acid methyl ester (see supporting information) suggesting that 26 is the structure of berkelic acid and that the stereochemistry at both C-15 and C-17 in the natural product is thermodynamically controlled.
Scheme 6.
Reagents and conditions: a) (R)-(−)-3, Dowex 50WX8-400-H+, MeOH, 25 °C, 60 h; b) CH2N2, ether (30% of 27a, 22% of 27b).
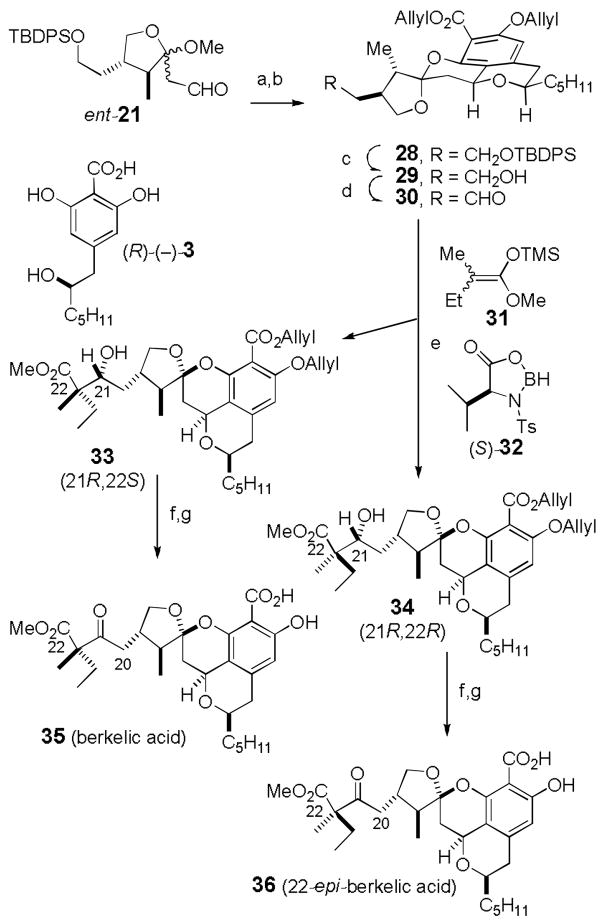
The methyl ester is not a suitable protecting group for the benzoic acid because it can’t be cleaved in the presence of the side chain methyl ester.[9] Therefore we condensed ent-21 and (R)-(−)-3 with Dowex 50WX8-400-H+ in MeOH for 60 h and treated the crude tetracyclic salicylic acid with the partially deprotected side chain with allyl bromide and K2CO3 in DMF to give 28 (32%) and 29 (20%) without allylation of the primary alcohol of 29 (see Scheme 7).[16] Desilylation of 28 with TBAF/HOAc afforded alcohol 29 in 86% yield so that the overall yield of 29 from ent-21 is 48%. Dess-Martin oxidation of 29 afforded aldehyde 30 in 88% yield. Aldol reactions under strongly basic conditions did not work well. Reaction of 30 with trimethylsilyl ketene acetal 31[17] and LiCl and N-methylimidazole[18] afforded an inseparable mixture of all four possible isomers. Fortunately, reaction of 30 and 31 as described by Kiyooka[19] using (S)-32, which is prepared in situ from N-Ts-(S)-valine and BH3•THF, was selective for the Si-face affording only two of the four aldol adducts from which pure 33 and 34 were each isolated in 40% yield. A similar reaction using N-Ts(R)-valine resulted in reduction to give primary alcohol 29. Apparently, the stereochemical preferences of 30 and (S)-32 are matched, while those of 30 and (R)-32 are mismatched resulting in reduction instead of aldol reaction.[20]
Scheme 7.
Reagents and conditions: a) Dowex 50WX8-400-H+, MeOH, 25 °C, 60 h; b) allyl bromide, K2CO3, DMF (32% of 28 and 20% of 29 from ent-21); c) TBAF/AcOH in THF, 12 h (86%); d) Dess-Martin (88%); e) N-Ts-(S)-valine, BH3•THF, CH2Cl2, 0 °C, 30 min, 25 °C, 30 min, cool to −78 °C, add 30, add 31, −78 °C, 4 h (40% of 33 and 40% of 34); f) Dess-Martin (85% from 33, 77% from 34); g) Pd(Ph3P)4 (0.2 equiv), HCO2H (40 equiv), Et3N (40 equiv), THF, 25 °C, 15 h (78% of 35, 72% of 36).
Dess-Martin oxidation (85%) of the less polar isomer 33 and deprotection (78%) of both allyl groups with Pd(Ph3P)4, Et3N, and HCO2H afforded berkelic acid (35). The 1H and 13C NMR spectral data of 35 in both CDCl3 and CD3OD are identical to those of natural berkelic acid. A similar sequence from the more polar isomer 34 afforded 36. The spectral data of 36 are similar to those of berkelic acid, but there are significant differences, most notably in the 1H NMR spectra for H-20 and the methyl and ethyl groups on C-22.
The Kiyooka aldol reaction leads to two, rather than four, aldol products making it possible to isolate pure 33 and 34. Unfortunately, this reaction controls the stereochemistry at the alcohol center (C-21), which is lost in the Dess-Martin oxidation, rather than at C-22. We prepared a series of analogous compounds from a model aldehyde and established their stereochemistry unambiguously. Comparison of the differences between the spectra of 33 and 34 with those between the model compounds as is fully described in the supporting information leads to the tentative C-22 stereochemistry assignment shown.[21]
The optical rotation of synthetic berkelic acid (35), [α]D22 –115.5 (c = 0.55, MeOH), has the same sign as that of the natural product, [α]D22 – 83.5 (c = 0.0113, MeOH), indicating that the absolute stereochemistry is as shown. The differing numerical values may result from the low sample concentration used for the natural product.
In conclusion, we have completed the first synthesis of (−)-berkelic acid (35), confirming Fürstner’s reassignment of the stereochemistry at C-18 and C-19, establishing the absolute stereochemistry, and tentatively assigning the stereochemistry at C-22. The synthesis proceeds with a longest linear sequence of only 13 steps in 2% overall yield using the novel condensation of (R)-(−)-3 and ent-21 to efficiently and stereospecifically construct the tetracyclic core.
Supplementary Material
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Footnotes
We are grateful to the National Institutes of Health (GM-50151) for generous financial support. We thank Prof. D. Stierle for copies of spectral data of berkelic acid and berkelic acid methyl ester and Mr. Artem Shvartsbart for experimental assistance.
References
- 1.Stierle AA, Stierle DB, Kelly K. J Org Chem. 2006;71:5357–5360. doi: 10.1021/jo060018d. [DOI] [PubMed] [Google Scholar]
- 2.Bullimore BK, McOmie JFW, Turner AB, Galbraith MN, Whalley WB. J Chem Soc C. 1967:1289–1293. [Google Scholar]
- 3.Rödel T, Gerlach H. Liebigs Ann Chem. 1997:213–216. [Google Scholar]
- 4.Zhou J, Snider BB. Org Lett. 2007;9:2071–2074. doi: 10.1021/ol0704338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The mechanism of the reaction of 3 with 8 is fully discussed in our model study.[4] For a review of the oxa-Pictet-Spengler reaction, see: Larghi EL, Kaufman TS. Synthesis. 2006:187–220.
- 6.Kim P, Olmstead MM, Nantz MH, Kurth MJ. Tetrahedron Lett. 2000;41:4029–4032. [Google Scholar]
- 7.PCMODEL version 8.0 from Serena Software was used with MMX. Calculations were carried out on analogues with the pentyl side chain replaced by a methyl group to minimize irrelevant conformational complexity. We suspect that the relative energies of 10 and 12 are too low because the calculations do not fully reflect the additional stability of 9 and 11 due to the anomeric effect.
- 8.For another model study, see: a) Marsini MA, Huang Y, Lindsey CC, Wu KL, Pettus TRR. Org Lett. 2008;10:1477–1480. doi: 10.1021/ol8003244.Huang Y, Pettus TRR. Synlett. 2008:1353–1356. doi: 10.1055/s-2008-1072750.
- 9.Buchgraber P, Snaddon TN, Wirtz C, Mynott R, Goddard R, Fürstner A. Angew Chem. 2008;120:8578–8582. doi: 10.1002/anie.200803339. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:8450–8454. doi: 10.1002/anie.200803339. [DOI] [PubMed] [Google Scholar]
- 10.a) Dol GC, Kamer PCJ, van Leeuwen PWNM. Eur J Org Chem. 1998:359–364. [Google Scholar]; b) Sienkowska MJ, Farrar JM, Zhang F, Kusuma S, Heiney PA, Kaszynski P. J Mater Chem. 2007;17:1399–1411. [Google Scholar]
- 11.Bharathi P, Zhao H, Thayumanavan S. Org Lett. 2001;3:1961–1964. doi: 10.1021/ol016064b. [DOI] [PubMed] [Google Scholar]
- 12.Prepared by hydrolytic kinetic resolution: Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN. J Am Chem Soc. 2002;124:1307–1315. doi: 10.1021/ja016737l.
- 13.Jiang ZY, Wang YG. Chem Lett. 2003;32:568–569. [Google Scholar]
- 14.Hanessian S, Gomtsyan A, Malek N. J Org Chem. 2000;65:5623–5631. doi: 10.1021/jo000388g. [DOI] [PubMed] [Google Scholar]
- 15.Calculations were carried out on analogues with both the pentyl and TBDPSOCH2CH2 side chains replaced by methyl groups to minimize irrelevant conformational complexity.
- 16.For the protection of a salicylic acid with two allyl groups, see: Migita A, Shichijo Y, Oguri H, Watanabe M, Tokiwano T, Oikawa H. Tetrahedron Lett. 2008;49:1021–1025.
- 17.Iida A, Nakazawa S, Okabayashi T, Horii A, Misaki T, Tanabe Y. Org Lett. 2006;8:5215–5218. doi: 10.1021/ol0619361. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara H, Inoguchi H, Fukushima M, Hoshi T, Suzuki T. Tetrahedron Lett. 2006;47:5371–5373. [Google Scholar]
- 19.a) Kiyooka S-i, Kaneko Y, Komura M, Matsuo H, Nakano M. J Org Chem. 1991;56:2276–2278. [Google Scholar]; b) Kiyooka S-i, Shahid KA. Tetrahedron: Asymmetry. 2000;11:1537–1542. [Google Scholar]; c) Kiyooka S-i, Shahid KA, Goto F, Okazaki M, Shuto Y. J Org Chem. 2003;68:7967–7978. doi: 10.1021/jo034901c. [DOI] [PubMed] [Google Scholar]; d) Kiyooka S-i, Hena MA. J Org Chem. 1999;64:5511–5523. doi: 10.1021/jo990342r. [DOI] [PubMed] [Google Scholar]; e) Fujiyama R, Goh K, Kiyooka S-i. Tetrahedron Lett. 2005;46:1211–1215. [Google Scholar]
- 20.For another example of very different yields with matched and mismatched aldehydes, see: Kiyooka S-i, Maeda H, Hena MA, Uchida M, Kim C-S, Horiike M. Tetrahedron Lett. 1998;39:8287–8290.
- 21.In his synthesis of berkelic acid methyl ester, Fürstner states that the spectral data of the stereoisomer corresponding to 36, not 35, match well with those of the natural berkelic acid methyl ester. However, he later states that confident assignment of the configuration of the stereochemistry at C-22 mandates direct comparison with an authentic sample. The spectral data for our sample of 35 correspond well to those of natural berkelic acid, while those of 36 do not, but the stereochemical assignment of 35 is based on comparison to analogues as described in the supporting information and thus is not fully secure.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.