Abstract
Aims
The caspases are thought to be central mediators of the apoptotic program, but recent data indicate that apoptosis may also be mediated by caspase-independent mechanisms such as apoptosis-inducing factor (AIF). The role of AIF-induced apoptosis in heart, however, is currently not well understood. The aim of this study was to investigate the presence of and conditions for AIF-induced cardiac apoptosis in vitro.
Methods and results
Hypertrophic cardiomyocyte (H-CM) cultures were prepared from the hearts of Dahl salt-sensitive rats fed a high salt diet. Apoptotic stimulation induced by hypoxia/reoxygenation or staurosporine (1 µM) enhanced AIF release in H-CMs compared with non-hypertrophic cardiomyocytes (N-CMs). Caspase inhibition using zVAD.fmk (25 µM) or overexpression of CrmA using recombinant adenovirus only partially protected N-CMs from apoptosis (63 ± 0.93%) and provided no significant protection against apoptosis in hypertrophic cells (23 ± 1.03%). On the other hand, poly-ADP-ribose polymerase inhibition using 4-AN (20 µM) during apoptotic stimulation blocked the release of AIF from mitochondria and significantly improved cell viability in hypertrophied cardiomyocytes (74 ± 1.18%).
Conclusion
A caspase-dependent, apoptotic pathway is important for N-CM death, whereas a caspase-independent, AIF-mediated pathway plays a critical role in H-CMs.
Keywords: Apoptosis-inducing factor, Caspase, Cardiomyocytes, Hypertrophy, PARP
1. Introduction
Apoptosis, or programmed cell death, plays an important role in cardiovascular disease.1–3 Although the caspases are thought to be central elements in the apoptotic programme, recent data indicate that apoptosis may also be mediated by caspase-independent mechanisms involving pro-apoptotic mitochondrial factors, such as apoptosis-inducing factor (AIF).4–9 AIF, an NADH-oxidase, is produced as a 67 kDa precursor protein containing an N-terminal mitochondrial localization sequence.10–12 Cell-free system experiments have shown that AIF is released from mitochondria and translocates to the nucleus, where it induces chromatin condensation and DNA cleavage.10 Knockout of AIF in the mouse is lethal before birth, and AIF-deficient embryonic stem (ES) cells are relatively resistant to apoptosis induced by serum deprivation compared with wild-type ES cells.9 AIF-induced apoptosis, therefore, appears to play a role during development. In heart, exposing in vitro cardiomyocytes to oxidant stress (e.g. hydrogen peroxide) releases both AIF and cytochrome c from the mitochondria,13 which indicates that both caspase-independent and -dependent apoptotic pathways are activated. AIF-induced apoptosis has also been demonstrated in ischaemia/reperfusion-induced myocardial injury and transverse aortic constriction-induced heart failure in vivo.13–15 Even so, the significance of AIF-induced apoptosis in heart is poorly understood at this time.
Cardiac hypertrophy is a complex and heterogeneous response to specific hypertrophic stimuli characterized by an increase in cardiomyocyte size. In various human and animal models of heart failure, the evidence suggests that the transition from pathological hypertrophy to heart failure may involve an increase in cardiac apoptosis.16 Studies by our laboratory and other investigators also suggest that the pathologically hypertrophic heart may be more sensitive to apoptotic stimulation.17–20 In this regard, we previously showed that pathological hypertrophy is characterized by significant alterations in genes involved in the apoptosis pathway.20 This suggests the presence of a milieu promoting apoptosis in hypertrophic cardiomyocytes (H-CMs). It also suggests that an increased propensity to undergo apoptosis may play a role during the transition to heart failure.19,20 We have recently demonstrated the activation of caspase-independent apoptosis, particularly AIF-induced apoptosis, in H-CMs.21 However, the association between apoptosis, especially AIF-induced apoptosis, and cardiac hypertrophy is yet to be clarified. In this study, we investigate the molecular mechanism and potential role of caspase-independent apoptosis in both H-CMs and non-hypertrophic cardiomyocytes (N-CMs). We hypothesize that AIF is an important mediator of apoptosis in heart, particularly in H-CMs.
2. Methods
2.1. Generation of hypertrophic and non-hypertrophic adult cardiomyocyte cultures
Pathological cardiac hypertrophy was generated in female Dahl salt-sensitive (DSS) rats (Harlan Sprague Dawley, Indianapolis, IN, USA) as described previously.20 We have previously published extensively using DSS rat models, including utilizing H-CM culture.19 Normal cardiomyocyte and hypertrophied cardiomyocyte cultures were prepared from the hearts of DSS rats according to a previously published protocol.19 A detailed method is provided in the Supplementary material online, Methods. The investigations conform with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, 1996) and were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center.
2.2. Induction of apoptosis and analysis of cell viability and apoptosis
Apoptosis was induced by adding staurosporine (1 µM) to cell cultures or by hypoxia/reoxygenation (H/R), and apoptosis was quantified by annexin V staining as described previously.19,22 Cell viability was determined using propidium iodide exclusion and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide] assay. Methodological details and the sources for antibodies and chemicals are provided in the Supplementary material online, Methods.
2.3. Caspase inhibition using recombinant adenovirus
Recombinant adenovirus expressing CrmA (AdCrmA) was used as described previously.23 The cytokine response modifier (CrmA), a 38 kDa protein, is a selective inhibitor of Group I and most Group III caspases, and achieves its anti-apoptotic activity by inhibiting a group of proteolytic enzymes involved in regulating cell death by apoptosis. CrmA has been shown to be an anti-apoptotic factor in ventricular myocytes during prolonged hypoxia.23 Briefly, 24 h after myocyte isolation, cells were infected with AdCrmA virus for 4 h, the viral suspension was removed, and cells incubated for an additional 20 h before experimentation. Adenovirus expressing nuclear lacZ was used as a control infection. We used the titre of 100 multiplication of infection (m.o.i.), which resulted in >80% infection efficiency.
2.4. Subcellular fractionation and immunoblot
Subcellular fractionations were obtained according to the previously published protocol.22,24 Details of the subcellular fraction method are provided in the Supplementary material online, Methods.
2.5. Caspase and poly-ADP-ribose polymerase activity assays
Cell-free caspase and poly-ADP-ribose polymerase (PARP) activity was measured according to the published protocol.19 A detailed method is provided in the Supplementary material online, Methods.
2.6. Statistics
Data are expressed as mean ± SEM of from four to six independent myocyte cultures. Statistical analyses between two groups and among the groups were performed with an unpaired Student's t-test and ANOVA with the Bonferroni method, respectively. Probability (P) values of <0.05 were considered significant.
3. Results
3.1. N-CM and H-CM cultures and their stability
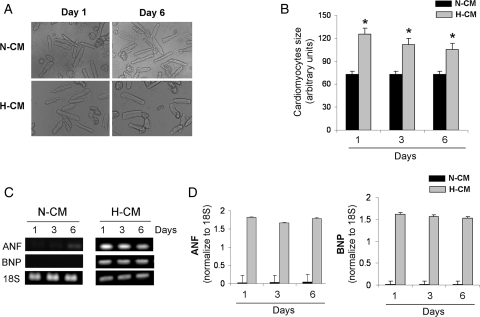
To demonstrate the stability of N-CMs and H-CMs in culture, we analysed the decline of cell viability under normoxic conditions (see Supplementary material online, Figure S1A). There was a stable and similar cell loss of <10% over 8 days in N-CM and H-CM cultures. In addition, to confirm that H-CMs remain hypertrophic, we analysed cell size in adult cardiomyocytes obtained on the day of isolation (day 1) or day 6 (Figure 1A and B). H-CMs were significantly larger (71%) than N-CMs on day 1 and remained significantly hypertrophic (43% larger) on day 6. Next, to demonstrate that H-CMs maintain the biochemical signature of hypertrophy, we measured atrial natriuretic factor (ANF) and brain natriuretic peptic (BNP) expression levels, which are well-established markers for hypertrophy (Figure 1C and D). Both markers were significantly activated at days 1 and 6 in H-CMs, but barely detectable in N-CMs. In addition, stability with respect to AIF and cytochrome c release was assessed at 0, 6, 12, and 24 h by western blot. There was no cytosolic release of AIF or cytochrome c in the absence of apoptotic induction in either H-CMs or N-CMs (see Supplementary material online, Figure S1B and C).
Figure 1.
Adult rat N-CM and H-CM cultures and their stability. (A) Morphology of H-CMs and N-CMs at days 1 and 6. (B) Quantitative analysis of cardiomyocyte size at days 1 and 6. n = 6. *P < 0.05. (C) PCR analysis of hypertrophic markers, ANF and BNP, at days 1, 3, and 6 in H-CMs and N-CMs. 18S was used as an internal control. (D) Quantitative analysis of ANF (left panel) and BNP (right panel) at days 1, 3, and 6 in H-CMs and N-CMs. n = 6.
3.2. Apoptosis in H-CMs is induced predominately through AIF
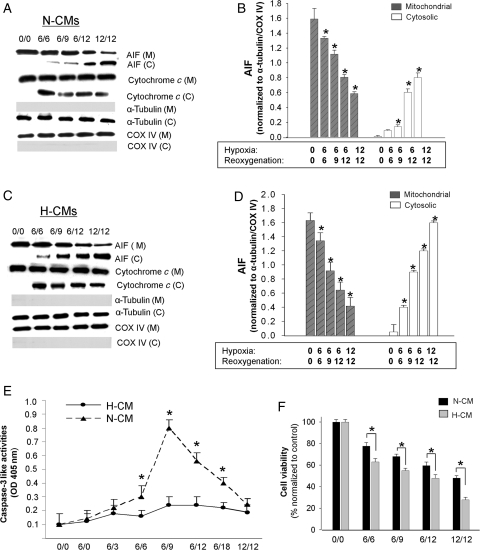
To examine apoptotic mechanisms, we first studied the activation of cytochrome c and AIF by two apoptotic stimuli: H/R and staurosporine (1 µM). Our previous study showed that cytochrome c activation peaked in N-CMs after 6 h of hypoxia followed by 6 h of reoxygenation (6H/6R).22 On the basis of this observation, we tested the following H/R conditions: 6H/6R, 6H/9R, 6H/12R, and 12H/12R. In N-CMs, cytochrome c release was evident after 6H/6R (Figure 2A), at which time cytosolic AIF was barely detectable. Cytosolic AIF increased with the duration of H/R and peaked at 12H/12R, whereas mitochondrial AIF decreased correspondingly over the durations tested (Figure 2A and B). There was a significantly greater release of AIF from the mitochondria in H-CMs exposed to the same apoptotic stimuli, which reached a 2.1-fold greater increase compared with N-CMs after 12H/12R (Figure 2C and D). To confirm that the degree of release of AIF from mitochondria is greater in H-CMs, we also examined cytosolic AIF from H-CMs and N-CMs on the same blot (see Supplementary material online, Figure S2A and B). These data confirm that H-CMs respond to H/R with a more prominent activation of AIF release.
Figure 2.
Time course of AIF and cytochrome c release from mitochondria in N-CMs and H-CMs after H/R. Western blot analysis of subcellular fractions in N-CMs (A) and H-CMs (C) after different H/R conditions. To confirm the absence of significant contamination in subcellular fractions, we probed for COX IV and α-tubulin, which are internal controls for mitochondrial and cytosolic fractions, respectively. Quantitative analysis of mitochondrial and cytosolic AIF levels after different H/R conditions in N-CMs (B) and H-CMs (D). Cytosolic and mitochondrial AIF levels were normalized to α-tubulin and COX IV levels, respectively. n = 4, *P < 0.05 compared with the control. (E) Quantitative analysis of caspase-3-like activities in N-CMs (closed triangle) and H-CMs (closed circle) after different H/R conditions. Caspase-3-like activities were measured using the specific caspase substrate DEVD-pNa. OD, optical density. n = 6. *P < 0.05. (F) Quantitative analysis of cell viability after H/R in N-CMs (dark shaded) and H-CMs (light shaded). n = 6, *P < 0.05.
We also examined caspase activation using H/R as a hypertrophic stimulus. Although a significant increase in caspase-3-like activities was observed after 6H/9R in N-CMs, no H/R condition activated caspase-3-like activities in H-CMs (Figure 2E). Examining earlier time points, such as 0H/0R, 6H/0R, and 6H/3R, also failed to reveal activated caspase-3-like activities in H-CMs. These findings suggest that H/R-induced apoptosis in H-CMs may not be a caspase-mediated process. H/R also decreased cell viability in both H-CMs and N-CMs with a significantly greater reduction in hypertrophic cells (Figure 2F), suggesting that AIF-induced apoptosis is more prominent in H-CMs.
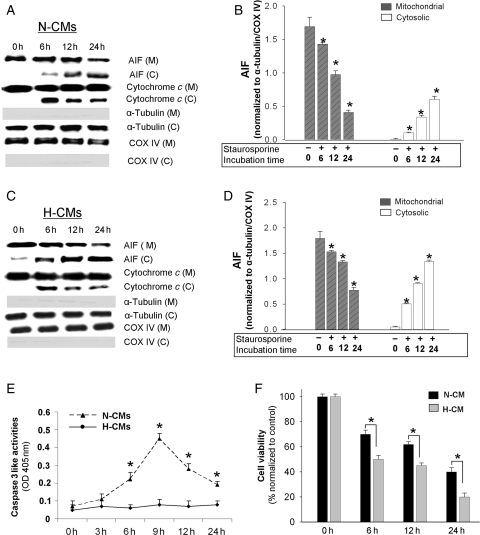
We next used staurosporine to assess whether the increased activation of AIF in H-CMs is specific to H/R or is sensitive to other apoptotic stimulations as well. In N-CMs, exposure to staurosporine resulted in a release of cytochrome c at 6 h that did not increase with further exposure (see Supplementary material online, Figure S3A and B). There was a delayed AIF release observed after 24 h in both N-CMs (Figure 3A and B) and H-CMs (Figure 3C and D). Although there was a significant activation of caspase-3-like activities that peaked at 9 h in N-CMs, H-CMs did not show any significant activation of caspase-3-like activities (Figure 3E). Finally, like H/R, staurosporine treatment significantly decreased cell viability (Figure 3F), with H-CMs showing a significantly greater reduction than N-CMs.
Figure 3.
Time course of AIF and cytochrome c release from mitochondria in N-CMs and H-CMs after staurosporine treatment. Western blot analysis of subcellular fractions in N-CMs (A) and H-CMs (B) after staurosporine treatment. Quantitative analysis of mitochondrial and cytosolic AIF after staurosporine treatment in N-CMs (C) and H-CMs (D). Cytosolic and mitochondrial AIF levels were normalized to α-tubulin and COX IV levels, respectively. n = 4, *P < 0.05 compared with the control. (E) Quantitative analysis of caspase-3-like activities in N-CMs (closed triangle) and H-CMs (closed circle) after staurosporine treatment. Caspase-3-like activities were measured using the specific caspase substrate DEVD-pNa. n = 6. *P < 0.05. (F) Quantitative analysis of cell viability after staurosporine treatment in N-CMs (dark shaded) and H-CMs (light shaded). n = 6, *P < 0.05.
3.3. Caspase inhibition has no effect on the activation of AIF-induced apoptosis in H-CMs
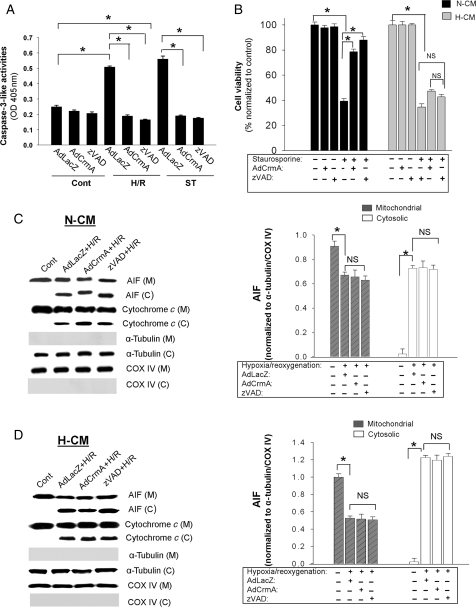
To further investigate the role of caspase, we examined the effect of apoptotic stimulation in the setting of caspase inhibition achieved by pre-treatment with zVAD.fmk, a broad-spectrum caspase inhibitor, or by overexpression of CrmA using recombinant adenovirus. CrmA has been shown to inhibit various caspases efficiently with an especially high predilection for caspase-3 and caspase-8.23 The results indicate that both zVAD.fmk pre-treatment and AdCrmA overexpression efficiently block the caspase-3-like activities induced by H/R and staurosporine in N-CMs compared with the AdLacZ controls (Figure 4A). In addition, caspase inhibition significantly attenuates the loss of cell viability caused by staurosporine treatment in N-CMs, although it did not restore viability to the baseline level. In direct contrast, caspase inhibition had no effect on the loss of cell viability after staurosporine in H-CMs (Figure 4B). This result is consonant with the fact, as noted earlier, that caspases were not activated in H-CMs by H/R or staurosporine simulation (Figures 2E and 3E). These findings strongly suggest that it is AIF-induced apoptosis, and not caspase-dependent apoptosis (as implied by cytochrome c release), which is the more important factor in determining cell viability after apoptotic stimulation in H-CMs.
Figure 4.
Effect of caspase inhibition on caspase-3-like activities and cell viability in cardiomyocytes after various apoptotic stimuli. (A) Inhibition of caspase-3-like activities induced by H/R and staurosporine using AdCrmA (100 m.o.i.) and zVAD.fmk (25 µM) in N-CMs. Caspase-3-like activities were measured after 6H/9R or 9 h exposure to staurosporine. (B) Effect of caspase inhibition on cell viability in N-CMs and H-CMs. Cell viability was assessed by MTT after 24 h exposure to 1 µM staurosporine. n = 6, *P < 0.05. (C, left panel) Western blot analysis of subcellular fractions in N-CMs with various caspase inhibitions (AdCrmA, 100 m.o.i. and zVAD.fmk, 25 µM) after 12H/12R. (C, right panel) Quantitative analysis of mitochondrial and cytosolic AIF levels with various caspase inhibitions after H/R in N-CMs. Cytosolic and mitochondrial AIF levels were normalized to α-tubulin and COX IV levels, respectively. n = 4, *P < 0.05. (D, left panel) Western blot analysis of subcellular fractions in H-CMs with various caspase inhibitions after H/R. (D, right panel) Quantitative analysis of mitochondrial and cytosolic AIF levels with various caspase inhibitions after H/R in H-CMs. Cytosolic and mitochondrial AIF levels were normalized to α-tubulin and COX IV levels, respectively. n = 4, *P < 0.05.
We next investigated the effect of caspase inhibition on AIF release from mitochondria after apoptotic stimulation. Neither mechanism of caspase inhibition, zVAD.fmk or AdCrmA, affected AIF release in either N-CMs (Figure 4C) or H-CMs (Figure 4D). Caspase inhibition also had no significant effect on the release of cytochrome c from mitochondria, which supports the notion that cytochrome c release is upstream of caspase activation, and consistent with our previous findings.22 Western blot analysis showed that the slight increase in cytochrome c in N-CMs after caspase inhibition was not statistically significant (see Supplementary material online, Figure S4A). These data indicate that in H-CMs, the dominant apoptotic mechanism involves AIF rather than caspases.
3.4. Concurrent inhibition of caspase and PARP-1 results in a more complete inhibition of apoptosis in both N-CMs and H-CMs
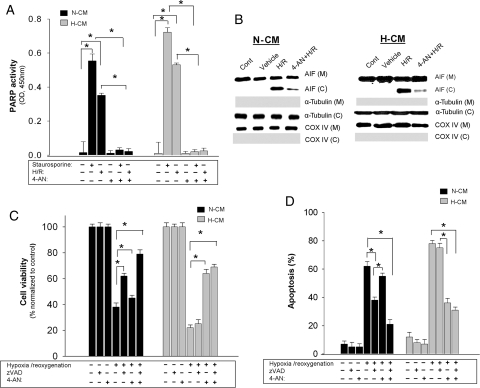
PARP-1 is an important regulator of AIF release in various cell types and has been shown to be a potentially important modulator of apoptosis in heart.25 Since AIF-induced apoptosis is predominant in H-CMs, we examined (i) PARP activation and (ii) the effect of PARP inhibition on the response to apoptotic stimuli. We found that apoptotic stimulation (12H/12R or 24 h of staurosporine) activated PARP in both N-CMs and H-CMs (Figure 5A). To confirm that PARP-1 activation is necessary for AIF release, we used a potent PARP inhibitor, 4-AN, which has been shown to inhibit PARP activation during cerebral ischaemia/perfusion.26,27 A significant reduction of AIF release was observed with 4-AN pre-treatment in both types of cardiomyocyte (Figure 5B) after H/R, suggesting that the release of AIF in cardiomyocytes is regulated by PARP. Neither 4-AN nor vehicle effected AIF release in either type of cardiomyocyte without an apoptotic stimulus (see Supplementary material online, Figure S4B).
Figure 5.
Effect of PARP inhibition in cardiomyocytes after various apoptotic stimuli. (A) PARP activity in N-CMs (dark shaded) and H-CMs (light shaded) after different apoptotic stimuli. n = 6, *P < 0.05. (B) Western blot analysis of subcellular fractions in N-CMs (left panel) and H-CMs (right panel) with PARP inhibition using 4-AN (20 µM) after H/R. (C and D) Quantitative analysis of cell viability (C) and apoptosis (D) after caspase inhibition (zVAD.fmk, 25 µM), PARP inhibition (4-AN, 20 µM), or both. Cell viability was measured by MTT assay and apoptosis was measured using annexin V staining. n = 6, *P < 0.05.
We also examined the effect of PARP inhibition on cell viability and apoptosis after 12H/12R. In N-CMs, there was a significant but only partial improvement in overall cell viability with either zVAD.fmk [62.34 ± 0.93%, P < 0.05 (H/R, 38.12 ± 0.53%)] or 4-AN (PARP, and therefore AIF, inhibition) pre-treatment (47.89 ± 0.98%, P < 0.05) (Figure 5C). However, concurrent inhibition with both 25 µM zVAD.fmk and 20 µM 4-AN resulted in an additive and more complete inhibition of apoptosis and significantly improved cell viability (79.92 ± 0.33%, P < 0.05). In H-CMs, inhibiting caspase with zVAD.fmk did not improve cell viability (23 ± 1.03%), but inhibiting AIF activation by pre-treatment with 4-AN significantly reduced apoptosis and improved overall cell viability (74 ± 1.18%, P < 0.05) (Figure 5C and D). These data suggest that apoptosis in H-CMs occurs predominantly through a caspase-independent, AIF-mediated process.
3.5. H-CMs constitute a milieu that provokes caspase-independent apoptosis
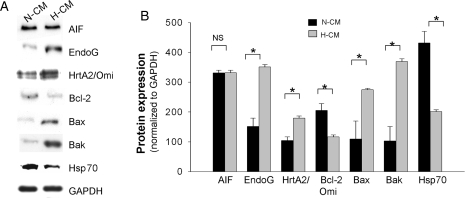
We have previously found that the heart contains high endogenous levels of a number of factors known to be involved in caspase-independent apoptotic pathways; these include AIF, EndoG, and HtrA2/Omi.21 In order to characterize the increased AIF-induced apoptosis observed here in H-CMs, we determined the expression level of other factors known to comprise caspase-independent, apoptotic pathways. In the whole cell lysate, hypertrophic heart tissues have increased levels of EndoG and HrtA2/Omi, as well as pro-apoptotic Bcl-2 family proteins, such as Bak and Bax. In contrast, the present experiments show that an anti-apoptotic factor, Bcl-2, a putative regulator of AIF release from mitochondria, is reduced by 1.5-fold in H-CMs compared with N-CMs (Figure 6A and B). Furthermore, we found that Hsp70 protein, which prevents the nuclear translocation of cytosolic AIF,28 was significantly down-regulated by 2.5-fold in H-CMs when compared with N-CMs (Figure 6A and B). Of note, our previous study in whole heart tissue lysate showed increased cytosolic AIF as well as increased processed HtrA2 in failing hearts but not in hearts with physiological or pathological hypertrophy; on the other hand, EndoG was not altered.21 However, in the present study, we observed increased EndoG in pure adult cardiomyocyte culture, which might be the possible effect of various culturing procedures on H-CMs. Also, it is possible that the difference in whole heart tissue was not evident because there is a higher level of EndoG in non-myocytes compared with cardiomyocytes (see Supplementary material online, Figure S5A). In an additional study, we observed no significant subcellular translocation of Bax, Bak, and Bcl-2 proteins at baseline in either N-CMs or H-CMs (see Supplementary material online, Figure S5B). These data suggest that unlike N-CMs, H-CMs constitute a milieu that is more sensitive to caspase-independent, apoptotic mechanisms.
Figure 6.
Expression of factors involved in caspase-independent apoptosis. (A) Representative western blots of various endogenous factors that modulate caspase-independent apoptosis in N-CMs and H-CMs. GAPDH is used as a loading control. (B) Quantitative analysis of these factors in N-CMs and H-CMs. Protein expressions were normalized to GAPDH expression. n = 4, *P < 0.05.
4. Discussion
In this study, we found that AIF-induced apoptosis is activated in cardiomyocytes, especially in H-CMs. In particular, caspase inhibition, either by zVAD.fmk or by overexpression of AdCrmA, had limited protective effect, suggesting that the mechanism of apoptosis in H-CMs is predominately caspase-independent. Supporting this notion, we found significant activation of AIF-induced apoptosis mediated by PARP-1 activation in H-CMs. Moreover, this is the first report to show that the inhibition of caspase-independent pathways is more effective than the inhibition of caspase-dependent pathways in H-CMs. It also implies that the increased sensitivity to apoptosis in H-CMs may be mediated by AIF.
The relationship between cardiac hypertrophy and apoptosis is complex and poorly understood. Our laboratory and other investigators have previously shown that hypertrophied cardiomyocytes are more sensitive to apoptotic stimulation and characterized by alterations in genes that promote apoptosis.17–20 We thus speculated that the changes in milieu that promote apoptosis in H-CMs may play a role during the transition to heart failure. The present study indicates that H-CMs are more prone to caspase-independent apoptosis, and provides evidence for the modulation of factors involved in caspase-independent apoptotic pathways. For example, we found that Hsp70 is down-regulated in H-CMs. Since Hsp70 has been reported to block apoptosis by preventing the nuclear translocation of released AIF in the cytosol,28 this suggests that hypertrophied cells are at a greater risk of AIF-induced apoptosis. In contrast to our finding, a previous study showed that Hsp70 expression increased in cardiac hypertrophy induced by isoproterenol infusion or aortic banding.29 We speculate that the difference may be related to different cell types and animal models used, and that such discrepancies perhaps reflect the complex regulation of apoptosis signalling pathways in different cell types or tissues. Further studies to elucidate the specific molecular mechanism involved in the increased propensity of H-CMs to undergo apoptosis are needed and are currently being conducted in our laboratory.
In our study, we observed the release of cytochrome c without significant activation of caspase activities in H-CMs. Two mechanisms may be responsible for this effect. First, caspase activation may be a multistep process requiring Apaf-1, pro-caspase-9, and dATP to form apoptosomes.30 An inability to form the apoptosome complex because a specific component is lacking may prevent downstream caspase activation. Secondly, it is possible that H-CMs possess increased levels of caspase inhibitors. Further studies are needed to better define this finding in H-CMs. In fact, although caspase inhibition has been shown to reduce the acute loss of myocardium in various animal models, other studies indicate that caspase inhibition alone might not be sufficiently effective.31 Several studies have shown that even in the presence of overall caspase inhibitors, such as zVAD.fmk, nuclear DNA fragmentation and significant tissue damage are still observed,6,32 since apoptosis can progress either by caspase-dependent and/or by caspase-independent mechanisms.9,33–35 Caspase-independent pathways, such as those mediated by PARP-1/AIF may contribute significantly to the induction of cardiac apoptosis, and it is thus important to better define the role of the caspase-independent pathway. We previously observed the activation of caspase-independent events, such as the release of AIF and HtrA2/Omi, during the end-stage of high salt diet-induced heart failure.21 In the present study, we observed a substantial release of AIF after apoptotic stimulation in H-CMs, indicating that the caspase-independent apoptotic pathway is both predominant and more critical than the caspase-mediated apoptotic pathway.
AIF and cytochrome c are both important for cell viability when they are located in mitochondria, but when released from the mitochondria they activate cell death programmes. Relatively little, however, is currently known about the release of AIF in heart. We previously found that caspase-dependent apoptosis, as determined by the mitochondrial release of cytochrome c, is activated by 6 h of hypoxia.22 In this study, we found that caspase-independent apoptosis, as determined by AIF release into cytosol, is also activated by 6 h of hypoxia, but an extension of the hypoxic period to 12 h promoted an even greater AIF release. Similarly, in staurosporine-induced apoptosis, we found that although the mitochondrial release of cytochrome c did not significantly increase beyond 6 h, the release of AIF in cytosol increased with a longer staurosporine incubation. This finding demonstrates the differences in the time course of caspase-dependent and -independent apoptosis, and suggests that caspase-independent apoptosis is activated in prolonged apoptotic stimulation compared with cytochrome c-mediated apoptosis.
We also demonstrated that PARP inhibition is an effective strategy for inhibiting apoptosis by blocking the release of AIF in H-CMs. PARP is a highly conserved, 116 kDa nuclear enzyme involved in DNA repair.36 PARP-1 has been shown to facilitate both the release of AIF from mitochondria and AIF nuclear translocation.36,37 Molnar et al.25 demonstrated the activation of PARP in the failing heart by showing an increased abundance of poly-ADP ribosylated proteins. We also observed increased PARP activity in response to apoptotic stimuli in both N-CMs and H-CMs, and PARP inhibition effectively blocked the release of AIF from mitochondria. This finding suggests that AIF is a downstream effector of caspase-independent apoptosis initiated by PARP-1. Supporting this notion, in a mouse model of heart failure induced by transverse aortic banding, the extent of AIF's mitochondrial-to-nuclear translocation was reduced by the inhibition of PARP-1 activation, using either isoindolinone-based PARP inhibitor (INO-1001) or PARP-1 genetic-deficient mice.15 Although the mechanism responsible for PARP-1-dependent release of AIF from mitochondria remains to be identified, it has been proposed that the cell death pathway initiated by PARP-1 activation is mediated by AIF.36
Mitochondria contain several apoptogenic factors such as cytochrome c, Smac/Diablo, HtrA2/Omi, AIF, and EndoG, which are released upon apoptotic stimulation.12,34,38,39 EndoG and AIF have been implicated in the induction of caspase-independent apoptosis in various cell types.12,34 We have mainly focused on the role of AIF in adult cardiomyocytes, since our laboratory and other investigators have found that AIF may play an important role in the development of heart failure.40 However, other studies suggest that there is a switch from caspase-dependent to caspase-independent death pathways after cardiac cell differentiation, and EndoG may play an important role in differentiated cardiomyocytes. Whether there is a preferential role of AIF and EndoG that may be cell type-dependent, or dependent on certain conditions, such as hypertrophy, as in our study, is not known.
Historically, two main forms of cell death have been distinguished: apoptosis and necrosis. Caspase activation has been considered the hallmark of apoptosis. However, it is now well recognized that programmed cell death can also be mediated by other apoptotic factors, such as AIF and EndoG, without the activation of caspases.5,34 In this study, for example, we consider AIF-mediated cell death as a caspase-independent, apoptotic process. In fact, necrosis, which has often been viewed as an accidental and uncontrolled cell death process, might also be a highly orchestrated type of programmed cell death, like apoptosis. In addition, autophagic cell death has features resembling apoptosis, including a possible association with the caspases41,42 and Bcl-2.43 Although we did not specifically take account of other cell death processes in this paper, we recognize that they have the potential to make a contribution to overall cell death in our model, especially in H-CMs, and that more work is needed to address this issue.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported in part by a grant from the National Institutes of Health (RO1 HL65742 to P.M.K.).
Supplementary Material
References
- 1.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 3.Kang PM, Izumo S. Apoptosis in heart: basic mechanisms and implications in cardiovascular diseases. Trends Mol Med. 2003;9:177–182. doi: 10.1016/s1471-4914(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 4.Abraham MC, Shaham S. Death without caspases, caspases without death. Trends Cell Biol. 2004;14:184–193. doi: 10.1016/j.tcb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 6.Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 8.Lockshin RA, Zakeri Z. Caspase-independent cell death? Oncogene. 2004;23:2766–2773. doi: 10.1038/sj.onc.1207514. [DOI] [PubMed] [Google Scholar]
- 9.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- 11.Penninger JM, Kroemer G. Mitochondria, AIF and caspases—rivaling for cell death execution. Nat Cell Biol. 2003;5:97–99. doi: 10.1038/ncb0203-97. [DOI] [PubMed] [Google Scholar]
- 12.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Zsengeller Z, Xiao CY, Szabo C. Mitochondrial-to-nuclear translocation of apoptosis-inducing factor in cardiac myocytes during oxidant stress: potential role of poly(ADP-ribose) polymerase-1. Cardiovasc Res. 2004;63:682–688. doi: 10.1016/j.cardiores.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Kim GT, Chun YS, Park JW, Kim MS. Role of apoptosis-inducing factor in myocardial cell death by ischemia-reperfusion. Biochem Biophys Res Commun. 2003;309:619–624. doi: 10.1016/j.bbrc.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, et al. Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 16.Doi R, Masuyama T, Yamamoto K, Doi Y, Mano T, Sakata Y, et al. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000;18:111–120. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, et al. Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng YJ, Ishikawa Y, Vatner DE, Wagner TE, Bishop SP, Vatner SF, et al. Apoptosis of cardiac myocytes in Gsalpha transgenic mice. Circ Res. 1999;84:34–42. doi: 10.1161/01.res.84.1.34. [DOI] [PubMed] [Google Scholar]
- 19.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H72–H80. doi: 10.1152/ajpheart.00556.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, et al. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- 21.Siu PM, Bae S, Bodyak N, Rigor DL, Kang PM. Response of caspase-independent apoptotic factors to high salt diet-induced heart failure. J Mol Cell Cardiol. 2007;42:678–686. doi: 10.1016/j.yjmcc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 23.Gurevich RM, Regula KM, Kirshenbaum LA. Serpin protein CrmA suppresses hypoxia-mediated apoptosis of ventricular myocytes. Circulation. 2001;103:1984–1991. doi: 10.1161/01.cir.103.15.1984. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Chen YS, Liu Z, Bodyak N, Rigor D, Bisping E, et al. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res. 2006;99:415–423. doi: 10.1161/01.RES.0000237387.05259.a5. [DOI] [PubMed] [Google Scholar]
- 25.Molnar A, Toth A, Bagi Z, Papp Z, Edes I, Vaszily M, et al. Activation of the poly(ADP-ribose) polymerase pathway in human heart failure. Mol Med. 2006;12:143–152. doi: 10.2119/2006-00043.Molnar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabra DG, Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective effect of 4-amino-1,8-napthalimide, a poly(ADP ribose) polymerase inhibitor in middle cerebral artery occlusion-induced focal cerebral ischemia in rat. Brain Res Bull. 2004;62:425–433. doi: 10.1016/j.brainresbull.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia-reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab. 2005;25:868–877. doi: 10.1038/sj.jcbfm.9600081. [DOI] [PubMed] [Google Scholar]
- 28.Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 2003;22:6669–6678. doi: 10.1038/sj.onc.1206794. [DOI] [PubMed] [Google Scholar]
- 29.Kee HJ, Eom GH, Joung H, Shin S, Kim JR, Cho YK, et al. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res. 2008;103:1259–1269. doi: 10.1161/01.RES.0000338570.27156.84. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 31.Okamura T, Miura T, Takemura G, Fujiwara H, Iwamoto H, Kawamura S, et al. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res. 2000;45:642–650. doi: 10.1016/s0008-6363(99)00271-0. [DOI] [PubMed] [Google Scholar]
- 32.Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Blink E, Maianski NA, Alnemri ES, Zervos AS, Roos D, Kuijpers TW. Intramitochondrial serine protease activity of Omi/HtrA2 is required for caspase-independent cell death of human neutrophils. Cell Death Differ. 2004;11:937–939. doi: 10.1038/sj.cdd.4401409. [DOI] [PubMed] [Google Scholar]
- 34.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 36.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 37.Pieper AA, Brat DJ, Krug DK, Watkins CC, Gupta A, Blackshaw S, et al. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu HR, Gao E, Hu A, Tao L, Qu Y, Most P, et al. Role of Omi/HtrA2 in apoptotic cell death after myocardial ischemia and reperfusion. Circulation. 2005;111:90–96. doi: 10.1161/01.CIR.0000151613.90994.17. [DOI] [PubMed] [Google Scholar]
- 39.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 40.Joza N, Oudit GY, Brown D, Benit P, Kassiri Z, Vahsen N, et al. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol. 2005;25:10261–10272. doi: 10.1128/MCB.25.23.10261-10272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorski SM, Chittaranjan S, Pleasance ED, Freeman JD, Anderson CL, Varhol RJ, et al. A SAGE approach to discovery of genes involved in autophagic cell death. Curr Biol. 2003;13:358–363. doi: 10.1016/s0960-9822(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 42.Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443–1455. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- 43.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.