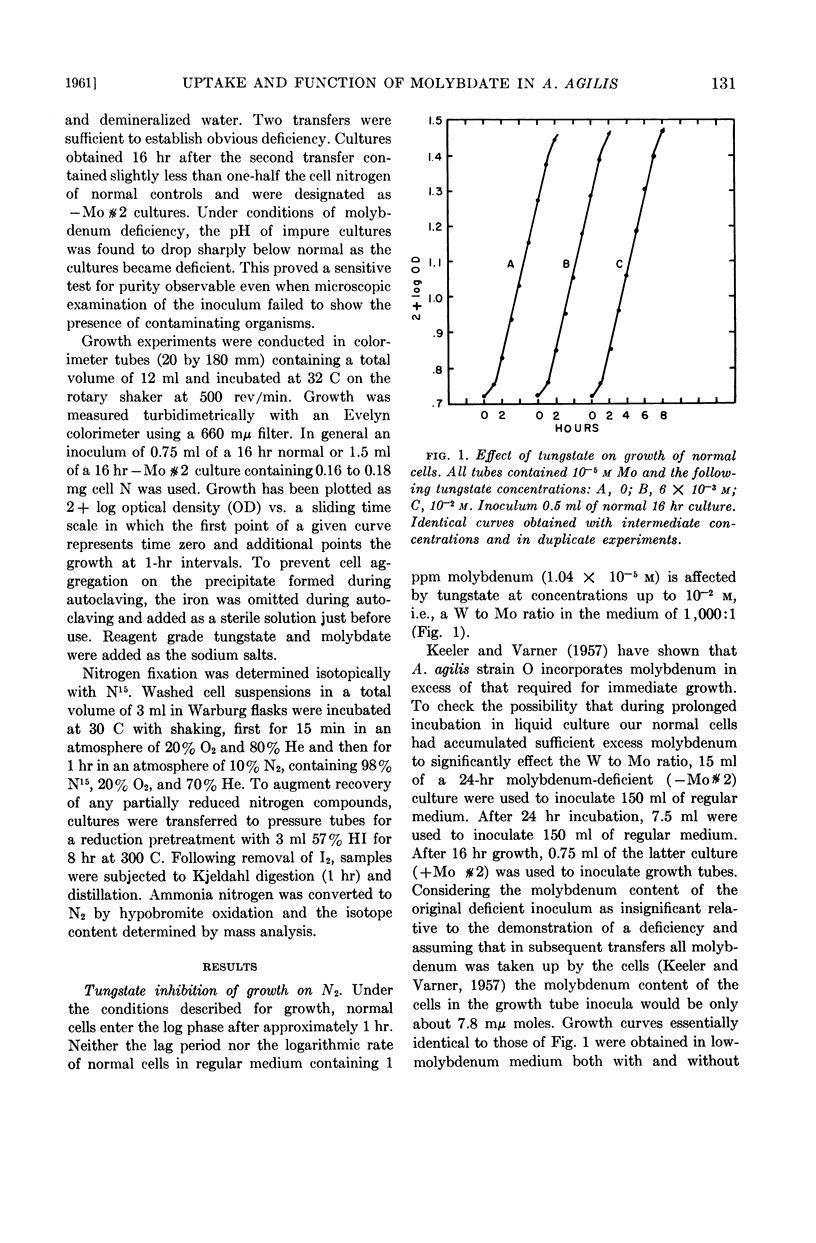
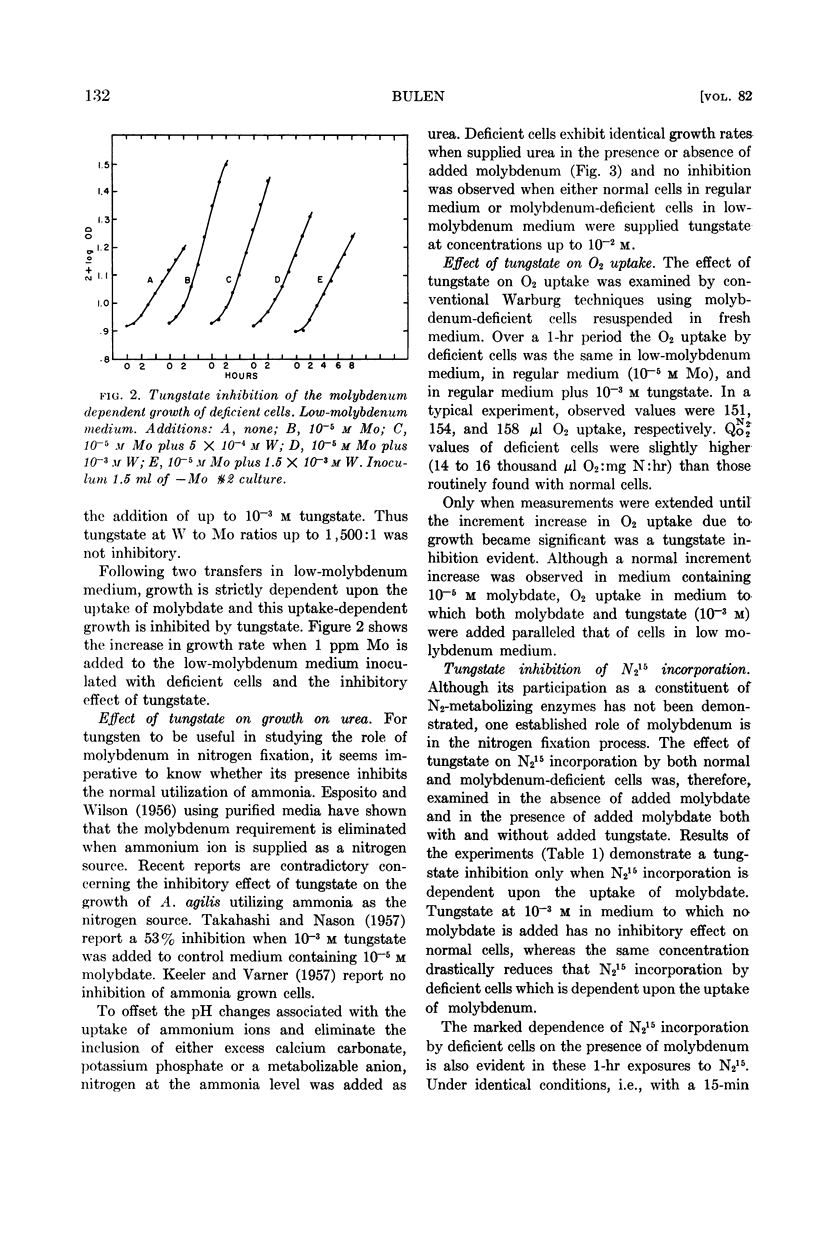
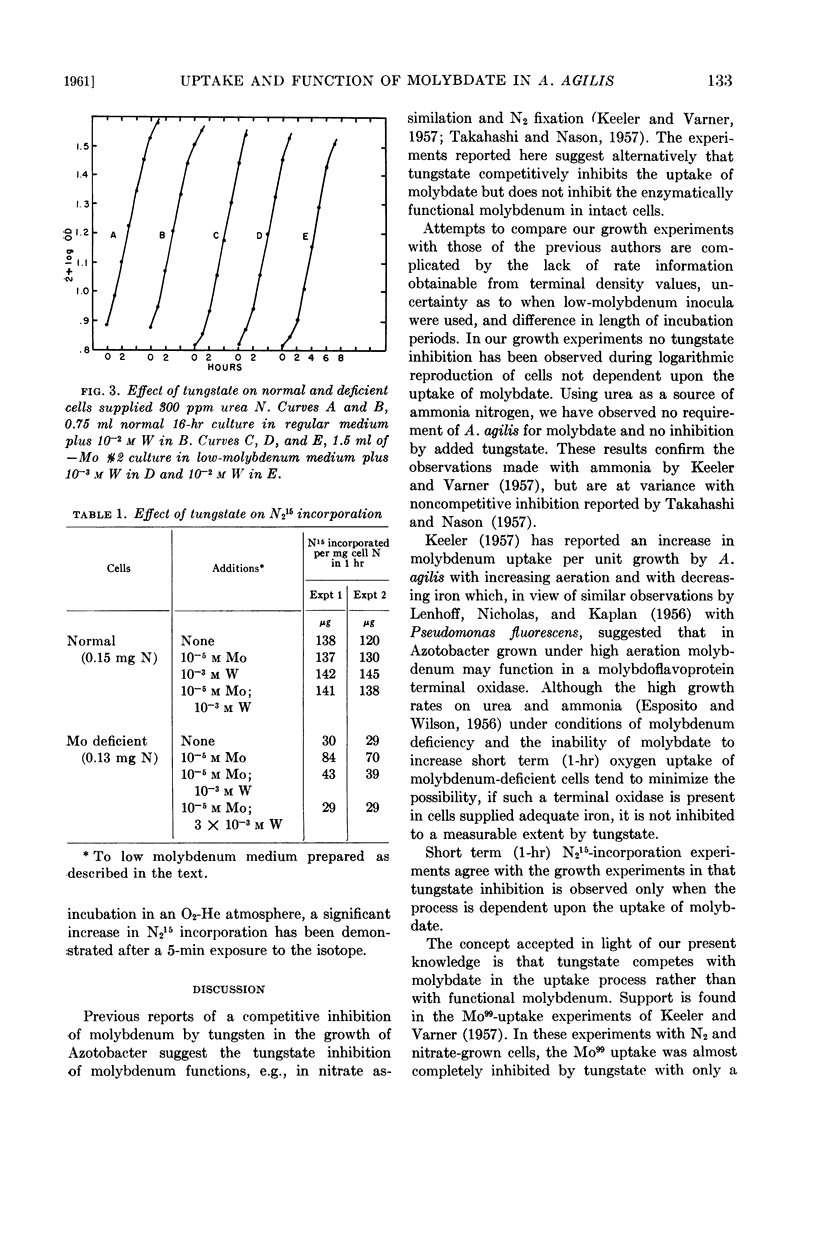
Abstract
Bulen, William A. (Charles F. Kettering Foundation, Yellow Springs, Ohio). Effect of tungstate on the uptake and function of molybdate in Azotobacter agilis. J. Bacteriol. 82:130–134. 1961.—The reported competitive inhibition of molybdate by tungstate was investigated in an effort to elucidate molybdenum functions associated with nitrogen fixation by Azotobacter agilis (A. vinelandii). Growth, respiration, and N215-incorporation experiments with normal and molybdenum-deficient cells indicated that tungstate inhibits the uptake of molybdate but does not compete with the metabolically functional molybdenum of cells metabolizing N2. Neither a molybdenum requirement nor a tungstate inhibition was observed with cells metabolizing urea.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burk D., Lineweaver H. THE INFLUENCE OF FIXED NITROGEN ON AZOTOBACTER. J Bacteriol. 1930 Jun;19(6):389–414. doi: 10.1128/jb.19.6.389-414.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPOSITO R. G., WILSON P. W. Trace metal requirements of Azotobacter. Proc Soc Exp Biol Med. 1956 Dec;93(3):564–567. doi: 10.3181/00379727-93-22820. [DOI] [PubMed] [Google Scholar]
- HIGGINS E. S., RICHERT D. A., WESTERFELD W. W. Molybdenum deficiency and tungstate inhibition studies. J Nutr. 1956 Aug 10;59(4):539–559. doi: 10.1093/jn/59.4.539. [DOI] [PubMed] [Google Scholar]
- HIGGINS E. S., RICHERT D. A., WESTERFELD W. W. Tungstate antagonism of molybdate in Aspergillus niger. Proc Soc Exp Biol Med. 1956 Jul;92(3):509–511. doi: 10.3181/00379727-92-22527. [DOI] [PubMed] [Google Scholar]
- KEELER R. F. Influence of culture conditions on uptake and distribution of molybdenum in Azotobacter vinelandii. J Bacteriol. 1957 Apr;73(4):582–583. doi: 10.1128/jb.73.4.582-583.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- LENHOFF H. M., NICHOLAS D. J., KAPLAN N. O. Effects of oxygen, iron, and molybdenum on routes of electron transfer in Pseudomonas fluorescens. J Biol Chem. 1956 Jun;220(2):983–995. [PubMed] [Google Scholar]
- MAHLER H. R., MACKLER B., GREEN D. E. Studies on metalloflavoproteins. III. Aldehyde oxidase: a molybdoflavoprotein. J Biol Chem. 1954 Sep;210(1):465–480. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Mechanism of action of nitrate reductase from Neurospora. J Biol Chem. 1954 Nov;211(1):183–197. [PubMed] [Google Scholar]
- RICHERT D. A., WESTERFELD W. W. Isolation and identification of the xanthine oxidase factor as molybdenum. J Biol Chem. 1953 Aug;203(2):915–923. [PubMed] [Google Scholar]
- TAKAHASHI H., NASON A. Tungstate as competitive inhibitor of molybdate in nitrate assimilation and in N2 fixation by Azotobacter. Biochim Biophys Acta. 1957 Feb;23(2):433–435. doi: 10.1016/0006-3002(57)90351-7. [DOI] [PubMed] [Google Scholar]
- TOTTER J. R., BURNETT W. T., Jr, MONROE R. A., WHITNEY I. B., COMAR C. L. Evidence that molybdenum is a nondialyzable component of xanthine oxidase. Science. 1953 Nov 6;118(3071):555–555. doi: 10.1126/science.118.3071.555. [DOI] [PubMed] [Google Scholar]


