Abstract
Background
We examined potential reasons (sociodemographics, psychological distress, health behavior, chronic health conditions, access to medical care) for increased prevalence of lower-body functional limitations (LBFL) among long-term (≥5 years) cancer survivors.
Methods
Using 2005-2007 National Health Interview Survey data, we defined LBFL as reporting difficulty/inability to perform at least 1 of 5 activities (walking 1/4 of a mile; walking up and down 10 steps without rest; standing for 2 hours; stooping, crouching, or kneeling; and lifting 10 lbs). Increased prevalence of LBFL was compared between long-term survivors of each of 11 cancer types reported by ≥50 respondents (n=2,143) and persons without cancer history (controls; n=72,618).
Results
Among cancer survivors, 57.0% had a LBFL versus 26.6% of controls. The unadjusted prevalence of LBFL varied by cancer type, ranging from 44.9% (lymphoma survivors) to 88.8% (lung cancer survivors). Long-term lung (odds ratio [OR]:7.91), uterine (OR:2.41), thyroid (OR:2.27), cervical (OR:1.76), ovarian (OR:1.75), and breast (OR:1.35) cancer survivors had increased odds of reporting a LBFL than controls after adjusting for sociodemographic factors (all p<0.05). Differences in prevalence of arthritis and lower-back pain and in access to medical care explained differences in LBFL prevalence between controls and long-term breast, cervical, ovarian, and uterine cancer survivors. Long-term bladder, colorectal, lymphoma, melanoma, and prostate cancer survivors were equally likely to report a LBFL as controls.
Conclusions
Treatment of arthritis and lower back pain and increasing access to medical care might help reduce the risk of LBFL and improve quality of life among specific long-term cancer survivors.
Keywords: dysfunction, arthritis, low-back pain, survivors
Introduction
About 1.4 million people will be diagnosed with cancer in the United States in 2008.1 This number is expected to double by the year 2050. The number of long-term cancer survivors (i.e., five or more years post diagnosis) also is increasing rapidly due to increased screening and improved treatment. It is estimated that nearly 11 million Americans were alive with a cancer history in 2004.1 As a result, the number of cancer survivors, including long-term survivors, is expected to increase dramatically in the next few decades.
Although cancer is increasingly being viewed as a chronic illness and many cancer survivors are in good health, there is the potential for experiencing adverse medical and psychosocial outcomes after cancer diagnosis, including functional limitations (inability to perform a physical action, task, or activity in an efficient, typically expected or competent manner).2 Functional status predicts survival, chemotherapy toxicity, postoperative morbidity, and mortality among cancer survivors.3 Because functional limitations represent an early and key transition in the disablement pathway,4, 5 they constitute an early target for interventions or for screening to slow or prevent disability6, 7 and thereby reduce the risk of disability, morbidity, and mortality among cancer survivors.
There are several potential reasons for the increased prevalence of LBFL among cancer survivors. First, cancer survivors are likely to be different in terms of their sociodemographic factors (e.g., age, income) from persons without a cancer history, and these differences may convey increased vulnerability to LBFL.8 Second, receipt of cancer treatment may lead to complications affecting various organs, including effects on the musculoskeletal, cardiovascular, and respiratory systems, which may subsequently lead to functional limitations.9 Third, cancer survivors are more likely to have chronic conditions and diseases than those without a cancer history, which may or may not be the result of types of cancer treatment received. Many of these conditions and diseases are risk factors for functional limitations.8 Fourth, access to and contact with the medical system may increase the likelihood of detecting functional limitations.8 Fifth, psychosocial factors such as depression, increased stress, and poor mental health status, which are risk factors for functional limitations,8 also may be elevated among cancer survivors. Sixth, cancer survivors may be more likely to engage in behaviors that increase the prevalence of functional limitations, such as smoking, or they may be less likely to engage in behaviors that reduce the prevalence of functional limitations, such as physical activity.8, 10
The aim of this analysis was to identify factors associated with increased prevalence of LBFL by comparing long-term cancer survivors with persons without a cancer history (controls). Because of differences in likelihood of LBFL across cancers,11 we examined a national sample of 11 specific types of long-term cancer survivors. Identification of these factors could lead to implementation of evidence-based interventions aimed at reducing the likelihood of LBFL and thereby preventing inability to carry out routine activities as part of daily life among long-term survivors with specific types of cancers.
Methods
Data source
We used data from the 2005-2007 National Health Interview Survey (NHIS).12 The NHIS is a continuous face-to-face household interview that covers a wide variety of health-related topics. It uses a complex, stratified sampling design to provide estimates for the civilian non-institutionalized US population. Data were collected about all family members in about 40,000 households annually. More detailed information is obtained from one randomly sampled adult from households. Participants in this study were limited to respondents who were part of the “sample adult” component of the survey who were asked about their cancer history. Data from the sample adult survey were self-reported, except when respondents were physically incapable of responding themselves. In this study, proxy-reported data accounted for 1.3% of all data (2.9% among long-term cancer survivors and 1.3% among respondents without cancer history). To obtain more stable estimates among survivors with specific types of cancer, we combined three years (2005-2007) of data. Response rates for the sample adult component of the NHIS in 2005, 2006, and 2007 were 69.0%, 70.8%, and 67.8%, respectively.
Lower body functional limitations
Five items from the Nagi physical performance scale assessed LBFL (0=no difficulties to 1=difficulty), which were summed to form the outcome measure (ranging from 0 to 5).13 This scale, which assesses lower-extremity strength and basic motor functions, has been used in studies of cancer survivors14, 15 and measures difficulties with walking a quarter of a mile; walking up and down 10 steps without rest; standing for 2 hours; stooping, crouching, or kneeling; and lifting 10 pounds.16 Subjects who expressed any difficulty or inability to perform an activity at the time of the interview were considered to be limited in that activity.17 For this study, we defined LBFL as reporting difficulty with or inability to perform at least one of the five physical activities.17
Cancer history
Cancer history was based on the question “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” If the respondent reported a history of cancer, they were asked the site of the cancer and the type of cancer from a list of 30 different types of cancer. Participants could report up to three cancer sites. Based on the age at the interview and age at the first cancer diagnosis, we calculated the number of years since cancer diagnosis. We excluded persons who reported more than one cancer.
We limited the analysis of cancer-specific data to 11 types of cancer (bladder, breast, cervical, colorectal, lung, lymphoma, melanoma, ovarian, prostate, thyroid, and uterine), which had been reported by at least 50 respondents in the survey and were diagnosed 5 or more years prior to the interview.15, 18 In addition to persons who reported more than one cancer, we excluded persons diagnosed with other cancers, more recent cancer survivors (<5 years since their diagnosis), and non-melanoma and unknown-type skin cancers from the analysis.15, 19 The comparison (control) group included persons without a cancer history.
Factors potentially associated with increased LBFL prevalence
We examined factors that may account for any observed association between cancer history and LBFL, including: 1) sociodemographic factors, 2) chronic conditions, 3) access to medical care, 4) health behaviors, and 5) psychological distress. Factors included in the analysis were patterned after other studies in the general population8, 20 and in cancer survivors.11, 15 First, sociodemographic factors included age group, race/Hispanic origin, gender, income categories, family size, receipt of government assistance because of low income, educational attainment, marital status, and home ownership. Second, chronic conditions consisted of current body-mass index categories (BMI)2 and 11 chronic conditions based on participants’ self-reported diagnosis by a physician. The chronic conditions included those previously associated with functional decline,8 namely having diabetes, current asthma, ever had coronary heart disease, ever had a myocardial infarction, ever had other heart disease, had hypertension on more than one occasion, ever had a stroke, ever had arthritis (osteoarthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia), general joint symptoms (pain, aching, or stiffness in or around a joint) during the past 30 days, lower-back pain during past three months, and current trouble with vision even when wearing glasses or contact lenses. Third, access to medical care consisted of having health care insurance at the time of the interview, being unable to afford prescription medicines, mental health care, dental care, or eye glasses during the past 12 months, being unable to see a doctor during the 12 months prior to the interview because of cost, and reporting delay in receipt of medical care during the past 12 months due to inability to a) get through on the telephone, b) get an appointment soon enough, c) get in to see a doctor soon enough, d) get to the clinic/doctor’s office when it was open, and e) find transportation to get there. Fourth, health behaviors included smoking status, amount of alcohol use during the past week, and current participation in moderate or vigorous physical activity at least two times per week. Fifth, nonspecific psychological distress was measured using the K6, which asks about the frequency of six symptoms of mental illness or nonspecific psychological distress.21 Using a 5-point response scale for each symptom, scores could range from 6 to 30, with higher scores indicating greater frequency of symptom experience. A score of 13 or above on the K6 was used to indicate serious psychological distress.22
Statistical analysis
The NHIS collects data through a complex sampling design, involving stratification, clustering and multistage sampling. In order to obtain nationally representative estimates, all data were weighted by adjusting for the probability of inclusion in the sample, taking into consideration survey non-response as well as the sex, age, and race/ethnicity of survey respondents. We used logistic regression to examine whether long-term cancer survivors had greater odds of prevalent LBFL compared to persons without cancer history and also to identify factors potentially associated with increased LBFL prevalence among the former population. Variance estimates for proportions and logistic regression models were calculated using the Taylor series approximation. All p values were two-sided.
The sociodemographic factors, chronic conditions, access to medical care, health behavior, and psychological distress factors were included as groups of variables in the logistic regression models to examine their effects on the prevalence odds ratio associated with a specific cancer diagnosis. Because of the differences in sociodemographic factors between persons with and without a cancer history, we examined in separate models the effect of each of the four other factors (chronic conditions, access to medical care, health behaviors, and psychological distress) in separate models after adjustment for sociodemographic factors. A change in the prevalence odds ratios was considered evidence for the effect of that factor on the association between cancer history and LBFL prevalence23 after adjusting for sociodemographic factors. We examined specific variables within a particular factor when that factor showed a significant reduction in odds ratio (at least 20%) for cancer history status; we report these data for specific variables in the text of the Results section. We used SAS 9.1 and SUDAAN 9.1 to take into account the complex sampling design.
Results
The 2005-2007 NHIS dataset included 79,096 adults who completed the “sample adult” interview. A total of 4,335 (5.5%) respondents were excluded because they met one or more of the following exclusion criteria: a) their only cancer was non-melanoma (n=1004) or unknown-type (n=494) skin cancer, b) they were diagnosed with cancer more recently than five years before the interview (n=2,191), c) their cancer history was unknown (n=229), or d) their cancer type was reported by fewer than 50 respondents (n=576). Data about 74,761 adults were included in the analysis. Of these, 2,143 (2.7%) were long-term cancer survivors, and 72,618 were never diagnosed with cancer (controls). Average time since diagnosis was 15.6 years (95% CI: 14.9–16.2). Of the long-term cancer survivors, 4.5 percent were diagnosed prior to age 18.
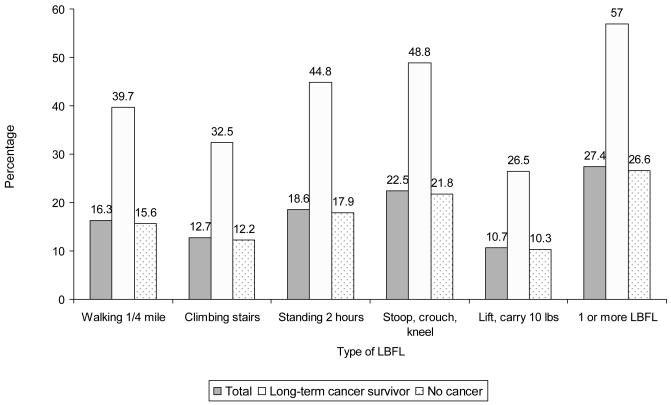
Table 1 describes selected characteristics of the study population. In unadjusted analysis, persons who were older, were female, had lower incomes, had limited access to medical care, were current or former smokers, were not participating in physical activity at least twice a week, had a greater number of chronic conditions, or who reported serious psychological distress each had increased odds of LBFL. Of the long-term cancer survivors, 57.0% (95% CI: 54.6–59.4) reported one or more LBFL compared to 26.6% (95% CI: 26.1–27.0) of persons without a cancer history. Long-term cancer survivors were 3.66 times (95% CI: 3.32–4.05) more likely to report having at least one LBFL compared to the control group in unadjusted analysis. As shown in Figure 1, the most frequent LBFL mentioned by cancer survivors was the inability to stoop, crouch, or kneel (48.8%), followed by standing for 2 hours (44.8%), and walking ¼ mile (39.7%). The prevalence of LBFL for each of the five types of LBFL was consistently higher among long-term cancer survivors compared to the control group Figure 2 displays the distribution of LBFL prevalence in the two groups.
Table 1.
Selected characteristics of the study population and unadjusted association (odds ratio, 95% confidence interval) with prevalence of lower-body functional limitations, 2005-2007.
| Long-term cancer survivors* (n=7,241,117) |
No cancer history (n=204,111,198) |
Odds ratio |
95% confidence interval |
|
|---|---|---|---|---|
| Characteristic | Percentage | Percentage | ||
| Sociodemographics | ||||
| Age** | ||||
| 8-39 | 8.9 | 42.6 | 1.00 | |
| 40-64 | 39.1 | 43.7 | 3.00 | 2.85 - 3.15 |
| 65-84 | 46.0 | 12.2 | 9.02 | 8.46 - 9.62 |
| 85+ | 6.1 | 1.5 | 25.38 | 22.27 – 28.92 |
| Sex** | ||||
| Male | 32.2 | 48.7 | 1.00 | |
| Female | 67.8 | 51.3 | 1.57 | 1.52 - 1.65 |
| Race** | ||||
| White | 89.4 | 81.0 | 1.00 | |
| African American | 7.1 | 12.2 | 0.97 | 0.92 - 1.03 |
| Other | 3.5 | 6.8 | 0.68 | 0.62 - 0.74 |
| Family income*** | ||||
| $0 - $34,999 | 33.6 | 28.6 | 2.24 | 2.10 - 2.40 |
| $35,000 - $74,999 | 27.4 | 27.6 | 1.28 | 1.19 - 1.38 |
| $75,000 and over | 18.6 | 23.8 | 1.00 | |
| Unknown | 20.4 | 20.0 | 1.31 | 1.21 - 1.41 |
| Health care access | ||||
| Usual place for medical care | ||||
| Yes | 92.0 | 82.1 | 0.50 | 0.47 - 0.53 |
| No | 5.9 | 15.8 | 1.00 | |
| More than one place | 1.3 | 1.1 | 1.17 | 0.97 - 1.42 |
| Unknown | 0.8 | 1.0 | 0.69 | 0.53 - 0.90 |
| One or more system barriers** | ||||
| Yes | 11.8 | 9.5 | 2.51 | 2.37 - 2.67 |
| No | 88.2 | 89.5 | 1.00 | |
| Unknown | 1.0 | 1.0 | 0.97 | 0.76 - 1.22 |
| Behavior | ||||
| Smoking** | ||||
| Current | 19.0 | 19.1 | 1.47 | 1.40 - 1.55 |
| Former | 36.9 | 19.8 | 2.03 | 1.93 - 2.13 |
| Never | 43.1 | 61.1 | 1.00 | |
| Other/unknown | 1.0 | 1.4 | 1.01 | 0.81 - 1.26 |
| Physical activity >= 2 times/week** | ||||
| Yes | 47.6 | 53.2 | 1.00 | |
| No | 51.2 | 45.5 | 1.79 | 1.71 - 1.87 |
| Other/Unknown | 1.2 | 1.3 | 0.73 | 0.58 - 0.90 |
| Chronic conditions | ||||
| Arthritis (vs. no)** | 45.9 | 19.3 | 9.18 | 8.74 – 9.65 |
| Asthma (vs. no)** | 10.4 | 7.1 | 2.55 | 2.38 – 2.74 |
| Coronary heart disease (vs. no)** | 12.4 | 3.7 | 6.67 | 6.05 - 7.35 |
| Diabetes (vs. no)** | 15.0 | 7.1 | 4.66 | 4.35 – 5.00 |
| General joint symptoms (vs. no)** | 48.3 | 27.9 | 4.67 | 4.35 – 5.00 |
| Having trouble seeing (vs. no)** | 16.8 | 9.0 | 4.45 | 4.20 – 4.72 |
| Hypertension (vs. no)** | 43.4 | 21.5 | 4.44 | 4.21 – 4.63 |
| Lower-back pain (vs. no)** | 37.7 | 26.3 | 4.63 | 4.44 – 4.82 |
| Myocardial infarction (vs. no)** | 8.8 | 2.8 | 6.81 | 6.09 – 7.61 |
| Other heart disease (vs. no)** | 16.5 | 6.4 | 3.99 | 3.71 – 4.28 |
| Stroke (vs. no)** | 6.7 | 2.1 | 10.19 | 8.86 – 11.72 |
| Body mass index | ||||
| <18.5 | 2.6 | 1.7 | 1.42 | 1.20 - 1.68 |
| 18.5 – 24.9 | 34.4 | 35.7 | 1.00 | |
| 25.0 – 29.9 | 33.4 | 33.5 | 1.37 | 1.30 – 1.44 |
| 30.0+ | 29.6 | 29.1 | 2.58 | 2.46 – 2.72 |
| Nonspecific psychological distress** | ||||
| Yes | 11.1 | 7.8 | 4.44 | 4.14 - 4.76 |
| No | 87.1 | 90.7 | 1.00 | |
| Unknown | 1.8 | 1.5 | 1.63 | 1.37 - 1.93 |
Types of cancers which had been reported by at least 50 respondents: bladder, breast, cervical, colorectal, lung, lymphoma, melanoma, ovarian, prostate, thyroid, uterine.
p<0.05 between survivors and controls
Adjusted for number of family members.
Figure 1.
Lower-body functional limitations (LBFL) by type among long-term survivors and persons without cancer history, United States 2005-2007.
Figure 2.
Distribution of lower-body functional limitations (LBFL) among long-term survivors and persons without cancer history, United States 2005-2007.
Factors associated with increased LBFL among long-term survivors of specific cancer types
The unadjusted prevalence of LBFL varied considerably by cancer type, ranging from a low of 44.9 percent among lymphoma survivors to a high of 88.8 percent among lung cancer survivors (Table 2). When adjusting for sociodemographic factors, long-term breast, cervical, lung, ovarian, thyroid, uterine, and multiple/other cancer survivors had increased odds of reporting LBFL than controls (Model 1, Table 3). Long-term bladder, colorectal, lymphoma, melanoma, and prostate cancer survivors were equally as likely to report at least one LBFL as respondents in the control group after adjusting for sociodemographic factors.
Table 2.
Frequency (unweighted and weighted), prevalence of lower-body functional limitations (LBFL, 95% confidence interval), and unadjusted prevalence odds ratio (and 95% confidence interval) by type of long-term cancer survivor, 2005-2007.*
| Type of cancer |
Unweighted frequency |
Weighted frequency |
Prevalence of LBFL |
95% confidence interval |
Odds ratio** |
95% confidence interval |
|---|---|---|---|---|---|---|
| Bladder | 60 | 177,280 | 51.6 | 36.1 – 66.8 | 2.94 | 1.56 – 5.55 |
| Breast | 583 | 1,372,577 | 60.6 | 56.1 – 64.9 | 4.24 | 3.53 – 5.10 |
| Cervical | 299 | 784,913 | 47.1 | 40.5 – 53.8 | 2.46 | 1.88 – 3.22 |
| Colorectal | 199 | 469,900 | 61.8 | 54.1 – 68.9 | 4.46 | 3.25 – 6.13 |
| Lung | 53 | 125,412 | 88.8 | 77.1 – 94.9 | 21.97 | 9.34 – 51.71 |
| Lymphoma | 77 | 246,780 | 44.9 | 32.6 – 57.8 | 2.25 | 1.34 – 3.79 |
| Melanoma | 197 | 562,446 | 46.5 | 38.3 – 54.9 | 2.46 | 1.71 – 3.36 |
| Ovarian | 95 | 207,565 | 52.8 | 41.3 – 64.0 | 3.09 | 1.95 – 4.92 |
| Prostate | 337 | 904,315 | 59.3 | 52.7 – 65.5 | 4.02 | 3.08 – 5.25 |
| Thyroid | 67 | 201,500 | 57.6 | 42.3 – 71.7 | 3.76 | 2.03 – 6.98 |
| Uterus | 194 | 518,419 | 67.3 | 58.9 – 74.7 | 5.68 | 3.95 – 8.17 |
Types of cancers which had been reported by at least 50 respondents.
Versus persons without cancer
Table 3.
Odds ratios* (95% confidence intervals) for five groups of factors examining odds of lower-body functional limitations among long-term survivors of 11 types of cancer compared to persons without a cancer history, 2005-2007.**
| Model 1: adjusted for socio- demographics |
Model 2: adjusted for sociodemographics and access to care |
Model 3: adjusted for sociodemographics and chronic conditions |
Model 4: adjusted for sociodemographics and health behavior |
Model 5: adjusted for sociodemographics and psychological distress |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of cancer |
Odds ratio |
95% confidence interval |
Odds ratio |
95% confidence interval |
Odds ratio |
95% confidence interval |
Odds ratio |
95% confidence interval |
Odds ratio |
95% confidence interval |
| Bladder | 1.23 | 0.64 – 2.38 | 1.05 | 0.61 – 1.82 | 0.77 | 0.41 – 1.45 | 1.13 | 0.59 – 2.15 | 1.25 | 0.62 – 2.54 |
| Breast | 1.35 | 1.12 – 1.63 | 1.38 | 1.14 – 1.67 | 1.34 | 1.05 – 1.70 | 1.36 | 1.13 – 1.64 | 1.37 | 1.13 – 1.66 |
| Cervical | 1.76 | 1.30 – 2.37 | 1.34 | 0.98 – 1.81 | 1.12 | 0.75 – 1.68 | 1.52 | 1.12 – 2.06 | 1.58 | 1.14 – 2.19 |
| Colorectal | 1.28 | 0.89 – 1.85 | 1.27 | 0.89 – 1.80 | 1.19 | 0.81 – 1.74 | 1.21 | 0.85 – 1.77 | 1.27 | 0.87 – 1.86 |
| Lung | 7.91 | 3.45 – 18.15 | 8.78 | 3.72 – 20.75 | 8.55 | 3.50 – 20.88 | 6.82 | 2.93 – 15.84 | 7.72 | 3.35 – 17.81 |
| Lymphoma | 1.19 | 0.68 – 2.09 | 1.17 | 0.69 – 2.01 | 1.15 | 0.57 – 2.29 | 1.22 | 0.70 – 2.12 | 1.16 | 0.67 – 2.02 |
| Melanoma | 1.31 | 0.94 – 1.83 | 1.18 | 0.82 – 1.69 | 1.21 | 0.78 – 1.90 | 1.35 | 0.96 – 1.88 | 1.30 | 0.92 – 1.83 |
| Ovarian | 1.75 | 1.04 – 2.94 | 1.46 | 0.89 – 2.42 | 1.03 | 0.52 – 2.05 | 1.61 | 0.95 – 2.71 | 1.42 | 0.85 – 2.36 |
| Prostate | 1.24 | 0.93 – 1.64 | 1.29 | 0.98 – 1.69 | 1.38 | 0.98 – 1.94 | 1.24 | 0.94 – 1.64 | 1.28 | 0.96 – 1.69 |
| Thyroid | 2.27 | 1.15 – 4.48 | 2.10 | 0.97 – 4.54 | 2.10 | 1.04 – 4.23 | 2.35 | 1.19 – 4.62 | 2.19 | 1.13 – 4.25 |
| Uterus | 2.41 | 1.63 – 3.58 | 2.11 | 1.40 – 3.18 | 1.50 | 0.94 – 2.40 | 2.18 | 1.49 – 3.20 | 2.69 | 1.54 – 3.33 |
Versus persons without cancer
Types of cancers which had been reported by at least 50 respondents.
For long-term breast cancer survivors, adding the access to care variables (Model 2, Table 3) to Model 1 did not reduce the odds ratio appreciably, suggesting that access to care variables did not explain differences in LBFL prevalence relative to controls. Also, the odds ratio changed little for breast cancer survivors when each of the other three groups of factors was added separately to Model 1 (Table 3). However, adjusting for the presence of arthritis alone in Model 1 showed that breast cancer survivors were equally likely to report at least one LBFL compared to controls (OR: 1.22; 95% CI: 0.99–1.51).
Long-term cervical cancer survivors had increased odds of reporting a LBFL compared to controls (Model 1, Table 3). Adding the access to medical care factors (Model 2) to Model 1 reduced the odds of lower-body functional limitations for cervical cancer survivors compared to controls as did adding chronic conditions. Inability to afford prescription medicines, mental health care, dental care, or eye glasses during the past 12 months and presence of lower-back pain during the past 30 days reduced the odds ratio to 1.18 (95% CI: 0.86-1.62) relative to Model 1 for cervical cancer survivors compared to controls. The three health behaviors (Model 4) and psychosocial distress (Model 5) reduced the odds ratio much less than the access to care and chronic condition variables.
Model 1 (Table 3) shows that lung cancer survivors had increased odds of reporting a LBFL compared to controls. Access to medical care (Model 2), chronic conditions (Model 3), and psychosocial distress (Model 5) did not reduce the odds ratio for lung cancer survivors. The health behavior factors (Model 5) reduced the odds ratio to 6.82 relative to Model 1. This was mainly due to increased smoking among lung cancer survivors (OR: 7.17; 95% CI: 3.11–17.92). However, lung cancer survivors had increased odds of reporting a LBFL relative to controls.
Women with ovarian cancer had increased odds of reporting a LBFL (Model 1, Table 3). The largest reduction in odds ratio relative to Model 1 was observed when adding chronic conditions (Model 3), followed by the psychological distress (Model 5), access to care (Model 2), and health behavior factors (Model 4). The access to care variables responsible for the reduction in odds ratio to 1.45 (95% CI: 0.90–2.34) included the inability to afford prescription medicines, mental health care, dental care, or eye glasses during the past 12 months. Among the chronic conditions, the presence of arthritis alone reduced the odds ratio to 1.32 (95% CI: 0.72–2.43) when added to Model 1. When the presence of arthritis and reduced access to medical care were all added to Model 1, the odds ratio was reduced to 1.14 (95% CI: 0.64-2.04).
Thyroid cancer survivors had increased odds of reporting a LBFL than controls (Model 1, Table 3). The odds ratio for thyroid cancer survivors changed little when adjusting for any of the other factors.
Uterine cancer survivors had increased odds of reporting a LBFL (Model 1, Table 3) than controls. Access to medical care (Model 2), health behavior (Model 4), and psychological distress (Model 5) did not affect this odds ratio appreciably. However, increased presence of arthritis (OR: 1.80; 95% CI: 1.20-2.72) and lower-back pain (OR: 2.00; 95% CI: 1.28-3.11) each reduced the odds ratio relative to Model 1 for uterine cancer survivors. Arthritis and lower-back pain combined reduced the odds ratio to 1.61 (95% CI: 1.01-2.57) for uterine cancer survivors compared to controls when added to Model 1.
Discussion
Among the long-term survivors of 11 types of cancer included in our sample, 57.0% had one or more LBFL compared with 26.6% of controls. LBFL prevalence and associated factors varied by cancer type. There were no differences in LBFL among long-term bladder, colorectal, lymphoma, melanoma, and prostate cancer survivors compared to controls after adjustment for sociodemographic factors. Increased odds of LBFL among long-term lung, uterine, and thyroid cancer survivors were not explained by factors we examined. Increased odds of LBFL among long-term cervical, ovarian, and breast cancer survivors were partly explained by having arthritis, lower-back pain and access to care.
Having arthritis partly explained LBFL among women diagnosed with breast, ovarian, and uterine cancer. Arthritis is one of the most prevalent chronic conditions and one of the leading causes of disability and functional limitations in the general population.24 Interventions to prevent LBFL among cancer survivors might include increasing physical activity to increase muscle strength and for breast cancer survivors, in particular, modifying systemic breast cancer treatment, such as taxanes and aromatase inhibitors, which are known to increase the risk of joint-like symptoms.25, 26 Depressive symptoms also are associated with LBFL among persons with arthritis;27, 28 therefore, diagnosing and treating symptoms of depression early could reduce the risk of developing LBFL among breast, ovarian, or uterine cancer survivors who also have arthritis. Pro-inflammatory cytokines also may play a role in some cancers, arthritis, and LBFL,29, 30 but such information was not available in the NHIS data.
Lower-back pain partly explained the increased odds of LBFL among women with cervical, ovarian, and uterine cancer. Radiotherapy may play a role in low back pain among women with cervical cancer,31, 32 although conflicting reports have been published.33 Pelvic radiation may result in chronic radiation enteritis, symptoms of which include diarrhea, incontinence, abdominal bloating/discomfort and can negatively affect the quality of physical, psychological and social aspects of life among women with ovarian and uterine cancer.34 It also appears that lack of access to medical care continues to be an issue after diagnosis of cervical cancer as it typically is before diagnosis.35
The Institute of Medicine recommends that every cancer survivor has a comprehensive care summary and follow-up plan once they complete their primary cancer care.36 The plan should address post-treatment needs to improve the survivor’s health and quality of life. This plan could include arthritis, lower-back pain, and access to medical care that were associated with an increased odds of LBFL. Primary care physicians and oncologists should ensure that cancer survivors with LBFL benefit as soon as possible from rehabilitation to regain physical functioning by focusing on the impact of arthritis, lower-back pain, and access to medical care.
Limitations of the study include the restriction that only non-institutionalized adults were eligible to participate in the NHIS, so cancer survivors whose physical limitation resulted in institutionalization were not included in this study. Second, because some cancers associated with significant loss of lower-body function also are more rapidly fatal, the association between long-term cancer survivorship and LBFL may be underestimated. Third, due to the cross-sectional study design, in some cases it is difficult to evaluate the temporal direction of the association between factors associated with the presence of LBFL and cancer occurrence depending on the time frame referred to in a question about chronic conditions. However, results about the increased prevalence of functional limitations among cancer survivors were unchanged in two studies when comparing a cross-sectional approach with a longitudinal approach.11, 37 Future studies should use a prospective approach to discern the temporal relationship of arthritis and lower-back pain among women who developed breast, cervical, ovarian, and uterine cancer. Fourth, our analysis relied on self-reported cancer history. Accuracy of self-reported cancer history has been found to vary by cancer type, with breast cancer being reported most accurately and cervical cancer most likely to be underreported.38 As a result, our findings pertaining to LBFL may have been underestimated. Self-reported chronic conditions, however, were of adequate accuracy.39 Fifth, cancer treatment data were not available in the NHIS, and therefore we cannot attribute LBFL to cancer survivors’ treatment. However, we did examine the association between LBFL and common conditions possibly resulting from cancer treatment, such as heart disease, arthritis, joint problems, and asthma. Finally, limitations in pulmonary function and joint mobility or upper body strength also might result in other functional limitations that are not necessarily specific to the lower body. Therefore, overall functional limitations could be even greater than the LBFL reported using the 5-item Nagi scale.
In conclusion, arthritis, lower-back pain, and access to medical care can be targeted to reduce the prevalence of LBFL and improve quality of life of long-term survivors of breast, cervical, ovarian, and uterine cancers, possibly with the aid of a comprehensive assessment and/or survivorship care plan. Additional factors associated with elevated LBFL prevalence should be examined for long-term survivors of lung, thyroid, and uterine cancer.
Supplementary Material
Online Table 1. Prevalence (95% confidence interval [CI]) of lower-body functional limitations by type of cancer, 2005-2007.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior and Outreach Core. This research was supported in part by grants from the National Cancer Institute (CA112159, CA91842). The funders did not have any role in the design of the study; the analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Contributor Information
Mario Schootman, Washington University School of Medicine Departments of Medicine and Pediatrics Division of Health Behavior Research, Box 8504 Saint Louis, MO 63108 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
Rebecca Aft, Washington University School of Medicine Department of Surgery Saint Louis, MO 63110 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110 and John Cochran VA Medical Center, Saint Louis, MO
Donna B. Jeffe, Washington University School of Medicine Departments of Medicine and Pediatrics Division of Health Behavior Research Saint Louis, MO 63108 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
References
- 1.American Cancer Society . Cancer Facts & Figures 2008. American Cancer Society; Atlanta, GA: 2008. [Google Scholar]
- 2.World Health Organization . International Classification of Functioning, Disability, and Health (ICF) Geneva, Switzerland: 2002. [Google Scholar]
- 3.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 4.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 5.Wolinsky F, Johnson R. The use of health services by older adults. J Gerontol. 1991;46:S345–S357. doi: 10.1093/geronj/46.6.s345. [DOI] [PubMed] [Google Scholar]
- 6.Wolinsky F, Miller D, Andresen E, Malmstrom T, Miller J. The effect of sub-clinical status in functional limitation and disability on adverse health outcomes three years later. J Gerontol A Biol Sci Med Sci. 2007;62:101–106. doi: 10.1093/gerona/62.1.101. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 8.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal P. Complications of cancer and cancer treatment. In: Lenhard R, Osteen R, Gansler T, editors. Clinical Oncology. The American Cancer Society; Atlanta, GA: 2001. pp. 231–249. [Google Scholar]
- 10.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98:521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics . Data File Documentation, National Health Interview Survey, 2007 (machine readable data file and documentation) National Center for Health Statistics, Centers for Disease Control and Prevention; Hyattsville, Maryland: 2008. [Google Scholar]
- 13.Miller DK, Wolinsky FD, Malmstrom TK, Andresen EM, Miller JP. Inner city, middleaged African Americans have excess frank and subclinical disability. J Gerontol A Biol Sci Med Sci. 2005;60:207–212. doi: 10.1093/gerona/60.2.207. [DOI] [PubMed] [Google Scholar]
- 14.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003;58:M1119–M1124. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 15.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16:197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Nagi S. An epidemiology of disability among adults in the United States. Milbank Q. 1976;54:439–467. [PubMed] [Google Scholar]
- 17.Schootman M, Andresen E, Wolinsky F, Malmstrom T, Miller J, Miller D. Neighborhood conditions and risk of incident lower-body functional limitations among middleaged African Americans. Am J Epidemiol. 2006;163:450–458. doi: 10.1093/aje/kwj054. [DOI] [PubMed] [Google Scholar]
- 18.Keating N, Nørredam M, Landrum M, Huskamp H, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53:2145–2152. doi: 10.1111/j.1532-5415.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:M82–M91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 20.Balfour JL, Kaplan GA. Neighborhood environment and loss of physical function in older adults: evidence from the Alameda County Study. Am J Epidemiol. 2002;155:507–515. doi: 10.1093/aje/155.6.507. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 22.Kessler R, Berglund P, Glanz M, et al. Estimating the prevalence and correlates of serious mental illness in community epidemiological surveys. In: Manderscheid R, Henderson M, editors. Mental Health, United States, 2002. U.S. Department of Health and Human Services; Rockville, MD: 2004. pp. 155–164. [Google Scholar]
- 23.Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivariate Behav Res. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- 24.Theis KA, Helmick CG, Hootman JM. Arthritis burden and impact are greater among U.S. women than men: intervention opportunities. J Womens Health. 2007;16:441–453. doi: 10.1089/jwh.2007.371. [DOI] [PubMed] [Google Scholar]
- 25.Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223–234. doi: 10.1016/j.breast.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Callender MA, Antonarakis ES. Rheumatoid arthritis masked by docetaxel chemotherapy in a patient with ovarian carcinoma. J Clin Rheumatol. 2008;14:121. doi: 10.1097/RHU.0b013e31816b8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlop D, Semanik P, Song J, Manheim L, Shih V, Chang R. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52:1274–1282. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patten SB, Williams JVA, Lavorato DH, Modgill G, Jetté N, Eliasziw M. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen Hosp Psychiatry. 2008;30:407–413. doi: 10.1016/j.genhosppsych.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Smolen J, Aletaha D. The burden of rheumatoid arthritis and access to treatment: a medical overview. Eur J Health Econ. 2008;8:39–47. doi: 10.1007/s10198-007-0087-9. [DOI] [PubMed] [Google Scholar]
- 31.Bye A, Trope C, Loge JH, Hjermstad M, Kaasa S. Health-related quality of life and occurrence of intestinal side effects after pelvic radiotherapy--evaluation of long-term effects of diagnosis and treatment. Acta Oncol. 2000;39:173–180. doi: 10.1080/028418600430734. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Samsioe G, Iosif C. Quality of life in long-term survivors of cervical cancer. Maturitas. 1999;32:95–102. doi: 10.1016/s0378-5122(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 33.Korfage IJ, Essink-Bot M-L, Mols F, van de Poll-Franse L, Kruitwagen R, van Ballegooijen M. Health-related quality of life in cervical cancer survivors: a population-based survey. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2008.06.1905. In press. [DOI] [PubMed] [Google Scholar]
- 34.Abayomi J. The impact of chronic radiation enteritis on quality of life, following radiotherapy for cervical or endometrial cancer. J Hum Nutr Diet. 2008;21:373–374. [Google Scholar]
- 35.Zapka J, Taplin S, Solberg L, Manos M. A framework for improving the quality of cancer care: The case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 36.Institute of Medicine . From Cancer Patient to Cancer Survivor: Lost in Translation. National Academies Press; Washington, DC: 2005. [Google Scholar]
- 37.Michael Y, Kawachi I, Berkman L, Holmes M, Colditz G. The persistent impact of breast carcinoma on functional health status. Cancer. 2000;89:2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Parikh-Patel A, Allen M, Wright WE, California Teachers Study Steering Committee Validation of self-reported cancers in the California Teachers Study. Am J Epidemiol. 2003;157:539–545. doi: 10.1093/aje/kwg006. [DOI] [PubMed] [Google Scholar]
- 39.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table 1. Prevalence (95% confidence interval [CI]) of lower-body functional limitations by type of cancer, 2005-2007.