Abstract
Posttraumatic stress disorder (PTSD) can result from a traumatic experience that elicits emotions of fear, helpless or horror. Most individuals remain asymptomatic or symptoms quickly resolve, but in a minority intrusive imagery and nightmares, emotional numbing and avoidance, and hyperarousal persist for decades. PTSD is associated with psychiatric and medical co-morbidities, increased risk for suicide, and with poor social and occupational functioning. Psychotherapy and pharmacotherapy are common treatments. Whereas, research supports the efficacy of the cognitive behavioral psychotherapies, there is insufficient evidence to unequivocally support the efficacy of any specific pharmacotherapy. Proven effective pharmacologic agents are sorely needed to treat core and targeted PTSD symptoms, and for prevention. This review describes current and emerging pharmacotherapies that advance these goals.
1.0 Background
Vignettes of behavior in literature from Homer's Iliad to the writings of 19th century physicians, Charcot and Janet, provide credible descriptions of the impact of traumatic stress on the human mind 1-3. However, the behavior described was not acknowledged by the medical community as a distinct clinical entity until 1980, when behavioral changes that follow life-threatening events were first codified into the diagnostic criteria for posttraumatic stress disorder (PTSD) and were included in the Diagnostic and Statistical Manual of Mental Disorders Version III (DSM-III). The original diagnostic criteria were revised in 1987 (DSM-III-R) and 1994 (DSM-IV), but remained unchanged during criteria review in 2000 (DSM-IV-TR) 4. Posttraumatic stress disorder is unique among the mental health disorders in that like infectious disease that requires exposure to a pathogenic vector as well as a vulnerable host to precipitate illness, an external stimulus, i.e. a life-threatening or traumatic event is thought to precede and precipitate the emergence of PTSD symptoms in susceptible individuals unlucky enough to be so exposed. This external stimulus has been incorporated into the DSM diagnostic criteria as Criterion A, defined as having experienced both a traumatic stress (“experienced or witnessed actual or threatened death, injury or threat to the physical integrity of self or others”) and having reacted with “intense fear, helplessness or horror”. In societies where rates of exposure have been measured, the lifetime prevalence of experiencing a traumatic event is reported to be between 17% and 89% 5-13. The DSM-IV definition does not recognize inherent pathogenic differences in the “vector”, e.g. differences in type of trauma exposure or in repeated versus single exposure. However, there is some evidence that duration and intensity of trauma exposure, and the risk for PTSD as a result of that exposure follow a dose-response relationship, and that some types of events are more “pathogenic” than others 8, 9, 14-18.
The behavioral manifestations that result from exposure to a Category A event in vulnerable individuals constitute additional diagnostic criteria for PTSD including intrusive recollections of the traumatic event (Criteria B), avoidance and emotional numbing (Criteria C), and hyperarousal, e.g. irritability, difficulty sleeping and increased startle reactivity (Criteria D). These PTSD symptoms must be present for at least one month (Criteria E) and must cause distress and impairment in various areas of functioning (Criteria F) 4. As defined in DSM-IV, PTSD symptom duration between one and three months is considered to be acute PTSD and duration of three months or longer is considered to be chronic PTSD. The disorder can also be delayed in onset, defined as commencement of symptoms occurring at least six months after trauma exposure. The common lexicon provided by DSM diagnostic criteria has formed a basis for the design and execution of the currently available epidemiological, neurobiological and treatment research on PTSD.
A recent assessment of symptoms following comparable traumatic events, terrorist bombings in Oklahoma City, United States and in Nairobi, Kenya, demonstrated remarkable cross-cultural similarity in PTSD symptom expression 19, 20. The incidence of PTSD by gender across sites was also similar, with females showing higher rates of illness, a finding common to virtually all epidemiological studies of PTSD. Large-scale epidemiological studies of lifetime and 12-month prevalence of PTSD have been completed in Europe, Australia and North America, with data showing lifetime rates of PTSD ranging from 1.9% (Europe) to 6.8% (North America) 5, 10, 18, 21. To date there are no large scale epidemiological studies in areas of the world recently impacted by war and massive natural disasters, e.g. sections of the Middle East, Africa and Asia, where rates of trauma exposure and lifetime prevalence of PTSD may well exceed those found in Europe, Australia and the Americas 22, 23.
In common with infectious vectors, data show that PTSD “vectors”, i.e. rates and types of DSM-IV Category A events, differ by locale and by demographic factors, such as age and gender; this variation is thought to account in part for discrepancies in PTSD lifetime and 12-month prevalence found in the epidemiologic literature 8, 9, 18. There are ongoing attempts to define the relationship between type and number of Category A trauma event(s) and PTSD outcome. Studies have shown that many individuals who have experienced at least one trauma event have experienced multiple events, that adults with a PTSD diagnosis report elevated rates of prior trauma events, and that some types of events appear to be more “pathogenic”, i.e, more highly associated with subsequent PTSD than others 9, 17, 18, 24-28.
However, a recent longitudinal epidemiological study highlights the complexity of the Category A – PTSD outcome relationship by showing that PTSD susceptibility impacts trauma exposure risk and by highlighting the importance of “host” vulnerability/resilience factors for conditional risk for PTSD over time 29. In this study of young adults the original cohort was subsequently interviewed over a 10 year timeframe. The respondents who had developed PTSD from a prior, index event had the highest likelihood of exposure to further traumatic events during the 10 year follow-up period, and respondents who had experienced a prior trauma but did not have PTSD had an intermediate likelihood of trauma exposure when compared to the reference group, subjects with no prior trauma exposure 29. Also, a preexisting diagnosis of major depression was associated with an elevated rate of trauma exposure during the 10-year follow-up period, thus pointing to various “host” characteristics that impact exposure risk and possible PTSD outcome 29. Even after controlling for relevant confounding factors, the conditional risk of PTSD during the 10 year follow-up period was significantly higher among trauma-exposed individuals who had experienced prior PTSD, relative to those who had experienced no prior trauma. In contrast, the conditional risk of PTSD during the follow-up period of individuals who had experienced prior trauma but not PTSD was not significantly elevated 29. These data are consistent with studies that show the genetic contribution to PTSD, which is about 20-30%, to be complex; that the likelihood of exposure to traumatic events is not random, but is influenced by both individual and familial risk factors, some of which increase vulnerability to the exposure of life-threat events, and others that increase PTSD risk in individuals exposed [See also Baker et al. 30 for review] 16, 31-35.
Longitudinal studies have grown in number since the late 1980s, and although they continue to be only a fraction (between 2.5% and 3.1%) of all PTSD research, they have contributed unique information about the natural history of the disorder 36. Specifically these studies have shown that there are a number of post-trauma symptom trajectories and that in fact, consistent with other data showing considerable inter-individual variability in vulnerability/resilience factors, most individuals exposed to a trauma event do not succumb to chronic PTSD. These studies show that post-trauma PTSD symptoms are common, that most trauma-exposed individuals who initially have symptoms show a time-dependent post-event progressive decline in PTSD symptoms, and that individuals who do go on to develop PTSD have a distinctly different symptom course than those who recover 36 37. Longitudinal studies replicate findings from cross sectional and genetic research that show that individual traits such as intelligence, gender, and environmental factors such as childhood adversity are predictors of the vulnerability/resilience profile for chronic PTSD following trauma exposure 36, 38-40.
In individuals who meet criteria for the core PTSD symptoms, co-occurring psychiatric disorders are more common than not, with the vast majority of large epidemiological studies reporting lifetime rates of co-morbid psychiatric disorders at 80% or greater 5, 7, 11. The most common co-morbidities are major depression, other anxiety disorders, and substance use disorders. But other psychiatric disorders, e. g. bipolar disorder and psychoses are also observed at higher rates in individuals with PTSD than without. These co-morbid disorders must be assessed and accounted for in the choice of psychopharmacologic interventions 41-45. Given that prior research has largely been cross-sectional, it is as yet unclear whether co-morbidities that accompany PTSD are more commonly triggered along with PTSD symptoms after exposure to a trauma event, emerge gradually after PTSD development, or are pre-existing conditions that constitute heritable risk factors for vulnerability. A recent longitudinal study that attempts to address this question found that virtually all individuals diagnosed with PTSD at age 26 had prior mental health diagnoses (93% lifetime) 46. Individuals assessed at age 26 who had not yet developed PTSD were then followed prospectively over a four year timeframe between ages 26 and 32; 96% of members of that cohort who developed PTSD had a prior diagnosed mental disorder, 77% before the age of 15 46. However since PTSD had not been assessed and diagnosed in this cohort along with other mental health disorders during the timeframe from birth to age 26, conclusions about whether trauma exposure, and PTSD were primary, or secondary to the co-morbidities was not ascertainable within the research design employed 46.
PTSD is associated with high levels of impairment across social and occupational domains 41, 47. The work loss index and role disability of individuals with PTSD is high, rivaling that of neurological disorders and exceeding that of diabetics 48. Annual productively loss from PTSD in the United States is estimated to be over three billion US dollars 49. In contrast to other anxiety disorders studied, PTSD is independently and significantly associated with both suicidal ideation and suicide attempts after controlling for covariates such as co-morbidity 50, 51.
Studies in which the prevalence of partial, or sub-threshold PTSD was examined found it to be substantial. A large Canadian epidemiological study assessing for current PTSD found the incidence to be 5.0% (women) and 1.7% (men), but the incidence of partial PTSD to be even higher at 5.7% and 2.2% for women and men respectively 52. Individuals with sub-threshold PTSD showed similar levels of social and occupational impairment as those meeting full criteria 52, 53. Functional impairment, rates of co-morbid disorders, and rates of suicidal ideation were shown to increase linearly with increasing number of PTSD symptoms, and individuals with sub-threshold PTSD had increased suicidal ideation even after controlling for the presence of co-morbid major depressive disorder 53.
Current theories or models that provide a framework for conceptualizing PTSD are: 1) A fear conditioning model, which involves an associative link between the aversive event, a psychophysiological response and memory encoding, and the subsequent involuntary activation of the memory in the form of PTSD symptoms 54, 2) A biological theory based on pre-clinical and neuroendocrine studies that show a persistent central hyperarousal, abnormalities in glucocorticoid feedback and inadequate or ineffective stress-system (e.g. serotonin, neuropeptide Y) modulation [see Charney 55 for review] and 3) a psycho-neuroanatomical model, that suggests traumatic recollections are initially encoded as unelaborated emotional memories and later have to be transformed into autobiographical or episodic memories through encoding 56. None of these models provides an etiological explanation for PTSD, but they represent the state-of-the-art currently available to inform rationale for pharmaceutical treatments and development of novel interventions 55, 57. In addition to the above explanatory models, there is an emerging understanding that there is population variability in trauma response as a result of “host” vulnerability/resilience factors. Moreover, there may be critical post-trauma timeframes for the resolution of symptoms, and that if the biological dynamics are fully understood, these timeframes may provide opportunities for interventions that might abort emergence of chronic symptoms. Toward this end, intervention research has been directed at psychotherapeutic and pharmaceutical strategies such as prophylaxis, i.e. prevention and early intervention, as well as at treatments for chronic PTSD 58. Therefore in this manuscript, pharmacologic initiatives for development of drugs for prophylaxis, in the form of prevention and early intervention will be discussed along with the scientific rationale and initiatives for development of interventions for treatment of chronic PTSD.
2.0 Medical Need
In comparison to pharmaceutical development for DSM diagnoses such as depression and psychosis, that were formally codified earlier in the 20th century, the number and breadth of PTSD pharmaceutical treatment studies has been relatively few, and the need for expanded treatment options is especially great. Serotonin re-uptake inhibitors (SSRIs), the most commonly prescribed medications for chronic PTSD are not effective in about 40% of patients, and even in partial responders, additional medications such as sedating antidepressants or sedative/hypnotics are frequently needed to target refractory symptoms, such as sleep disturbance, nightmares, or panic/anxiety symptoms. Moreover, despite being relatively safe, the use of SSRIs can be limited by potential side effects; especially sexual or gastrointestinal side effects or less commonly fatigue, akasthisia or other adverse experiences.
There is a clear need for a broader array of effective medications for chronic PTSD as a whole, as well as for effective medications to target refractory symptoms, such as sleep or panic anxiety. Additionally, development of novel psychotherapeutic and pharmaceutical interventions for early intervention would also be beneficial, as would adjunctive medications that might improve upon the efficacy of currently accepted cognitive behavioral interventions for chronic PTSD, such as cognitive processing therapy (CPT) or prolonged exposure therapy (PE). Unfortunately, drug development in PTSD suffers from a drawback common to that of other mental disorders; whereas there have been spectacular advances in our understanding of the brain and its function over the past half century, the causal neurobiology of these disorders, including PTSD is yet to be elucidated 55, 57.
3.0 Existing Treatment
Within the past three years a number of extensive and systematic reviews of the efficacy of pharmacotherapy and other PTSD treatments have been published including manuscripts from The Institute of Medicine (IOM), the National Institute of England (NICE), and the Australian Center for Public Health), among others 59-62. The entities that reviewed currently used psychotherapies agree that various cognitive behavioral treatments, especially PE and CPT, have proven efficacy in treatment of PTSD. However, adequately powered and well-designed PTSD pharmaceutical studies are few; only 37 were deemed acceptable for review by the IOM and the Cochrane Review. And overall, there is less concordance about the proven efficacy of pharmacotherapy than of psychotherapy; IOM and NICE argue that there is insufficient evidence to show proven efficacy of any drug or drug class, whereas the Cochrane Reviews and some others endorse the use of pharmacotherapy as a first line treatment 59-62. However, the Cochrane Review concludes that there is no clear evidence to show that any particular class of medication is more effective or better tolerated than any other, while noting also that the greatest number of trials, as well as the largest, showing efficacy to date have been with SSRIs 60-62.
In actual practice, medications are commonly used in the treatment of PTSD 63, 64. A review of US Department of Veterans Affairs pharmacy records in fiscal year 2004 revealed that most veterans diagnosed with PTSD received psychotropic medication (80%); and among these, 89% were prescribed antidepressants, 61% anxiolytics or sedative-hypnotics, and 34% antipsychotics 63. Pharmacotherapy is nearly as common in the civilian sector. Data from the Marketscan database, a database that compiles claims from US private insurers show that of adult mental health care users with PTSD, sixty percent received a psychotropic medication. Among those who received medications, 74.3 % received antidepressants, 73.7% received anxiolytics or sedative hypnotics, and 21% received antipsychotics. Having a co-morbid diagnosis was the most robust predictor of medication use for both the veteran and civilian groups, and whereas disease-specific use for both PTSD and co-morbid disorders was common, substantial use seemed to be unrelated to diagnosis, thus was likely to be targeting specific symptoms (e.g. insomnia, anxiety, nightmares, intrusive imagery), rather than diagnosed illness 63, 64. The overall cost of the illness and treatment interventions is difficult to determine, however. Although a large number of mental health economic studies have been conducted, none have focused specifically on interventions for PTSD 65, 66.
Here, we define therapeutic treatments as those that reduce current symptoms, either as a whole, or in a targeted manner, e.g. treatment of insomnia, or nightmares. To date pharmacotherapy has predominantly targeted chronic PTSD; only recently have prophylactic or adjunctive treatments been investigated. Of the published pharmaceutical trials, only 37 randomized clinical controlled trials (RCTs), all of which target chronic PTSD symptoms, were deemed of sufficient quality for review by the IOM and Cochrane Reviews 61, 62. Drug classes reviewed include antidepressants, e.g. SSRIs, monoamine oxidase inhibitors, tricyclics, as well as alpha-adrenergic blockers, anticonvulsants, benzodiazepines, novel antipsychotics, and others such as d-cycloserine and inositol 61, 62.
Most of the compounds in the RCTs reviewed are thought to act by dampening the generalized arousal and anxiety experienced by PTSD patients 67. Below, we review some of these therapeutic classes. Later in the manuscript, under scientific rationale we also review some novel agents under investigation in pre-clinical or early clinical trials that may ultimately prove valuable as pharmacologic agents if brought to market.
3.1 Serotonin reuptake inhibitors
The putative mechanism of action of SSRIs is the blockade of the reuptake of serotonin in certain synapses in the brain. As with most currently used pharmaceutical agents, investigation of their efficacy in PTSD was initiated after studies showed their efficacy in depression and other anxiety disorders. Data from studies of two SSRIs, sertraline and paroxetine have been submitted to the Federal Drug Administration (FDA), and the data were deemed adequate to gain indication for use of these SSRIs in treatment of chronic PTSD 68-71. In practice, SSRIs are typically the first line treatment, and the various SSRIs, e.g. sertraline, paroxetine, citalopram, fluoxetine, escitalopram are often prescribed interchangeably for treatment of chronic PTSD based on factors, such as cost and side effect profile. Some PTSD symptoms, such as irritability appear to respond better to SSRIs than others, such as sleep disturbance. Often sexual side effects are limiting, especially in young men. SSRIs have not shown uniform efficacy across studies; symptom improvement has varied across different populations and/or trauma types, e.g. by gender and between civilian and veteran cohorts 62,72. In a recently published study in which an SSRI was prescribed as an adjunct to enhance response to psychotherapy, it showed no added treatment benefit 73.
3.2 Monoamine oxidase inhibitors
Monoamine oxidase inhibitors (MAOI) act by preventing the breakdown of monoamine neurotransmitters and thereby increase their availability. There are four RCTs examining the effects of two MAOIs, in the treatment of PTSD, phenelzine, a non-selective MAOI and brofaromine, a selective MAOI-A inhibitor 6, 74-76. The studies, which showed mixed efficacy, were small and of variable design quality. Thus interpretability of their efficacy is difficult. In practice, MAOIs are rarely prescribed for use in PTSD because of fears of the side effect profile of these drugs. Selegiline, the reversible and relatively selective MAOI-B inhibitor that can be delivered via a transdermal patch, would potentially circumvent some of the dietary side effect concerns. A meta-analysis of clinical trials of seleginine in dysthymia and major depression failed to show efficacy despite positive attributes, such as neuroprotective effects. Its efficacy has not been studied in PTSD.
3.3 Novel Antipsychotics
Clinically, antipsychotic medications are frequently used as adjunct treatment in civilian and veterans with PTSD, especially in patients with mental health co-morbidities or who have refractory symptoms, e.g. sleep disturbance 63, 64. Serious adverse effects, e.g. tardive dyskinesias, weight gain and altered glucose tolerance are potential limiting factors, especially since chronic PTSD may be associated with metabolic syndrome and risk for cardiac disease 77-79. There have been eight published RCTs of two different antipsychotics, risperidone and olanzepine, in PTSD 80-87. The studies were small, of variable design quality and have generally focused on special features of PTSD, such as refractoriness to treatment or associated psychotic features 81, 83, 85. In six trials of risperidone it was used as an augmentation to other medications, rather than as a primary treatment. All except one of these studies showed risperidone to be effective used in this way 80-84, 87. Based on these findings, a large, multi-site trial of risperidone is now underway in the United States in veterans to determine its effectiveness as an adjunct treatment in individuals with treatment resistant, chronic PTSD.
3.4 Anticonvulsants
Anticonvulsants are used in PTSD to treat co-morbid disorders, such as bipolar affective disorder (BAD), or to target symptoms such as impulsive anger. BAD is observed at higher rates in individuals with PTSD; the combination of disorders is especially destabilizing; and unfortunately the co-morbidity is easy to miss clinically. Anticonvulsants, e.g. valproic acid, are generally effective in restoring emotional stability and improving social functioning when prescribed to patients with PTSD and BAD. Unfortunately there are no RCTs of the use of anticonvulsants in co-morbid PTSD-BAD. A study of valproate in the treatment of impulsive aggression that included PTSD subjects had insufficient numbers for analysis of efficacy in treating core PTSD symptoms 88. More recently, a study of valproate in the treatment of core PTSD symptoms showed no efficacy 89. RCTs of three other anticonvulsants, tiagabine, topiramate, and lamotrigine have been completed with variable results, negative for tiagabine and topiramate and positive for lamotrigine, although the lamotrigine study was too small (n = 15) to show statistical significance or to estimate an effect size 89-92.
3.5 Alpha adrenergic blocking agents
There are two small RCTs and a cross-over study of prazosin, an alpha1-adrenergic blocker showing suggested efficacy for nightmares and sleep disturbance in PTSD 93-95. The goal of these studies was not evaluation of overall PTSD symptoms, but evaluation of targeted symptoms, which showed good outcomes, e.g. a reduction in nightmares, and polysomnographic evidence of an increase in total sleep time, REM sleep time, and mean REM period duration, without sedative-like effects. High doses of prazosin are sometimes required; limiting side effects include orthostatic hypotension and sometimes headache. Based on findings from these smaller studies, a large, multi-site trial of prazosin is now underway in United States veterans. An RCT of guanfacine, an alpha- blocking agent failed to show efficacy for global or targeted symptoms of adults with PTSD 96.
3.6 Benzodiazepines
Clinically, benzodiazepines are sometimes used to target panic/anxiety symptoms, or to target sleep difficulties. Given the high co-morbidity of PTSD with substance abuse disorders, a complete assessment of co-morbidities and a consideration of the addictive potential of the benzodiazepines being prescribed are necessary. There are few RCTs of the use of this class of drugs in PTSD; one a clinical trial of alprazolam in treatment of chronic PTSD, and the other for use of the benzodiazepines, alprazolam or clonezepam, in the prophylaxis of PTSD 97, 98.
3.7 Other antidepressants
When SSRIs fail to alleviate PTSD symptoms or when depressive symptoms accompanying PTSD are unresponsive, the common practice is to prescribe other antidepressants, including dual serotonin reuptake inhibitors, tricyclic and tetracyclic antidepressants, or atypical antidepressants, e.g. buproprion. These antidepressants are variably effective, and are limited by side effects common to their class. Unfortunately RCTs of these antidepressants in the treatment of PTSD are few; there are three trials of tricyclics, two of venlafaxine, one of mirtazepine and one of nefazodone 62. All RCTs show modest improvements in PTSD symptoms, but all are small or have limiting design and/or data handling issues that limit their interpretability. For example, in two studies of venlafaxine that show some improvement in symptoms, dropout rates exceeded 30% 99, 100
3.8 Miscellaneous
Small RCTs of d-cycloserine and inositol to treat global symptoms of chronic PTSD showed no efficacy. There are a number of ongoing trials of the use of d-cycloserine as an adjunct treatment for PE. See scientific rationale for greater detail.
4.0 Current Research Goals
Research into novel PTSD treatment strategies have been directed at a number of different goals. These include: 1) Mitigation of global reduction of symptoms and general anxiety levels in individuals with chronic PTSD, predicated upon normalizing putative underlying pathological processes, 2) Mitigation of targeted symptoms, e.g. sleep, nightmares, irritability, predicated upon modifying biological processes driving those symptoms, 3) Use of medication as an augmentation strategy to various types of psychotherapy, e.g. as an additional treatment to enhance reduction in global symptoms 101, or as an adjunct to improve the learning expected to take place in exposure therapy 102.
Adjunctive treatments are defined in this manuscript as synergistic treatments that aid efficacy and response time to other pharmacological treatments or psychological therapies, in particular exposure therapy, such as PE designed to facilitate the extinction of fear memory 103. Rothbaum and Davis describe PTSD as a disorder characterized by a “failure of fear extinction after trauma” 104. In animals and humans, a conditioned fear association occurs when a conditioned stimulus (CS) and an aversive unconditioned stimulus (US) are presented in close temporal proximity. Thus the subject learns that the CS “predicts” the occurrence of the US. In the case of PTSD, environmental cues during trauma are associated with the pain and fear of the traumatic event, and these cues continue to evoke strong fear reactions long after the initial trauma has receded. In the laboratory this phenomenon is modeled in humans and animals by pairing a tone or light with noxious stimuli such as an electrical shock. Once the association between the CS and US has been learned, the presentation of the CS alone will invoke a conditioned fear response (e.g. autonomic activation, exaggerated startle response, avoidance behavior). The phenomenon of fear extinction occurs when the learned CS is then presented without the occurrence of the US, hence the subject learns that the CS no longer predicts the presence of the US and subsequent fear responses to the CS are inhibited or “extinguished”. It is this phenomenon that is hypothesized to be disrupted in PTSD patients, which continue to show pronounced signs of anxiety, avoidance, and arousal in response to trauma reminders.
Research on the biological basis of fear extinction has exploded [see Quirk and Mueller 105 for review]; however, in this manuscript we will focus on systemic studies of drugs that facilitate extinction learning.
As noted in the background section, there is an emerging awareness that pharmacologic interventions given in the immediate or acute post-trauma timeframe may be effective as a prophylaxis, in preventing symptom consolidation and aborting the development of chronic PTSD. Conceptually there are two intervals: the immediate (0-48 hour) and the acute (within a few weeks post trauma) periods when prophylactic interventions are indicated 58. However, ethical issues emerge in the use of both psycho- and pharmaco-therapy as prevention/early intervention strategies. Given our current inability to easily and accurately predict symptom trajectory, the specific benefit/risk ratio for any specific treatment must be carefully considered. Since prevention and early intervention is hampered by incomplete knowledge about host factors that predict trajectories of adaptation to various traumas and the biological underpinnings of that adaptation, longitudinal studies of these phenomena are sorely needed 58. Moreover, the ethical issues of memory blockade (especially when legal proceedings will follow the traumatic event) have also produced a flurry of debate [See Henry et al. 106 and letter responses]. Some drugs that are being evaluated as prophylactic treatments have proven amnesic properties (e.g. ketamine), while others have been used in the clinic for many years with low cognitive side effects (e.g. propranolol).
Presently, the prime rationales for intervention in the acute aftermath of trauma have been to either dampen arousal and/or enhance biological resilience. Many stress systems (e.g. norepinephrine, CRF, corticosterone) have been shown in preclinical studies to strengthen fear memories; hence have been considered targets for inhibitors to attenuate memory strength without cognitive impairment. Drugs given for early intervention would theoretically be effective if they aid in reducing fear memory consolidation and/or attenuate strong coupling of the memory with intense physiological responses 107. Certainly there are individual differences in how people respond and adapt to traumatic stress, and understanding these mechanisms may help us develop novel treatments. Toward that end, recent work has focused on potential epigenetic contributors to the biological plasticity underlying fear learning and extinction 32, 108, 109. Based on the pre-clinical work demonstrating that chromatin-modifying enzymes may play a role in fear memories, Nestler and his colleagues postulate that post-trauma resilient behavior represents a distinct, active neurobiological process 32, 110. If these epigenetic mechanisms prove correct, they may offer viable targets for drug development. Post-trauma resilient behavior may also depend on modulatory neurochemicals and neuropeptides, e.g. serotonin, neuropeptide Y and others. For example, there is some support that mutations in the human serotonin transporter gene (SLC6A4), located in the 5′ region (5 HTTLPR) are associated with increased risk for depression and anxiety disorders 111-116, anxiety traits 117, differential acquisition of conditioned fear and increased amygdala excitability 118, 119, and that they may be associated with measures of resilience 120 and vulnerability to PTSD 121, 122.
5.0 Scientific Rationale
In contrast to the section “existing treatment”, and the table in the section “competitive environment”, we focus this section is on scientific rationale in support of novel treatment targets currently limited to preclinical testing and/or early proof of principle clinical trials.
5.1 Steroids/Glucocorticoids
Glucocorticoids serve as modulators of the corticotropin releasing factor (CRF) signal, positively increasing CRF in limbic circuits in response to environmental stress 123, 124. Although there is a large literature on glucocorticoids and the HPA axis in PTSD, the literature remains controversial and conflicted. There is some evidence for abnormalities including peripheral hypocortisolemia and enhanced hypothalamic-pituitary-adrenal (HPA) axis negative feedback but findings are not consistent, consequently the authors of a recent review suggest the need for cerebrospinal fluid (CSF) studies 125-127. Baker et al. have shown normal plasma, but elevated cerebrospinal fluid (CSF) cortisol concentrations in chronic PTSD, as well as a positive correlation between CSF CRF and CSF cortisol concentrations 128. These findings have yet to be replicated. However, based on preclinical models and upon clinical hypotheses, both glucocorticoid agonists and antagonists have been proposed.
5.1.1 Agonists
Pre-clinical models suggest that ability to mount a glucocorticoid response to stress may predict resilience to PTSD 129. Data from some human studies show plasma cortisol levels to be negatively correlated with PTSD symptom severity 130. Hypothesized mechanisms underlying possible glucocorticoid facilitation of resilience are via either facilitation of extinction or impairment of fear memory retrieval, given that glucocorticoid receptor agonists increase extinction and impair fear memory recall in rodent models of fear learning 131, 132. Moreover surgical patients given preoperative hydrocortisone showed a reduction in PTSD symptoms post operatively 133, providing clinical support to the hypothesis that cortisol release during trauma may be important for preventing chronic PTSD. A very small double-blind placebo controlled cross-over study (n=3) also showed some promising effects of hydrocortisone in reducing trauma memories 134, which may be related to its effects in blocking memory recall or fear extinction 135. Hydrocortisone treatment is currently under examination in at least 3 clinical trials as adjunctive treatment with extinction based therapies (clinicaltrials.gov).
5.1.2 Glucocorticoid Antagonists
In rodents glucocorticoid antagonists have been shown to disrupt fear memory consolidation when given immediately after training, hence in theory they have prophylactic utility 136, 137 when given immediately after trauma. We must be cautious however as glucocorticoids have been shown to facilitate extinction in pre-clinical studies as indicated above 131, thus the post trauma timeframe in which these treatments are being administered, immediately after trauma or during the extinction phase of trauma, may be crucial 135. Based on the administration timeframe, they may be beneficial or harmful. Small studies (n = 30) of psychotic depression and bipolar disorder thus far using a non-selective glucocorticoid receptor antagonist Mifepristone (RU-486), otherwise known as the morning after pill, are reported to show promise in reducing cognitive disruption and psychosis 138, 139. There are currently 4 listed clinical trials for mifepristone treatment in PTSD, with one recently completed. Outstanding issues in regard to use of glucocorticoid antagonists for PTSD remain. These include: 1) Their site of action, i.e. the pituitary versus the brain, has not been clarified. 2) The findings from these small depression studies are yet to be replicated in large, or multi-site trials. 3) Lastly, there are no published reports of the efficacy of glucocorticoid antagonists in PTSD.
5.2.0 Glutamate signaling
5.2.1 Ionotropic glutamate receptor ligands
Preclinical studies have demonstrated that glutamate transmission in the amygdala is necessary for fear extinction (FPS). [For review see Meyers and Davis 140]. Much research has centered around the finding that d-cycloserine (DCS), a partial NMDA (N-methyl-D-aspartic acid) receptor agonist acting on the glycine modulator site, significantly enhances extinction when given during or immediately after extinction training 141, with a recent meta-analysis across animal and human literature confirming the DCS effects in enhancing extinction across multiple studies 142. Some caution must be taken, as one preclinical study indicates that DCS may facilitate reconsolidation when the subject is given a limited number of exposures 121. Thus more research is required in understanding the critical factors (number of sessions, dose, time of treatment, most responsive population) that impact the DCS efficacy as adjunctive therapy 143-145. DCS has shown some efficacy as an adjunctive treatment in certain anxiety disorders [For review see Norberg et al.142], but it is also important to note that based on the clinical findings thus far, DCS may not be effective under all circumstances (e.g. over prolonged treatment periods, in sub-clinical populations, when long intervals are between therapy sessions) 142. Despite the high level of interest, to our knowledge, no study has yet been published on DCS efficacy in exposure therapy for PTSD although many are ongoing. There is also still much research needed to identify treatment and therapy strategies that optimize efficacy of behavioral therapy for PTSD 146.
The NMDA receptor antagonist ketamine has been proposed as a possible acute treatment for distress and depression in PTSD patients. This hypothesis is based on some efficacy in small trials of depression showing rapid depression reduction lasting up to 1 week post infusion 147, 148. Like other non-competitive NMDA antagonists, ketamine interferes with memory consolidation 149, thus might be useful as an initial acute treatment for early PTSD. Currently there is at least one proof of principle clinical trial underway to test the utility of this drug class (Clinical trial identifier NCT00749203) in PTSD. However, others have questioned the risk/benefit of the competitive NMDA class as treatments due the side effect profile of drugs of this class including ketamine, which at higher doses includes neurotoxicity, psychosis and cognitive disruption 150. Other NR2B receptor subunit antagonists are also under investigation, e.g. CP101,606, HON0001, with the hope of mitigating these potential side effects [For review see Layton et al. 151]. Other possible glutamatergic cognitive enhancers that may benefit extinction therapy are positive allosteric modulators of AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptors 152. However, such compounds require much more preclinical research and confirmation to support their use in humans.
5.2.2 Metabatropic glutamate receptor ligands
Overall, reductions in the glutamate signal have also been a potential targets for anxiolytic effects 153. Such reductions can occur via activation of pre-synaptic regulatory receptors such as metabatropic glutamate 2/3 receptors (mGluR2/3) or blockade of post synaptic mGluR5 receptors 153. The mGlu2/3 receptors are group II metabotropic receptors that when activated inhibit adenylate cyclase and suppress synaptic excitability via pre- and post-synaptic mechanisms 154. These receptors are highly expressed in the cortex, amygdala and hippocampus, and thus are likely modulators of anxiety and fear responses.
In the late 90's the mGluR2/3 agonist LY35450 was shown to inhibit anxiety-like behavior in a number of rodent models 155 as well as to block the neuroendocrine response to stress 156. In rodents and in non-anxious humans, acute LY345740 treatment has been shown to block expression of learned fear 155, 157. mGlur2/3 agonists may reduce anxious responses via inhibition of basolateral amygdala output 158. The potent blockade of learned fear behaviors and basolateral amygdala signaling suggests it may be useful for disorders like PTSD that involve contributions of cued fear responses to symptom provocation. Small proof of concept studies using a pro-drug of LY34570, LY544344, had shown promising results in GAD patients, however, pre-clinical findings of reduced seizure threshold have set back the development of these compounds 159.
Preclinical data over the last 10 years have shown clear evidence that mGluR5 antagonism or genetic deletion produces-anxiolytic-like effects on rodent behavior, both in unconditioned and conditioned stress models 160. Fenobam is an inverse agonist for the mGluR5 receptor which has shown preliminary anxiolytic effects in early clinical trials, however it also produced some perceptual changes including hallucinations and vertigo, thus was dropped from further development 161. Preclinical studies have indicated that mGluR5 antagonists may have psychotomimetic effects as they potentiate NMDA receptor antagonist induced disruptions in sensorimotor gating, and reduce social isolation 162. However, a recent small non placebo controlled crossover study in Fragile X patients showed that fenobam treatment produced small but significant increases in sensorimotor gating, suggesting that underlying pathology may dictate the effects of fenobam on perceptual indices 163. Although these results are intriguing, the adverse effect profile of mGluR5 agonists both pre-clinically and clinically suggest caution. Much more data are needed before testing in PTSD patient populations, which may be particularly sensitive to pro-psychosis side effects.
5.3.0 Monaminergic signaling
5.3.1 Reduction of Sympathetic arousal
Preclinical data have long suggested that noradrenergic activation strengthens emotional memory, thus leading to the hypothesis that noradrenergic blockade may help uncouple emotional memory from its strong physiological responses 164. A number of agents are currently under investigation in clinical trials.
Propranolol
Initial un-blinded pilot studies supported the potential use of propranolol as a preventative treatment 165, 166. However thus far, a larger double blind randomized placebo controlled study in post trauma surgical patients has not found propranolol to be effective as a preventative treatment for either depression or PTSD 167. A recent small study showed efficacy to reduce physiological symptoms as adjunctive therapy in script driven trauma imagery 168, which is supported by its capacity in preclinical models to block fear memory reconsolidation 169. There are currently 9 listed clinical trials (clinicaltrials.gov). Three studies for propranolol use as a preventative or therapeutic treatment for PTSD are listed as completed as of Jan 2009, thus we will know much more about the utility of this compound soon.
Dopamine-beta hydroxylase is an enzyme involved in catecholamine synthesis that is critical to conversion of dopamine to norepinephrine. Plasma DBH has been shown to be lower in subjects with PTSD but not combat controls 170. In a smaller study, PTSD subjects with psychosis were found to have higher plasma DBH than PTSD subjects without 171. Thus it is not clear if DBH abnormalities contribute to PTSD. However, due to the possible increased responsiveness of PTSD subjects to noradrenergic activation [e.g. see Southwick et al. for review 172], Nepicastat, a potent, selective, orally active inhibitor of dopamine-beta-hydroxylase is currently under study in a multisite trial for efficacy in treatment of chronic PTSD.
5.4 Neuropeptides
6.4.1 Neuropeptide Y (NPY)
Neuropeptide Y acts on a number of receptors, Y1, Y2, Y3, Y4 and Y5 that exert a number of physiological effects including anxiolysis, modulation of feeding and cardiovascular function. NPY signaling may be reduced in PTSD subjects 173. In rodents, NPY appears to counteract anxiogenic effects of corticotropin releasing factor, which is observed to be excessively released in PTSD patients compared to controls 174. In rats, central infusions of NPY have been shown to facilitate extinction, which may be via Y1 receptor activation since Y1 receptor antagonists are known to disrupt extinction 175. Given the evidence supporting anxiolytic effects of Y1 receptor activation 176 these data give preliminary support for further research and development of a Y1 agonist for use in the treatment of PTSD symptoms as well as a prophylactic agent to foster resilience to trauma. One possible drawback to Y1 agonists as a chronic treatment however could be weight gain due to possible orexigenic side effects 177. Although there are selective peptide Y1 agonists, no small molecule Y1 agonists have been reported to our knowledge.
5.4.2 CRF
PTSD patients have been shown to exhibit excess CRF CSF concentrations 174, 178, 179. Exactly which specific symptoms of PTSD may be most reflected in these CRF alterations is unclear, however. None of these studies found a clear relationship between CRF levels and clinically rated PTSD symptoms. Although it is generally presumed that the observed CRF hypersecretion in PTSD patients occurs only after trauma, this assumption has yet to be tested. It is also possible that excess CRF release is a predisposing factor for PTSD, similar to findings of reduced hippocampal volume in twin studies of PTSD 180. Indeed some pre-clinical studies (see below) indicate CRF receptor activation enhances fear learning, hence individuals with high CRF tone might develop stronger trauma related memories than those with lower CRF release.
Based on evidence for CRF dysfunction in depression and anxiety patients, CRF1 and CRF2 receptors, as well as the CRF binding protein, which may modulate ligand availability for receptor signaling, have all been proposed as pharmacotherapeutic targets for depression and anxiety disorders 181-183. Potent, orally active small molecule CRF1 antagonists have now been developed and are ready for human investigation 184. Thus far clinical trials in depression have been disappointing. Although early, small clinical trials 185 were promising, more recent double blind placebo controlled studies for depression have been disappointing 186. The side effect profile of these compounds is also a consideration; some compounds have been associated with liver damage. Given that CRF release is likely to be strongest immediately after trauma, and it is known to potentiate fear memory acquisition, it is possible that CRF1 antagonists may be effective as a prophylactic treatment.
5.4.3 Oxytocin
The neuropeptide oxytocin is a nine amino acid peptide made in the hypothalamus and released into the blood from the posterior lobe of the pituitary gland. Oxytocin is synthesized in neurons in the hypothalamus that send processes to a variety of brain regions including the amygdala, which express some of the highest concentrations of oxytocin receptors in the nervous system 187. In multiple clinical studies 188, 189 190 presentation of fear stimuli to normal patients, including aversive pictures such as emotional facial expressions, increased activity in the amygdala. This hyper-activation was blocked by nasal administration of oxytocin. Preclinical studies have shown that oxytocin may have anxiolytic effects as well as attenuate learned fear behaviors 191, 192. A small study (n=15/group) in Vietnam veterans with PTSD showed some promise for intranasal oxytocin to reduce measures of arousal during exposure to personal trauma scripts 193. Oxytocin is only 3 fold more potent at oxytocin versus vasopressin receptors, however, and prolonged stimulation of peripheral vasopressin receptors leads to antidiuresis, increasing the risk for hyponatremia and diminishing the therapeutic utility of this drug over time 194. These studies suggest that development of highly selective oxytocin receptor agonists may produce therapeutic efficacy in treating PTSD and avoid the pronounced side effects of oxytocin itself. Oxytocin selective agonists are currently being developed by a number of companies.
5.5 Cannabinoids
The endogenous cannabinoid (CB) system, in particular the CB1 receptor, represents a potential therapeutic target for the treatment of a variety of learned fear-related disorders, including PTSD 195. CB1 agonists have been shown to facilitate extinction while CB1 antagonism or gene deletion disrupts extinction 196 197 198. However, there may be less of an effect if there is previous experience with CB1 agonism or under chronic treatment regimens 199. Hence, like DCS, research on the most effective parameters and design regimens are necessary to better understand if these drugs show promise for facilitating extinction therapies.
6.0 Competitive Environment
Although there are few RCTs of compounds for PTSD, and yet fewer in which design and study execution allow for clear interpretation of results, nearly every class of potential compounds active in the central nervous system has been tried clinically and/or is represented in open label trials or case reports. Discussion of compounds in this manuscript is limited to those with published RCTs (Existing Treatment), or are compounds in active pre-clinical development or in early clinical trials (Scientific Rationale). Table 1 includes drugs currently marketed. Table 2 lists compounds under development.
Table 1.
Marketed drugs with potential utility in PTSD
| Compound | Company (originator) |
Structure | Mechanism of action | Side effects | Phase | Comments | Indication |
|---|---|---|---|---|---|---|---|
| Serotonergic | |||||||
| Sertraline | Pfizer | 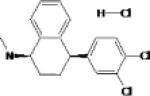 |
Selective serotonin reuptake inhibitor (SSRI) Minor inhibitor of dopamine reuptake |
Highest rate of nausea and diarrhea of SSRIs Headache Sexual side effects |
Marketed | FDA approved for PTSD Only medication approved for long term treatment of PTSD |
Posttraumatic stress disorder Major depression Social anxiety Panic Disorders Premenstrual syndrome Obsessive Compulsive Disorder |
| Fluoxetine | Eli Lilly & Co. | 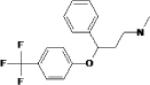 |
Atypical serotonin reuptake inhibitor (SSRI) Weak inhibition at the dopamine transporter |
Nausea, Diarrhea, Headache, Sexual side effects Dopiminergic action may contribute to activation, akasthesia |
Marketed | Commonly used in PTSD Long half-life and nonlinear kinetics FDA approved for children age 8 and over |
Major Depressive Panic Disorder Bulimia Premenstrual syndrome Obsessive- compulsive disorder |
| Paroxetine | Novo Nordisk | 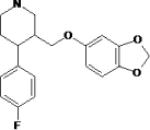 |
Most potent selective serotonin reuptake inhibitor (SSRI Weak inhibitor of norepinephrine reuptake |
Nausea, Diarrhea Headache Sexual side effects Sexual side effects, Rare fatigue somnolence |
Marketed | FDA approved for PTSD FDA warning may increase rate of birth defects taken in 1st trimester |
Posttraumatic stress disorder Depressive and Anxiety Disorders Obsessive- compulsive disorder Premenstrual syndrome |
| Citalopram hydrobromide |
Lundbeck | 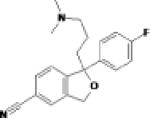 |
Selective serotonin reuptake inhibitor (SSRI) |
SSRI side effects may be fewer than with other SSRIs |
Marketed | Used in PTSD Overdose concerns; seizures and QT interval prolongation |
Major depression, |
| Escitalopram | Forest Labs and Lundbeck |
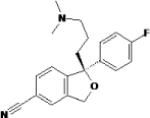 |
Newest and most selective SSRI Pharmacologically active S-isomer of citalopram |
SSRI side effects may be fewer than with other SSRIs including citalopram |
Marketed | Relatively fast onset of action Poster presentation ISTSS, 2005: Similar PTSD efficacy compared to sertraline |
Major depression, general Generalized anxiety disorder Obsessive- compulsive disorder (registered) |
|
Dual reuptake intake |
|||||||
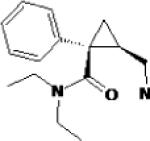
| Duloxetine | Eli Lilly & Co. | 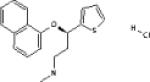 |
Potent serotonin and norepinephrine reuptake inhibitor Weak dopamine reuptake inhibitor |
High rates of nausea during treatment initiation Insomnia Weight gain Increase in eye pressure |
Marketed | Beneficial effects on physical pain PTSD clinical trial in progress |
Major depression, Neuropathy, diabetic Generalized anxiety disorder Fibromyalgia (filed) Stress urinary incontinence (approved, Europe) |
| Venlafaxine | Wyeth Pharmaceu- ticals |
 |
Potent serotonin and norepinephrine reuptake inhibitor Weak dopamine reuptake inhibitor |
Nausea Vomiting Can increase HR, BP and eye pressure |
Marketed | Extended release formulation Significant treatment withdrawal symptoms |
Major depression Generalized anxiety disorder Social anxiety Panic Disorder |
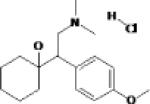
| Milnacipran | Pierre Fabre Forest |
 |
Potent norepinephrine and serotonin reuptake inhibitor: 3/1 ratio |
Nausea Vomiting Can increase HR, BP and eye pressure |
Marketed | Beneficial effects on physical pain Antidepressant efficacy similar to SSRIs |
Fibromyalgia Depression, bipolar (Phase II) Major depressive disorder (Phase II) |
| Monominergic | |||||||
| Prazosin | Pfizer | 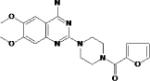 |
Selective alpha-1 adrenergic blocking agent |
Orthostatic hypotension Syncope Headache Additive hypotensive effects |
Marketed | Possible efficacy for nightmares and sleep in PTSD Large multi-site trial underway |
Hypertension Heart failure |
| Mirtazapine | 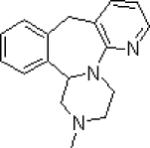 |
Alpha-2 adrenergic blocking agent Also blocks 5HT2 and 5HT3 receptors Potent antagonist at peripheral and central histamine receptors |
Sedation at lower doses Weight gain Headache Additive hypotensive effects |
Commonly used in PTSD Efficacy in PTSD not well studied |
Major Depression | ||
| Propranolol | 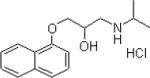 |
Nonselective, beta- adrenergic blocking agent |
Sedation at lower doses Lethargy Bradycardia Rare mental disturbance including hallucinations |
Propranolol has been proposed as a PTSD prevention agent: studies are ongoing |
Hypertension Angina Pectoris Other cardiac indications |
||
| Steriod | |||||||
| Mifepristone | Exelgyn |  |
Glucocorticoid and progesterone receptor antagonist |
Nausea Vomiting Abdominal pain |
Marketed | There are ongoing clinical trials of glucocorticoid antagonists in depression; their use has been proposed in PTSD Their access to the CNS has not been determined |
Medical abortion |
|
Anti-convulsant /mood stabilizers |
|||||||
| Lamotrigine | Glaxo-Smith Kline |
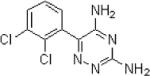 |
Inhibits voltage sensitive Na channels, thereby stabilizing membranes Modulation of pre-synpatic release of excitatory amino acids |
Nausea Dizziness Double vision Headache |
Marketed | Has mood stabilizing properties Rare adverse event: Stevens Johnson Syndrome Positive effect in one small (n=15) clinical trial in PTSD |
Partial onset seizures Primary generalized tonic- clonic seizures mono- or adjunct therapy Lennox Gastaut Syndrome seizures Migraine prophylaxis |
| Valproate | Abbott Laboratories |
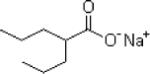 |
Blockade of voltage gated Na channels and T-type calcium channels Enhances GABA effects through GABA transaminase inhibition Also inhibits histone deacetylase |
Nausea Sedation Tremors Weight gain |
Marketed | Has mood stabilizing properties May be useful in PTSD with co- morbid BAD Contraindicated in pregnancy |
Partial onset seizures Primary generalized tonic- clonic seizures mono- or adjunct therapy Lennox Gastaut Syndrome seizures Migraine prophylaxis |
| Antipsychotics: | |||||||
| Risperidone | Johnson & Johnson |
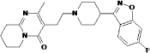 |
5-HT2A and dopamine D2 antagonist |
Somnolence Extra- pyramidal symptoms More rarely later state depressive symptoms |
Marketed | Possible benefit as adjunctive treatment in chronic PTSD Large multi-site trial underway Metabolic effects (increased blood glucose, weight gain) common to atypical antipsychotics |
Schizophrenia Acute mania Mixed manic episodes Autism, irritability |
Table 2.
Compounds in development for PTSD
| Compound | Company | Structure | Mechanism of action | Side effects | Phase | Comments | Indication |
|---|---|---|---|---|---|---|---|
| Nepicastat | Synosia Therapeutics |
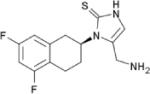 |
Selective dopamine β-hydroxylase inhibitor |
Nausea Diarrhea Dizziness Headache Full side effect profile to be established in multisite trial |
Phase II Clinical Trials |
Crosses the blood brain barrier Large multi-site trial underway |
Post-traumatic stress disorder (Phase II) Addiction, cocaine (Phase II) |
| D-cycloserine | Searle (Monsanto; now Pfizer) Eli Lily & Co. |
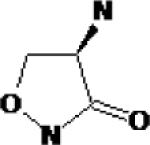 |
Partial NMDA (glycine) receptor agonist |
Anxiety, disorientation, confusion in chronic dosing |
Marketed | Possible benefit as adjunctive treatment in prolonged exposure treatment for PTSD Clinical trials underway |
Previously used as an antibiotic against Mycobacterium tuberculosis |
| Rufinamide |
Easai Synosia Therapeutics |
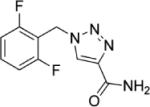 |
Inhibits voltage sensitive Na channels, thereby stabilizing membranes |
Somnolence Nausea Vomiting Headache Fatigue Dizziness |
Marketed (Europe) |
Has mood stabilizing properties Possible better adverse event profile than lamotrigine No report of Stevens Johnson to date |
Lennox Gastaut Syndrome seizures Generalized Anxiety Disorder (Phase II) |
| NK1 antagonist GR 205171 |
Merck & Co | 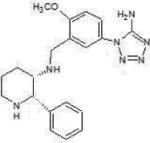 |
Neurokinin-1 inhibitor Substance P (SP) antagonist mediates effect via NK1 receptor |
Not known | Marketed | Antidepressant anxiolytic and Antiemetic properties Cerebrospinal fluid SP is increased in PTSD NIH sponsored clinical trial in PTSD |
Acute and delayed chemotherapy induced nausea and vomiting |
| CRF antagonist | Glaxo Smith Kline Neurocrine Biosciences, Johnson and Johnson, Pfizer, Bristol Meyers Squibb |
Not available | Corticotropin-releasing hormone (CRF) R1 antagonist |
Not known Hepatic side effects have limited previous CRF antagonist trials |
Phase I and II Clinical Trials |
Cerebrospinal fluid CRF is increased in PTSD |
Major Depression Phase II Clinical Trial (Compound 561679) Anxiety-Depression Phase I Clinical Trial (Compound 586529) |
7.0 Potential Development Issues
Currently available medications for treatment of PTSD provide limited benefit. The first line of treatment is antidepressants, usually SSRIs. Response is often incomplete; response rates hover around 60%, and less than 20-30% of patients fully remit 85, 200. Despite the obvious need for more effective PTSD treatments, drug development has largely been focused on compounds for other indications, such as depression or other anxiety disorders. Clinical trials for PTSD are most often initiated after drugs are developed for these other indications, often years later, when the drug is nearing patent expiration. The lag in PTSD drug development may be due to the lack of reliable pre-clinical models, to slow acceptance of the PTSD diagnosis as a diagnostic entity, to a failure to recognize the prevalence of the disorder or to a perception that PTSD treatment trials are fraught with difficulty and that compounds studied are unlikely to show efficacy.
Indeed there may be issues specific to PTSD that proffer special challenges for PTSD research, some that are a characteristic of the population and others related to limited knowledge of the disorder. First, the tendency of PTSD patients to avoid trauma reminders offers a special challenge in patient recruitment. PTSD subjects may be more likely to drop out than subjects with other diagnoses, at least until a solid clinical alliance is made. Secondly, the placebo rate, at about 25%, comparable to that of depression, is high, thus requiring relatively large cohorts to show statistical efficacy 6, 62. And, deficits in our knowledge about subgroups, defined by demographics such as gender and ethnicity, or characteristics such as type and duration of trauma, longevity of symptoms or co-occurring psychiatric morbidities add to variability across studies, consequently impact the ability to interpret results and prove efficacy. Many of these impediments can only be remedied by research.
Other drug development issues, also common to other mental disorders are pharmacokinetic, in particular the transport of compounds to the CNS past the blood brain barrier. Transport systems include carrier- and receptor-mediated systems such as cationic and anionic influx and efflux systems (e.g., P-glycoproteins, multidrug resistance proteins, organic anion transporters) and others 201-204.
8.0 Expert Opinion
Despite epidemiological research showing PTSD to be a common mental disorder, development of pharmaceutical agents to treat PTSD symptoms has lagged that depression and other anxiety disorders, explained in part by its late inclusion in the DSM. Over the past few years, however, United States government funding for PTSD research, including for large scale clinical trials has expanded, a result of the recognition of increased rates of PTSD and suicidal behavior among troops returning from wars in Iraq and Afghanistan 205, 206. As with other mental disorders, drug development for PTSD is hampered by an incomplete understanding of the natural history of the disorder, and by a lack of knowledge of the etiology and biological correlates of core PTSD symptoms and associated insomnia, depression and panic/anxiety that frequently fail to respond to current treatments.
Heightened interest and increased funding have provided for expanded PTSD research; large scale, multi-site clinical trials are underway on drugs to treat core PTSD symptoms (nepicastat), to treat targeted symptoms (prazosin) and as adjunct treatment in recalcitrant cases (risperidone). These trials should provide definitive answers on the efficacy of these compounds. In addition, there are a plethora of smaller scale trials of drugs with new mechanisms of action; these include trials with dual reuptake inhibitors, glucocorticoids, glucocortiocoid antagonists, and with stress system modulators, such as NPY. Moreover, there is a paradigm shift toward prophylaxis, i.e. attempted intervention with preventive and early intervention pharmacotherapies.
In order to achieve the goal of development of effective pharmacologic treatments for PTSD, improved knowledge of the natural history of PTSD and of etiological factors is crucial. Prospective, longitudinal studies that incorporate biological as well as psychosocial measures would help accomplish these goals. At present, there are least four such studies underway, three in military populations and one in police.
In addition, better pre-clinical models would be helpful. There may be fewer subtypes of PTSD, and less biological variability across the diagnosis than will ultimately be found in depression or schizophrenia. If so, this may bode well for drug development. And although there are as yet no universally agreed upon animal model for PTSD, there are a number in development based on fear learning that may be helpful in better understanding underlying biology and will prove to be effective models for translational drug testing.
In summary, current drugs used to treat PTSD or moderately effective at best. Much work is needed to develop effective treatment and prevention strategies.
Acknowledgments
Declaration of interest
The authors (DGB, VBR, CMN) wish to acknowledge support from the VA Center of Excellence for Stress and Mental Health (CESAMH). In addition, they wish to acknowledge VACO Merit Review, HSR&D, and Cooperative Study Program (DGB) and NIH MH074697 (VBR) grant support and DOD (Navy, Marine) grant and contract support (DGB).
DGB has received grants from Organon. VBR has received grants from Addex Pharmaceuticals, Ferring Pharmaceuticals, Pfizer and Organon.
Contributor Information
Dewleen G. Baker, University of California San Diego 9500 Gilman Drive (0603V) La Jolla, California 92093-0603V USA.
Caroline M. Nievergelt, Department of Psychiatry University of California San Diego 9500 Gilman Drive La Jolla, CA 92093-0838, USA cnievergelt@ucsd.edu.
Victoria B. Risbrough, Department of Psychiatry University of California San Diego 9500 Gilman Drive La Jolla, CA 92093-0838, USA vrisbrough@ucsd.edu.
References
- 1.Homer The Iliad: The Fitzgerald Translation: Farrar, Straus and Giroux. 2004 [Google Scholar]
- 2.Janet P. Psychological healing; a historical and clinical study. G. Allen & Unwin ltd.; The Macmillan company; London: New York: 1925. [Google Scholar]
- 3.van der Kolk BA, van der Hart O. Pierre Janet and the breakdown of adaptation in psychological trauma. Am J Psychiatry. 1989 Dec;146(12):1530–40. doi: 10.1176/ajp.146.12.1530. [DOI] [PubMed] [Google Scholar]
- 4.APA . Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4 ed. American Psychiatric Association; Washington, DC: 2000. section 309.81: Posttraumatic stress disorder. [Google Scholar]
- 5.Creamer M, Burgess P, McFarlane AC. Post-traumatic stress disorder: findings from the Australian National Survey of Mental Health and Well-being. PsycholMed. 2001;31(7):1237–47. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- 6.Baker DG, Diamond BI, Gillette G, Hamner M, Katzelnick D, Keller T, et al. A double-blind, randomized, placebo-controlled, multi-center study of brofaromine in the treatment of post-traumatic stress disorder. Psychopharmacology (Berl) 1995 Dec;122(4):386–9. doi: 10.1007/BF02246271. [DOI] [PubMed] [Google Scholar]
- 7.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991 Mar;48(3):216–22. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 8.Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatr Scand. 2005 Apr;111(4):291–9. doi: 10.1111/j.1600-0447.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 9.Darves-Bornoz JM, Alonso J, de Girolamo G, de Graaf R, Haro JM, Kovess-Masfety V, et al. Main traumatic events in Europe: PTSD in the European study of the epidemiology of mental disorders survey. J Trauma Stress. 2008 Oct;21(5):455–62. doi: 10.1002/jts.20357. [DOI] [PubMed] [Google Scholar]
- 10.Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(420):21–7. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995 Dec;52(12):1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 12.Perkonigg A, Kessler RC, Storz S, Wittchen HU. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand. 2000 Jan;101(1):46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- 13.Norris FH, Murphy AD, Baker CK, Perilla JL, Rodriguez FG, Rodriguez Jde J. Epidemiology of trauma and posttraumatic stress disorder in Mexico. J Abnorm Psychol. 2003 Nov;112(4):646–56. doi: 10.1037/0021-843X.112.4.646. [DOI] [PubMed] [Google Scholar]
- 14.Green BL, Grace MC, Lindy JD, Gleser GC, Leonard A. Risk factors for PTSD and other diagnoses in a general sample of Vietnam veterans. Am J Psychiatry. 1990 Jun;147(6):729–33. doi: 10.1176/ajp.147.6.729. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe J, Chrestman KR, Ouimette PC, Kaloupek D, Harley RM, Bucsela M. Trauma-related psychophysiological reactivity in women exposed to war-zone stress. J Clin Psychol. 2000 Oct;56(10):1371–9. doi: 10.1002/1097-4679(200010)56:10<1371::AID-JCLP8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002 Oct;159(10):1675–81. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 17.Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry. 2005 Jun;162(6):1188–91. doi: 10.1176/appi.ajp.162.6.1188. [DOI] [PubMed] [Google Scholar]
- 18.Van Ameringen M, Mancini C, Patterson B, Boyle MH. Post-traumatic stress disorder in Canada. CNS Neurosci Ther. 2008 Fall;14(3):171–81. doi: 10.1111/j.1755-5949.2008.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North CS, Pfefferbaum B, Narayanan P, Thielman S, McCoy G, Dumont C, et al. Comparison of post-disaster psychiatric disorders after terrorist bombings in Nairobi and Oklahoma City. Br J Psychiatry. 2005 Jun;186:487–93. doi: 10.1192/bjp.186.6.487. [DOI] [PubMed] [Google Scholar]
- 20.North CS, Suris AM, Davis M, Smith RP. Toward validation of the diagnosis of posttraumatic stress disorder. Am J Psychiatry. 2009 Jan;166(1):34–41. doi: 10.1176/appi.ajp.2008.08050644. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 2008;29:115–29. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- 22.Tanios CY, Abou-Saleh MT, Karam AN, Salamoun MM, Mneimneh ZN, Karam EG. The epidemiology of anxiety disorders in the Arab world: A review. J Anxiety Disord. 2008 Oct 31; doi: 10.1016/j.janxdis.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Fernando GA. Assessing mental health and psychosocial status in communities exposed to traumatic events: Sri Lanka as an example. Am J Orthopsychiatry. 2008 Apr;78(2):229–39. doi: 10.1037/a0013940. [DOI] [PubMed] [Google Scholar]
- 24.Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993 Feb;150(2):235–9. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- 25.Kulka R, Schlenger W, Fairbank J, Hough R, Jordan B, Marmar C. Trauma and the Vietnam war generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; New York, NY: 1990. [Google Scholar]
- 26.Zaidi LY, Foy DW. Childhood abuse experiences and combat-related PTSD. J Trauma Stress. 1994 Jan;7(1):33–42. doi: 10.1007/BF02111910. [DOI] [PubMed] [Google Scholar]
- 27.King DW, King LA, Foy DW, Gudanowski DM. Prewar factors in combat-related posttraumatic stress disorder: structural equation modeling with a national sample of female and male Vietnam veterans. J Consult Clin Psychol. 1996 Jun;64(3):520–31. doi: 10.1037//0022-006x.64.3.520. [DOI] [PubMed] [Google Scholar]
- 28.Galea S, Resnick H, Ahern J, Gold J, Bucuvalas M, Kilpatrick D, et al. Posttraumatic stress disorder in Manhattan, New York City, after the September 11th terrorist attacks. J Urban Health. 2002 Sep;79(3):340–53. doi: 10.1093/jurban/79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslau N, Peterson EL, Schultz LR. A second look at prior trauma and the posttraumatic stress disorder effects of subsequent trauma: a prospective epidemiological study. Arch Gen Psychiatry. 2008 Apr;65(4):431–7. doi: 10.1001/archpsyc.65.4.431. [DOI] [PubMed] [Google Scholar]
- 30.Baker D, Risbrough V, Schork N. Post traumatic stress disorder: genetic and environmental risk factors. In: Lukey BJ, Tepe V, editors. Biobehavioral resilience to stress. CRC Press; Boca Raton: 2008. [Google Scholar]
- 31.Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, et al. A high risk twin study of combat-related PTSD comorbidity. Twin Res. 2003 Jun;6(3):218–26. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- 32.Jang KL, Taylor S, Stein MB, Yamagata S. Trauma exposure and stress response: exploration of mechanisms of cause and effect. Twin Res Hum Genet. 2007 Aug;10(4):564–72. doi: 10.1375/twin.10.4.564. [DOI] [PubMed] [Google Scholar]
- 33.Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, et al. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2008 Jan;105(13):109–15. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherrer JF, Xian H, Lyons MJ, Goldberg J, Eisen SA, True WR, et al. Posttraumatic stress disorder; combat exposure; and nicotine dependence, alcohol dependence, and major depression in male twins. Compr Psychiatry. 2008 May-Jun;49(3):297–304. doi: 10.1016/j.comppsych.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: informing clinical conceptualizations and promoting future research. Am J Med Genet C Semin Med Genet. 2008 May 15;148(2):127–32. doi: 10.1002/ajmg.c.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peleg T, Shalev AY. Longitudinal studies of PTSD: overview of findings and methods. CNS Spectr. 2006 Aug;11(8):589–602. doi: 10.1017/s109285290001364x. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell ML, Elliott P, Lau W, Creamer M. PTSD symptom trajectories: from early to chronic response. Behav Res Ther. 2007 Mar;45(3):601–6. doi: 10.1016/j.brat.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Brewin CR. Risk factor effect sizes in PTSD: what this means for intervention. J Trauma Dissociation. 2005;6(2):123–30. doi: 10.1300/J229v06n02_11. [DOI] [PubMed] [Google Scholar]
- 39.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000 Oct;68(5):748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 40.Kremen WS, Koenen KC, Boake C, Purcell S, Eisen SA, Franz CE, et al. Pretrauma cognitive ability and risk for posttraumatic stress disorder: a twin study. Arch Gen Psychiatry. 2007 Mar;64(3):361–8. doi: 10.1001/archpsyc.64.3.361. [DOI] [PubMed] [Google Scholar]
- 41.Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. 12-Month comorbidity patterns and associated factors in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(420):28–37. doi: 10.1111/j.1600-0047.2004.00328.x. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman M, McGlinchey JB, Chelminski I, Young D. Diagnostic co-morbidity in 2300 psychiatric out-patients presenting for treatment evaluated with a semi-structured diagnostic interview. Psychol Med. 2008 Feb;38(2):199–210. doi: 10.1017/S0033291707001717. [DOI] [PubMed] [Google Scholar]
- 43.Neria Y, Olfson M, Gameroff MJ, Wickramaratne P, Pilowsky D, Verdeli H, et al. Trauma exposure and posttraumatic stress disorder among primary care patients with bipolar spectrum disorder. Bipolar Disord. 2008 Jun;10(4):503–10. doi: 10.1111/j.1399-5618.2008.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perkonigg A, Pfister H, Stein MB, Hofler M, Lieb R, Maercker A, et al. Longitudinal course of posttraumatic stress disorder and posttraumatic stress disorder symptoms in a community sample of adolescents and young adults. Am J Psychiatry. 2005 Jul;162(7):1320–7. doi: 10.1176/appi.ajp.162.7.1320. [DOI] [PubMed] [Google Scholar]
- 45.Sareen J, Cox BJ, Goodwin RD, G JGA. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress. 2005 Aug;18(4):313–22. doi: 10.1002/jts.20040. [DOI] [PubMed] [Google Scholar]
- 46.Koenen KC, Moffitt TE, Caspi A, Gregory A, Harrington H, Poulton R. The developmental mental-disorder histories of adults with posttraumatic stress disorder: a prospective longitudinal birth cohort study. J Abnorm Psychol. 2008 May;117(2):460–6. doi: 10.1037/0021-843X.117.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Druss BG, Hwang I, Petukhova M, Sampson NA, Wang PS, Kessler RC. Impairment in role functioning in mental and chronic medical disorders in the United States: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2008 Feb 19; doi: 10.1038/mp.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Disability and quality of life impact of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(420):38–46. doi: 10.1111/j.1600-0047.2004.00329.x. [DOI] [PubMed] [Google Scholar]
- 49.Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, et al. Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43(3):150–62. doi: 10.1159/000054884. [DOI] [PubMed] [Google Scholar]
- 50.Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the National Comorbidity Survey. J Nerv Ment Dis. 2005 Jul;193(7):450–4. doi: 10.1097/01.nmd.0000168263.89652.6b. [DOI] [PubMed] [Google Scholar]
- 51.Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJ. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007 Apr;69(3):242–8. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- 52.Stein MB, Walker JR, Hazen AL, Forde DR. Full and partial posttraumatic stress disorder: findings from a community survey. Am J Psychiatry. 1997 Aug;154(8):1114–9. doi: 10.1176/ajp.154.8.1114. [DOI] [PubMed] [Google Scholar]
- 53.Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL. Comorbidity, impairment, and suicidality in subthreshold PTSD. Am J Psychiatry. 2001 Sep;158(9):1467–73. doi: 10.1176/appi.ajp.158.9.1467. [DOI] [PubMed] [Google Scholar]
- 54.Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA. Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999 Oct;4(4):234–41. doi: 10.153/SCNP00400234. [DOI] [PubMed] [Google Scholar]
- 55.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003;(417):38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 56.Brewin CR, Dalgleish T, Joseph S. A dual representation theory of posttraumatic stress disorder. Psychol Rev. 1996 Oct;103(4):670–86. doi: 10.1037/0033-295x.103.4.670. [DOI] [PubMed] [Google Scholar]
- 57.Agid Y, Buzsaki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007 Mar;6(3):189–201. doi: 10.1038/nrd2217. [DOI] [PubMed] [Google Scholar]
- 58.Litz BT. Early intervention for trauma: where are we and where do we need to go? A commentary. J Trauma Stress. 2008 Dec;21(6):503–6. doi: 10.1002/jts.20373. [DOI] [PubMed] [Google Scholar]
- 59.Forbes D, Creamer M, Phelps A, Bryant R, McFarlane A, Devilly GJ, et al. Australian guidelines for the treatment of adults with acute stress disorder and post-traumatic stress disorder. Australian and New Zealand Journal of Psychiatry. 2007;41(8):637–48. doi: 10.1080/00048670701449161. [DOI] [PubMed] [Google Scholar]
- 60.National Collaborating Centre for Mental Health, National Institute for Health and Clinical Excellence . Post-traumatic stress disorder : the management of PTSD in adults and children in primary and secondary care. Published by Gaskell and the British Psychological Society; London: 2005. [PubMed] [Google Scholar]
- 61.Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006;(1):CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Institute of Medicine (IOM) Treatment of posttraumatic stress disorder: an assessment of the evidence. National Academies Press; Washington, D.C.: 2008. Committee on Treatment of Posttraumatic Stress Disorder. [Google Scholar]
- 63.Mohamed S, Rosenheck RA. Pharmacotherapy of PTSD in the U.S. Department of Veterans Affairs: diagnostic- and symptom-guided drug selection. J Clin Psychiatry. 2008 Jun;69(6):959–65. doi: 10.4088/jcp.v69n0611. [DOI] [PubMed] [Google Scholar]
- 64.Harpaz-Rotem I, Rosenheck RA, Mohamed S, Desai RA. Pharmacologic treatment of posttraumatic stress disorder among privately insured Americans. Psychiatr Serv. 2008 Oct;59(10):1184–90. doi: 10.1176/ps.2008.59.10.1184. [DOI] [PubMed] [Google Scholar]
- 65.McCrone P, Knapp M, Cawkill P. Posttraumatic stress disorder (PTSD) in the Armed Forces: health economic considerations. J Trauma Stress. 2003 Oct;16(5):519–22. doi: 10.1023/A:1025722930935. [DOI] [PubMed] [Google Scholar]
- 66.McCrone P, Weich S. The costs of mental health care: paucity of measurement. In: Tansella M, Thornicroft G, editors. Mental health outcome measures. 2nd ed. Gaskell; London: 2001. p. ix. 325 p. [Google Scholar]
- 67.Alderman CP, McCarthy LC, Marwood AC. Pharmacotherapy for post-traumatic stress disorder. Expert Review of Clinical Pharmacology. 2009;2(1):77–86. doi: 10.1586/17512433.2.1.77. [DOI] [PubMed] [Google Scholar]
- 68.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000 Apr 12;283(14):1837–44. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 69.Davidson J, Pearlstein T, Londborg P, Brady KT, Rothbaum B, Bell J, et al. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry. 2001 Dec;158(12):1974–81. doi: 10.1176/appi.ajp.158.12.1974. [DOI] [PubMed] [Google Scholar]
- 70.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001 Dec;158(12):1982–8. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 71.Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001 Nov;62(11):860–8. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 72.Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007 May;68(5):711–20. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- 73.Simon NM, Connor KM, Lang AJ, Rauch S, Krulewicz S, LeBeau RT, et al. Paroxetine CR augmentation for posttraumatic stress disorder refractory to prolonged exposure therapy. J Clin Psychiatry. 2008 Mar;69(3):400–5. doi: 10.4088/jcp.v69n0309. [DOI] [PubMed] [Google Scholar]
- 74.Katz RJ, Lott MH, Arbus P, Crocq L, Herlobsen P, Lingjaerde O, et al. Pharmacotherapy of post-traumatic stress disorder with a novel psychotropic. Anxiety. 1994;1(4):169–74. doi: 10.1002/anxi.3070010404. [DOI] [PubMed] [Google Scholar]
- 75.Kosten TR, Frank JB, Dan E, McDougle CJ, Giller EL., Jr Pharmacotherapy for posttraumatic stress disorder using phenelzine or imipramine. J Nerv Ment Dis. 1991 Jun;179(6):366–70. doi: 10.1097/00005053-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 76.Shestatzky M, Greenberg D, Lerer B. A controlled trial of phenelzine in posttraumatic stress disorder. Psychiatry Res. 1988 May;24(2):149–55. doi: 10.1016/0165-1781(88)90057-1. [DOI] [PubMed] [Google Scholar]
- 77.Boscarino JA. Psychobiologic predictors of disease mortality after psychological trauma: implications for research and clinical surveillance. J Nerv Ment Dis. 2008 Feb;196(2):100–7. doi: 10.1097/NMD.0b013e318162a9f5. [DOI] [PubMed] [Google Scholar]
- 78.Baker DG, Geracioti TDJ, Kasckow JW, Zoumakis E, Chrousos GP. Cytokines and Post Traumatic Stress Disorder. In: Kronfol Z, editor. Cytokines and mental health. Kluwer Academic Publishers; Boston ; London: 2003. [Google Scholar]
- 79.Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA, et al. The Association of Posttraumatic Stress Disorder and Metabolic Syndrome: A Study of Increased Health Risk in Veterans. BMC Med. 2009 Jan 9;7(1):1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padala PR, Madison J, Monnahan M, Marcil W, Price P, Ramaswamy S, et al. Risperidone monotherapy for post-traumatic stress disorder related to sexual assault and domestic abuse in women. Int Clin Psychopharmacol. 2006 Sep;21(5):275–80. doi: 10.1097/00004850-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Bartzokis G, Lu PH, Turner J, Mintz J, Saunders CS. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 2005 Mar 1;57(5):474–9. doi: 10.1016/j.biopsych.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 82.Reich DB, Winternitz S, Hennen J, Watts T, Stanculescu C. A preliminary study of risperidone in the treatment of posttraumatic stress disorder related to childhood abuse in women. J Clin Psychiatry. 2004 Dec;65(12):1601–6. doi: 10.4088/jcp.v65n1204. [DOI] [PubMed] [Google Scholar]
- 83.Hamner MB, Faldowski RA, Ulmer HG, Frueh BC, Huber MG, Arana GW. Adjunctive risperidone treatment in post-traumatic stress disorder: a preliminary controlled trial of effects on comorbid psychotic symptoms. Int Clin Psychopharmacol. 2003 Jan;18(1):1–8. doi: 10.1097/00004850-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Monnelly EP, Ciraulo DA, Knapp C, Keane T. Low-dose risperidone as adjunctive therapy for irritable aggression in posttraumatic stress disorder. J Clin Psychopharmacol. 2003 Apr;23(2):193–6. doi: 10.1097/00004714-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry. 2002 Oct;159(10):1777–9. doi: 10.1176/appi.ajp.159.10.1777. [DOI] [PubMed] [Google Scholar]
- 86.Butterfield MI, Becker ME, Connor KM, Sutherland S, Churchill LE, Davidson JR. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int Clin Psychopharmacol. 2001 Jul;16(4):197–203. doi: 10.1097/00004850-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Rothbaum BO, Killeen TK, Davidson JR, Brady KT, Connor KM, Heekin MH. Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant civilian posttraumatic stress disorder. J Clin Psychiatry. 2008 Apr;69(4):520–5. doi: 10.4088/jcp.v69n0402. [DOI] [PubMed] [Google Scholar]
- 88.Hollander E, Tracy KA, Swann AC, Coccaro EF, McElroy SL, Wozniak P, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003 Jun;28(6):1186–97. doi: 10.1038/sj.npp.1300153. [DOI] [PubMed] [Google Scholar]
- 89.Davis LL, Davidson JR, Ward LC, Bartolucci A, Bowden CL, Petty F. Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. J Clin Psychopharmacol. 2008 Feb;28(1):84–8. doi: 10.1097/JCP.0b013e318160f83b. [DOI] [PubMed] [Google Scholar]
- 90.Davidson JR, Brady K, Mellman TA, Stein MB, Pollack MH. The efficacy and tolerability of tiagabine in adult patients with post-traumatic stress disorder. J Clin Psychopharmacol. 2007 Feb;27(1):85–8. doi: 10.1097/JCP.0b013e31802e5115. [DOI] [PubMed] [Google Scholar]
- 91.Hertzberg MA, Butterfield MI, Feldman ME, Beckham JC, Sutherland SM, Connor KM, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999 May 1;45(9):1226–9. doi: 10.1016/s0006-3223(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 92.Tucker P, Trautman RP, Wyatt DB, Thompson J, Wu SC, Capece JA, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007 Feb;68(2):201–6. doi: 10.4088/jcp.v68n0204. [DOI] [PubMed] [Google Scholar]
- 93.Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008 Mar 15;63(6):629–32. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003 Feb;160(2):371–3. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 95.Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007 Apr 15;61(8):928–34. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 96.Neylan TC, Lenoci M, Samuelson KW, Metzler TJ, Henn-Haase C, Hierholzer RW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006 Dec;163(12):2186–8. doi: 10.1176/appi.ajp.163.12.2186. [DOI] [PubMed] [Google Scholar]
- 97.Braun P, Greenberg D, Dasberg H, Lerer B. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990 Jun;51(6):236–8. [PubMed] [Google Scholar]
- 98.Gelpin E, Bonne O, Peri T, Brandes D, Shalev AY. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996 Sep;57(9):390–4. [PubMed] [Google Scholar]
- 99.Davidson J, Rothbaum BO, Tucker P, Asnis G, Benattia I, Musgnung JJ. Venlafaxine extended release in posttraumatic stress disorder: a sertraline- and placebo-controlled study. J Clin Psychopharmacol. 2006 Jun;26(3):259–67. doi: 10.1097/01.jcp.0000222514.71390.c1. [DOI] [PubMed] [Google Scholar]
- 100.Davidson J, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006 Oct;63(10):1158–65. doi: 10.1001/archpsyc.63.10.1158. [DOI] [PubMed] [Google Scholar]
- 101.Rothbaum BO, Cahill SP, Foa EB, Davidson JR, Compton J, Connor KM, et al. Augmentation of sertraline with prolonged exposure in the treatment of posttraumatic stress disorder. J Trauma Stress. 2006 Oct;19(5):625–38. doi: 10.1002/jts.20170. [DOI] [PubMed] [Google Scholar]
- 102.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006 Aug 15;60(4):369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 103.Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–5. [PubMed] [Google Scholar]
- 104.Rothbaum BO, Davis M. Applying Learning Principles to the Treatment of Post-Trauma Reactions. Ann NY Acad Sci. 2003 December 1;1008(1):112–21. doi: 10.1196/annals.1301.012. 2003. [DOI] [PubMed] [Google Scholar]
- 105.Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2007;33(1):56. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henry M, Fishman JR, Youngner SJ. Propranolol and the prevention of post-traumatic stress disorder: is it wrong to erase the “sting” of bad memories? Am J Bioeth. 2007 Sep;7(9):12–20. doi: 10.1080/15265160701518474. [DOI] [PubMed] [Google Scholar]
- 107.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002 Apr;12(2):205–10. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 108.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008 Oct 16;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008 Oct 15;64(8):691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007 Oct 19;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 111.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003 Jul 18;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 112.Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004 Oct;9(10):908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 113.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005 Feb;10(2):220–4. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- 115.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005 May;62(5):529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 116.Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005 Jan;35(1):101–11. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 117.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005 Feb;62(2):146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 118.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996 Nov 29;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 119.Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav Neurosci. 2001 Apr;115(2):358–64. [PubMed] [Google Scholar]
- 120.Stein MB, Campbell-Sills L, Gelernter J. Genetic variation in 5HTTLPR is associated with emotional resilience. Am J Med Genet B Neuropsychiatr Genet. 2009 Jan 16; doi: 10.1002/ajmg.b.30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee JLC, Milton AL, Everitt BJ. Reconsolidation and Extinction of Conditioned Fear: Inhibition and Potentiation. J Neurosci. 2006 September 27;26(39):10051–6. doi: 10.1523/JNEUROSCI.2466-06.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007 Nov;164(11):1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 123.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007 Jul;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 124.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005 Dec;28(12):629–35. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 125.Yehuda R, Halligan SL, Grossman R, Golier JA, Wong C. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol Psychiatry. 2002 Sep 1;52(5):393–403. doi: 10.1016/s0006-3223(02)01357-4. [DOI] [PubMed] [Google Scholar]
- 126.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006 Jul;1071:137–66. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 127.Shalev AY, Videlock EJ, Peleg T, Segman R, Pitman RK, Yehuda R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: a longitudinal study. Part I: HPA axis responses. Int J Neuropsychopharmacol. 2008 May;11(3):365–72. doi: 10.1017/S1461145707008127. [DOI] [PubMed] [Google Scholar]
- 128.Baker DG, Ekhator NN, Kasckow JW, Dashevsky BA, Horn PS, Bednarik L, et al. Increased basal serial cerebrospinal fluid cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162(5):992–4. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- 129.Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA Axis Response to Stress Influences Susceptibility to Posttraumatic Stress Response in Rats. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.12.003. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 130.Olff M, Güzelcan Y, de Vries G-J, Assies J, Gersons BPR. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31(10):1220. doi: 10.1016/j.psyneuen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 131.Yang YL, Chao PK, Lu KT. Systemic and Intra-Amygdala Administration of Glucocorticoid Agonist and Antagonist Modulate Extinction of Conditioned Fear. Neuropsychopharmacology. 2005 Oct 5; doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 132.de Quervain DJF, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: A novel therapeutic approach. European Journal of Pharmacology. 2008;583(23):365. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 133.Schelling G, Kilger E, Roozendaal B, de Quervain DJF, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biological Psychiatry. 2004;55(6):627. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 134.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Low-Dose Cortisol for Symptoms of Posttraumatic Stress Disorder. Am J Psychiatry. 2004 August 1;161(8):1488–90. doi: 10.1176/appi.ajp.161.8.1488. 2004. [DOI] [PubMed] [Google Scholar]
- 135.Cai W-H, Blundell J, Han J, Greene RW, Powell CM. Postreactivation Glucocorticoids Impair Recall of Established Fear Memory. J Neurosci. 2006 September 13;26(37):9560–6. doi: 10.1523/JNEUROSCI.2397-06.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joseph M, Andreano LC. Glucocorticoid Release and Memory Consolidation in Men and Women. Psychological Science. 2006;17(6):466–70. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 137.Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(8):1213. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 138.Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and Biological Effects of Mifepristone Treatment for Psychotic Depression. Neuropsychopharmacology. 2005;31(3):628. doi: 10.1038/sj.npp.1300884. [DOI] [PubMed] [Google Scholar]
- 139.Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Nicol Ferrier I. Improvements in Neurocognitive Function and Mood Following Adjunctive Treatment with Mifepristone (RU-486) in Bipolar Disorder. Neuropsychopharmacology. 2004;29(8):1538. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]
- 140.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2006;12(2):120. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 141.Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71(3):379–92. doi: 10.1016/s0091-3057(01)00698-0. JID - 0367050 2002 3. [DOI] [PubMed] [Google Scholar]
- 142.Norberg MM, Krystal JH, Tolin DF. A Meta-Analysis of D-Cycloserine and the Facilitation of Fear Extinction and Exposure Therapy. Biological Psychiatry. 2008;63(12):1118. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 143.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008 Mar 15;63(6):544–9. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 144.Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007 Apr;45(4):663–72. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 145.Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007 Sep;41(6):466–71. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 146.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46(1):5. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 147.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry. 2006 August 1;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. 2006. [DOI] [PubMed] [Google Scholar]
- 148.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000 Feb 15;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 149.Parwani A, Weiler M, Blaxton T, Warfel D, Hardin M, Frey K, et al. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacology. 2005;183(3):265–74. doi: 10.1007/s00213-005-0177-2. [DOI] [PubMed] [Google Scholar]
- 150.Tsai GE. Searching for Rational Anti N-methyl-D-aspartate Treatment for Depression. Arch Gen Psychiatry. 2007 September 1;64(9):1099–100. doi: 10.1001/archpsyc.64.9.1099. 2007. [DOI] [PubMed] [Google Scholar]
- 151.Layton ME, Kelly MJ, 3rd, Rodzinak KJ. Recent advances in the development of NR2B subtype-selective NMDA receptor antagonists. Curr Top Med Chem. 2006;6(7):697–709. doi: 10.2174/156802606776894447. [DOI] [PubMed] [Google Scholar]
- 152.Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of Extinction Learning for Contextual Fear Memory by PEPA: A Potentiator of AMPA Receptors. J Neurosci. 2007 January 3;27(1):158–66. doi: 10.1523/JNEUROSCI.3842-06.2007. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nordquist R, Steckler T, Wettstein J, Mackie C, Spooren W. Metabotropic glutamate receptor modulation, translational methods, and biomarkers: relationships with anxiety. Psychopharmacology. 2008;199(3):389. doi: 10.1007/s00213-008-1096-9. [DOI] [PubMed] [Google Scholar]
- 154.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 Receptor Agonist as a Novel Approach to Treat Anxiety/Stress. Stress. 2003;6(3):189–97. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- 155.Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998 Feb;284(2):651–60. [PubMed] [Google Scholar]
- 156.Scaccianoce S, Matrisciano F, Del Bianco P, Caricasole A, Di Giorgi Gerevini V, Cappuccio I, et al. Endogenous activation of group-II metabotropic glutamate receptors inhibits the hypothalamic-pituitary-adrenocortical axis. Neuropharmacology. 2003 Apr;44(5):555–61. doi: 10.1016/s0028-3908(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 157.Grillon C, Cordova J, Levine L, Morgan IIIC. Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist ( LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology. 2003;168(4):446. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- 158.Muly CE, Mania I, Guo J, Rainnie DG. Group II metabotropic glutamate receptors in anxiety circuitry: Correspondence of physiological response and subcellular distribution. The Journal of Comparative Neurology. 2007;505(6):682–700. doi: 10.1002/cne.21525. [DOI] [PubMed] [Google Scholar]
- 159.Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and Tolerability of an mGlu2//3 Agonist in the Treatment of Generalized Anxiety Disorder. Neuropsychopharmacology. 2007;33(7):1603. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- 160.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacology & Therapeutics. 2007;115(1):116. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 161.Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol. 1982 Apr;2(2):129–33. [PubMed] [Google Scholar]
- 162.Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002 Dec;43(8):1199–209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 163.Berry-Kravis EM, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, et al. A pilot open-label single-dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009 January 6; doi: 10.1136/jmg.2008.063701. 2009:jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995 Sep 28;377(6547):295–6. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 165.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002 Jan 15;51(2):189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 166.Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003 Nov 1;54(9):947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 167.Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. Journal of Traumatic Stress. 2007;20(6):923–32. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 168.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatric Research. 2008;42(6):503. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 169.Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006 Jul;1071:521–4. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- 170.Mustapic M, Pivac N, Kozaric-Kovacic D, Dezeljin M, Cubells JF, Mueck-Seler D. Dopamine beta-hydroxylase (DBH) activity and -1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B(8):1087–9. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- 171.Hamner MB, Gold PB. Plasma dopamine beta-hydroxylase activity in psychotic and non-psychotic post-traumatic stress disorder. Psychiatry Research. 1998;77(3):175–81. doi: 10.1016/s0165-1781(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 172.Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997 Aug;54(8):749–58. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 173.Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, et al. Neuropeptide Y in the Amygdala Induces Long-Term Resilience to Stress-Induced Reductions in Social Responses But Not Hypothalamic-Adrenal-Pituitary Axis Activity or Hyperthermia. J Neurosci. 2008 January 23;28(4):893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999 Apr;156(4):585–8. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 175.Gutman AR, Yang Y, Ressler KJ, Davis M. The Role of Neuropeptide Y in the Expression and Extinction of Fear-Potentiated Startle. J Neurosci. 2008 November 26;28(48):12682–90. doi: 10.1523/JNEUROSCI.2305-08.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. European Journal of Pharmacology. 1999;368(23):143. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 177.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, et al. Role of Dorsomedial Hypothalamic Neuropeptide Y in Modulating Food Intake and Energy Balance. J Neurosci. 2009 January 7;29(1):179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997 May;154(5):624–9. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003 Dec 15;54(12):1382–8. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 180.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002 Nov;5(11):1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999 Jan;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 182.Grigoriadis DE. The corticotropin-releasing factor receptor: a novel target for the treatment of depression and anxiety-related disorders. Expert Opinion on Therapeutic Targets. 2005;9(4):651–84. doi: 10.1517/14728222.9.4.651. [DOI] [PubMed] [Google Scholar]
- 183.Valdez GR, Zorrilla EP, Koob GF. Homeostasis within the corticotropin-releasing factor system via CRF2 receptor activation: A novel approach for the treatment of anxiety. Drug Development Research. 2005;65(4):205–15. [Google Scholar]
- 184.Dyck B, Grigoriadis DE, Gross RS, Guo Z, Haddach M, Marinkovic D, et al. Potent, orally active corticotropin-releasing factor receptor-1 antagonists containing a tricyclic pyrrolopyridine or pyrazolopyridine core. J Med Chem. 2005 Jun 16;48(12):4100–10. doi: 10.1021/jm050070m. [DOI] [PubMed] [Google Scholar]
- 185.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000 May-Jun;34(3):171–81. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 186.Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-Week Randomized, Placebo-Controlled Trial of CP-316,311 (a Selective CRH1 Antagonist) in the Treatment of Major Depression. Am J Psychiatry. 2008 May 1;165(5):617–20. doi: 10.1176/appi.ajp.2008.07071199. 2008. [DOI] [PubMed] [Google Scholar]
- 187.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences of the United States of America. 1992 July 1;89(13):5981–5. doi: 10.1073/pnas.89.13.5981. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005 Dec 7;25(49):11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin Attenuates Amygdala Responses to Emotional Faces Regardless of Valence. Biological Psychiatry. 2007;62(10):1187–90. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 190.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. J Neurosci. 2008 June 25;28(26):6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS Region-Specific Oxytocin Receptor Expression: Importance in Regulation of Anxiety and Sex Behavior. J Neurosci. 2001 April 1;21(7):2546–52. doi: 10.1523/JNEUROSCI.21-07-02546.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kovács GL, Vécsei L, Telegdy G. Opposite action of oxytocin to vasopressin in passive avoidance behavior in rats. Physiology & Behavior. 1978;20(6):801. doi: 10.1016/0031-9384(78)90309-8. [DOI] [PubMed] [Google Scholar]
- 193.Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Research. 1993;48(2):107. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- 194.Seifer DB, Sandberg EC, Ueland K, Sladen RN. Water intoxication and hyponatremic encephalopathy from the use of an oxytocin nasal spray. A case report. J Reprod Med. 1985 Mar;30(3):225–8. [PubMed] [Google Scholar]
- 195.Viveros MP, Marco EM, Llorente R, Lopez-Gallardo M. Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast. 2007;2007:52908. doi: 10.1155/2007/52908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 197.Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing Cannabinoid Neurotransmission Augments the Extinction of Conditioned Fear. Neuropsychopharmacology. 2004;30(3):516. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 198.Pamplona F, Prediger R, Pandolfo P, Takahashi R. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology. 2006;188(4):641. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- 199.Lin H-C, Mao S-C, Chen P-S, Gean P-W. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learning & Memory. 2008 December 2008;15(12):876–84. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- 200.Zohar J, Amital D, Miodownik C, Kotler M, Bleich A, Lane RM, et al. Double-blind placebo-controlled pilot study of sertraline in military veterans with posttraumatic stress disorder. J Clin Psychopharmacol. 2002 Apr;22(2):190–5. doi: 10.1097/00004714-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 201.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005 Aug;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 202.de Boer AG, Gaillard PJ. Strategies to improve drug delivery across the blood-brain barrier. Clin Pharmacokinet. 2007;46(7):553–76. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- 203.de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol. 2007;47:323–55. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- 204.Thoeringer CK, Wultsch T, Shahbazian A, Painsipp E, Holzer P. Multidrug-resistance gene 1-type p-glycoprotein (MDR1 p-gp) inhibition by tariquidar impacts on neuroendocrine and behavioral processing of stress. Psychoneuroendocrinology. 2007 Sep-Nov;32(810):1028–40. doi: 10.1016/j.psyneuen.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004 Jul 1;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 206.Milliken CS, Auchterlonie JL, Hoge CW. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007 Nov 14;298(18):2141–8. doi: 10.1001/jama.298.18.2141. [DOI] [PubMed] [Google Scholar]


