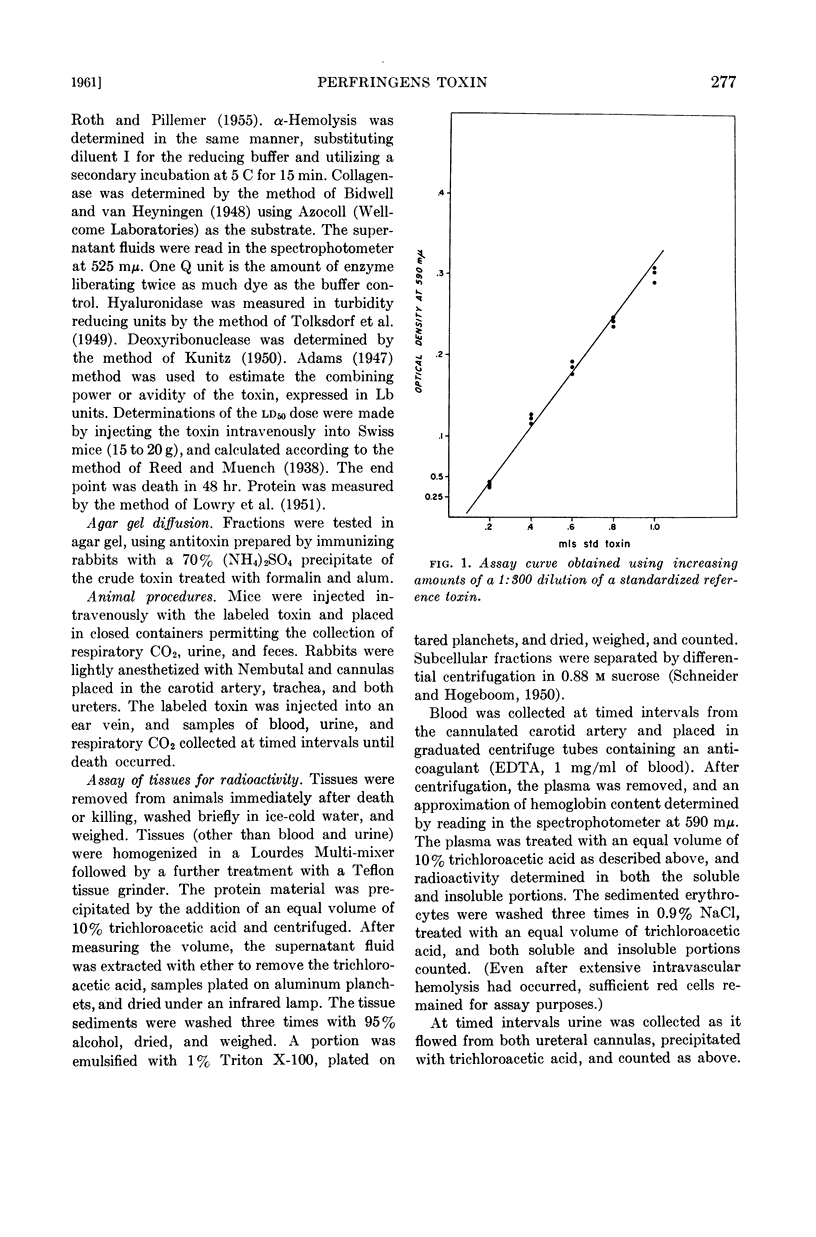
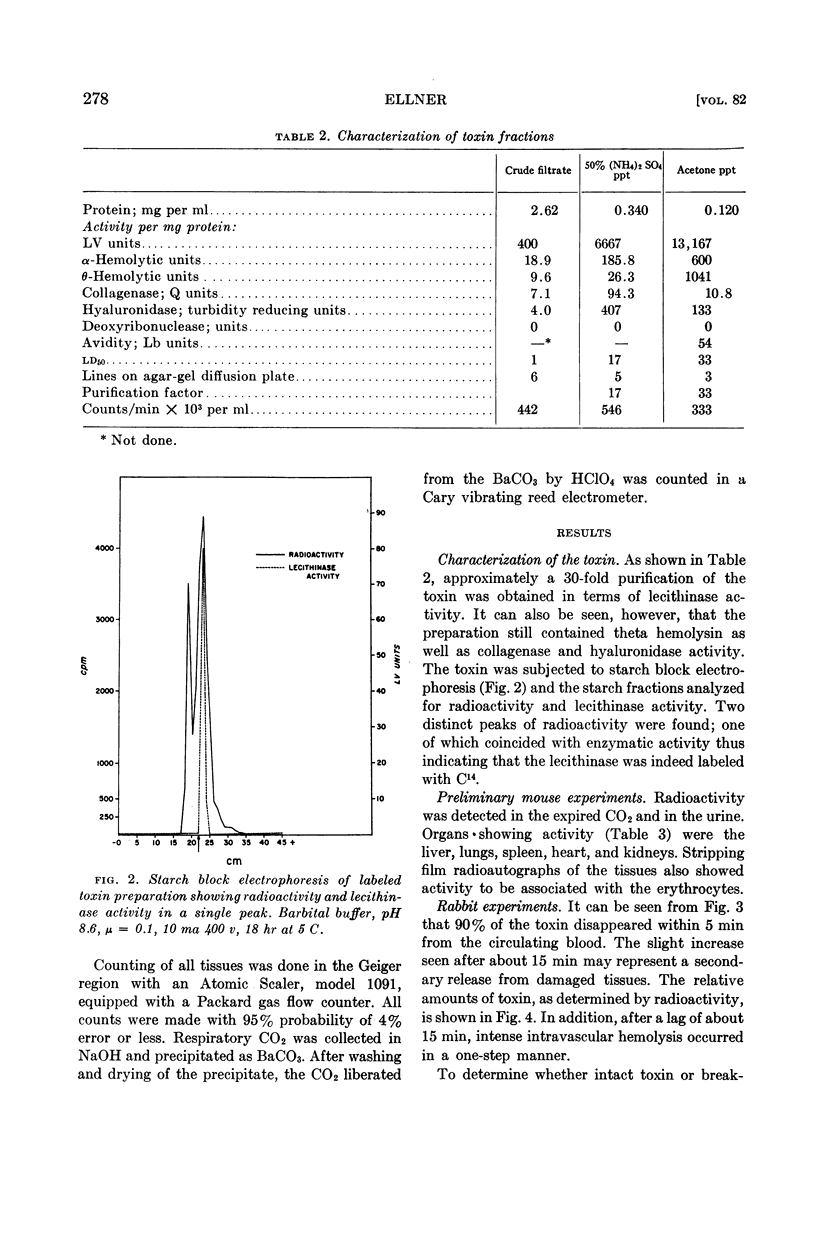
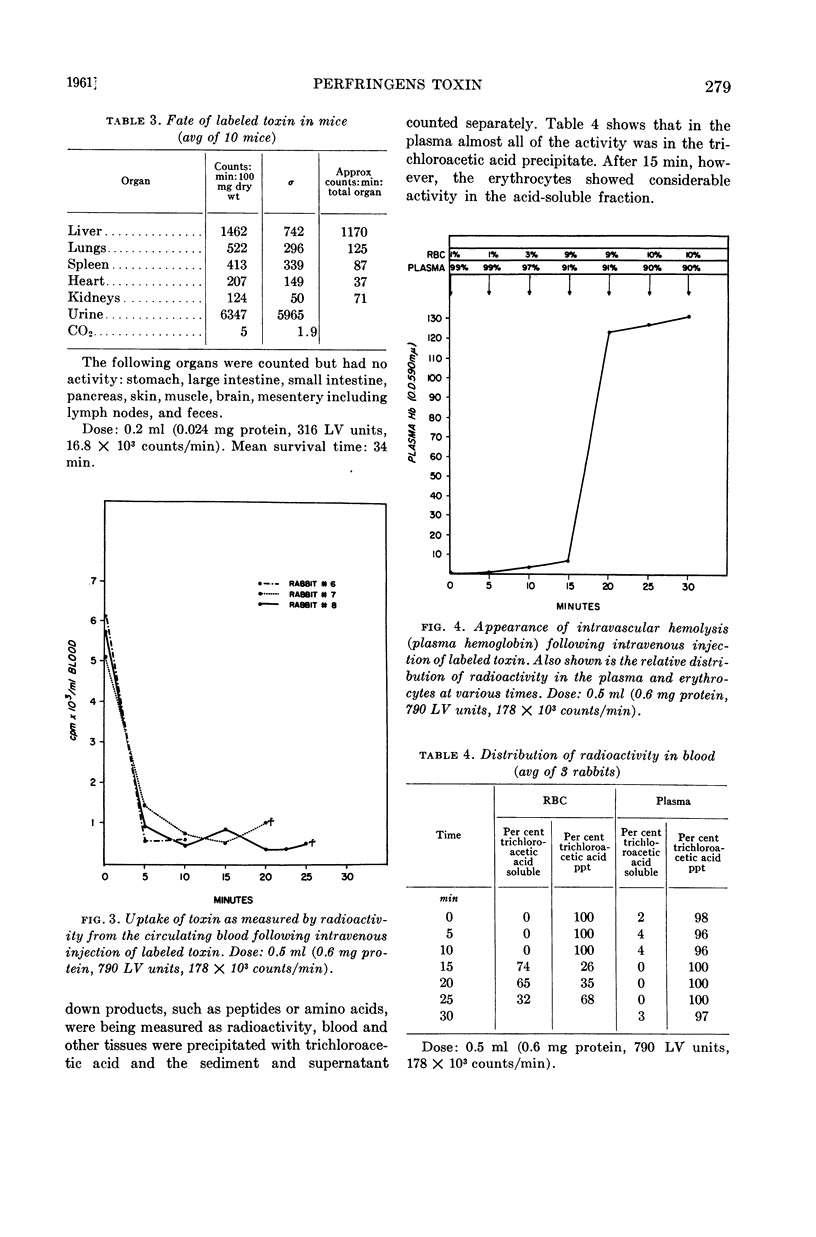
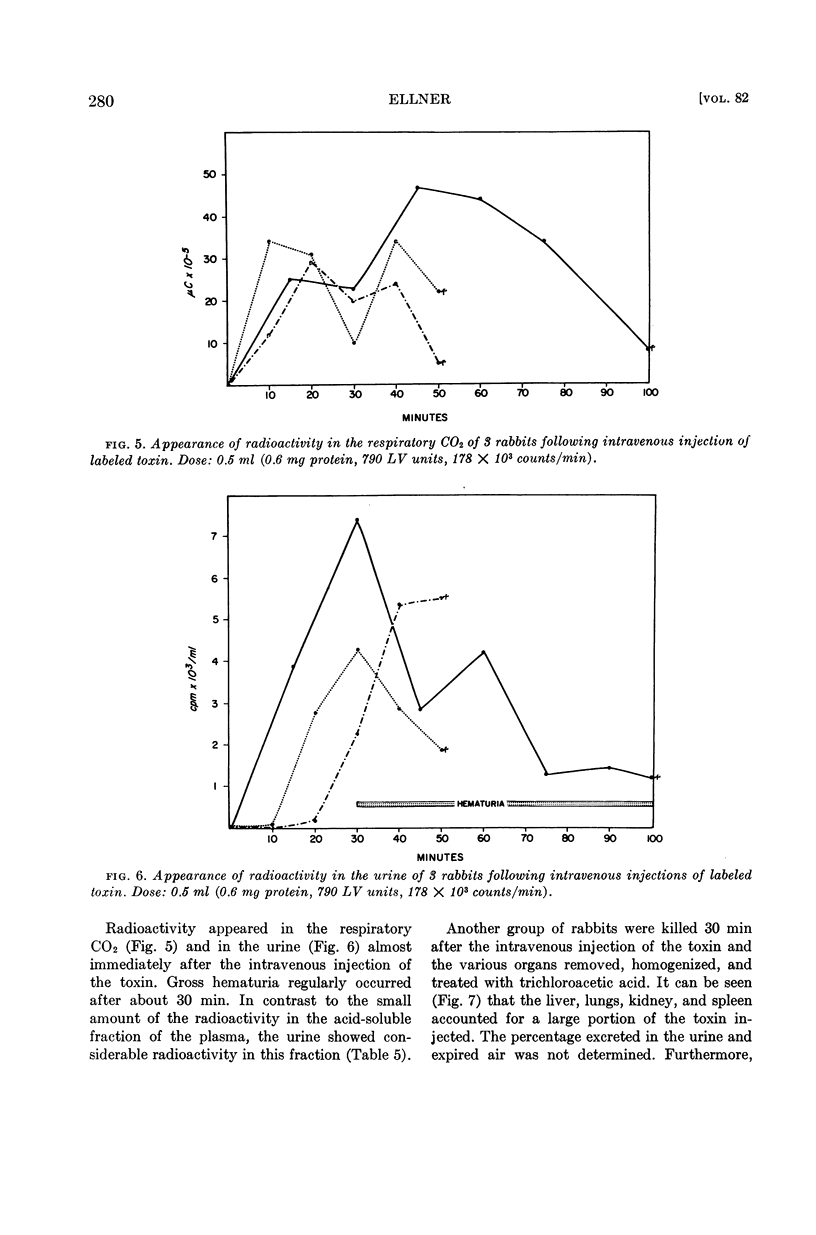
Abstract
Ellner, Paul D. (University of Florida, Gainesville). Fate of partially purified C14-labeled toxin of Clostridium perfringens. J. Bacteriol. 82:275–283. 1961.—A study was made of the fate of Clostridium perfringens toxin in susceptible animals. Labeled toxin was prepared by growing C. perfringens type A in a complex casein hydrolyzate medium containing a tryptic hydrolyzate of C14-labeled algal protein. The toxin was purified about 30-fold (in terms of lecithinase activity) by ammonium sulfate and acetone precipitations. The tagged toxin was injected intravenously into mice and rabbits, and the disappearance from the blood stream, deposition in organs and appearance in urine and expired air determined by measurement of radioactivity. Experimental data showed that toxin disappears rapidly from the bloodstream following intravenous injection, with radioactivity appearing in the urine and expired air shortly thereafter (10 to 20 min). The organs primarily responsible for the uptake of toxin from the blood are the liver (72%), lungs (15%), kidney (8%), and spleen (5%). The toxin is not bound to skeletal muscle. Fractionation of the liver into subcellular particles by centrifugation showed the radioactivity to be concentrated in the mitochondrial fraction. These experiments indicate that the toxin is rapidly removed from the circulating blood, is metabolized, and breakdown products excreted in the urine, with 1 carbon fragments eliminated as CO2 in the expired air.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIKAT B. K., DIBLE J. H. The pathology of Clostridium welchii infection. J Pathol Bacteriol. 1956 Apr;71(2):461–476. doi: 10.1002/path.1700710220. [DOI] [PubMed] [Google Scholar]
- BERG M., LEVINSON S. A. Hyperglycemia and histochemical changes induced in dogs by Clostridium perfringens toxin. AMA Arch Pathol. 1959 Jul;68(1):83–93. [PubMed] [Google Scholar]
- BERG M., LEVINSON S. A., WANG K. J. Effect of experimental shock induced by Clostridium perfringens toxin on the kidneys of dogs. AMA Arch Pathol. 1951 Feb;51(2):137–153. [PubMed] [Google Scholar]
- Bidwell E., Van Heyningen W. E. The biochemistry of the gas gangrene toxins: 5. The kappa-toxin (collagenase) of Clostridium welchii. (with addendum by P. A. Charlwood). Biochem J. 1948;42(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- ELLNER P. D. A medium promoting rapid quantitative sporulation in Clostridium perfringens. J Bacteriol. 1956 Apr;71(4):495–496. doi: 10.1128/jb.71.4.495-496.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner P. D. An Improved Technique for the Growth of Chlorella in CO(2). Plant Physiol. 1959 Nov;34(6):638–640. doi: 10.1104/pp.34.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURR W. E., Jr, BOURDEAU R. V., ROACH H. D., LAUFMAN H. In vivo effects of Clostridium welchii lecithinase. Surg Gynecol Obstet. 1952 Oct;95(4):465–471. [PubMed] [Google Scholar]
- HABERMANN E. Uber die Giftkomponenten des Gasbranderregers Clostridium Welchii Ty. A. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1959;235(6):513–534. [PubMed] [Google Scholar]
- JAYKO L. G., LICHSTEIN H. C. Nutritional factors concerned with growth and lecithinase production by Clostridium perfringens. J Infect Dis. 1959 Mar-Apr;104(2):142–151. doi: 10.1093/infdis/104.2.142. [DOI] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACFARLANE M. G., DATTA N. Observations on the immunological and biochemical properties of liver mitochondria with reference to the action of Clostridium welchii toxin. Br J Exp Pathol. 1954 Apr;35(2):191–202. [PMC free article] [PubMed] [Google Scholar]
- MASOUREDIS S. P. Behavior of intravenously administered I 131 diphtheria toxin in the guinea pig. J Immunol. 1959 Apr;82(4):319–327. [PubMed] [Google Scholar]
- ROTH F. B., PILLEMER L. Purification and some properties of Clostridium welchii type A theta toxin. J Immunol. 1955 Jul;75(1):50–56. [PubMed] [Google Scholar]
- ROTH F. B., PILLEMER L. The separation of alpha toxin (lecithinase) from filtrates of Clostridium welchii. J Immunol. 1953 Jun;70(6):533–537. [PubMed] [Google Scholar]
- Van Heyningen W. E., Bidwell E. The biochemistry of the gas gangrene toxins: 4. The reaction between the alpha-toxin (lecithinase) of Clostridium welchii and its antitoxin. Biochem J. 1948;42(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- WILLIS A. T., HOBBS G. Some new media for the isolation and identification of Clostridia. J Pathol Bacteriol. 1959 Apr;77(2):511–521. doi: 10.1002/path.1700770223. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Nathanson I. T., Aub J. C. PHYSIOLOGIC ACTION OF CLOSTRIDIUM WELCHII (TYPE A) TOXINS IN DOGS. J Clin Invest. 1947 May;26(3):394–403. doi: 10.1172/JCI101821. [DOI] [PMC free article] [PubMed] [Google Scholar]


